Submitted:
16 September 2023
Posted:
18 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
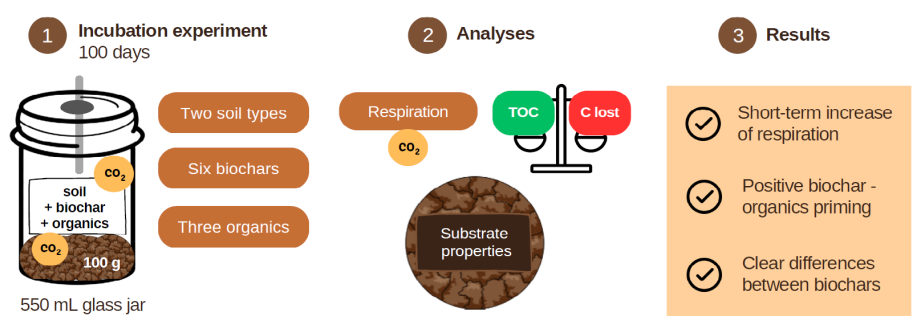
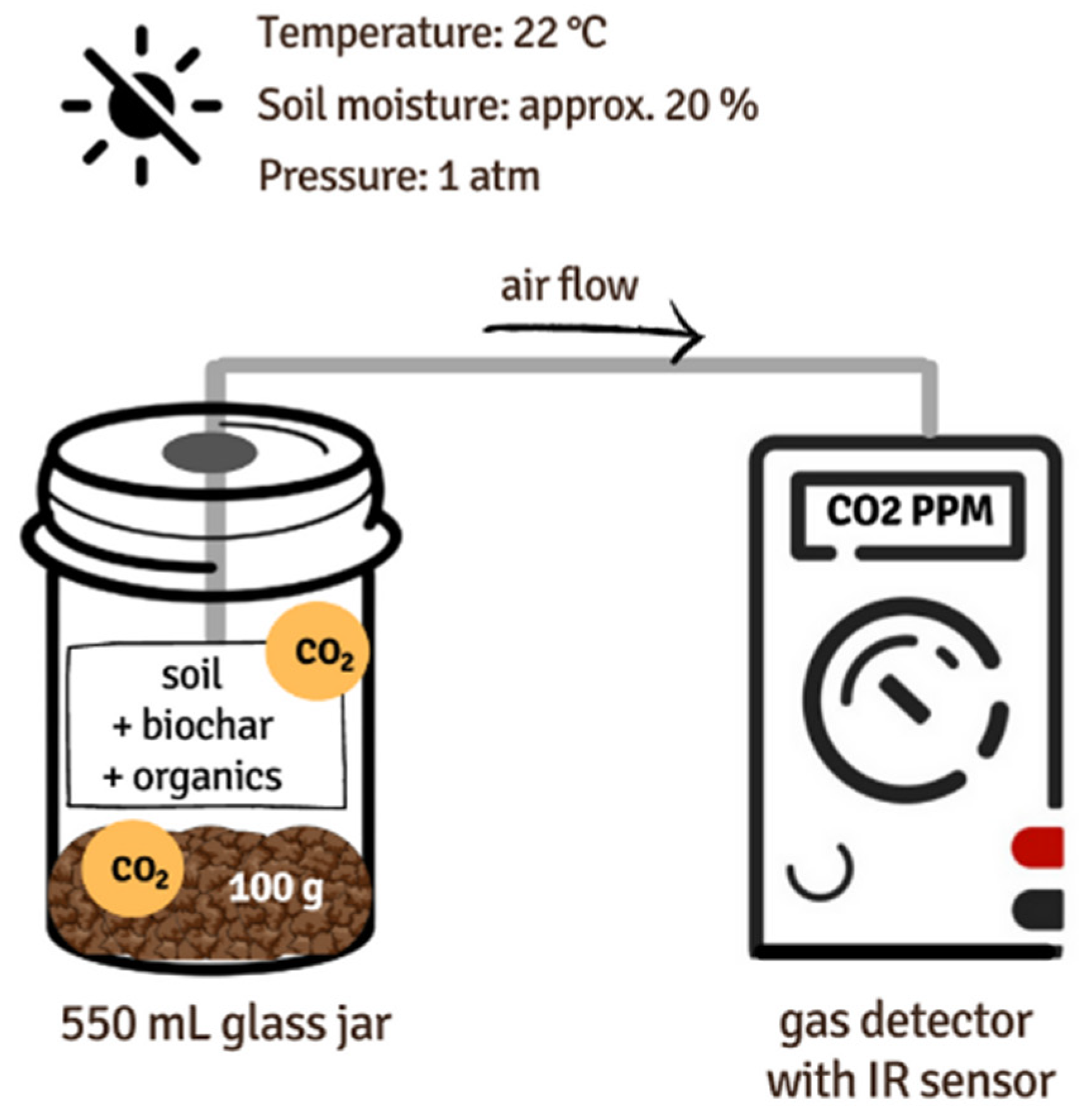
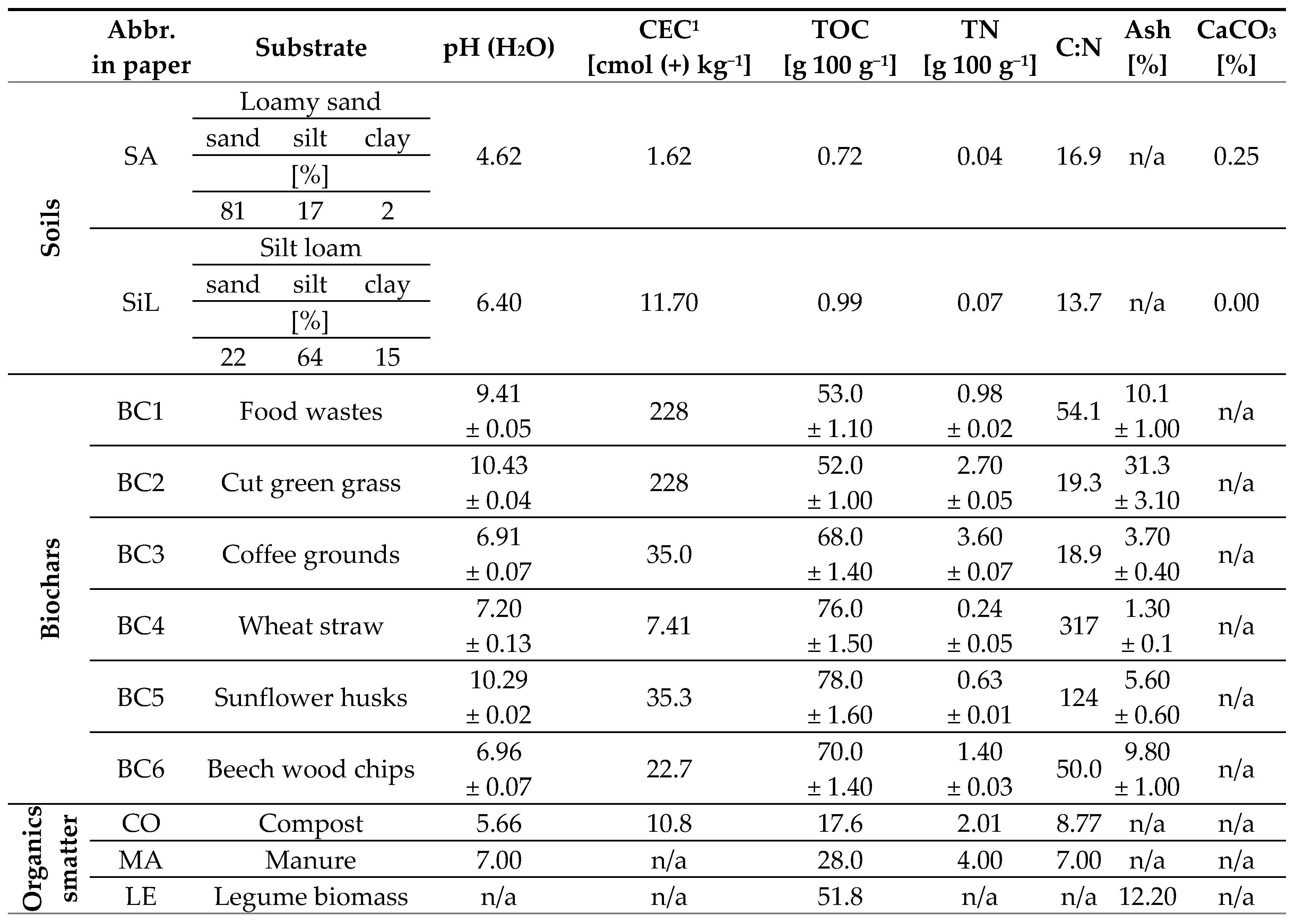
2.1. Incubation experiment setup
2.2. Analysis of substrates
2.3. Respiration measurements
2.4. Carbon loss estimation
3. Results
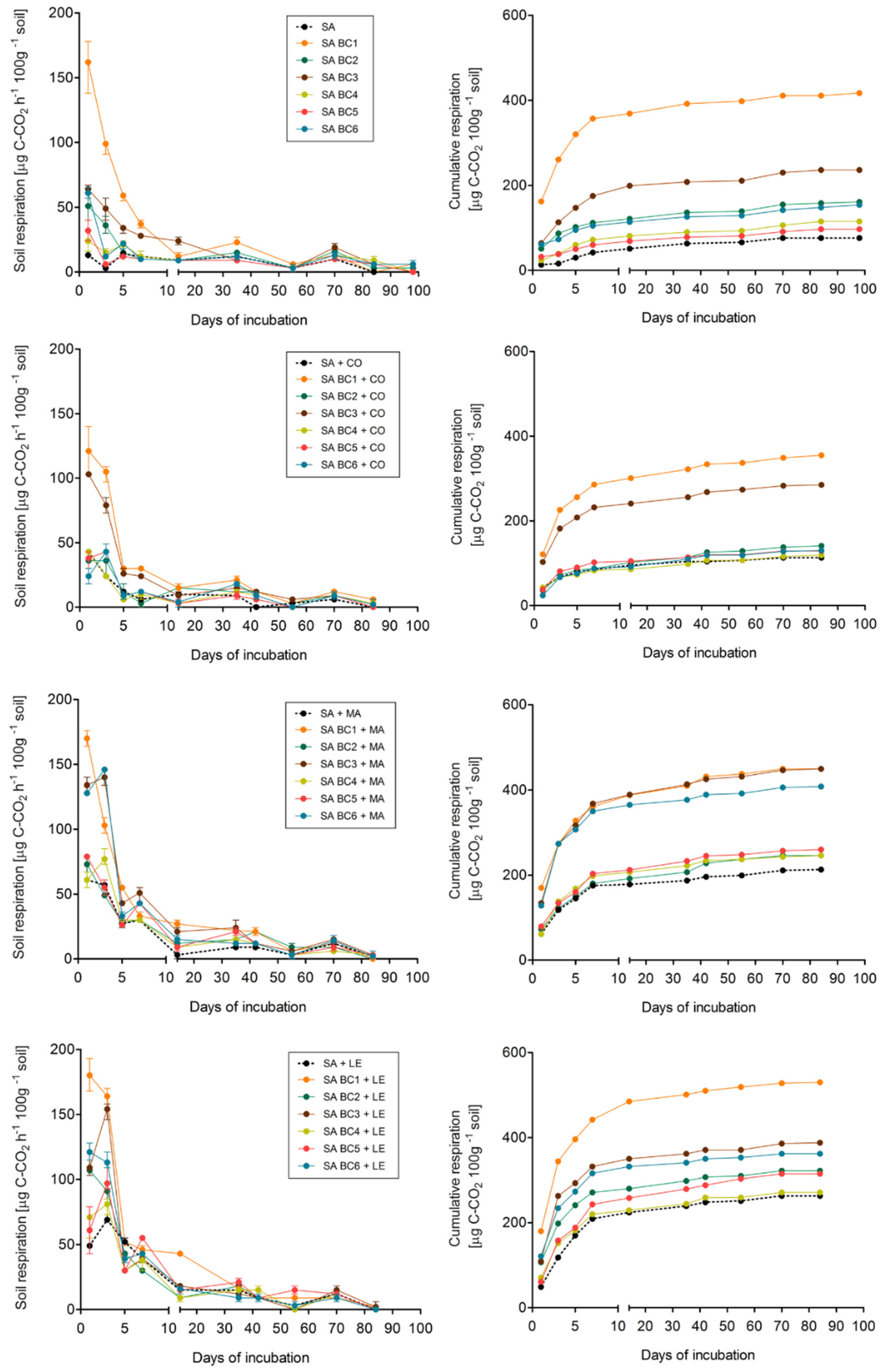
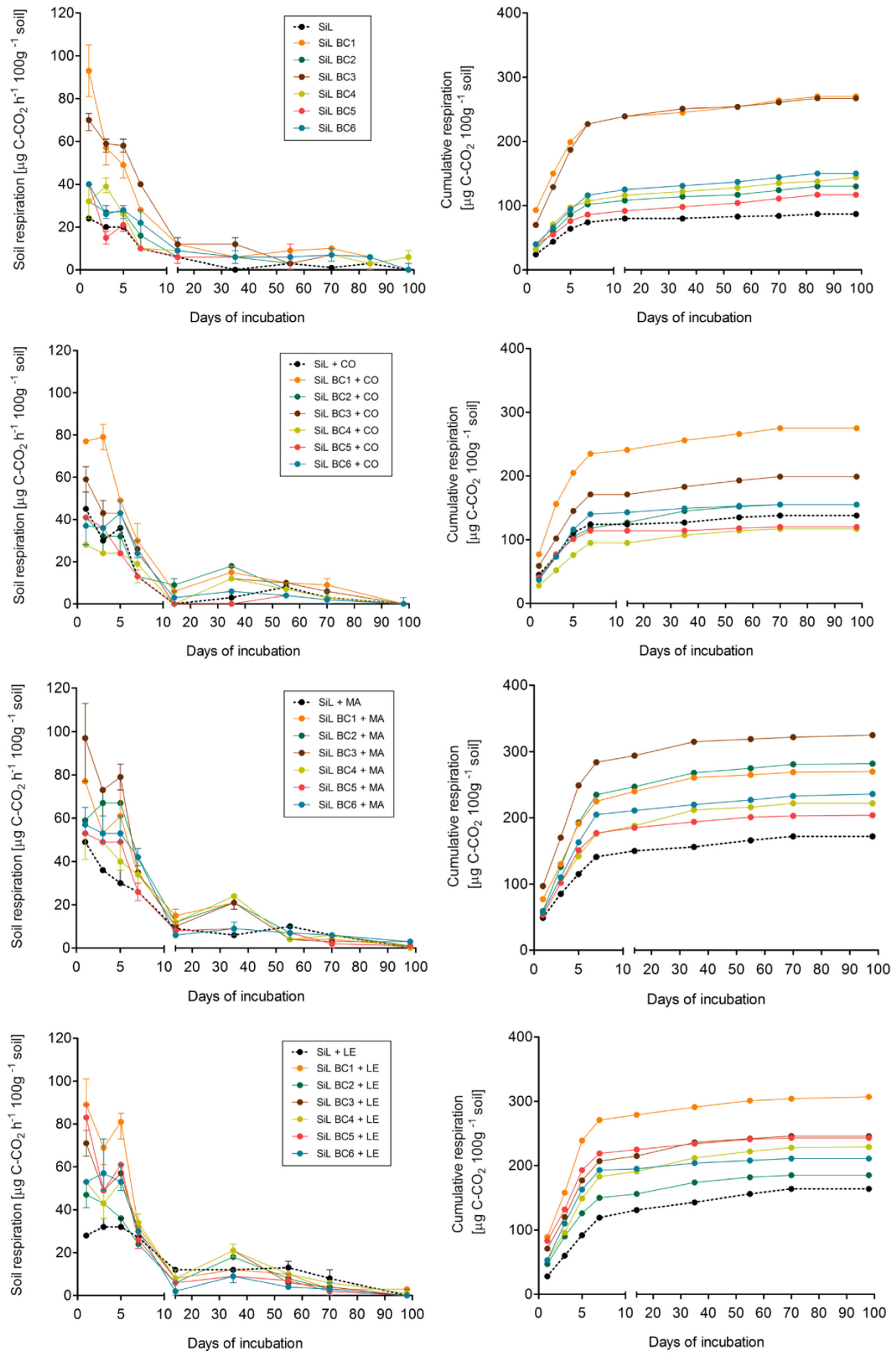
3.1. Effect of soil and biochar type on respiration
3.2. Effect of exogenous organic matter on soil respiration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Budai, A.; Rasse, D.P.; Lagomarsino, A.; Lerch, T.Z.; Paruch, L. Biochar Persistence, Priming and Microbial Responses to Pyrolysis Temperature Series. Biol Fertil Soils 2016, 52, 749–761. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, L.; Cheng, H.; Yue, S.; Li, S. Effects of Biochar Application on CO2 Emissions from a Cultivated Soil under Semiarid Climate Conditions in Northwest China. Sustainability 2017, 9, 1482. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The Role of Biochar and Biochar-Compost in Improving Soil Quality and Crop Performance: A Review. Applied Soil Ecology 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Latawiec, A.; Królczyk, J.; Bogacz, A.; Kawałko, D.; Bednik, M.; Dudek, M. Biochar Improves Maize Growth but Has a Limited Effect on Soil Properties: Evidence from a Three-Year Field Experiment. Sustainability 2021, 13, 3617. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A Review of Biochar and Its Use and Function in Soil. In Advances in Agronomy; Elsevier, 2010; Vol. 105, pp. 47–82. ISBN 978-0-12-381023-6. [Google Scholar]
