Submitted:
16 September 2023
Posted:
18 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
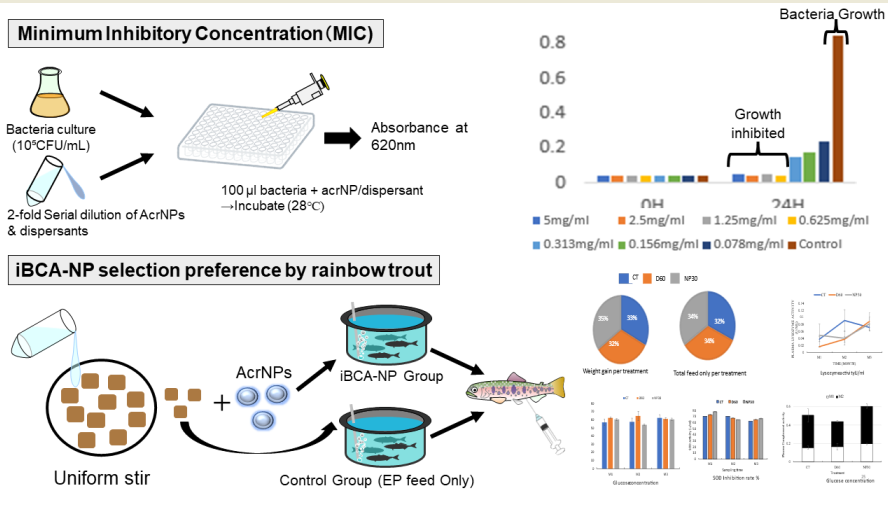
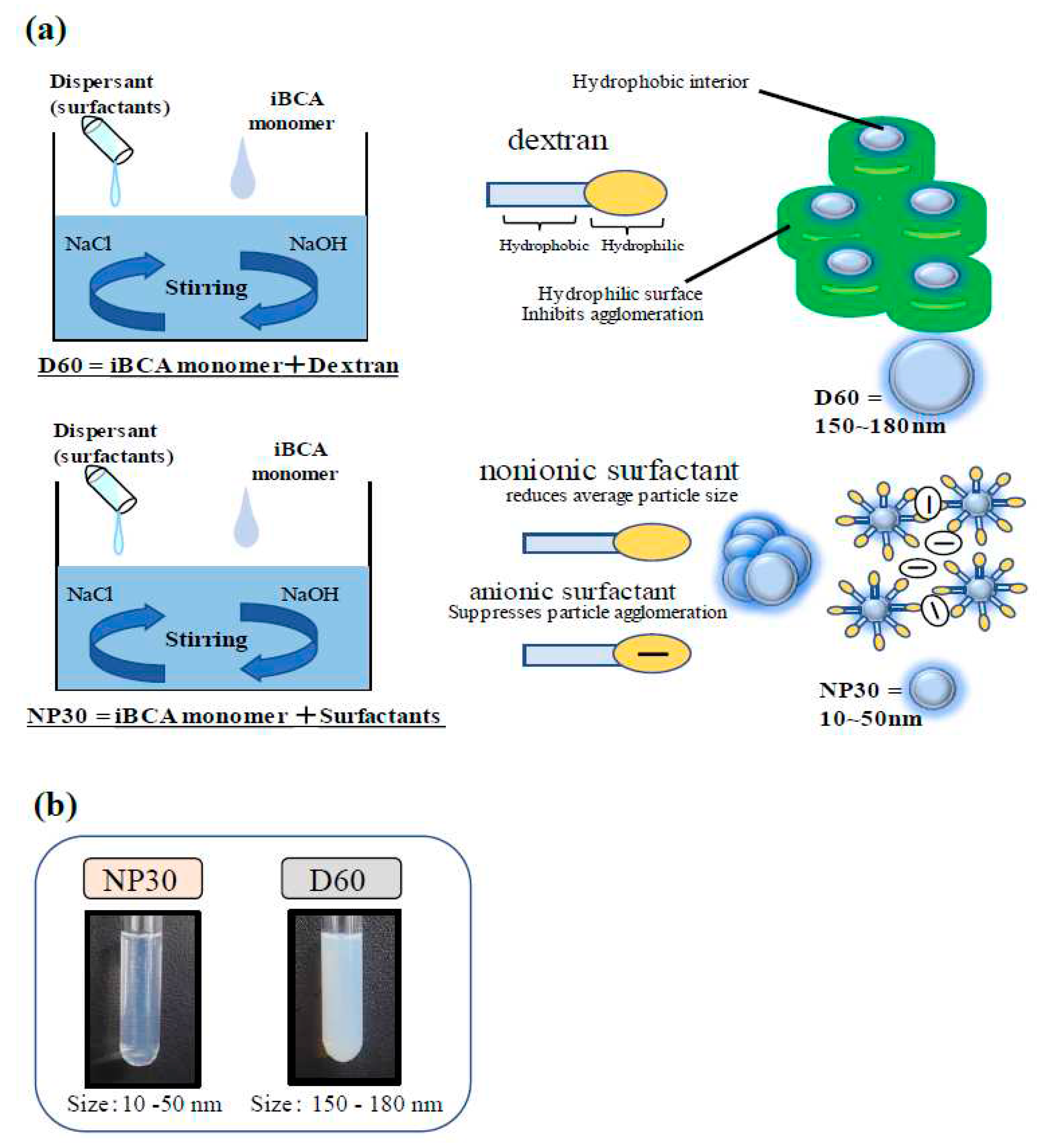
2.1. Isobutyl Cyanoacrylate Nanoparticle Preparation
2.1.1. Characterization of the Synthesized iBCA-NPs
2.2. Bacterial Pathogens
2.2.1. Bacterial Culture Medium for MIC Assay
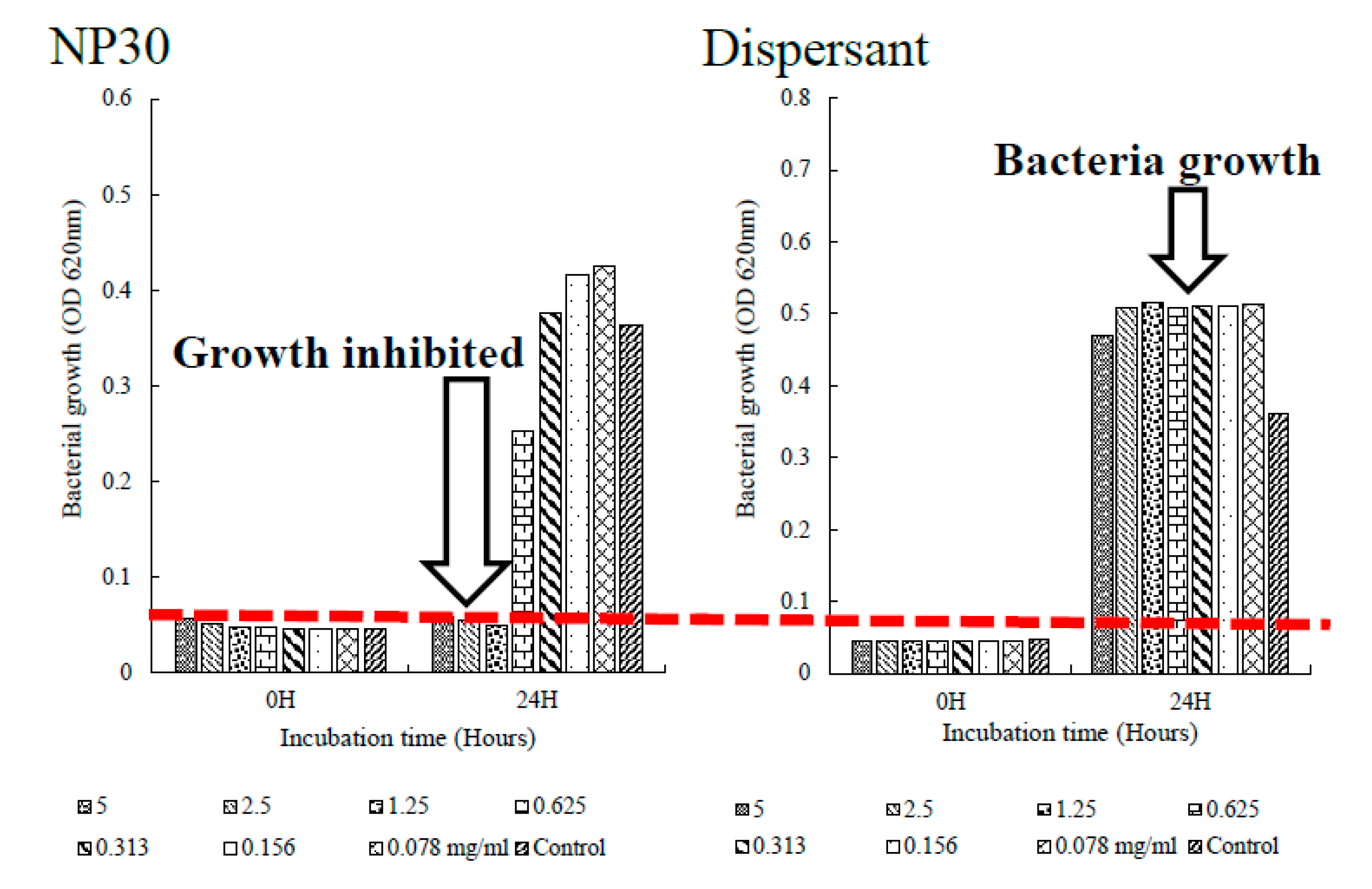
2.3. MIC Measurement by Micro Liquid Dilution Method
2.3.1. Minimum Bactericidal Concentration (MBC) Assay
2.4. Selection Preference and Growth Performance of iBCA-NPs-Fed Rainbow Trout
2.4.1. Experimental Design
2.4.2. Sampling
2.4.3. Growth Performance
2.4.4. Blood Plasma Lysozyme Activity and Complement Titer
2.4.5. Stress and Oxidative Stress Biomarkers
2.5. Statistical Analysis
3. Results
3.1. Physicochemical Characteristics of iBCA-NPs
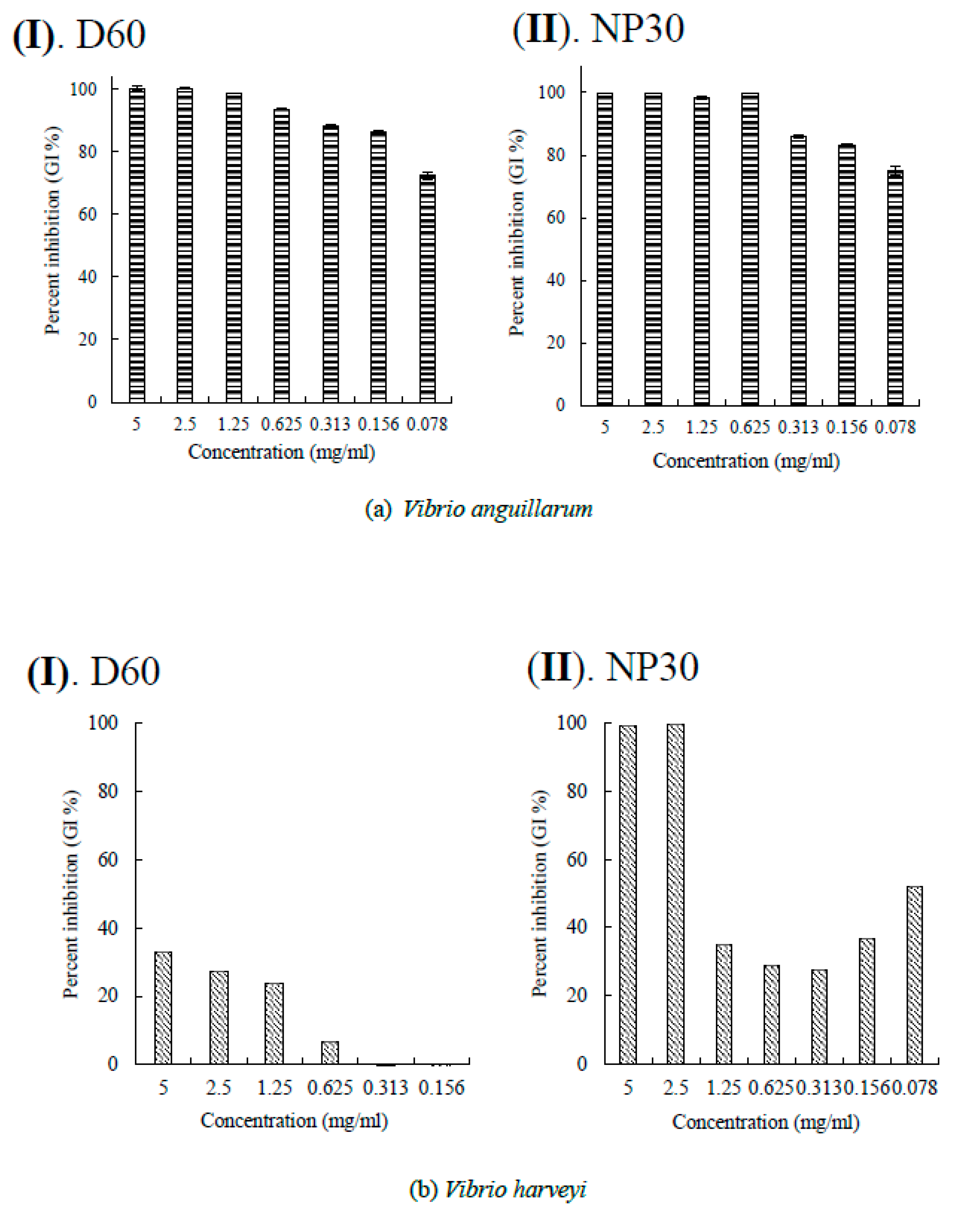
3.2. Antibacterial Effect of iBCA-NPs
3.2.1. Minimum Inhibitory Concentration (MIC)
3.2.2. Minimum Bactericidal Concentration (MBC)
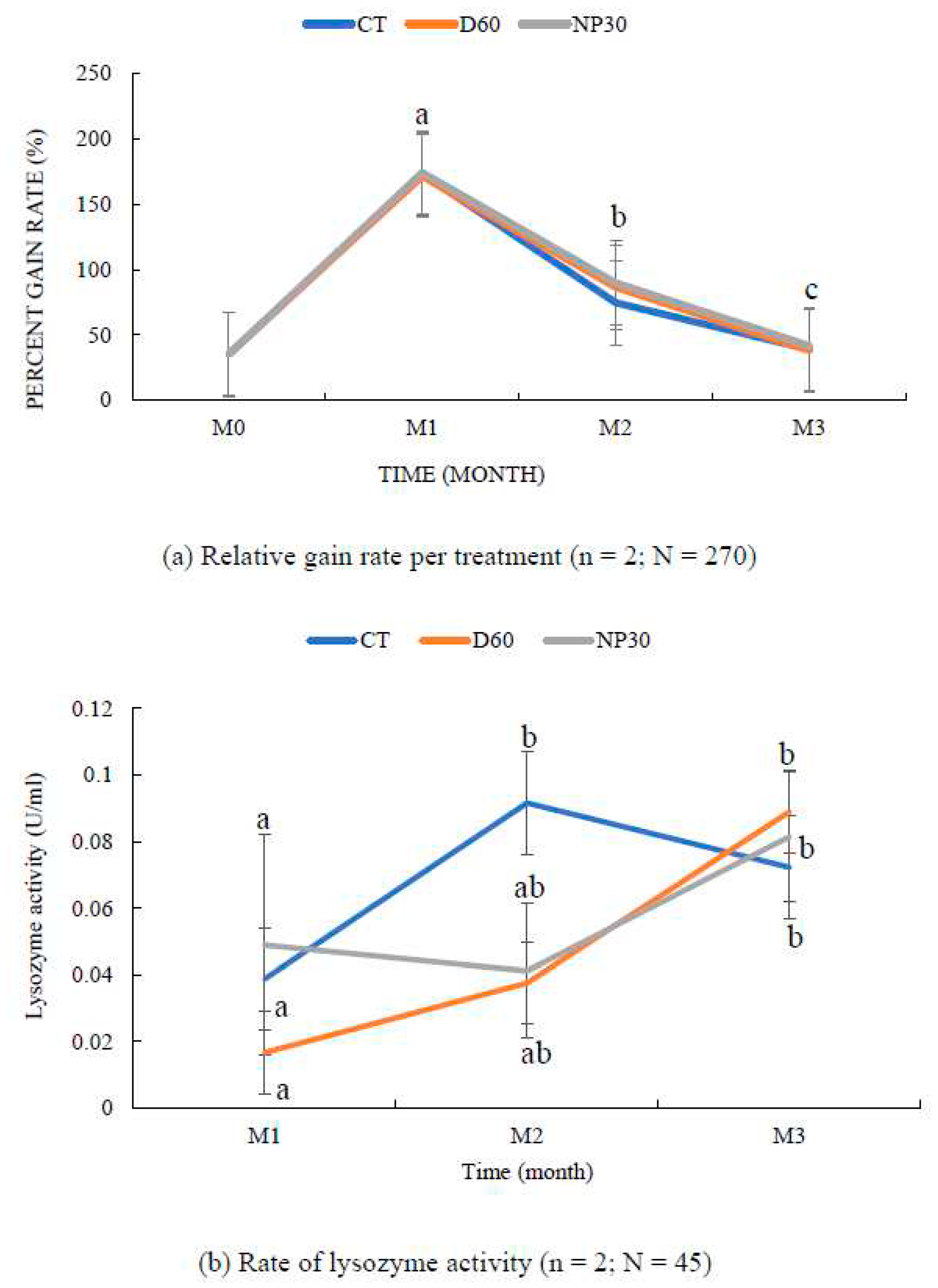
3.3. Growth Performance and Safety Evaluations in Rainbow Trout
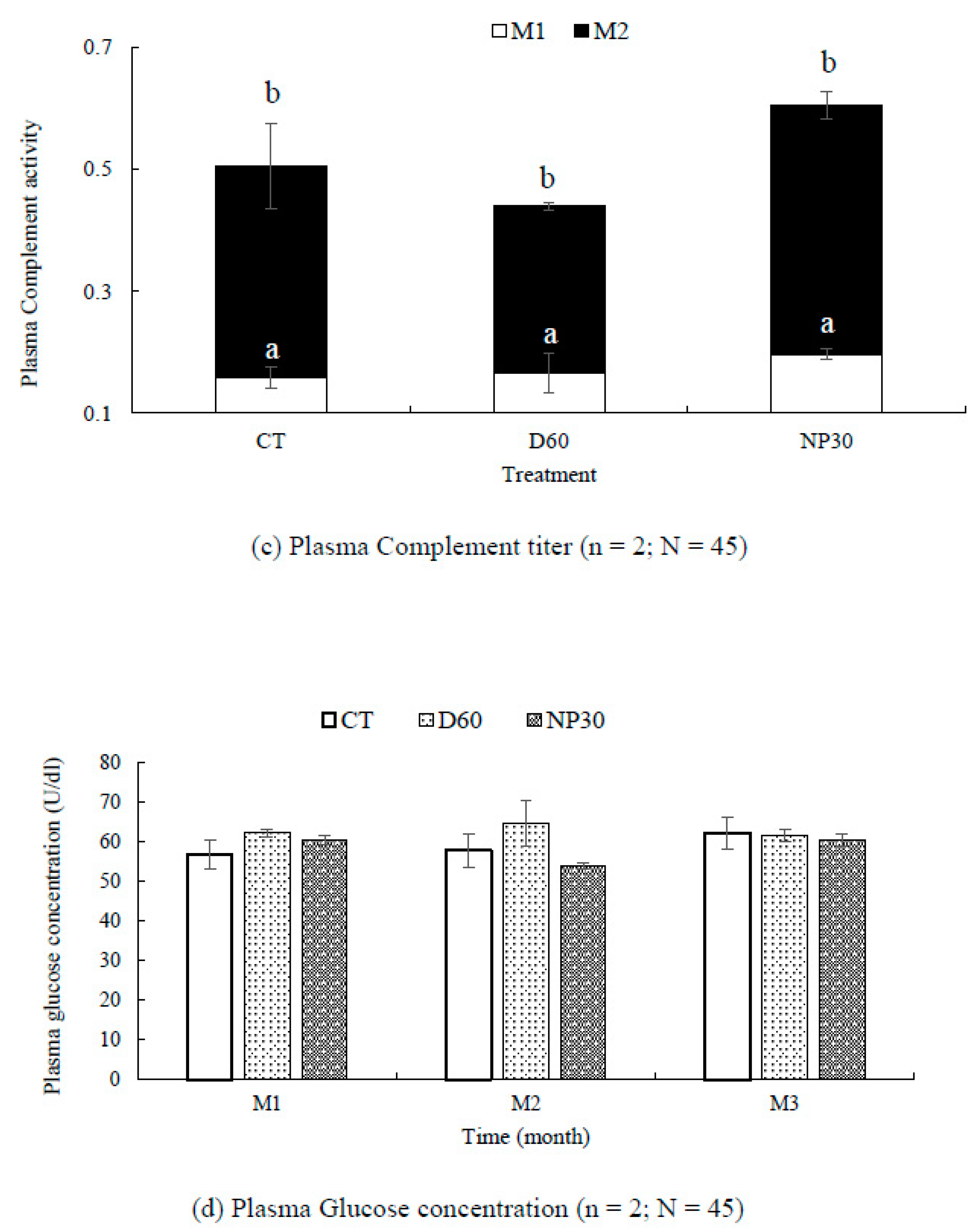
3.4. Innate Defense Capability
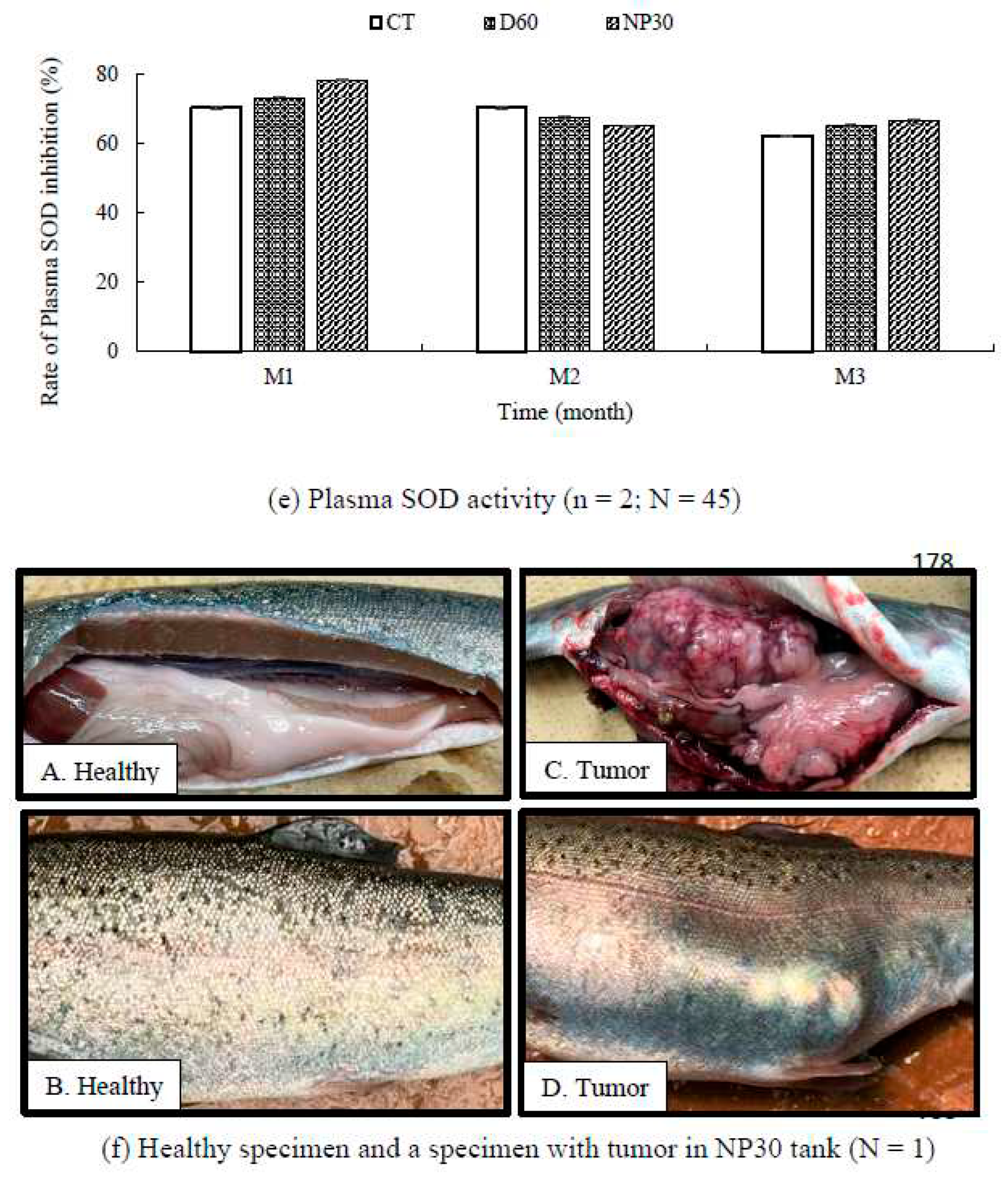
3.5. Stress-Related Biomarkers
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, J.T.; Bellinger, D.C.; Connor, W.E.; Kris-Etherton, P.M.; Lawrence, R.S.; Savitz, D.A.; Shaywitz, B.A.; Teutsch, S.M.; Gray, G.M. A Quantitative Risk-Benefit Analysis of Changes in Population Fish Consumption. Am. J. Prev. Med. 2005, 29, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Pradeepkiran, J.A. Aquaculture Role in Global Food Security with Nutritional Value: A Review. Transl. Anim. Sci. 2019, 3, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Garlock, T.; Asche, F.; Anderson, J.; Ceballos-Concha, A.; Love, D.C.; Osmundsen, T.C.; Pincinato, R.B.M. Aquaculture: The Missing Contributor in the Food Security Agenda. Glob. Food Secur. 2022, 32, 100620. [Google Scholar] [CrossRef]
- Sebastião, F. de A.; Lemos, E.G.M.; Pilarski, F. Validation of Absolute Quantitative Real-Time PCR for the Diagnosis of Streptococcus Agalactiae in Fish. J. Microbiol. Methods 2015, 119, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Stentiford, G.D.; Sritunyalucksana, K.; Flegel, T.W.; Williams, B.A.P.; Withyachumnarnkul, B.; Itsathitphaisarn, O.; Bass, D. New Paradigms to Help Solve the Global Aquaculture Disease Crisis. PLoS Pathog. 2017, 13, e1006160. [Google Scholar] [CrossRef]
- Assefa, A.; Abunna, F. Maintenance of Fish Health in Aquaculture: Review of Epidemiological Approaches for Prevention and Control of Infectious Disease of Fish. Vet. Med. Int. 2018, 2018, 5432497. [Google Scholar] [CrossRef]
