1. Introduction
Chronic obstructive pulmonary disease (COPD) ranks globally as the fourth most common cause of death [
1]. Deterioration of the respiratory symptoms beyond normal daily symptoms called COPD exacerbation which leads to increased morbidity [
2,
3] and mortality [
1,
4].
In a recent study based in the United Arab Emirates (UAE) [
5], the quantitative real-time polymerase chain reaction (qPCR) test demonstrated an overall respiratory viral detection of 37.2% (507/1362) where influenza virus and human rhinovirus (HRV) were the most prevalent (20.0% and 10.7% respectively). The positive rate grew during the winter, peaking in December and falling to its lowest point in September [
5].
In developed countries with temperate temperatures, the epidemiology of respiratory viruses has been thoroughly investigated [
6,
7]. However, epidemiological research on respiratory virus detection in tropical and subtropical regions is scarce, but the epidemiological variety according to local climate and latitude has been well explored [
8,
9,
10].
Knowledge of the regional distribution of respiratory viruses is crucial for preventing and controlling local infection and global health decision-making [
11]. The epidemiology and clinical features of respiratory viral infections in the UAE are poorly understood.
It is unclear whether HRV infections can be associated with exacerbations in their own right or whether they predispose to secondary bacterial infection.
Rapid molecular methods, such as qPCR, should increase the yield of microbial detection and provide a better insight into the microbial association with COPD exacerbation [
12,
13,
14,
15,
16].
Our study's objective was to describe the association between HRV detection and COPD exacerbation and determine the molecular epidemiology, including the seasonality of HRV in Abu Dhabi, UAE over one year.
2. Materials and Methods
2.1. Patients and Sputum Samples
The sputum samples used in this study were collected from COPD patients in an emergency department in Abu Dhabi, UAE, over one year from November 2021 to October 2022. Ethical approval was obtained from Fatima College of Health Sciences (IRB-UAE-2021-125 and IRB356), and a consent form was obtained from every participant in this study.
Expectorated sputum samples from patients with recognized COPD were acquired for standard evaluation, and the patients were regularly checked for pulmonary exacerbation for the duration of this investigation. The expectorated sputum samples were taken within 24 hours from the onset of the exacerbation (E1) and 14 days after the exacerbation episode and taking the appropriate treatment (E2). Samples were obtained, processed, and stored in a -80oC freezer for retrospective analysis at the completion of the study. Frozen samples were transferred to the laboratory on dry ice.
2.2. Human Rhinovirus Detection
Nucleic acid extraction (RNA) from sputum samples and concentration measurements were performed according to our recent publications [
17,
18,
19].
Using the SuperScriptTM III PlatinumTM One-Step qRT-PCR Kit (Invitrogen, Thermo Fischer, UK), a master mix solution was prepared based on previously reported qPCR assays [
20,
21], with some modifications as described in
Table 1 where 11 μl aliquots of the amplification mix were transferred into 96 white LightCycler-480 multi-well plates (Roche Diagnostics Ltd., UK). HRV detection for the sputum specimens was carried out using specific primers [
18] that targeted the HRV genome’s highly conserved “5’ non-coding region” (5’-NCR) (Invitrogen, Thermo Fischer, UK).
The eluted nucleic acid from each sputum sample was transferred in 4 μl volumes into the wells of the plates containing the amplification mix. LightCycler-480 sealing foil was then used to re-seal the plates before centrifugation at 4000 rpm (2576 g). The plates were then placed in the LightCycler-480 (Roche Diagnostics, West Sussex, UK) for amplification according to the following real-time qPCR cycling conditions: [50 °C for 15-min, 95 °C for 2-min, 45 cycles of 95 °C for 15s then 60 °C for 30-s, and finally 60 °C for 30-s].
The absolute quantification strategy was used to analyze the samples. A positive result was assigned for the cycle threshold (Ct) values less than or equal to 40 cycles. On the other hand, results were considered negative for either Ct values of greater than 40 cycles or in the absence of detectable Ct [
17,
18,
22,
23].