- Li, S.; Chan, C.Y. Will Biochar Suppress or Stimulate Greenhouse Gas Emissions in Agricultural Fields? Unveiling the Dice Game through Data Syntheses. Soil Systems 2022, 6, 73. [Google Scholar] [CrossRef]
- Saarnio, S.; Heimonen, K.; Kettunen, R. Biochar Addition Indirectly Affects N2O Emissions via Soil Moisture and Plant N Uptake. Soil Biology and Biochemistry 2013, 58, 99–106. [Google Scholar] [CrossRef]
- Tang, Y.; Gao, W.; Cai, K.; Chen, Y.; Li, C.; Lee, X.; Cheng, H.; Zhang, Q.; Cheng, J. Effects of Biochar Amendment on Soil Carbon Dioxide Emission and Carbon Budget in the Karst Region of Southwest China. Geoderma 2021, 385, 114895. [Google Scholar] [CrossRef]
- Sarfaraz, Q.; Silva, L.S. da; Drescher, G.L.; Zafar, M.; Severo, F.F.; Kokkonen, A.; Dal Molin, G.; Shafi, M.I.; Shafique, Q.; Solaiman, Z.M. Characterization and Carbon Mineralization of Biochars Produced from Different Animal Manures and Plant Residues. Sci Rep 2020, 10, 955. [Google Scholar] [CrossRef]
- Bednik, M.; Medyńska-Juraszek, A.; Ćwieląg-Piasecka, I. Effect of Six Different Feedstocks on Biochar’s Properties and Expected Stability. Agronomy 2022, 12, 1525. [Google Scholar] [CrossRef]
