Submitted:
23 March 2024
Posted:
25 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
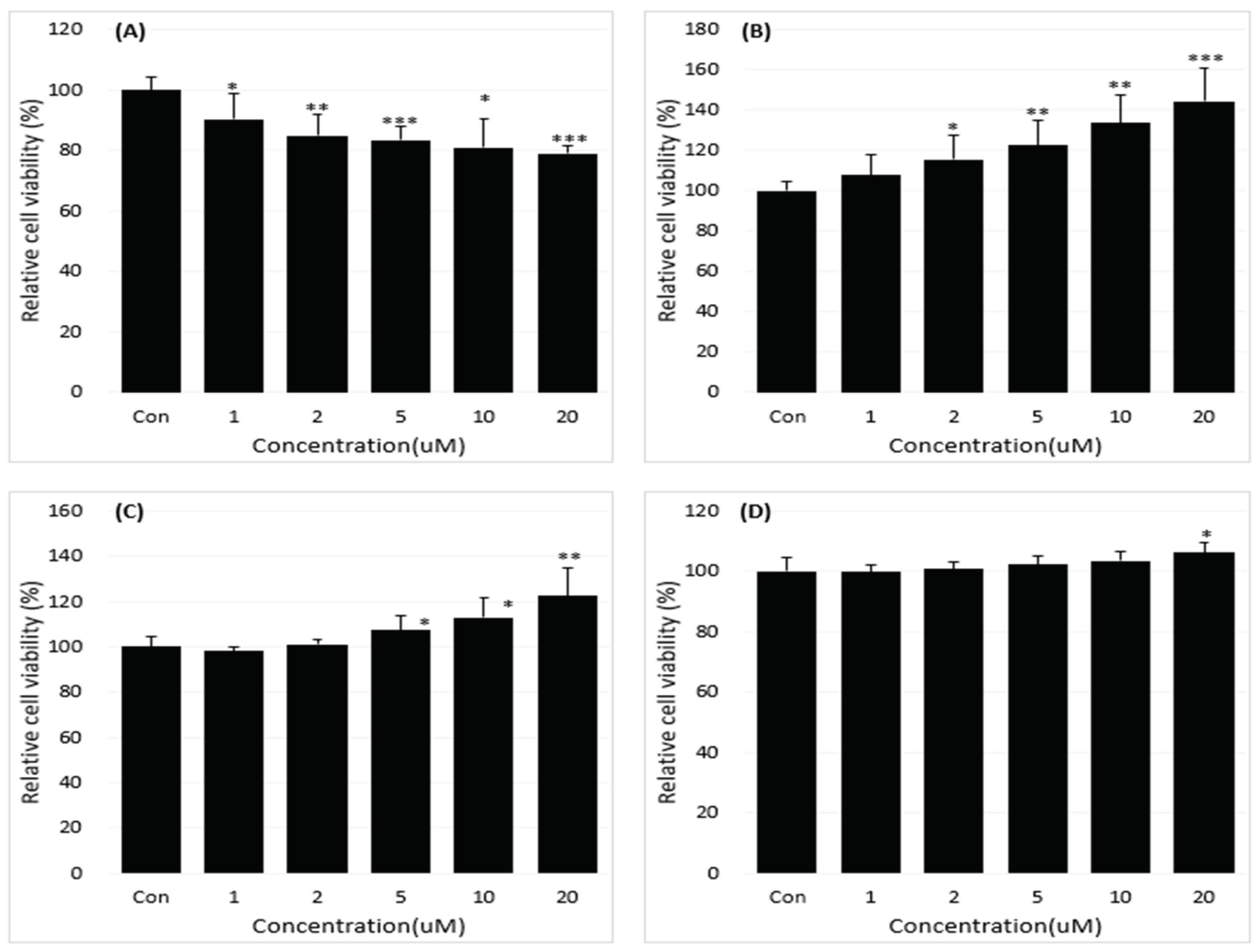
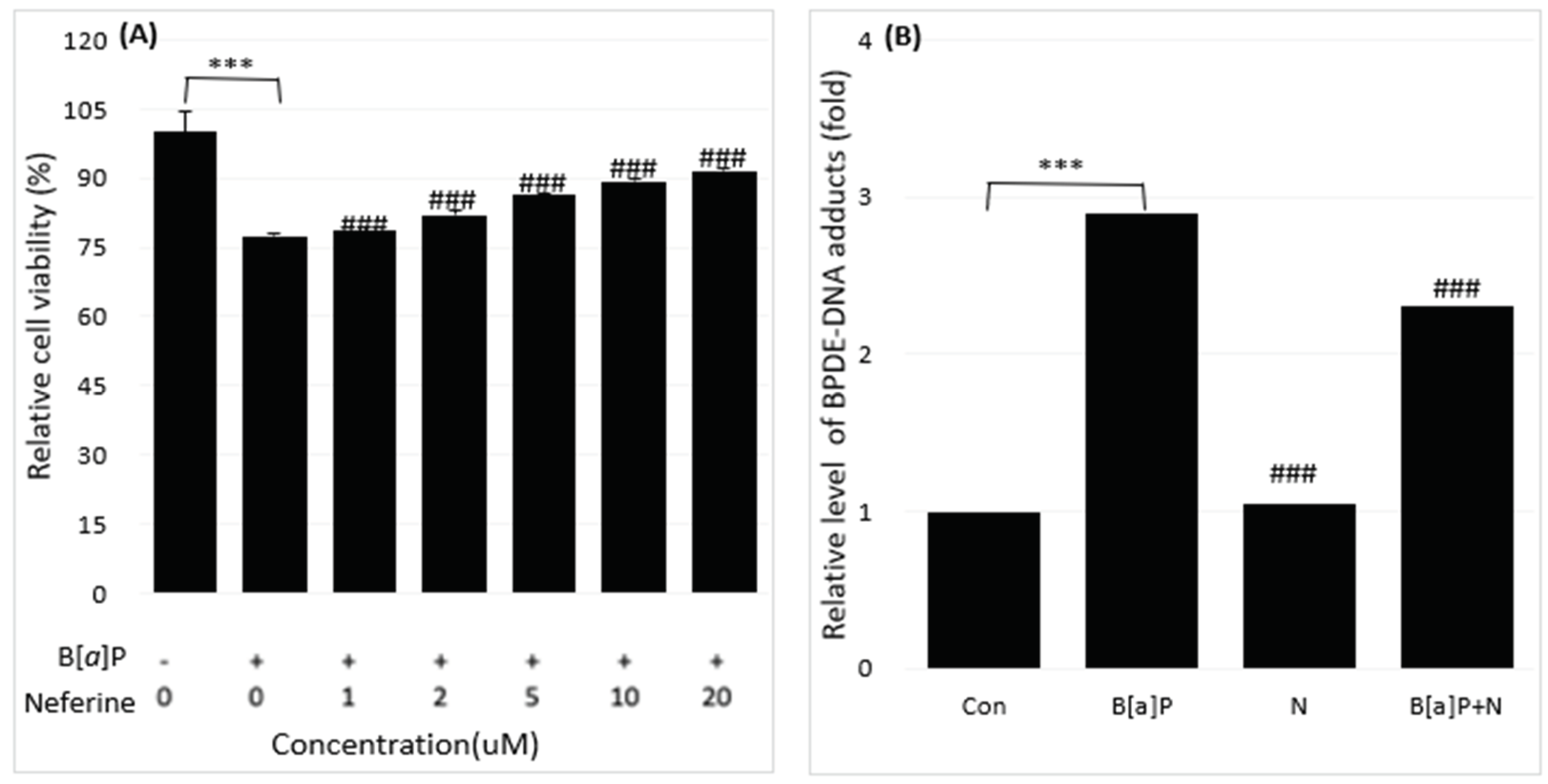
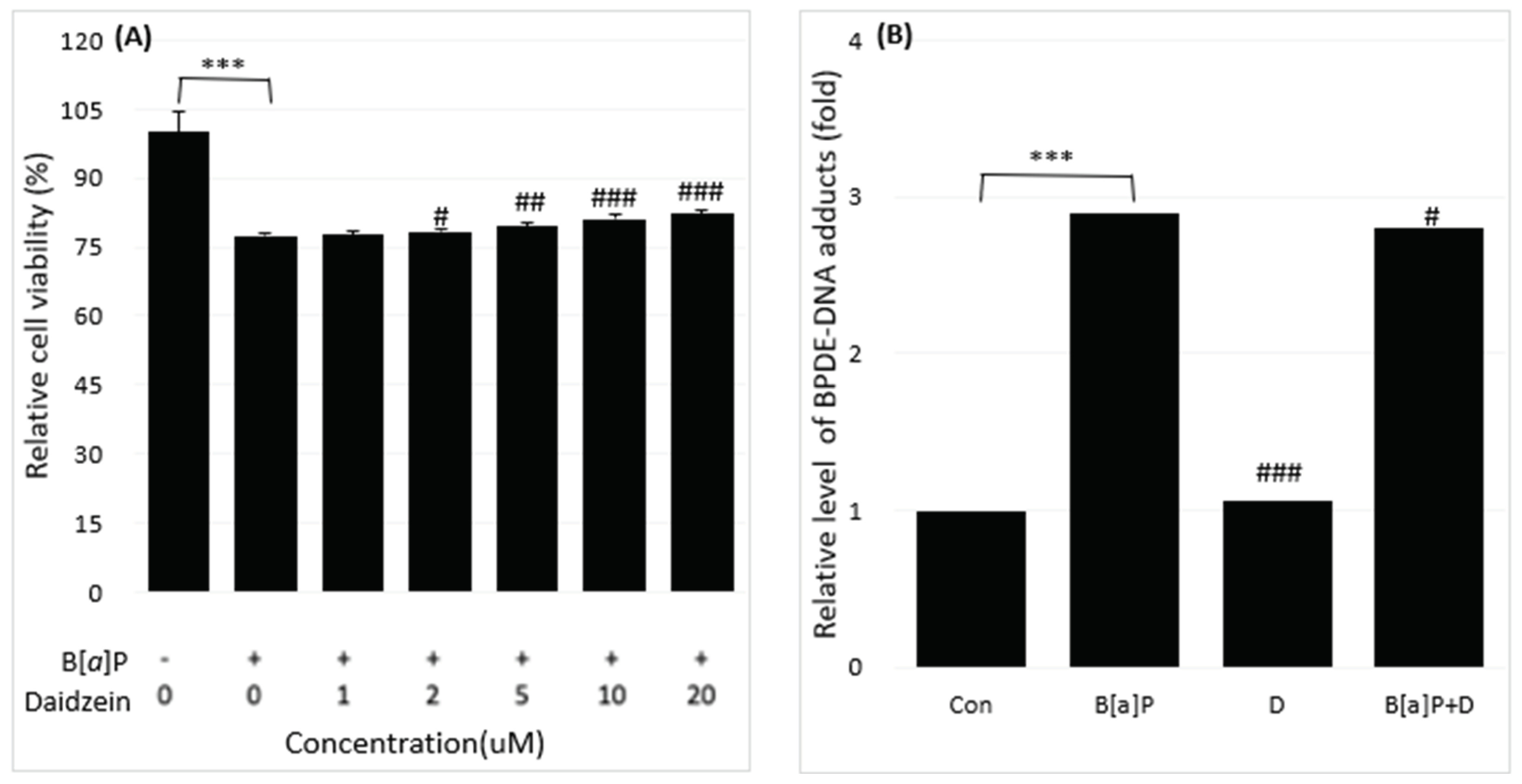
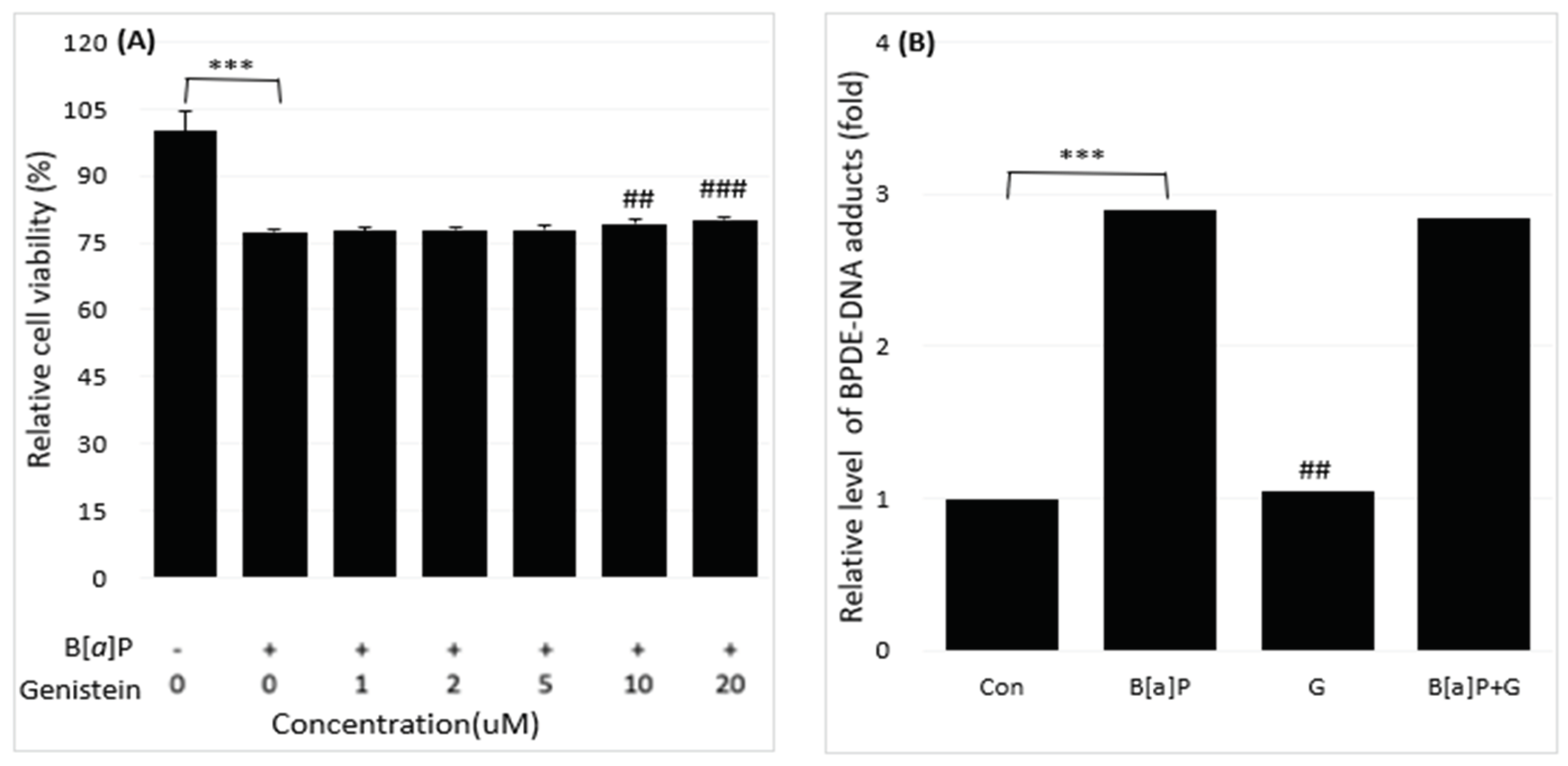
2.1. Reduction of B[a]P-Induced In Vitro Toxicity by Neferine, Daidzein, Genistein
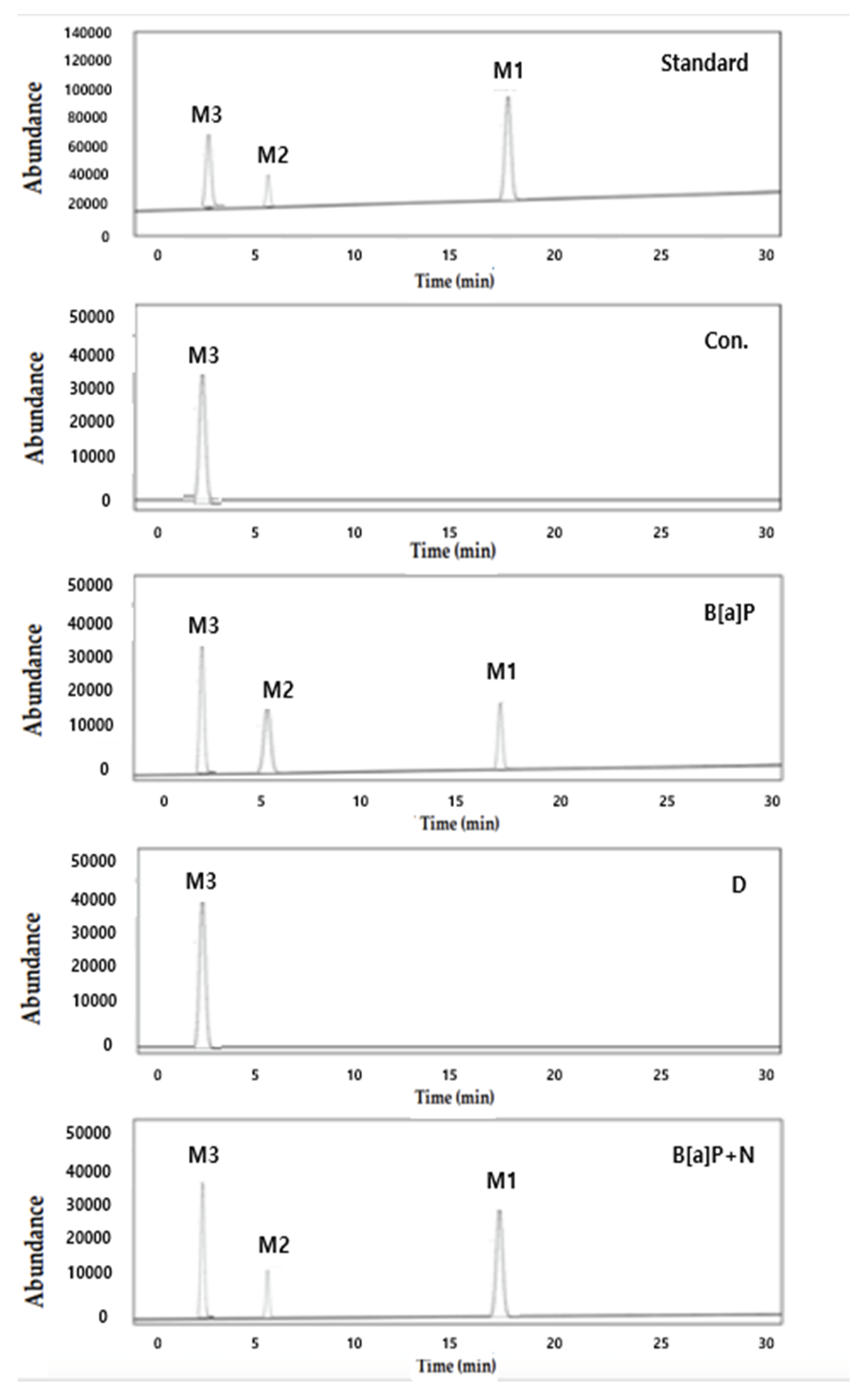
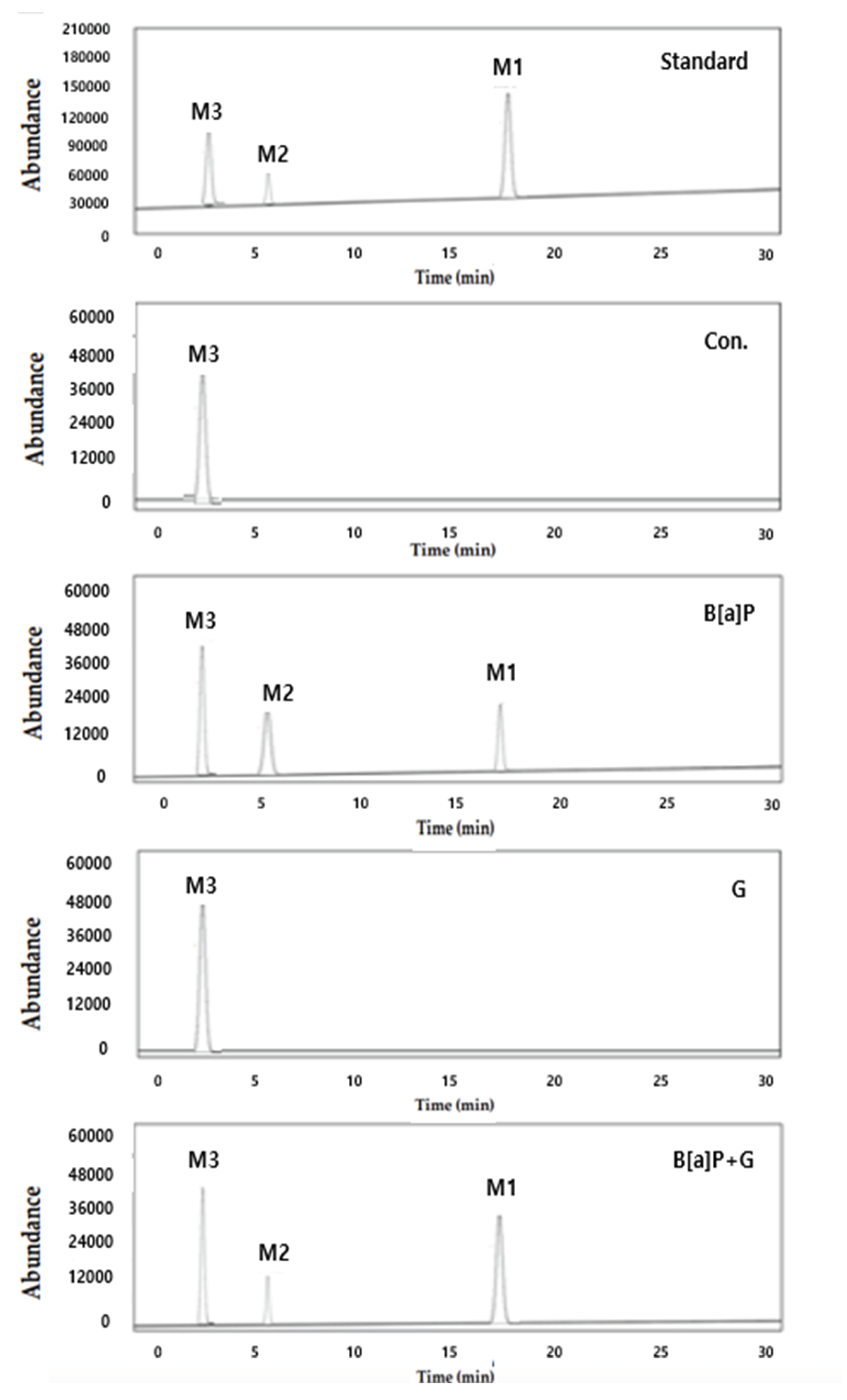
2.2. Reduction of Intracellular B[a]P Metabolites by Neferine, Daidzein, Genistein
2.3. Validation of Analytical Method for Neferine, Daidzein, Genistein Analysis
2.4. Comparison of Neferine, Daidzein and Genistein Concentrations
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Sample Preparations for Isolation of Neferine
3.3. Sample Preparations for Isolation of Daidzein, Genistein
3.4. HepG2 Cells Culture and Treatment
3.5. Cell Viability and Proliferation Assay
3.6. Cell Isolation
3.7. HPLC-UV Analysis for Method Validation
3.8. BPDE-DNA Adduct Formation Analysis
3.9. The Typical Intracellular Metabolites of B[a]P Were Measured by High Performance Liquid Chromatography (HPLC)
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Phillips, D.H.; Venitt, S. DNA and protein adducts in human tissues resulting from exposure to tobacco smoke. Int J Cancer. 2012, 131, 2733–2753. [Google Scholar] [CrossRef] [PubMed]
- Baird, W.M.; Hooven, L.A.; Mahadevan, B. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ Mol Mutagen. 2005, 45, 106–114. [Google Scholar] [CrossRef]