2.3. Statistical Analysis
Data were analyzed using SPSS Statistics v.26 (IBM Corporation, New York, NY, USA). The Shapiro-Wilk test for normality was applied. The probability value of p <0.05 was considered statistically significant.
A comparison of the proportions of positive samples for HRV between the E1 and E2 was conducted with the McNemar’s test for related samples.
3. Results
3.1. Study Participants
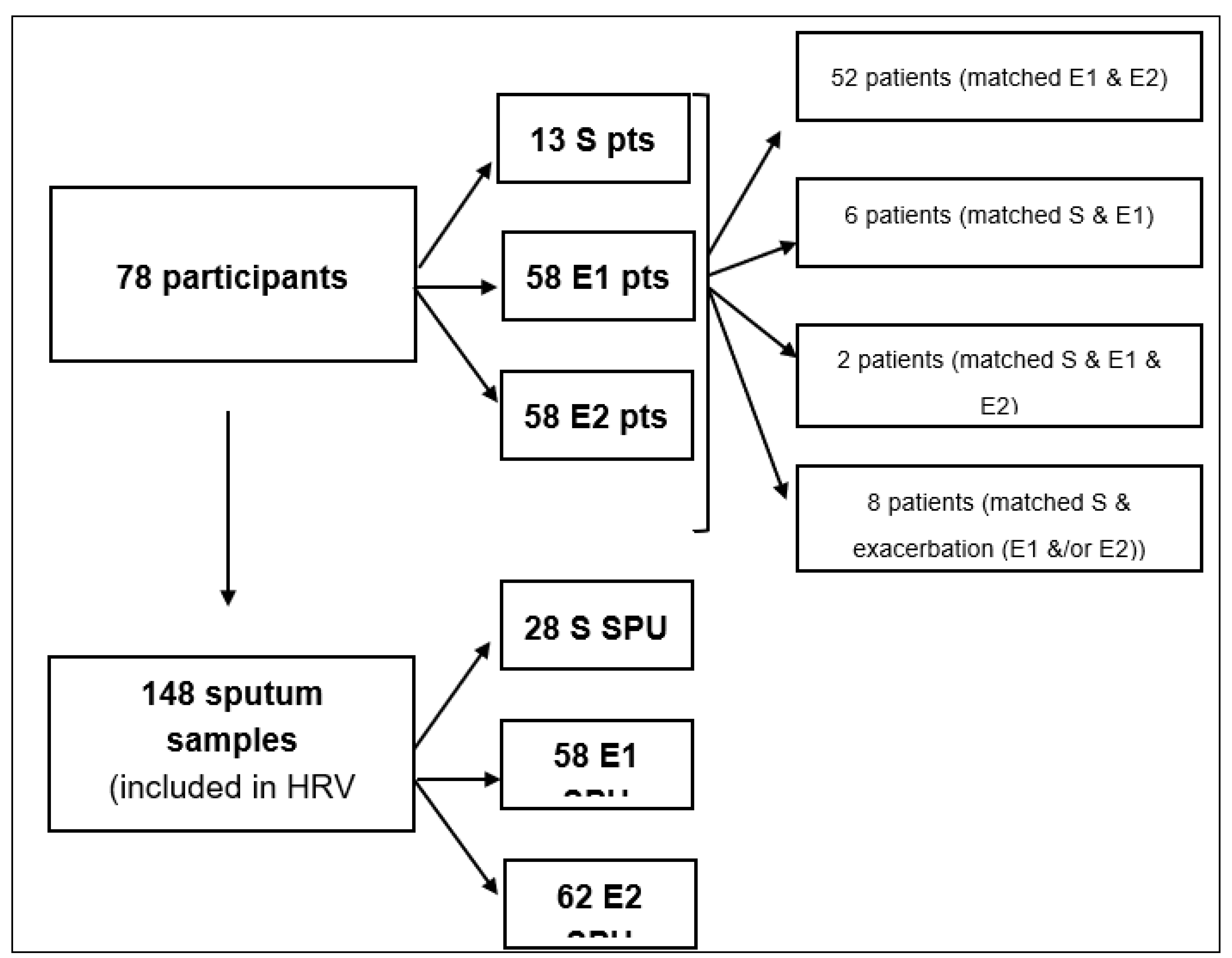
Seventy-eight COPD patients participated in the study, of which 58 (74%) patients presented with one or more exacerbation episodes. The participants' mean age (range) was 57.3 (45-77) years. The male gender of the participants represented 46/78 (59%). A total of 58 exacerbation events from 58 patients were included in the study.