- Mandal, S.; Sarkar, B.; Bolan, N.; Novak, J.; Ok, Y.S.; Van Zwieten, L.; Singh, B.P.; Kirkham, M.B.; Choppala, G.; Spokas, K.; et al. Designing Advanced Biochar Products for Maximizing Greenhouse Gas Mitigation Potential. Critical Reviews in Environmental Science and Technology 2016, 46, 1367–1401. [Google Scholar] [CrossRef]
- Zheng, N.; Yu, Y.; Li, Y.; Ge, C.; Chapman, S.J.; Yao, H. Can Aged Biochar Offset Soil Greenhouse Gas Emissions from Crop Residue Amendments in Saline and Non-Saline Soils under Laboratory Conditions? Science of The Total Environment 2022, 806, 151256. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, X.; Jiang, L.; Li, M.; Du, Z.; Zhou, G.; Shao, J.; Wang, X.; Xu, Z.; Hosseini Bai, S.; et al. Effects of Biochar Application on Soil Greenhouse Gas Fluxes: A Meta-Analysis. GCB Bioenergy 2017, 9, 743–755. [Google Scholar] [CrossRef]
- Cross, A.; Sohi, S.P. The Priming Potential of Biochar Products in Relation to Labile Carbon Contents and Soil Organic Matter Status. Soil Biology and Biochemistry 2011, 43, 2127–2134. [Google Scholar] [CrossRef]
- Lu, W.; Zha, Q.; Zhang, H.; Chen, H.Y.H.; Yu, J.; Tu, F.; Ruan, H. Changes in Soil Microbial Communities and Priming Effects Induced by Rice Straw Pyrogenic Organic Matter Produced at Two Temperatures. Geoderma 2021, 400, 115217. [Google Scholar] [CrossRef]
- Case, S.D.C.; McNamara, N.P.; Reay, D.S.; Whitaker, J. Can Biochar Reduce Soil Greenhouse Gas Emissions from a Miscanthus Bioenergy Crop? GCB Bioenergy 2014, 6, 76–89. [Google Scholar] [CrossRef]