- Saunders, C.R.; Das, S.K.; Ramesh, A.; Shockley, D.C.; Mukherjee, S. Benzo[a]pyrene-induced acute neurotoxicity in the F-344 rat: role of oxidative stress. J Appl Toxicol. 2006, 26, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Jahan, S.A.; Kabir, E.; Brown, R.J. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ Int. 2013, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.; Snawder, J.E.; Chen, I.C.; Slone, J.; Calafat, A.M.; Wang, Y.; Meng, L.; Alexander-Scott, M.; Breitenstein, M.; Johnson, B.; Meadows, J.; Fairfield, E.C. Exposure assessment of polycyclic aromatic hydrocarbons in refined coal tar sealant applications. Int J Hyg Environ Health. 2022, 242, 113971. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Inyang, F.; Hood, D.B.; Archibong, A.E.; Knuckles, M.E.; Nyanda, A.M. Metabolism, bioavailability, and toxicokinetics of benzo(alpha)pyrene in F-344 rats following oral administration. Exp Toxicol Pathol. 2001, 53, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xiang, B.; Jin, Y.; Li, C.; Ren, S.; Wu, Y.; Li, J.; Luo, Q. Hepatotoxic effects of inhalation exposure to polycyclic aromatic hydrocarbons on lipid metabolism of C57BL/6 mice. Environ Int. 2020, 134, 105000. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H. Reactive oxygen and mechanisms of inflammatory liver injury. J Gastroenterol Hepatol. 