Figure 1 illustrates the flow chart of COPD patients and sample groups included in the present study. Thirteen patients visited the department with a stable state of COPD and were excluded from the investigation due to the insignificant comparison.
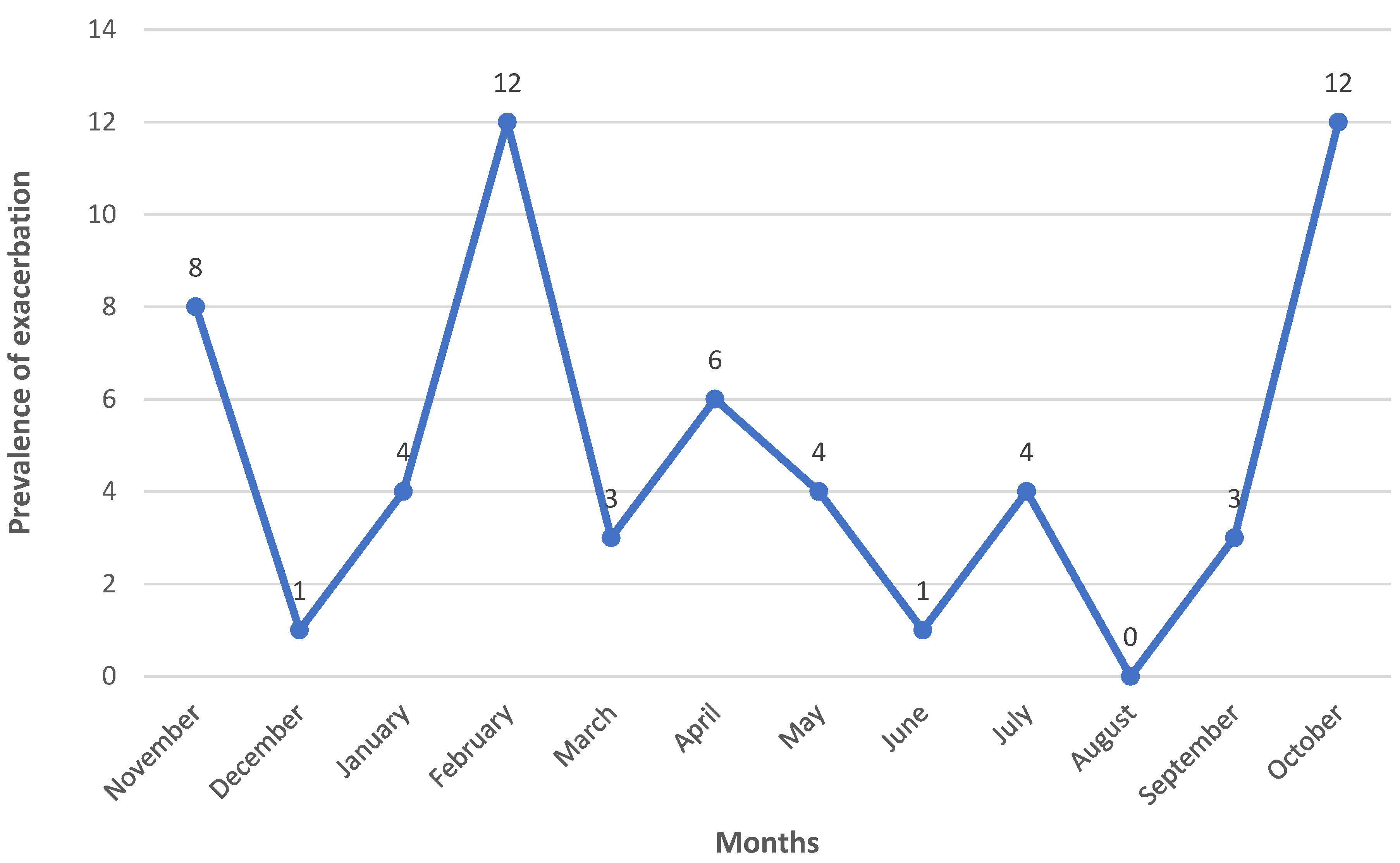
The number of pulmonary exacerbations (N=58) of COPD was recorded across the entire study interval (
Figure 2). The prevalence of COPD pulmonary exacerbation was higher throughout the winter and autumn months, followed by the spring months, with the lowest detection level occurring in the summer months.