- Rasul, M.; Cho, J.; Shin, H.-S.; Hur, J. Biochar-Induced Priming Effects in Soil via Modifying the Status of Soil Organic Matter and Microflora: A Review. Science of The Total Environment 2022, 805, 150304. [Google Scholar] [CrossRef]
- Bruun, S.; Clauson-Kaas, S.; Bobuľská, L.; Thomsen, I.K. Carbon Dioxide Emissions from Biochar in Soil: Role of Clay, Microorganisms and Carbonates: CO 2 Emissions from Biochar in Soil. Eur J Soil Sci 2014, 65, 52–59. [Google Scholar] [CrossRef]
- Fang, Y.; Singh, B.; Singh, B.P. Effect of Temperature on Biochar Priming Effects and Its Stability in Soils. Soil Biology and Biochemistry 2015, 80, 136–145. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, J.; Zhang, D.; Cheng, K.; Zhou, H.; Zhang, A.; Li, L.; Joseph, S.; Smith, P.; Crowley, D.; et al. Biochar Has No Effect on Soil Respiration across Chinese Agricultural Soils. Science of The Total Environment 2016, 554–555, 259–265. [Google Scholar] [CrossRef]
- Ding, X.; Li, G.; Zhao, X.; Lin, Q.; Wang, X. Biochar Application Significantly Increases Soil Organic Carbon under Conservation Tillage: An 11-Year Field Experiment. Biochar 2023, 5, 28. [Google Scholar] [CrossRef]
- Shakoor, A.; Arif, M.S.; Shahzad, S.M.; Farooq, T.H.; Ashraf, F.; Altaf, M.M.; Ahmed, W.; Tufail, M.A.; Ashraf, M. Does Biochar Accelerate the Mitigation of Greenhouse Gaseous Emissions from Agricultural Soil? - A Global Meta-Analysis. Environmental Research 2021, 202, 111789. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, S.; Zhang, T.; Zhao, X.; Chen, S.; Wang, Q. Priming of Soil Organic Carbon Decomposition Induced by Exogenous Organic Carbon Input: A Meta-Analysis. Plant Soil 2019, 443, 463–471. [Google Scholar] [CrossRef]