2000, 15, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.P.; Ramos, K.S. Impact of cellular metabolism on the biological effects of benzo[a]pyrene and related hydrocarbons. Drug Metab Rev. 2001, 33, 1–35. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, X.; Chang, Q.; Xu, J.; Huang, Y.; Guo, Q. Neferine, a bisbenzylisoquinline alkaloid attenuates bleomycin-induced pulmonary fibrosis. Eur. J. Pharmacol. 2010, 627, 304–312. [Google Scholar] [CrossRef]
- Mahbubur Rahman AHM. Traditional medicinal plants used in the treatment of different skin diseases of santals at abdullahpur village under akkelpur upazilla of joypurhat district, Bangladesh. Biomed. and Biotechnol. 2013, 1, 17–20. [Google Scholar]
- Chen, Y.; Fan, G.R.; Wu, H.L.; Wu, Y.T.; Mitchell, A. Separation, identification and rapid determination of liensine, isoliensinine and neferine from embryo of the seed of Nelumbo nucifera GAERTN by liquid chromatography coupled to diode array detector and tandem mass spectrometry. J. Pharm. Biomed. Anal. 2007, 43, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Poornima, P.; Weng, C.F.; Padma, V.V. Neferine, an alkaloid from lotus seed embryo, inhibits human lung cancer cell growth by MAPK activation and cell cycle arrest. Biofactors. 2014, 40, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Liggins, J.; Mulligan, A.; Runswick, S.; Bingham, S.A. Daidzein and genistein content of cereals. Eur. J. Clin Nutr. 2002, 56, 961–966. [Google Scholar] [CrossRef]