- Kibenge, F.S.B.; Godoy, M.G.; Fast, M.; Workenhe, S.; Kibenge, M.J.T. Countermeasures against Viral Diseases of Farmed Fish. Antiviral Res. 2012, 95, 257–281. [Google Scholar] [CrossRef]
- Surachetpong, W.; Roy, S.R.K.; Nicholson, P. Tilapia Lake Virus: The Story so Far. J. Fish Dis. 2020, 43, 1115–1132. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Abdel-Daim, M.M.; Van Doan, H. Probiotic Application for Sustainable Aquaculture. Rev. Aquac. 2019, 11, 907–924. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Ringø, E.; Ángeles Esteban, M.; Dadar, M.; Dawood, M.A.O.; Faggio, C. Host-Associated Probiotics: A Key Factor in Sustainable Aquaculture. Rev. Fish. Sci. Aquac. 2020, 28, 16–42. [Google Scholar] [CrossRef]
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. P T Peer-Rev. J. Formul. Manag. 2015, 40, 277–283. [Google Scholar]
- Watts, J.E.M.; Schreier, H.J.; Lanska, L.; Hale, M.S. The Rising Tide of Antimicrobial Resistance in Aquaculture: Sources, Sinks and Solutions. Mar. Drugs 2017, 15, 158. [Google Scholar] [CrossRef] [PubMed]
- Bondad-Reantaso, M.G.; MacKinnon, B.; Karunasagar, I.; Fridman, S.; Alday-Sanz, V.; Brun, E.; Le Groumellec, M.; Li, A.; Surachetpong, W.; Karunasagar, I.; et al. Review of Alternatives to Antibiotic Use in Aquaculture. Rev. Aquac. n/a. [CrossRef]
- Abdelkhalek, N.K.M.; Eissa, I.A.M.; Ahmed, E.; Kilany, O.E.; El-Adl, M.; Dawood, M.A.O.; Hassan, A.M.; Abdel-Daim, M.M. Protective Role of Dietary Spirulina Platensis against Diazinon-Induced Oxidative Damage in Nile Tilapia; Oreochromis Niloticus. Environ. Toxicol. Pharmacol. 2017, 54, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Adel, M.; Dadar, M.; Khajavi, S.H.; Pourgholam, R.; Karimí, B.; Velisek, J. Hematological, Biochemical and Histopathological Changes in Caspian Brown Trout (Salmo Trutta Caspius Kessler, 1877) Following Exposure to Sublethal Concentrations of Chlorpyrifos. Toxin Rev. 2017, 36, 73–79. [Google Scholar] [CrossRef]
- Matsuura, Y.; Terashima, S.; Takano, T.; Matsuyama, T. Current Status of Fish Vaccines in Japan. Fish Shellfish Immunol. 2019, 95, 236–247. [Google Scholar] [CrossRef]
- Newaj-Fyzul, A.; Al-Harbi, A.H.; Austin, B. Review: Developments in the Use of Probiotics for Disease Control in Aquaculture. Aquaculture 2014, 431, 1–11. [Google Scholar] [CrossRef]
- Shaalan, M.; Saleh, M.; El-Mahdy, M.; El-Matbouli, M. Recent Progress in Applications of Nanoparticles in Fish Medicine: A Review. Nanomedicine Nanotechnol. Biol. Med. 2016, 12, 701–710. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Hńfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J. Nanobiotechnology 2017, 15, 1–20. [Google Scholar] [CrossRef]