- Stegenta-Dąbrowska, S.; Sobieraj, K.; Koziel, J.A.; Bieniek, J.; Białowiec, A. Kinetics of Biotic and Abiotic CO Production during the Initial Phase of Biowaste Composting. Energies 2020, 13, 5451. [Google Scholar] [CrossRef]
- Kane, S.; Ryan, C. Biochar from Food Waste as a Sustainable Replacement for Carbon Black in Upcycled or Compostable Composites. Composites Part C: Open Access 2022, 8, 100274. [Google Scholar] [CrossRef]
- EBC, 2012-2022. European Biochar Certificate - Guidelines for a Sustainable Production of Biochar.' Carbon Standards International (CSI), Frick, Switzerland. (http://european-biochar.org). Version 10.2 from 8th Dec 2022 (acessed 10 March 2023).
- Dudek, M.; Łabaz, B.; Bednik, M.; Medyńska-Juraszek, A. Humic Substances as Indicator of Degradation Rate of Chernozems in South-Eastern Poland. Agronomy 2022, 12, 733. [Google Scholar] [CrossRef]
- Łabaz, B.; Kabała, C.; Dudek, M.; Waroszewski, J. Morphological Diversity of Chernozemic Soils in South-Western Poland. Soil Science Annual 2019, 70, 211–224. [Google Scholar] [CrossRef]
- Munera-Echeverri, J.L.; Martinsen, V.; Strand, L.T.; Zivanovic, V.; Cornelissen, G.; Mulder, J. Cation Exchange Capacity of Biochar: An Urgent Method Modification. Science of The Total Environment 2018, 642, 190–197. [Google Scholar] [CrossRef]