- Sirtori, C.R. Risks and benefits of soy phytoestrogens in cardiovascular diseases, cancer, climacteric symptoms and osteoporosis. Drug. Saf. 2001, 24, 665–682. [Google Scholar] [CrossRef] [PubMed]
- Adjakly, M.; Ngollo, M.; Boiteux, J.P.; Bignon, Y.J.; Guy, L.; Gallon, D.B. Genistein and daidzein: different molecular effects on prostate cancer. Anticancer Res. 2013, 33, 39–44. [Google Scholar] [PubMed]
- Sarao, L.; Kaur, S.; Malik, T.; Singh, A. Chapter 19 – Genistein and daidzein. Nutraceuticals and Health Care. 2022, 331–341. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Ji, X.; Li, Y.; He, J.; Shah, W.; Xue, X.; Feng, G.; Zhang, H.; Gao, M. Depletion of mitochondrial enzyme system in liver, lung, brain, stomach and kidney induced by benzo[a]pyrene. Environ Toxicol Pharmacol. 2016, 43, 83–93. [Google Scholar] [CrossRef]
- Jin, Y.; Miao, W.; Lin, X.; Wu, T.; Shen, H.; Chen, S.; Li, Y.; Pan, Q.; Fu, Z. Sub-chronically exposing mice to a polycyclic aromatic hydrocarbon increases lipid accumulation in their livers. Environ Toxicol Pharmacol. 2014, 38, 353–363. [Google Scholar] [CrossRef]
- Mumtaz, M.M.; George, J.D.; Gold, K.W.; Cibulas, W.; DeRosa, C.T. ATSDR evaluation of health effects of chemicals. IV. Polycyclic aromatic hydrocarbons (PAHs): understanding a complex problem. Toxicol Ind Health. 1996, 12, 742–971. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Han, H.; Yang, Y.; Jin, Y.; Wang, X.; Liu, X. Neferine prevented hyperglycemia-induced endothelial cell apoptosis through suppressing ROS/Akt/NF-κB signal. Endocrine. 