- Khosravi-Katuli, K.; Prato, E.; Lofrano, G.; Guida, M.; Vale, G.; Libralato, G. Effects of Nanoparticles in Species of Aquaculture Interest. Environ. Sci. Pollut. Res. 2017, 24, 17326–17346. [Google Scholar] [CrossRef]
- Fajardo, C.; Martinez-Rodriguez, G.; Blasco, J.; Mancera, J.M.; Thomas, B.; De Donato, M. Nanotechnology in Aquaculture: Applications, Perspectives and Regulatory Challenges. Aquac. Fish. 2022, 7, 185–200. [Google Scholar] [CrossRef]
- Bacchetta, C.; Ale, A.; Simoniello, M.F.; Gervasio, S.; Davico, C.; Rossi, A.S.; Desimone, M.F.; Poletta, G.; López, G.; Monserrat, J.M.; et al. Genotoxicity and Oxidative Stress in Fish after a Short-Term Exposure to Silver Nanoparticles. Ecol. Indic. 2017, 76, 230–239. [Google Scholar] [CrossRef]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique Cellular Interaction of Silver Nanoparticles: Size-Dependent Generation of Reactive Oxygen Species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef]
- Lenaerts, V.; Couvreur, P.; Christiaens-Leyh, D.; Joiris, E.; Roland, M.; Rollman, B.; Speiser, P. Degradation of Poly (Isobutyl Cyanoacrylate) Nanoparticles. Biomaterials 1984, 5, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Leonard, F.; Kulkarni, R.K.; Brandes, G.; Nelson, J.; Cameron, J.J. Synthesis and Degradation of Poly (Alkyl α-Cyanoacrylates). J. Appl. Polym. Sci. 1966, 10, 259–272. [Google Scholar] [CrossRef]
- Vauthier, C.; Dubernet, C.; Fattal, E.; Pinto-Alphandary, H.; Couvreur, P. Poly(Alkylcyanoacrylates) as Biodegradable Materials for Biomedical Applications. Adv. Drug Deliv. Rev. 2003, 55, 519–548. [Google Scholar] [CrossRef]
- Shirotake, S. A New Cyanoacrylate Colloidal Polymer with Novel Antibacterial Mechanism and Its Application to Infection Control. J. Nanomedicine Biotherapeutic Discov. 2014, 04, 1. [Google Scholar] [CrossRef]
- Sulheim, E.; Baghirov, H.; von Haartman, E.; Bøe, A.; Åslund, A.K.O.; Mørch, Y.; Davies, C. de L. Cellular Uptake and Intracellular Degradation of Poly(Alkyl Cyanoacrylate) Nanoparticles. J. Nanobiotechnology 2016, 14, 1. [Google Scholar] [CrossRef]
- Widyaningrum, D.; Iida, D.; Tanabe, Y.; Hayashi, Y.; Kurniasih, S.D.; Ohama, T. Acutely Induced Cell Mortality in the Unicellular Green Alga Chlamydomonas Reinhardtii (Chlorophyceae) Following Exposure to Acrylic Resin Nanoparticles. J. Phycol. 2019, 55, 118–133. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Sato, T.; Weightman, A.J.; Martin, T.A.; Fry, J.C.; Hiom, S.J.; Dymock, D.; Wade, W.G. Design and Evaluation of Useful Bacterium-Specific PCR Primers That Amplify Genes Coding for Bacterial 16S RRNA. Appl. Environ. Microbiol. 1998, 64, 795–799. [Google Scholar] [CrossRef]