- Fierer, N. 2013. Measuring Soil C Mineralization Rates. Laboratory protocol. https://labs.eemb.ucsb.edu/schimel/josh/Protocols/soil%20respiration.pdf (accessed 10 March 2023).
- Ameloot, N.; Graber, E.R.; Verheijen, F.G.A.; De Neve, S. Interactions between Biochar Stability and Soil Organisms: Review and Research Needs: Biochar Stability and Soil Organisms. Eur J Soil Sci 2013, 64, 379–390. [Google Scholar] [CrossRef]
- Pariyar, P.; Kumari, K.; Jain, M.K.; Jadhao, P.S. Evaluation of Change in Biochar Properties Derived from Different Feedstock and Pyrolysis Temperature for Environmental and Agricultural Application. Science of The Total Environment 2020, 713, 136433. [Google Scholar] [CrossRef]
- Farrell, M.; Kuhn, T.K.; Macdonald, L.M.; Maddern, T.M.; Murphy, D.V.; Hall, P.A.; Singh, B.P.; Baumann, K.; Krull, E.S.; Baldock, J.A. Microbial Utilisation of Biochar-Derived Carbon. Science of The Total Environment 2013, 465, 288–297. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chu, W.; Li, H.; Boyd, S.A.; Teppen, B.J.; Mao, J.; Lehmann, J.; Zhang, W. Quantification and Characterization of Dissolved Organic Carbon from Biochars. Geoderma 2019, 335, 161–169. [Google Scholar] [CrossRef]
- Ouyang, L.; Yu, L.; Zhang, R. Effects of Amendment of Different Biochars on Soil Carbon Mineralisation and Sequestration. Soil Res. 2014, 52, 46. [Google Scholar] [CrossRef]