3.2. HRV Detection in COPD Patients
HRV positivity in patients during exacerbation (E1) was 11/58 (19%) and 15/58 (26%) two weeks after the exacerbation episode (E2) (
Table 2). There was no significant difference in the HRV load in these patients.
To compare the proportion of HRV detection between E1 and E2 on a matched basis, fifty-two patients were investigated (
Table 3). At the exacerbation events, 10/52 (19%) patients were HRV-positive. Two weeks following the exacerbation (E2), the number of HRV-positive patients had increased to 14/52 (27%). This change resulted from 10 HRV-negative patients at the E1 state becoming HRV-positive at E2. Four patients were HRV-positive at E1 and E2, but six were initially (E1) HRV-positive becoming HRV-negative two weeks following the exacerbation (E2). Indeed, 8% more participants (compared to the total) were HRV-positive two weeks after the exacerbation (
Table 3). The related samples McNemar's test with continuity correction [
24] was used to conclude whether a significant difference exists in the proportion of HRV-positive at the E1 and E2 states. The ratio of HRV-positive increased from the E1 state value of 19% to 27% at E2 state, a statistically insignificant difference, χ2(1) = 0.562, p = 0.454 (
Table 3).
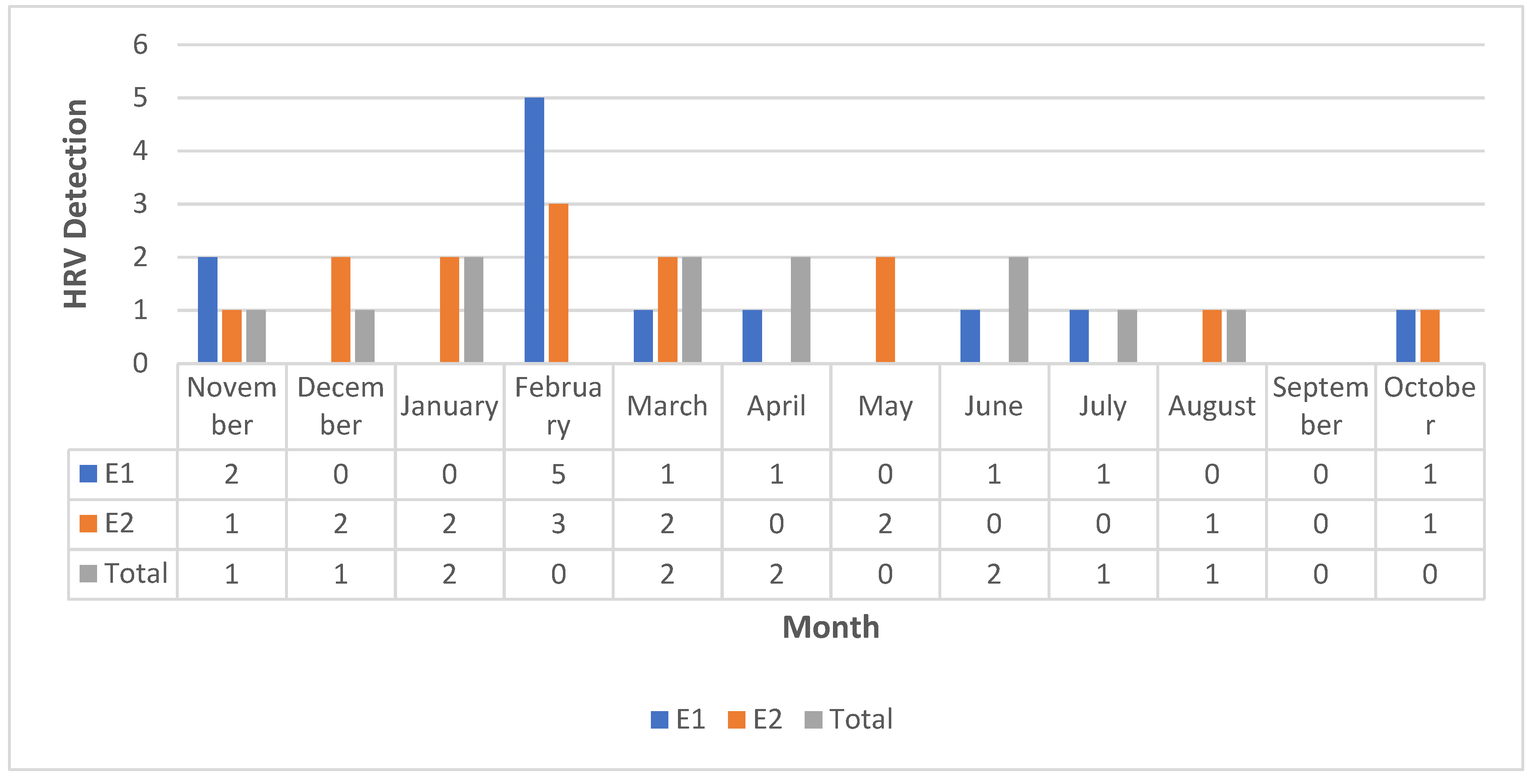
Based on a review of HRV detection during this study, it was evident that HRV was detected throughout the winter and spring, with the lowest detection level occurring in the summer months (
Figure 3). Four patients experienced HRV dual detection during the study (
Table 4).
4. Discussion
This study aimed to determine the rate of HRV detection and seasonality in COPD patients in Abu Dhabi, UAE, and to assess the association of HRV with COPD exacerbations.