- Ahmed, F.; Soliman, F.M.; Adly, M.A.; Soliman, H.A.M.; El-Matbouli, M.; Saleh, M. In Vitro Assessment of the Antimicrobial Efficacy of Chitosan Nanoparticles against Major Fish Pathogens and Their Cytotoxicity to Fish Cell Lines. J. Fish Dis. 2020, 43, 1049–1063. [Google Scholar] [CrossRef]
- Altschul, S.F.; Boguski, M.S.; Gish, W.; Wootton, J.C. Issues in Searching Molecular Sequence Databases. Nat. Genet. 1994, 6, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, T.; Kamaishi, T.; Ooseko, N.; Kurohara, K.; Iida, T. Pathogenicity of Motile and Non-Motile Edwardsiella Tarda to Some Marine Fish. Fish Pathol. 2005, 40, 133–135. [Google Scholar] [CrossRef]
- Nakamura, Y.; Takano, T.; Yasuike, M.; Sakai, T.; Matsuyama, T.; Sano, M. Comparative Genomics Reveals That a Fish Pathogenic Bacterium Edwardsiella Tarda Has Acquired the Locus of Enterocyte Effacement (LEE) through Horizontal Gene Transfer. BMC Genomics 2013, 14, 642. [Google Scholar] [CrossRef] [PubMed]
- Japanese Society of Antimicrobials for Animals The Determination Method of Minimum Inhibitory Concentration (MIC) of Antimicrobials against Bacteria Isolated from Animals (RevisedStandard Method of the Japanese Society of Antimicrobials for Animals in 2003). Proceeding Jpn. Soc. Antimicrob. Anim. 2003, 25, 63–73.
- Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing. CLSI Suppl. M100 Wayne PA CLSI 2019, 25.
- Vijayakumar, P.P.; Muriana, P.M. A Microplate Growth Inhibition Assay for Screening Bacteriocins against Listeria Monocytogenes to Differentiate Their Mode-of-Action. Biomolecules 2015, 5, 1178–1194. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathog. Basel Switz. 2021, 10, 165. [Google Scholar] [CrossRef]
- Du, W.-L.; Niu, S.-S.; Xu, Y.-L.; Xu, Z.-R.; Fan, C.-L. Antibacterial Activity of Chitosan Tripolyphosphate Nanoparticles Loaded with Various Metal Ions. Carbohydr. Polym. 2009, 75, 385–389. [Google Scholar] [CrossRef]
- Du, W.-L.; Xu, Y.-L.; Xu, Z.-R.; Fan, C.-L. Preparation, Characterization and Antibacterial Properties against E. Coli K(88) of Chitosan Nanoparticle Loaded Copper Ions. Nanotechnology 2008, 19, 085707. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and Antibacterial Activity of Chitosan Nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Demers, N.E.; Bayne, C.J. The Immediate Effects of Stress on Hormones and Plasma Lysozyme in Rainbow Trout. Dev. Comp. Immunol. 1997, 21, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Gupta, D.K.; Sharma, P.K. Condition Factor and Organosomatic Indices of Parasitized Rattus Rattus as Indicators of Host Health. J. Parasit. Dis. 2017, 41, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ighwela, K.A.; Ahmad, A.B.; Abol-Munafi, A.B. The Selection of Viscerosomatic and Hepatosomatic Indices for the Measurement and Analysis of Oreochromis Niloticus Condition Fed with Varying Dietary Maltose Levels.