- Ventura, M.; Alberti, G.; Panzacchi, P.; Vedove, G.D.; Miglietta, F.; Tonon, G. Biochar Mineralization and Priming Effect in a Poplar Short Rotation Coppice from a 3-Year Field Experiment. Biol Fertil Soils 2019, 55, 67–78. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Physicochemical and Sorptive Properties of Biochars Derived from Woody and Herbaceous Biomass. Chemosphere 2015, 134, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Bromm, T.; Glaser, B. Soil Organic Carbon Sequestration after Biochar Application: A Global Meta-Analysis. Agronomy 2021, 11, 2474. [Google Scholar] [CrossRef]
- Zong, Y.; Wang, Y.; Sheng, Y.; Wu, C.; Lu, S. Ameliorating Soil Acidity and Physical Properties of Two Contrasting Texture Ultisols with Wastewater Sludge Biochar. Environ Sci Pollut Res 2018, 25, 25726–25733. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Mavi, M.S. Co-Application of Biochar with Non-Pyrolyzed Organic Material Accelerates Carbon Accrual and Nutrient Availability in Soil. Environmental Technology & Innovation 2022, 25, 102128. [Google Scholar] [CrossRef]
- Amoakwah, E.; Arthur, E.; Frimpong, K.A.; Lorenz, N.; Rahman, M.A.; Nziguheba, G.; Islam, K.R. Biochar Amendment Impacts on Microbial Community Structures and Biological and Enzyme Activities in a Weathered Tropical Sandy Loam. Applied Soil Ecology 2022, 172, 104364. [Google Scholar] [CrossRef]
- Kolb, S.E.; Fermanich, K.J.; Dornbush, M.E. Effect of Charcoal Quantity on Microbial Biomass and Activity in Temperate Soils. Soil Science Soc of Amer J 2009, 73, 1173–1181. [Google Scholar] [CrossRef]