HRV positivity in patients during exacerbation (E1) and two weeks after the exacerbation episode (E2) were 11/58 (19%) and 15/58 (26%), respectively. The consequences of respiratory HRV infections on the progression of COPD require a study with a larger matched sample size. We could therefore speculate that the impact of HRV on exacerbation is probably underestimated in patients with COPD in this study. Nevertheless, the fact that the role of HRV infection in COPD has not been duly acknowledged should not be overlooked which stems from how patients undergo assessment at the point when they present (i.e., normally after the emergence of the exacerbation, thereby meaning that observation of the HRV could be unlikely).
The proportion of HRV detection increased from the E1 state value of 10/52 (19%) to 14/52 (27%) at E2 state when fifty-two COPD patients were investigated on a matched basis, p = 0.454. Nevertheless, it has not been possible to identify the nature of the relationship between HRV detection and COPD pulmonary exacerbation. HRV infection may result in COPD exacerbation and elevated airway inflammation. Still, the plausibility of a reversed relationship must also be entertained (namely, that individuals who suffer from COPD exacerbation could be more likely to have HRV infections). To figure out the precise relationship, a close longitudinal follow-up with repeated respiratory samples assessment is required. This will also assist in revealing whether HRV infection in COPD tends to be a re-infection or persistent in nature, especially since four patients experienced HRV twice detection during our study period.