- Morado, C.N.; Araújo, F.G.; Gomes, I.D. The Use of Biomarkers for Assessing Effects of Pollutant Stress on Fish Species from a Tropical River in Southeastern Brazil. Acta Sci. Biol. Sci. 2017, 39, 431–439. [Google Scholar] [CrossRef]
- Hashiguchi, K.; Kawai, K.; Imajoh, M.; Oshima, S. Influence of Fresh Water Bathing on Stress Response of Yellowtail <I>Seriola Quinqueradiata</I>. Aquac. Sci. 2015, 63, 79–87. [Google Scholar] [CrossRef]
- Mahboub, H.H.; Khedr, M.H.E.; Elshopakey, G.E.; Shakweer, M.S.; Mohamed, D.I.; Ismail, T.A.; Ismail, S.H.; Abdel Rahman, A.N. Impact of Silver Nanoparticles Exposure on Neuro-Behavior, Hematology, and Oxidative Stress Biomarkers of African Catfish (Clarias Gariepinus). Aquaculture 2021, 544, 737082. [Google Scholar] [CrossRef]
- Miwa, I.; Okudo, J.; Maeda, K.; Okuda, G. Mutarotase Effect on Colorimetric Determination of Blood Glucose with -D-Glucose Oxidase. Clin. Chim. Acta Int. J. Clin. Chem. 1972, 37, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Arcos, M.; Dille, J.; Rousse, C.; Godet, S.; Malet, L. Effect of the Concentration and the Type of Dispersant on the Synthesis of Copper Oxide Nanoparticles and Their Potential Antimicrobial Applications. ACS Omega 2021, 6, 18576–18590. [Google Scholar] [CrossRef] [PubMed]
- Agnew, W.; Barnes, A.C. Streptococcus Iniae: An Aquatic Pathogen of Global Veterinary Significance and a Challenging Candidate for Reliable Vaccination. Vet. Microbiol. 2007, 122, 1–15. [Google Scholar] [CrossRef]
- Eissa, A.E.; Abou-Okada, M.; Alkurdi, A.R.M.; El Zlitne, R.A.; Prince, A.; Abdelsalam, M.; Derwa, H.I.M. Catastrophic Mass Mortalities Caused by Photobacterium Damselae Affecting Farmed Marine Fish from Deeba Triangle, Egypt. Aquac. Res. 2021, 52, 4455–4466. [Google Scholar] [CrossRef]
- Handlinger, J.; Soltani, M.; Percival, S. The Pathology of Flexibacter Maritimus in Aquaculture Species in Tasmania, Australia. J. Fish Dis. 2003, 20, 159–168. [Google Scholar] [CrossRef]
- Kato, G.; Oka, K.; Matsumoto, M.; Kanemaru, M.; Yamamoto, M.; Sano, M. Prevalence of Infection with Nocardia Seriolae in Juvenile of Yellowtail Seriola Quinqueradiata Cultured in Owase Bay, Japan. Fish Pathol. 2020, 55, 1–7. [Google Scholar] [CrossRef]
- Mabrok, M.; Algammal, A.M.; Sivaramasamy, E.; Hetta, H.F.; Atwah, B.; Alghamdi, S.; Fawzy, A.; Avendaño-Herrera, R.; Rodkhum, C. Tenacibaculosis Caused by Tenacibaculum Maritimum: Updated Knowledge of This Marine Bacterial Fish Pathogen. Front. Cell. Infect. Microbiol. 2023, 12, 1068000. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Han, J.E.; Kwon, H.; Park, S.C.; Kim, J.H. Recent Insights into Aeromonas Salmonicida and Its Bacteriophages in Aquaculture: A Comprehensive Review. J. Microbiol. Biotechnol. 2020, 30, 1443–1457. [Google Scholar] [CrossRef] [PubMed]
- Sanches-Fernandes, G.M.M.; Sá-Correia, I.; Costa, R. Vibriosis Outbreaks in Aquaculture: Addressing Environmental and Public Health Concerns and Preventive Therapies Using Gilthead Seabream Farming as a Model System. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Baldisserotto, B.; Hosseini Shekarabi, S.P.; Shafiei, S.; Bashiri, M. Lactococcosis a Re-Emerging Disease in Aquaculture: Disease Significant and Phytotherapy. Vet. Sci. 2021, 8, 181. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, X.-H. Edwardsiella Tarda: An Intriguing Problem in Aquaculture. Aquaculture 2014, 431, 129–135. [Google Scholar] [CrossRef]