2014, 47, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, W.; Chen, Y.; Zhou, Q.; Xiao, P.; Tang, R.; Xue, J. Neferine attenuates acute kidney injury by inhibiting NF-κB signaling and upregulating Klotho expression. Frontiers in Pharmacology. 2019, 10, 1197. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.K.; Li, W.Q.; Xia, S.; Guo, L.; Miao, Y.; Zhang, B.K. Metabolic activation of the toxic natural products from herbal and dietary supplements leading to toxicities. Frontiers in pharmacology. 2021, 12, 758468. [Google Scholar] [CrossRef] [PubMed]
- Eid, W.; Abdel-Rehim, W. Neferine enhances the antitumor effect of mitomycin-C in hela cells through the activation of p38-MAPK pathway. J. Cell. Biochem. 2017, 118, 3472–3479. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Wang, R.; Li, S.; Yuan, Y. Neferine exerts antioxidant and anti-inflammatory effects on carbon tetrachloride-induced liver fibrosis by inhibiting the MAPK and NF-κB/IκBα pathways. Evid. Based Complementary Altern. Med. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Guolan, D.; Lingli, W.; Wenyi, H.; Wei, Z.; Baowei, C.; Sen, B. Anti-inflammatory effects of neferine on LPS-induced human endothelium via MAPK, and NF-κβ pathways. Die Pharmazie. 2018, 73, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Poornima, P.; Weng, C.F.; Padma, V.V. Neferine from Nelumbo nucifera induces autophagy through the inhibition of PI3K/Akt/mTOR pathway and ROS hyper generation in A549 cells. Food chem. 2013, 141, 3598–3605. [Google Scholar] [CrossRef]