Furthermore, the pattern of HRV detection seasonality observed in this study, where the rate peaked during winter and bottomed in the summer, might be attributed to the higher rate of observation of pulmonary exacerbations during the winter either in this study or previous researche [
25]. The increasing incidence of pulmonary exacerbations during winter may result in detrimental consequences such as increased morbidity in addition to the substantial burden on healthcare services. The precise cause of the seasonality exacerbations is not well known but thought to be partially due to the increased prevalence of respiratory viral infections during the cold and damp whether [
26]. The exact mechanism by which cold weather increases the susceptibility of viral infection is not well understood but could be attributed to the inhibition of respiratory defensive mechanisms by the cold air. A cold environment might favor the survival of HRV, which may facilitate transmission and cross-infection [
27]. Increased susceptibility to HRV infection may also be mediated by high airway inflammation. The possibility that the winter peak may, in part, relate to patients using less controller medication immediately before the period when they may be at the greatest risk of HRV-related COPD exacerbations. The seasonality of pulmonary exacerbations informs us about the triggers of these exacerbations and suggests possible strategies to reduce their number. Understanding the seasonal pattern of HRV infection may help determine how best to employ the investigated anti-rhinoviral agents or vaccines in patients presenting with symptoms of HRV respiratory infection in COPD patients.
It is not clear whether HRV infections can be associated with COPD exacerbations or whether they predispose to secondary bacterial infection. Moreover, it remains unclear what are the exact impacts of HRV infection on the bacterial communities and the clinical outcome of COPD patients.
In a recent study in the UAE [
5]., the positive respiratory viral rate grew during the winter, peaking in December and falling to its lowest point in September. In our study focusing on COPD, the highest HRV detection was in February, and the lowest was in September.
The increased frequency of HRV in the UAE may be attributable to the ease with which patients with a common cold can visit the emergency room. However, the prevalence of HRV may have been exaggerated. Many patients who tested positive for HRV at the emergency department may have bacterial coinfections or concomitant comorbidities.
The UAE is situated in a subtropical region and has no rainy season. The majority of the population of the UAE is exposed to a dry, airconditioned atmosphere for the bulk of the day, particularly during the summer months when temperatures vary from 39 to 45°C.
Despite this relatively controlled environment, the summer peak of influenza in the UAE could be explained by prolonged effective contact rates as a result of increased indoor activity and decreased relative humidity, both of which have been linked to the incidence of viral illnesses [
28,
29].
The main limitations of this study were the relatively small sample size for the matched patients and including only a single sampling time point during each event. As such, the statistical comparison results may not represent a larger population. However, the findings should add valuable information and have many advantages over the previous studies in this field.
5. Conclusions
According to our knowledge, this is the first study to characterize the relationship between HRV detection using qPCR and COPD exacerbations and the HRV seasonal trend in the UAE. In addition, the detection method's high sensitivity facilitated the collection of more accurate epidemiologic data. Very few research have supplied similar information for the Middle East to date. The seasonality of HRV detection and COPD exacerbation was comparable to that observed in temperate countries. This information can serve as a foundation for future, more comprehensive surveillance investigations of respiratory viruses in the UAE and the Middle East and their association with COPD exacerbations.
Author Contributions
The first and corresponding author was involved in all parts of the study and manuscript preparation. The M.A.M author was involved in the methodology and design part. All of the authors were involved in the literature search and writing.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval was obtained from Fatima College of Health Sciences (IRB-UAE-2021-125 and IRB356) and a consent form was obtained from every participant in this study.
Informed Consent Statement
a consent form was obtained from every participant in this study.
Data Availability Statement
NA.
Acknowledgments
The authors would like to acknowledge the participants and the healthcare team supported this study.
Conflicts of Interest
The authors reports no conflicts of interest in this work.
References
- GOLD, GLOBAL STRATEGY FOR THE DIAGNOSIS, MANAGEMENT, AND PREVENTION OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE. 2022.