- Álvarez-Chimal, R.; García-Pérez, V.I.; Álvarez-Pérez, M.A.; Tavera-Hernández, R.; Reyes-Carmona, L.; Martínez-Hernández, M.; Arenas-Alatorre, J.Á. Influence of the Particle Size on the Antibacterial Activity of Green Synthesized Zinc Oxide Nanoparticles Using Dysphania Ambrosioides Extract, Supported by Molecular Docking Analysis. Arab. J. Chem. 2022, 15, 103804. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, H.; Shen, Y.; Zhang, W.; Zhang, L. Antibacterial Activity of Silver Nanoparticles of Different Particle Size against Vibrio Natriegens. PloS One 2019, 14, e0222322. [Google Scholar] [CrossRef]
- Naqvi, Q.-U.-A.; Kanwal, A.; Qaseem, S.; Naeem, M.; Ali, S.R.; Shaffique, M.; Maqbool, M. Size-Dependent Inhibition of Bacterial Growth by Chemically Engineered Spherical ZnO Nanoparticles. J. Biol. Phys. 2019. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular Mechanisms of Membrane Targeting Antibiotics. Biochim. Biophys. Acta BBA - Biomembr. 2016, 1858, 980–987. [Google Scholar] [CrossRef]
- Li, X.-Z.; Plésiat, P.; Nikaido, H. The Challenge of Efflux-Mediated Antibiotic Resistance in Gram-Negative Bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef] [PubMed]
- Sarian, F.D.; Ando, K.; Tsurumi, S.; Miyashita, R.; Ute, K.; Ohama, T. Evaluation of the Growth-Inhibitory Spectrum of Three Types of Cyanoacrylate Nanoparticles on Gram-Positive and Gram-Negative Bacteria. Membranes 2022, 12, 782. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, I.; Favini-Stabile, S.; Dessen, A. Resistance to Antibiotics Targeted to the Bacterial Cell Wall. Protein Sci. Publ. Protein Soc. 2014, 23, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Espaillat, A.; Cava, F. Bacterial Strategies to Preserve Cell Wall Integrity Against Environmental Threats. Front. Microbiol. 2018, 9, 2064. [Google Scholar] [CrossRef]
- Baker-Austin, C. Chapter 6 - Antimicrobial Resistance in Vibrio Species. In Antimicrobial Resistance and Food Safety; Chen, C.-Y., Yan, X., Jackson, C.R., Eds.; Academic Press: San Diego, 2015; pp. 105–118. ISBN 978-0-12-801214-7. [Google Scholar]
- Guz, L.; Nowakiewicz, A.; Puk, K.; Zięba, P.; Gnat, S.; Matuszewski, Ł. Virulence and Antimicrobial Resistance Pattern of Aeromonas Spp. Colonizing European Pond Turtles Emys Orbicularis and Their Natural Environment. First Study from Poland. Anim. Open Access J. MDPI 2021, 11, 2772. [Google Scholar] [CrossRef]
- Gxalo, O.; Digban, T.O.; Igere, B.E.; Olapade, O.A.; Okoh, A.I.; Nwodo, U.U. Virulence and Antibiotic Resistance Characteristics of Vibrio Isolates From Rustic Environmental Freshwaters. Front. Cell. Infect. Microbiol. 2021, 11. [Google Scholar] [CrossRef]
- Loo, K.-Y.; Letchumanan, V.; Law, J.W.-F.; Pusparajah, P.; Goh, B.-H.; Ab Mutalib, N.-S.; He, Y.-W.; Lee, L.-H. Incidence of Antibiotic Resistance in Vibrio Spp. Rev. Aquac. 2020, 12, 2590–2608. [Google Scholar] [CrossRef]
- Trudel, M.V.; Vincent, A.T.; Attéré, S.A.; Labbé, M.; Derome, N.; Culley, A.I.; Charette, S.J. Diversity of Antibiotic-Resistance Genes in Canadian Isolates of Aeromonas Salmonicida Subsp. Salmonicida: Dominance of PSN254b and Discovery of PAsa8. Sci. Rep. 2016, 6, 35617. [Google Scholar] [CrossRef]
- Wu, L.; Tian, Y.; Pang, M.; Yang, Z.; Bao, H.; Zhou, Y.; Sun, L.; Wang, R.; Zhang, H. A Novel Vibriophage VB_VhaS_PcB-1G Capable of Inhibiting Virulent Vibrio Harveyi Pathogen. Aquaculture 2021, 542, 736854. [Google Scholar] [CrossRef]