- Jee, S.C.; Kim, M.; Sung, J.S. Modulatory effects of silymarin on benzo[a]pyrene-induced hepatotoxicity. Int. J. Mol. Sci. 2020, 21, 2369–2384. [Google Scholar] [CrossRef]
- Kim, M.; Jee, S.C.; Kim, K.S.; Kim, H.S.; Yu, K.N.; Sung, J.S. Quercetin and isorhamnetin attenuate benzo[a]pyrene-induced toxicity by modulating detoxification enzymes through the AhR and NRF2 signaling pathways. Antioxidants. 2021, 10, 787–801. [Google Scholar] [CrossRef]
- Jahan, N.; Chowdhury, A.; Li, T.; Xu, K.; Wei, F.; Wang, S. Neferine improves oxidative stress and apoptosis in benign prostate hyperplasia via Nrf2-ARE pathway. Redox Rep. 2021, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Jin, S.E.; Choi, R.J.; Kim, D.H.; Kim, Y.S.; Ryu, J.H.; Choi, J.S. Anti-amnesic activity of neferine with antioxidant and anti-inflammatory capacities, as well as inhibition of ChEs and BACE1. Life Sciences. 2010, 87, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Asokan, S.M.; Mariappan, R.; Muthusamy, S.; Velmurugan, B.K. Pharmacological benefits of neferine-A comprehensive review. Life Sciences. 2018, 199, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chou, H.; Li, L.; Li, H.; Cui, Z. Wound healing activity of neferine in experimental diabetic rats through the inhibition of inflammatory cytokines and nrf-2 pathway. Artif Cells Nanomed Biotehcnol. 