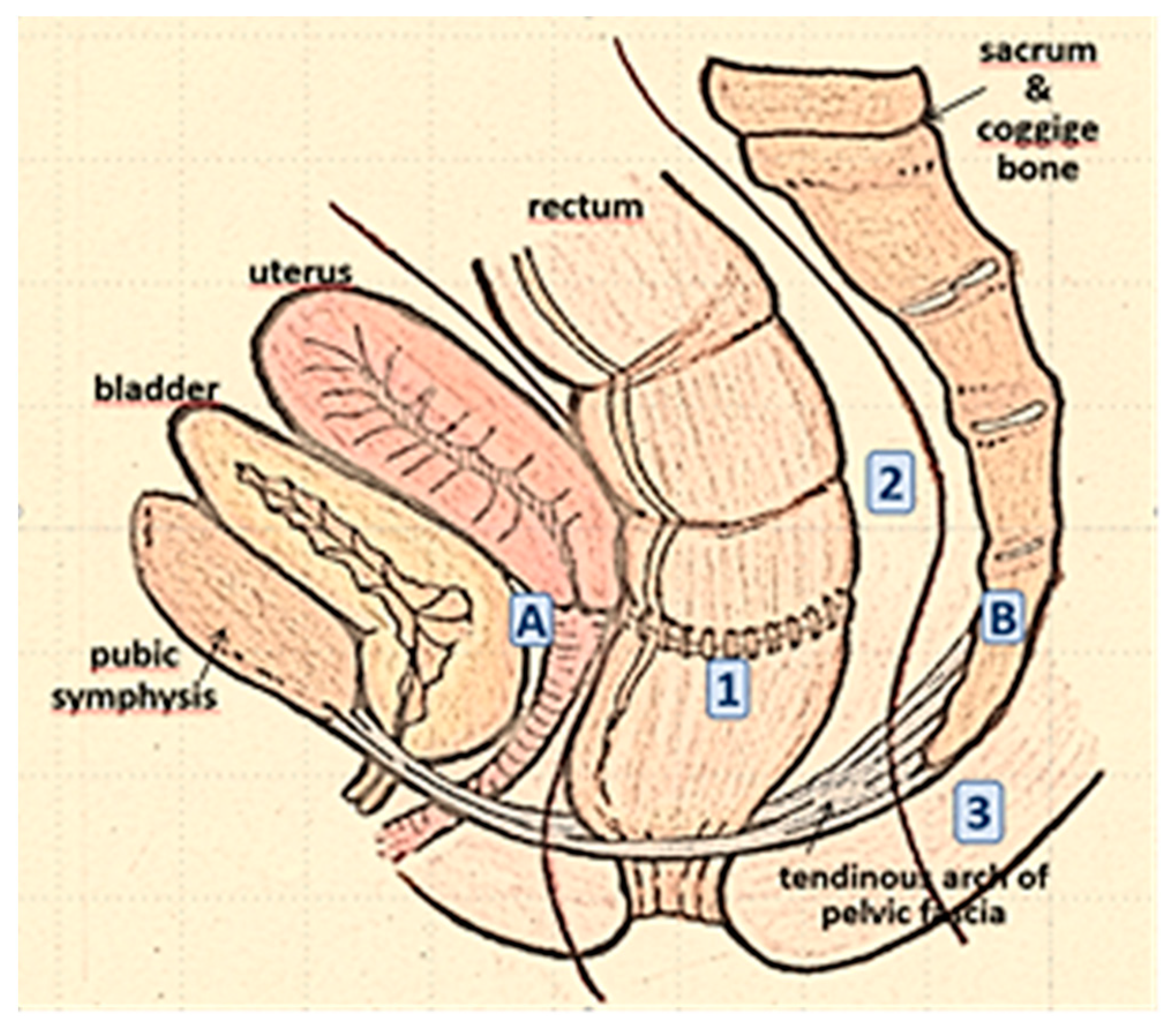
1. Introduction
Carcinoma or cancer of the rectum is a malignant tumor is a form of cancer which contributes signficantly to the spectrum of large intestine malignancies, accounting for approximately 30-35% of all cases within this category. Colorectal cancer, which encompasses both colon and rectal cancers, collectively constitutes around 10% of all diagnosed cancers. In Italy, this disease poses a substantial health burden, with an annual diagnosis rate of 12,000 individuals. It is estimated that 40 out of every 100,000 inhabitants are affected by rectal cancer, leading to an unfortunate yearly toll of 7,000 lives lost due to this disease1.