- Reichley, S.R.; Ware, C.; Steadman, J.; Gaunt, P.S.; García, J.C.; LaFrentz, B.R.; Thachil, A.; Waldbieser, G.C.; Stine, C.B.; Buján, N.; et al. Comparative Phenotypic and Genotypic Analysis of Edwardsiella Isolates from Different Hosts and Geographic Origins, with Emphasis on Isolates Formerly Classified as E. Tarda, and Evaluation of Diagnostic Methods. J. Clin. Microbiol. 2017, 55, 3466–3491. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.B.; Kanai, K.; Yoshikoshi, K. Serological Characterization of Atypical Strains of Edwardsiella Tarda Isolated from Sea Breams. Fish Pathol. 33, 265–274. [CrossRef]
- Divya, K.; Vijayan, S.; George, T.K.; Jisha, M.S. Antimicrobial Properties of Chitosan Nanoparticles: Mode of Action and Factors Affecting Activity. Fibers Polym. 2017, 18, 221–230. [Google Scholar] [CrossRef]
- Boyd, C.E.; Tucker, C.S. Pond Aquaculture Water Quality Management; Springer US: Boston, MA, 1998; ISBN 978-1-4613-7469-5. [Google Scholar]
- Zhao, J.; Wang, Z.; Liu, X.; Xie, X.; Zhang, K.; Xing, B. Distribution of CuO Nanoparticles in Juvenile Carp (Cyprinus Carpio) and Their Potential Toxicity. J. Hazard. Mater. 2011, 197, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, P. a.; Leeson, S.; Cho, C. y.; Bureau, D. p. Growth and Feed Utilization of Large Size Rainbow Trout (Oncorhynchus Mykiss) and Atlantic Salmon (Salmo Salar) Reared in Freshwater: Diet and Species Effects, and Responses over Time. Aquac. Nutr. 2004, 10, 401–411. [Google Scholar] [CrossRef]
- Zaineldin, A.I.; Hegazi, S.; Koshio, S.; Ishikawa, M.; Bakr, A.; El-Keredy, A.M.S.; Dawood, M.A.O.; Dossou, S.; Wang, W.; Yukun, Z. Bacillus Subtilis as Probiotic Candidate for Red Sea Bream: Growth Performance, Oxidative Status, and Immune Response Traits. Fish Shellfish Immunol. 2018, 79, 303–312. [Google Scholar] [CrossRef]
- Ighwela, K.A.; Ahmad, A.B.; Abol-Munafi, A.B. The Selection of Viscerosomatic and Hepatosomatic Indices for the Measurement and Analysis of Oreochromis Niloticus Condition Fed with Varying Dietary Maltose Levels. 2014, 3, 18–20. [Google Scholar]
- Bavia, L.; Santiesteban-Lores, L.E.; Carneiro, M.C.; Prodocimo, M.M. Advances in the Complement System of a Teleost Fish, Oreochromis Niloticus. Fish Shellfish Immunol. 2022, 123, 61–74. [Google Scholar] [CrossRef]
- Biller-Takahashi, J.D.; Urbinati, E.C. Fish Immunology. The Modification and Manipulation of the Innate Immune System: Brazilian Studies. An. Acad. Bras. Ciênc. 2014, 86, 1484–1506. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An Important Defence Molecule of Fish Innate Immune System. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Subbotkin, M.F.; Subbotkina, T.A. Effects of Environment and Physiological State of an Organism on the Activity and Content of Lysozyme in Fishes of the Family Cyprinidae: A Review. Inland Water Biol. 2018, 11, 184–194. [Google Scholar] [CrossRef]
- Holland, M.C.H.; Lambris, J.D. The Complement System in Teleosts. Fish Shellfish Immunol. 2002, 12, 399–420. [Google Scholar] [CrossRef] [PubMed]
- Sauer, D.J.; Heidinger, B.J.; Kittilson, J.D.; Lackmann, A.R.; Clark, M.E. No Evidence of Physiological Declines with Age in an Extremely Long-Lived Fish. Sci. Rep. 2021, 11, 9065. [Google Scholar] [CrossRef] [PubMed]