2020, 48, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Bai, H.; Shu, M.; Chen, M.; Khan, A.; Bai, Z. Antioxidative and antiphotoaging activities of neferine upon UV-A irradiation in human dermal fibroblasts. Biosci. 2018, 38, BSR20181414. [Google Scholar] [CrossRef] [PubMed]
- Somasundram, B.; Manogaran, P.; Vasudevan, M.; Viswanadha, V.P. Chemosensitizing effect of neferine on cisplatin-resistant colorectal cancer: Identification of potential candidate genes and pathways through whole transcriptome profiling. Phytomedicine Plus. 2022, 2, 100299. [Google Scholar] [CrossRef]
- Borradaile, N.M.; Dreu, L.E.; Wilcox, L.J.; Edwards, J.Y.; Huff, M.W. Soya phytoestrogens, genistein and daidzein, decrease apolipoprotein B secretion from HepG2 cells through multiple mechanisms. Biochem J. 2002, 366, 531–539. [Google Scholar] [CrossRef]
- Lu, R.; Zheng, Z.; Yin, Y.; Jiang, Z. Effect of genistein on cholesterol metabolism-related genes in HepG2 cell. J. Food Sci. 2019, 84, 2330–2336. [Google Scholar] [CrossRef]
- Nan, G.; Gao, Y.; Guo, L.; Meng, X.; Yang, G. Solid-liquid extraction of daidzein and genistein from soybean: Kinetic modeling of influential factors. Prep. Biochem. Biotechnol. 2018, 48, 946–953. [Google Scholar] [CrossRef]
- Maštovská, K.; Lehotay, S.J. Evaluation of common organic solvents for gas chromatographic analysis and stability of multiclass pesticide residues. J. Chromatogr. A. 2004, 1040, 259–272. [Google Scholar] [CrossRef]