- Wedzicha, J. A.; Seemungal, T. A. R. , COPD exacerbations: defining their cause and prevention. The Lancet 2007, 370(9589), 786–796. [Google Scholar] [CrossRef]
- Seemungal, T. A.; Donaldson, G. C.; Paul, E. A.; Bestall, J. C.; Jeffries, D. J.; Wedzicha, J. A. , Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine 1998, 157(5), 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Soler-Cataluna, J. J.; Martinez-Garcia, M. A.; Roman Sanchez, P.; Salcedo, E.; Navarro, M.; Ochando, R. , Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005, 60(11), 925–31. [Google Scholar] [CrossRef]
- Jeon, J. H.; Han, M.; Chang, H. E.; Park, S. S.; Lee, J. W.; Ahn, Y. J.; Hong, D. J. , Incidence and seasonality of respiratory viruses causing acute respiratory infections in the Northern United Arab Emirates. Journal of medical virology 2019, 91(8), 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Tanner, H.; Boxall, E.; Osman, H. , Respiratory viral infections during the 2009-2010 winter season in Central England, UK: Incidence and patterns of multiple virus co-infections. European Journal of Clinical Microbiology and Infectious Diseases 2012, 31(11), 3001–3006. [Google Scholar] [CrossRef] [PubMed]
- Ambrosioni, J.; Bridevaux, P. O.; Wagner, G.; Mamin, A.; Kaiser, L. , Epidemiology of viral respiratory infections in a tertiary care centre in the era of molecular diagnosis, Geneva, Switzerland, 2011-2012. Clinical Microbiology and Infection 2014, 20(9), O578–O584. [Google Scholar] [CrossRef]
- Paynter, S. , Humidity and respiratory virus transmission in tropical and temperate settings. Epidemiology and Infection 2015, 143(6), 1110–1118. [Google Scholar] [CrossRef]
- Tang, J. W.; Loh, T. P. , Correlations between climate factors and incidence-a contributor to RSV seasonality. Reviews in Medical Virology 2014, 24(1), 15–34. [Google Scholar] [CrossRef]
- Tamerius, J. D.; Shaman, J.; Alonso, W. J.; Bloom-Feshbach, K.; Uejio, C. K.; Comrie, A.; Viboud, C., Environmental Predictors of Seasonal Influenza Epidemics across Temperate and Tropical Climates. PLoS Pathogens 2013, 9, (3).
- Ratnam, I.; Black, J.; Leder, K.; Biggs, B. A.; Gordon, I.; Matchett, E.; Padiglione, A.; Woolley, I.; Karapanagiotidis, T.; Gherardin, T.; Demont, C.; Luxemburger, C.; Torresi, J. , Incidence and risk factors for acute respiratory illnesses and influenza virus infections in Australian travellers to Asia. Journal of Clinical Virology 2013, 57(1), 54–58. [Google Scholar] [CrossRef]
- Desai, H.; Eschberger, K.; Wrona, C.; Grove, L.; Agrawal, A.; Grant, B.; Yin, J.; Parameswaran, G. I.; Murphy, T.; Sethi, S. , Bacterial colonization increases daily symptoms in patients with chronic obstructive pulmonary disease. Annals of the American Thoracic Society 2014, 11(3), 303–309. [Google Scholar] [CrossRef]
- Sethi, S.; Murphy, T. F. , Infection in the pathogenesis and course of chronic obstructive pulmonary disease. New England Journal of Medicine 2008, 359(22), 2355–2365. [Google Scholar] [CrossRef]
- Alsayed, A. R.; Abed, A.; Khader, H. A.; Al-Shdifat, L. M. H.; Hasoun, L.; Al-Rshaidat, M. M. D.; Alkhatib, M.; Zihlif, M., Molecular Accounting and Profiling of Human Respiratory Microbial Communities: Toward Precision Medicine by Targeting the Respiratory Microbiome for Disease Diagnosis and Treatment. Int J Mol Sci 2023, 24, (4). [CrossRef]
- Alsayed, A. R.; Al-Dulaimi, A.; Alkhatib, M.; Al Maqbali, M.; Al-Najjar, M. A. A.; Al-Rshaidat, M. M. D., A comprehensive clinical guide for Pneumocystis jirovecii pneumonia: a missing therapeutic target in HIV-uninfected patients. Expert Rev Respir Med 2022, 16, (11-12), 1167-1190. [CrossRef]