- Abdelazim, A.M.; Saadeldin, I.M.; Swelum, A.A.-A.; Afifi, M.M.; Alkaladi, A. Oxidative Stress in the Muscles of the Fish Nile Tilapia Caused by Zinc Oxide Nanoparticles and Its Modulation by Vitamins C and E. Oxid. Med. Cell. Longev. 2018, 2018, e6926712. [Google Scholar] [CrossRef]
- Farag, M.R.; Abo-Al-Ela, H.G.; Alagawany, M.; Azzam, M.M.; El-Saadony, M.T.; Rea, S.; Di Cerbo, A.; Nouh, D.S. Effect of Quercetin Nanoparticles on Hepatic and Intestinal Enzymes and Stress-Related Genes in Nile Tilapia Fish Exposed to Silver Nanoparticles. Biomedicines 2023, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- García-Medina, S.; Galar-Martínez, M.; Cano-Viveros, S.; Ruiz-Lara, K.; Gómez-Oliván, L.M.; Islas-Flores, H.; Gasca-Pérez, E.; Pérez-Pastén-Borja, R.; Arredondo-Tamayo, B.; Hernández-Varela, J.; et al. Bioaccumulation and Oxidative Stress Caused by Aluminium Nanoparticles and the Integrated Biomarker Responses in the Common Carp (Cyprinus Carpio). Chemosphere 2022, 288, 132462. [Google Scholar] [CrossRef]
- Saddick, S.; Afifi, M.; Abu Zinada, O.A. Effect of Zinc Nanoparticles on Oxidative Stress-Related Genes and Antioxidant Enzymes Activity in the Brain of Oreochromis Niloticus and Tilapia Zillii. Saudi J. Biol. Sci. 2017, 24, 1672–1678. [Google Scholar] [CrossRef]
- Hood, E. Fullerenes and Fish Brains: Nanomaterials Cause Oxidative Stress. Environ. Health Perspect. 2004, 112, A568. [Google Scholar] [CrossRef]
- Ibrahim, A.Th.A.; Banaee, M.; Sureda, A. Genotoxicity, Oxidative Stress, and Biochemical Biomarkers of Exposure to Green Synthesized Cadmium Nanoparticles in Oreochromis Niloticus (L.). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 242, 108942. [Google Scholar] [CrossRef]
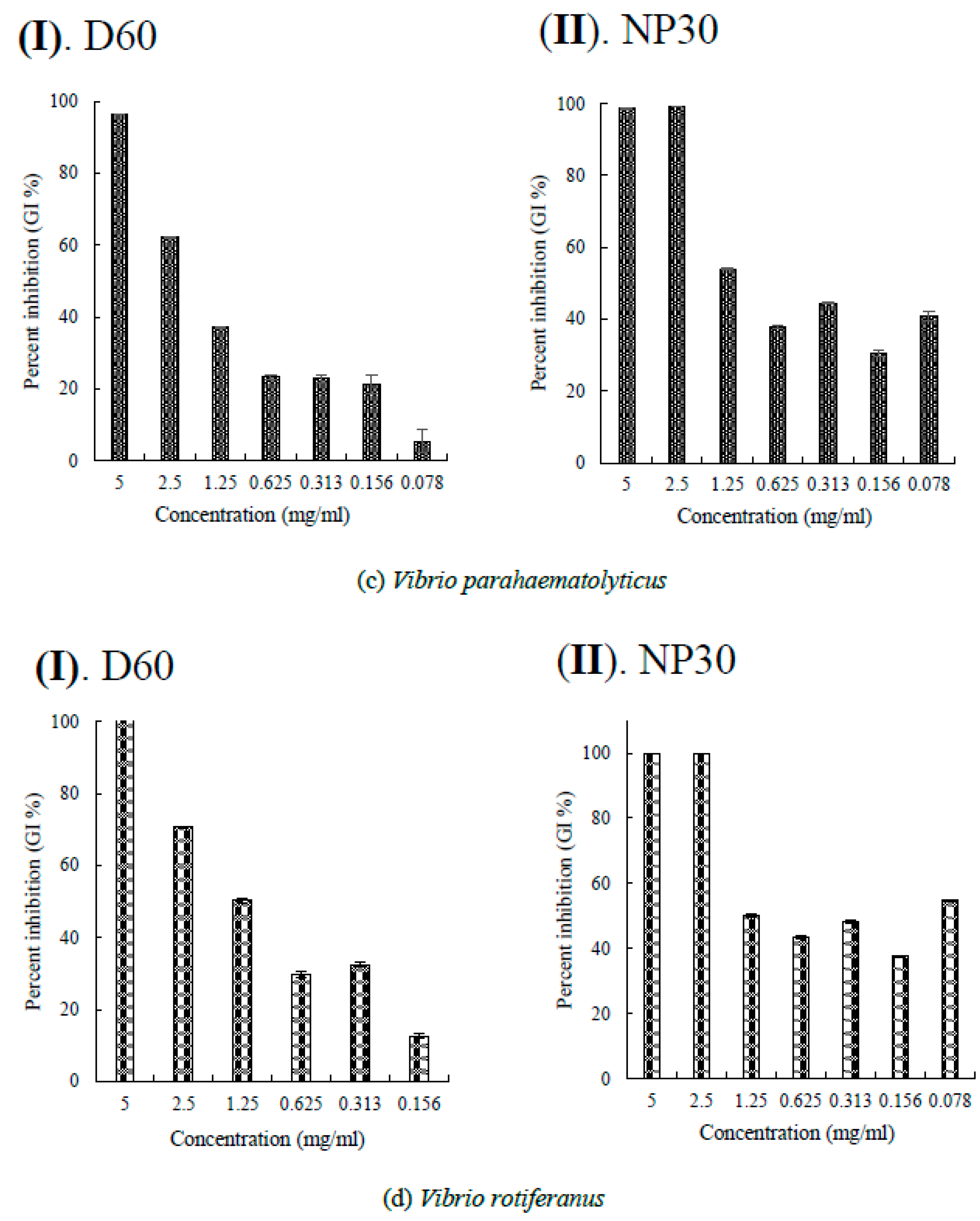
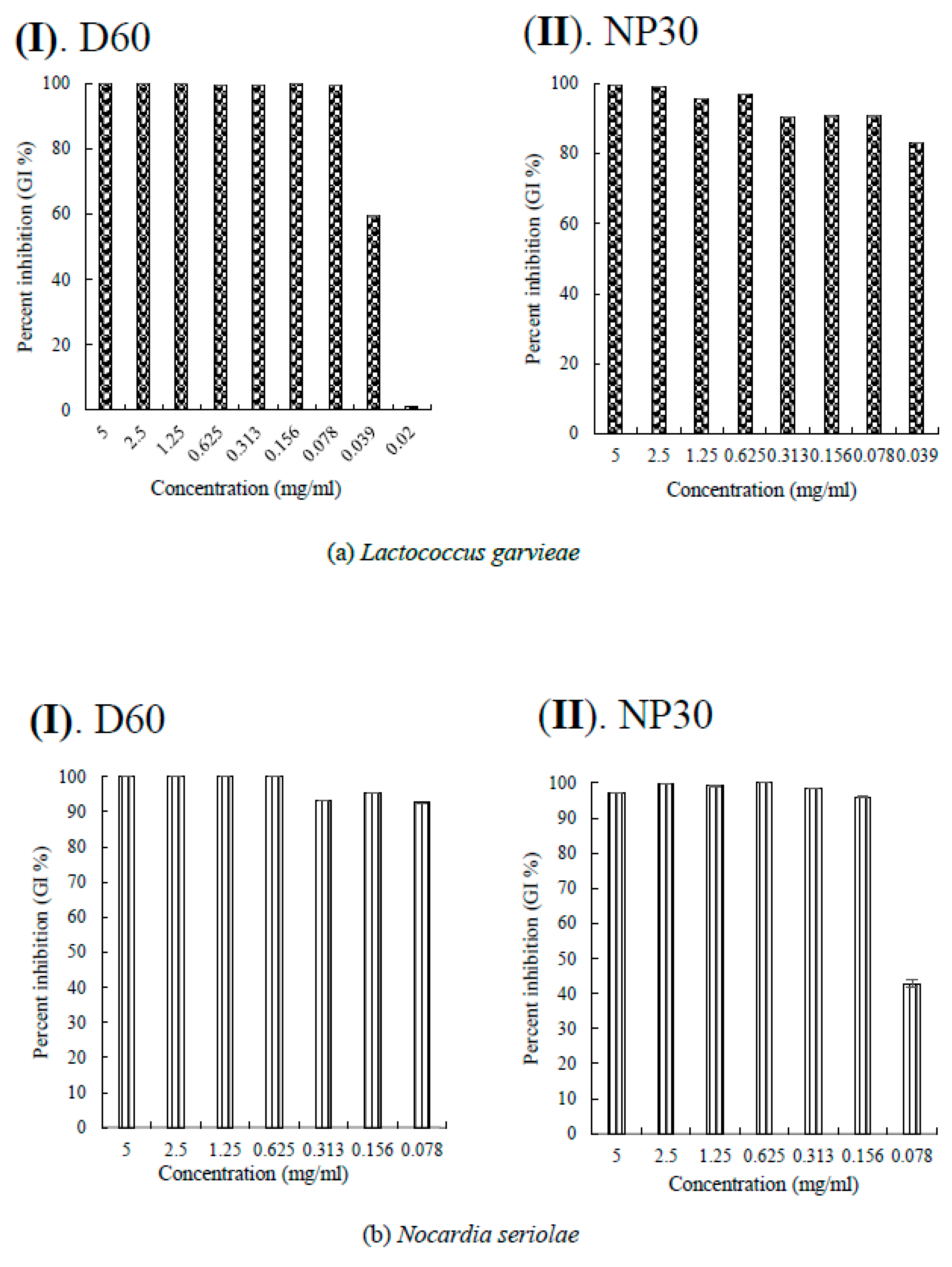
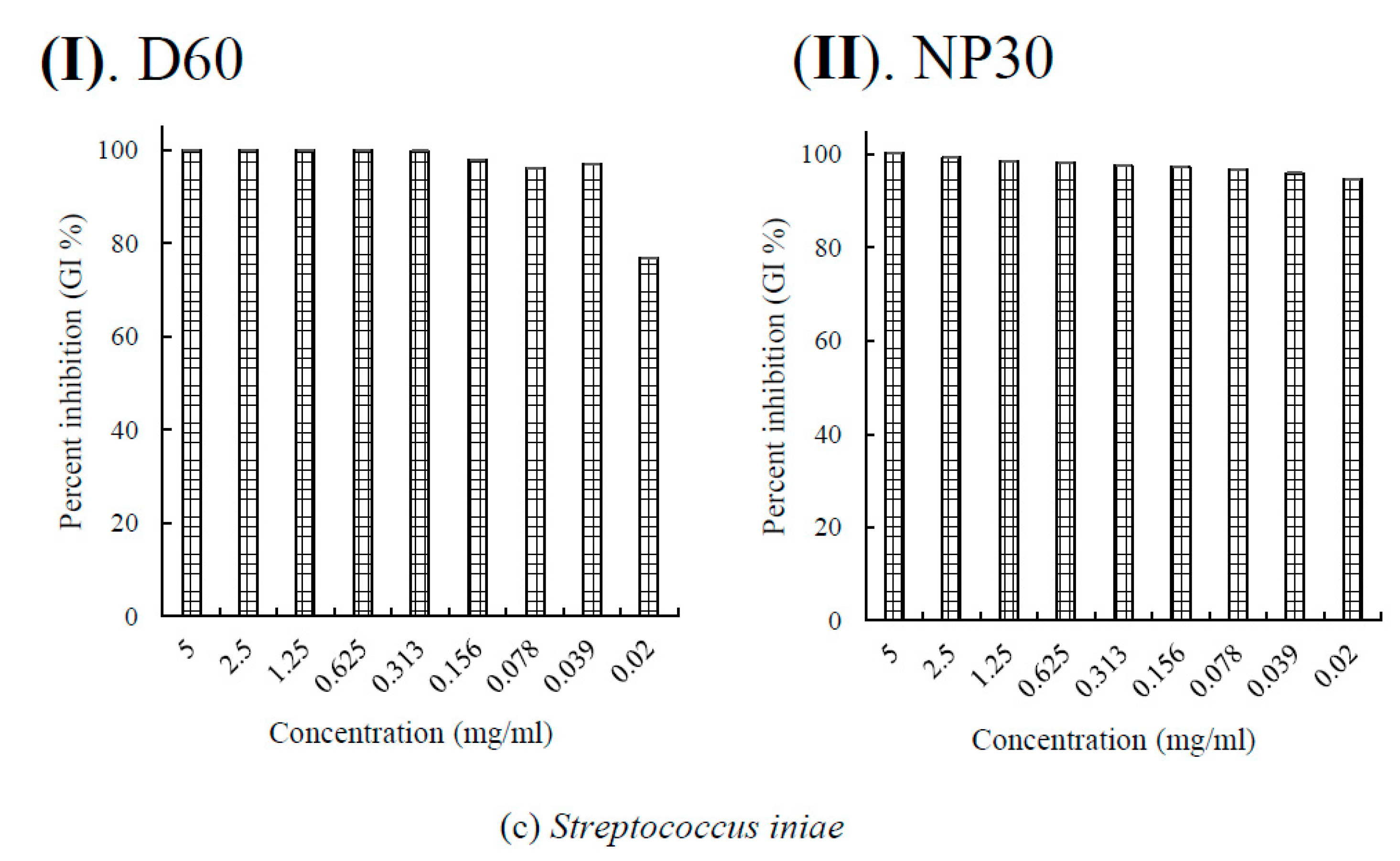
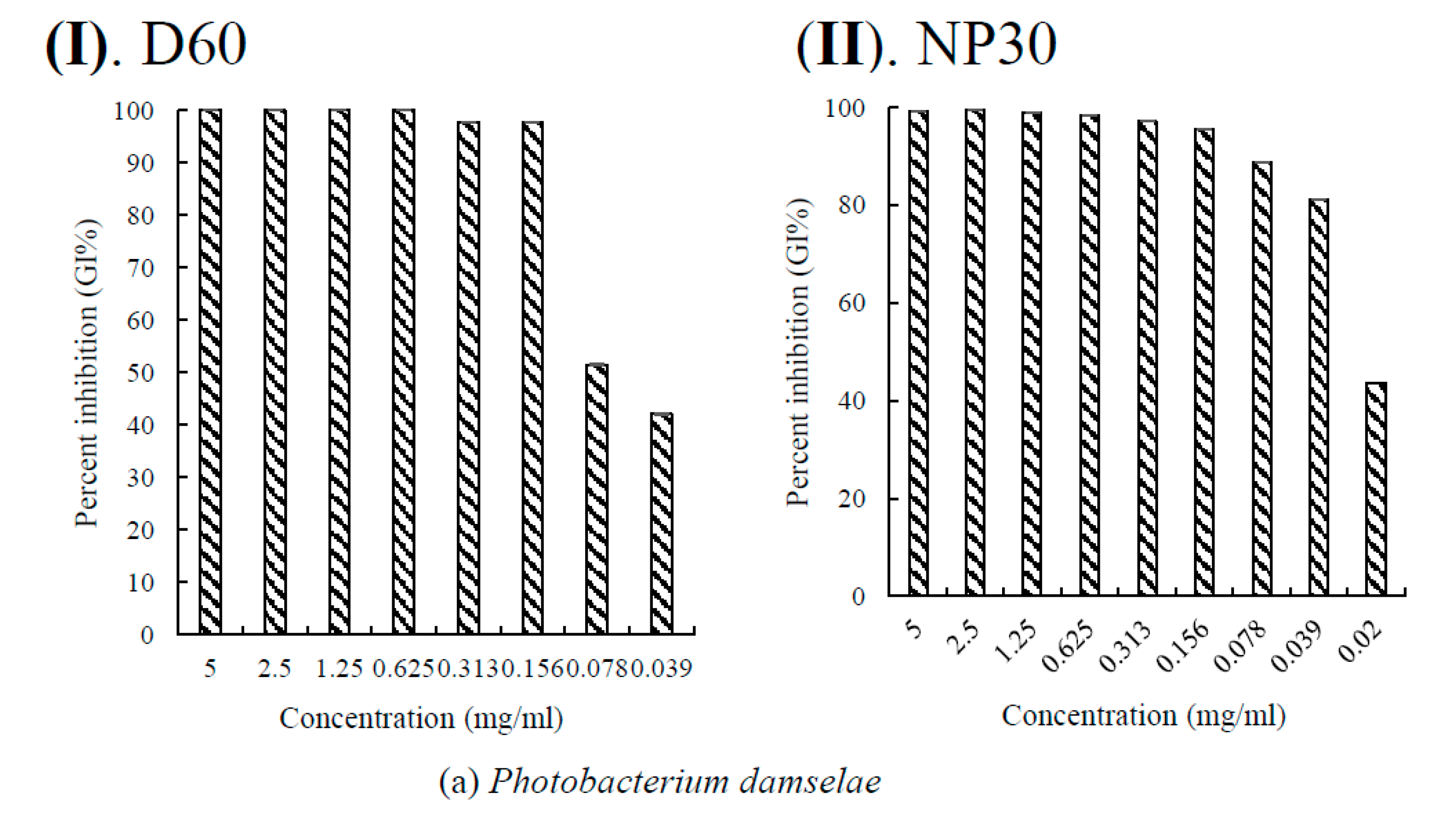
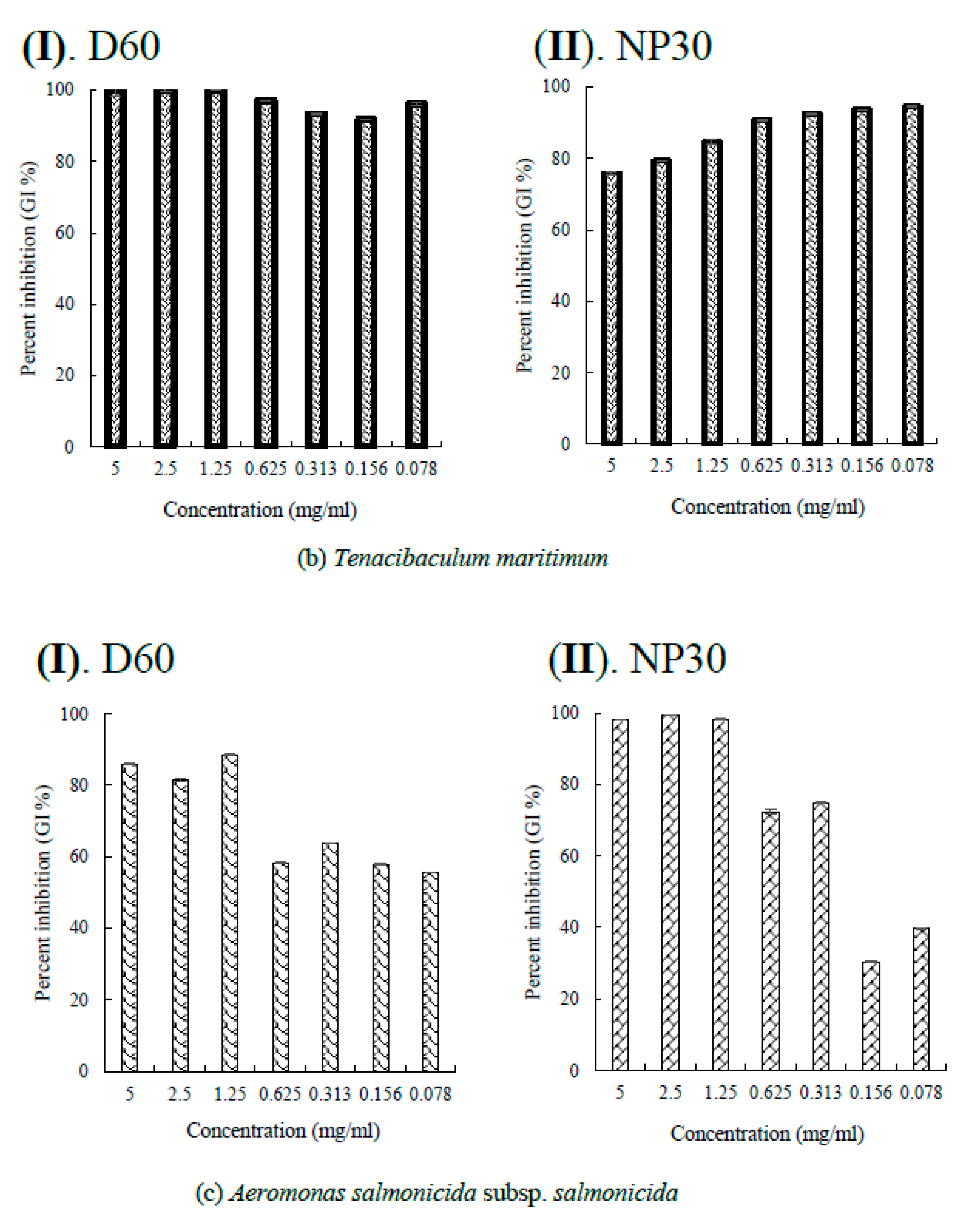
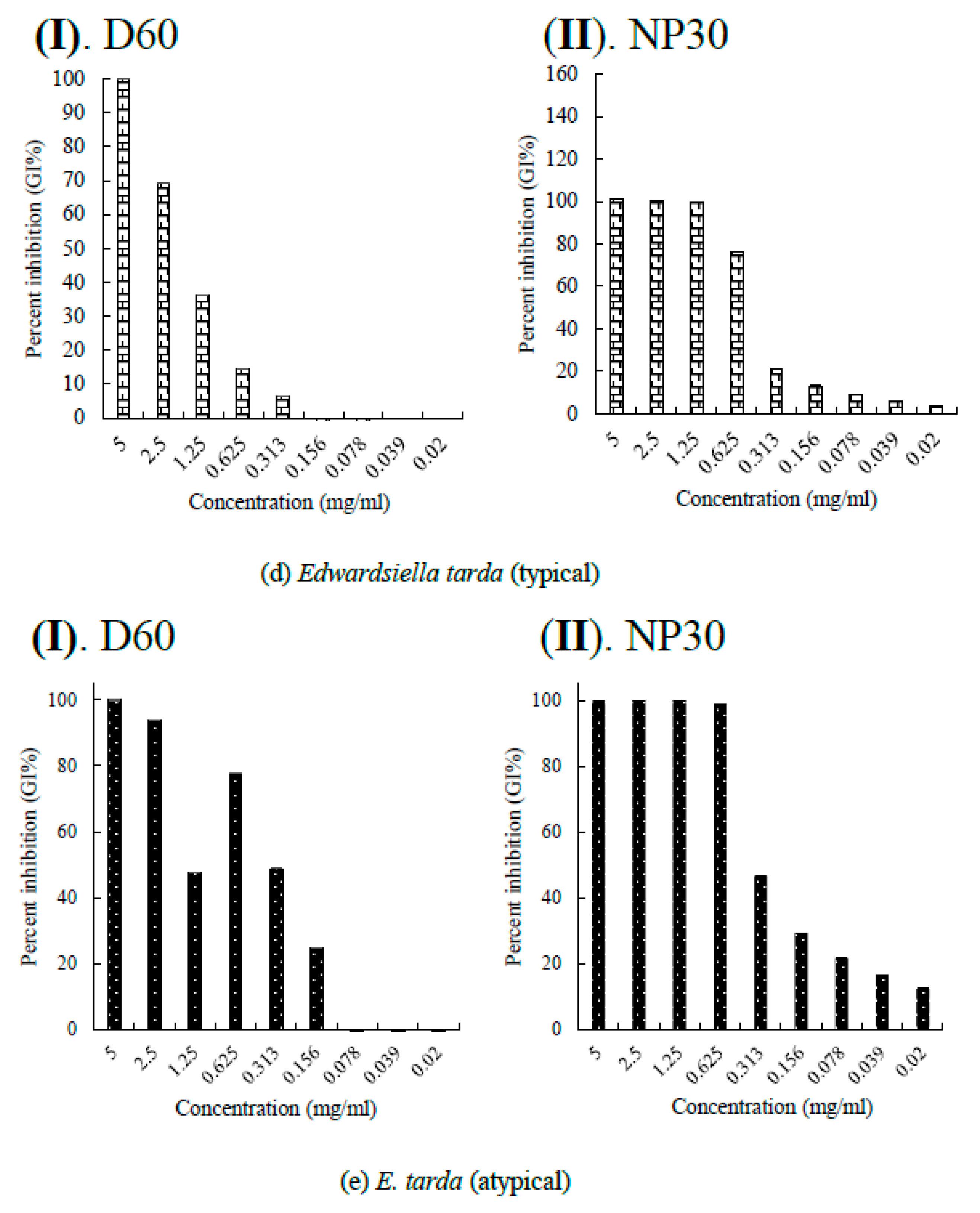

| Bacteria type | Species | NP30 (mg/ml) | D60 (mg/ml) | ||
|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | ||
| Gram-positive bacteria | Lactococcus garvieae | 0.156 | 5.00 | 0.078 | - |
| Streptococcus iniae | 0.078 | 0.078 | 0.078 | - | |
| Nocardia seriolae | 0.313 | 1.25 | 0.313 | - | |
| Gram-negative bacteria | Edwardsiella tarda (typical) | 1.25 | 2.50 | 5.00 | - |
| Edwardsiella tarda (atypical) | 1.25 | 2.50 | 5.00 | - | |
| Aeromonas salmonicida subsp. salmonicida | 1.25 | 2.50 | 5.00 | - | |
| Tenacibaculum maritimum | 0.078 | 0.313 | 0.078 | 0.625 | |
| Photobacterium damselae subsp. piscicida | 0.078 | 5.00 | 0.078 | - | |
| Vibrio anguillarium | 0.625 | 1.25 | 0.313 | 2.50 | |
| V. harveyi | 2.50 | 2.50 | - | - | |
| V. parahaemolyticus | 2.50 | 2.50 | 5.00 | - | |
| V. rotiferanus | 2.50 | 2.50 | 5.00 | - | |
| Parameters | Treatment | ||
|---|---|---|---|
| Control | D60 | NP30 | |
| Initial weight (g) | 35.05 ± 0.42 | 35.07 ± 0.42 | 35.12 ± 0.52 |
| Final weight (g) | 231.75 ± 6.61 | 244.76 ± 6.46 | 258.04 ± 7.65 |
| Relative gain rate (RGR %) | 561.20 ± 6.62 | 598.16 ± 40.48 | 634.78 ± 52.8 |
| Specific growth (% per day) | 2.25 ± 0.71 | 2.31 ± 0.7 | 2.37 ± 0.69 |
| Feed conversion ratio (FCR) | 1.51 ± 0.06 | 1.25 ± 0.31 | 1.26 ± 0.26 |
| Survival rate (%) | 98.89 ± 1.11 | 98.89 ± 1.11 | 100 ± 0.00 |
| Condition factor | 1.75 ± 0.02 | 1.92 ± 0.10 | 1.83 ± 0.06 |
| Hepatosomatic index | 1.03 ± 0.04 | 1.07 ± 0.05 | 1.06 ± 0.07 |
| Viscerosomatic index | 8.47 ± 0.09 | 8.46 ± 0.12 | 8.92 ± 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