- Ryu, G.H.; Weon, J.B.; Yang, W.S.; Ma, C.J. Simultaneous determination of four compounds in a Nelumbo nucifera seed embryo by HPLC-DAD. J. Spectrosc. 2017, 1–6. [Google Scholar] [CrossRef]
- Arau´jo, J.M.A.; Silva, M.V.; Chaves, J.B.P. Supercritical fluid extraction of daidzein and genistein isoflavones from soybean hypocotyl after hydrolysis with endogenous ß-glucosidases. Food Chem. 2007, 105, 266–272. [Google Scholar] [CrossRef]

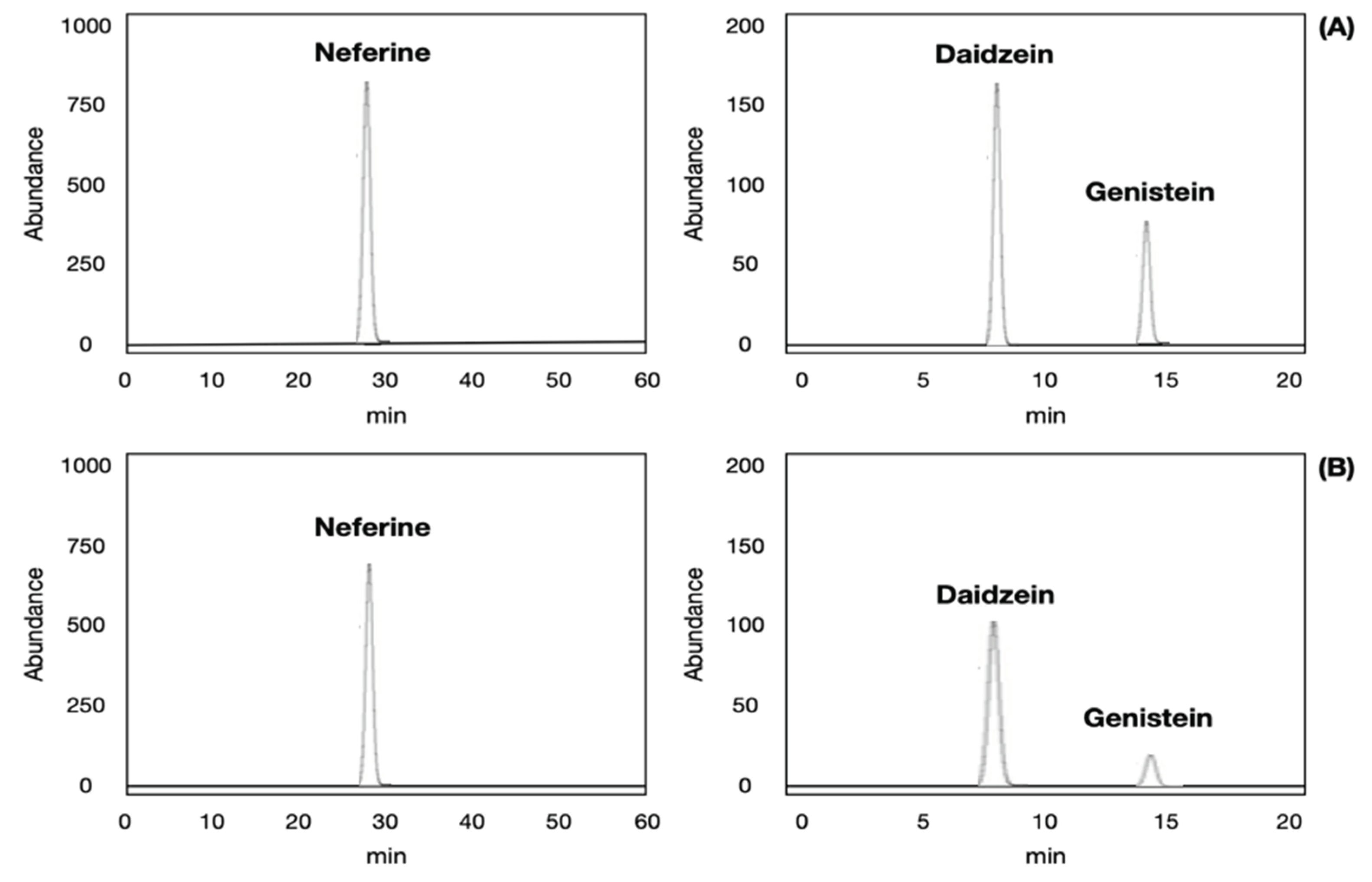
| Matrix type | Neferine, daidzein and genistein matrices | ||||
|---|---|---|---|---|---|
| LOD (μg/kg)1) |
LOQ (μg/kg)2) |
Linearity3) | Calibration equation | ||
| Lotus matrix (n = 3) |
Neferine | 0.12 | 0.36 | R2=0.9967 | y=0.0021x+0.007 |
| Soybean matrix (n = 3) |
Daidzein | 0.08 | 0.24 | R2=0.9958 | y=0.0012x-0.008 |
| Genistein | 0.09 | 0.27 | R2=0.9942 | y=0.0015x-0.005 | |
| Matrix type | Conc. | 3 (μg/kg) | 10 (μg/kg) | 40 (μg/kg) |
|---|---|---|---|---|
| Lotus matrix (n = 3) |
Neferine | 82.95±0.67 | 102.75±0.56 | 99.54±0.32 |
| Soybean matrix (n = 3) |
Daidzein | 93.02±2.45 | 111.46±5.67 | 86.72±0.11 |
| Genistein | 96.75±3.64 | 107.65±8.65 | 104.77±0.96 |
| Matrix type | Conc. | Intraday (n=3) | Interday (n=3) |
|---|---|---|---|
| Precision (%)1) | Precision (%) | ||
| Lotus matrix (n = 3) |
Neferine | 0.24 | 0.65 |
| Soybean matrix (n = 3) |
Daidzein | 1.26 | 3.42 |
| Genistein | 6.82 | 4.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