- Steinbeiss, S.; Gleixner, G.; Antonietti, M. Effect of Biochar Amendment on Soil Carbon Balance and Soil Microbial Activity. Soil Biology and Biochemistry 2009, 41, 1301–1310. [Google Scholar] [CrossRef]
- Kalu, S.; Simojoki, A.; Karhu, K.; Tammeorg, P. Long-Term Effects of Softwood Biochar on Soil Physical Properties, Greenhouse Gas Emissions and Crop Nutrient Uptake in Two Contrasting Boreal Soils. Agriculture, Ecosystems & Environment 2021, 316, 107454. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, K.; Han, L.; Chen, Y.; Liu, J.; Xing, B. Biochar Stability and Impact on Soil Organic Carbon Mineralization Depend on Biochar Processing, Aging and Soil Clay Content. Soil Biology and Biochemistry 2022, 169, 108657. [Google Scholar] [CrossRef]
| Description | Abbreviation | Dose equivalent [t ha−1] |
|---|---|---|
| Sandy soil without amendments | SA | - |
| Sandy soil with 6 types of biochar | SA BC1 - SA BC6 1 | 0.57 – 0.92 (2% v/w) |
| Sandy soil with 6 types of biochar and three types of organic matter | SA BC1- BC6 CO for compost SA BC1- BC6 MA for manure SA BC1- BC6 LE for legumes |
biochar: 0.57 – 0.92 (2% v/w) organics: 37.50 (1% w/w) |
| Silt loam soil without amendments | SiL | - |
| Silt loam soil with 6 types of biochar | SiL BC1 - SiL BC6 | 0.57 – 0.92 (2% v/w) |
| Silt loam soil with 6 types of biochar and three types of organic matter | SiL BC1- BC6 CO for compost SiL BC1- BC6 MA for manure SiL BC1- BC6 LE for legumes |
biochar: 0.57 – 0.92 (2% v/w) organics: 37.50 (1% w/w) |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