- Alsayed, A. R.; Abed, A.; Jarrar, Y. B.; Alshammari, F.; Alshammari, B.; Basheti, I. A.; Zihlif, M. , Alteration of the Respiratory Microbiome in Hospitalized Patients with Asthma–COPD Overlap during and after an Exacerbation. Journal of Clinical Medicine 2023, 12(6), 2118. [Google Scholar] [CrossRef]
- Al-Dulaimi, A.; Alsayed, A. R.; Maqbali, M. A.; Zihlif, M. , Investigating the human rhinovirus co-infection in patients with asthma exacerbations and COVID-19. Pharm Pract (Granada) 2022, 20(2), 2665. [Google Scholar] [CrossRef]
- Alsayed, A.; Al-Doori, A.; Al-Dulaimi, A.; Alnaseri, A.; Abuhashish, J.; Aliasin, K.; Alfayoumi, I. , Influences of bovine colostrum on nasal swab microbiome and viral upper respiratory tract infections - A case report. Respir Med Case Rep 2020, 31, 101189. [Google Scholar] [CrossRef] [PubMed]
- Alsayed, A. R.; Hasoun, L.; Khader, H. A.; Abu-Samak, M. S.; Al-Shdifat, L. M.; Al-Shaimari, B.; Al Maqbali, M. , Co-infection of COVID-19 patients with atypical bacteria: A study based in Jordan. Pharmacy Practice 2023, 21(1), 1–5. [Google Scholar] [CrossRef] [PubMed]
- Scheltinga, S.; Templeton, K.; Beersma, M.; Claas, E. , Diagnosis of human metapneumovirus and rhinovirus in patients with respiratory tract infections by an internally controlled multiplex real-time RNA PCR. Journal of clinical virology 2005, 33(4), 306–311. [Google Scholar] [CrossRef] [PubMed]
- Scheltinga, S.; Templeton, K.; Beersma, M.; Claas, E., Erratum to “Diagnosis of human metapneumovirus and rhinovirus in patients with respiratory tract infections by an internally controlled multiplex real-time RNA PCR”[J. Clin. Virol. 33 (2005) 306–311]. Journal of Clinical Virology 2005, 34, (3), 231.
- Alsayed, A. R.; Talib, W.; Al-Dulaimi, A.; Daoud, S.; Al Maqbali, M. , The first detection of Pneumocystis jirovecii in asthmatic patients post-COVID-19 in Jordan. Bosn J Basic Med Sci 2022, 22(5), 784–790. [Google Scholar] [CrossRef]
- Alsayed, A. R.; Abed, A.; Abu-Samak, M.; Alshammari, F.; Alshammari, B. , Etiologies of Acute Bronchiolitis in Children at Risk for Asthma, with Emphasis on the Human Rhinovirus Genotyping Protocol. Journal of Clinical Medicine 2023, 12(12), 3909. [Google Scholar] [CrossRef]
- Edwards, A. L. , Note on the “correction for continuity” in testing the significance of the difference between correlated proportions. Psychometrika 1948, 13(3), 185–187. [Google Scholar] [CrossRef]
- Ortiz, J. R.; Neuzil, K. M.; Victor, J. C.; Wald, A.; Aitken, M. L.; Goss, C. H. , Influenza-associated cystic fibrosis pulmonary exacerbations. Chest 2010, 137(4), 852–60. [Google Scholar] [CrossRef]
- Johansen, H.; Høiby, N. , Seasonal onset of initial colonisation and chronic infection with Pseudomonas aeruginosa in patients with cystic fibrosis in Denmark. Thorax 1992, 47(2), 109–111. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G. C.; Wedzicha, J. A. , The causes and consequences of seasonal variation in COPD exacerbations. International journal of chronic obstructive pulmonary disease 2014, 9, 1101. [Google Scholar] [CrossRef]
- Fisman, D. , Seasonality of viral infections: Mechanisms and unknowns. Clinical Microbiology and Infection 2012, 18(10), 946–954. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, Y. , Airborne spread of infectious agents in the indoor environment. American Journal of Infection Control 2016, 44(9), S102–S108. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).