One notable aspect of colorectal cancer is that a significant proportion of patients, approximately 80%2, have tumors that are amenable to curative surgery (R0 resection). This implies that the tumor can be surgically removed without any residual cancerous tissue. However, despite the initial success of surgical intervention, a concerning phenomenon arises: approximately 40% of these patients experience disease relapse, often occurring within the first three years following surgery3. This relapse, known as local recurrence (L.R.), is characterized by the reappearance of malignant lesions at or near the site of the previous surgical procedure. Local recurrence after curative surgery of rectum cancer remains a clinical challenge today that requires a multidisciplinary approach and careful selection of patients suitable for surgery. The main risk factors associated with the onset of local recurrences include incomplete removal of the mesorectum, the presence of a resection margin affected by the neoplasm (some authors report an 85% risk of local recurrence in these cases4), a disease-free margin of less than 2 mm5, tumor staging as B or C according to Dukes (with risks of 16.3% and 28.6%, respectively6), and onset in the lower third of the rectum7. Conversely, the use of surgical techniques such as Trans-Anal Endoscopic Microsurgery (TEM) and the presence of a disease-free margin of 6 mm or more have been associated with a lower risk of local recurrences8. Additionally, a higher risk of local recurrences has been observed in tumors with mucous histology9.
The pelvis is the primary site for local disease recurrence in rectal cancer: metastases at this level are mostly asymptomatic in many cases (50%) or may present with symptoms such as sacral and perineal pain or rectal bleeding10 and they are typically discovered during imaging exams performed during follow-up11. Advances in TME surgery and the use of adjuvant and neoadjuvant therapies have reduced pelvic recurrence rates from 20-30% to 6-10% in recent population studies. Pelvic recurrences typically manifest within the first 5 years post-surgery, with 70% occurring in the first 2 years and 85% in the first 3 years. Surgical intervention with R0 resection, achievable in over 50% of cases, remains the sole potentially curative option, offering a 5-year survival rate of around 30%12. However, these surgeries can be complex, costly, and associated with significant morbidity13. Consequently, only a limited number of patients can benefit from them.
The aim of this study is to frame the diagnostic-therapeutic problem of pelvic recurrence of rectum cancer, highlighting the current surgical standards and the possible role of the minimally invasive approach. It is necessary to identify an approach to the treatment of recurrences that may be shared. Although the method of presentation cannot be identical in the different patients, it is necessary to identify whether the approach using the laparoscopic technique, the robotic technique, the open-sky technique and/or chemotherapy or radiotherapy is better and when and how these resources can be harmonized. This retrospective examination of our surgical case study wishes to enter the debate to propose a possible line of action to adopt according to the site of the recurrence.
2. Materials and Methods
We examined, retrospectively, all the patients diagnosed with cancer of the rectum between 2008 and 2018 treated at the "Pietro Valdoni" Department of Surgery and monitored their follow-up for 5 years. The sample consists of 368 patients with rectal neoplasm, 136 females and 232 males, with an average age of 65.8 (the range was 37-86 years). Subsequently, we conducted a literature search to compare the results of our experience with those of other authors.
3. Results
Among the 368 patients, 103 had cancer located in the upper rectum (28%), 119 in the middle rectum (32.3%), 102 in the lower rectum (27.7%), 31 at the right/sigma junction (8.4%), and 13 had unknown primary location (3.5%).
Curative surgery was feasible in 288 of these patients (78.26%), with 75 patients (20.4%) receiving neoadjuvant chemotherapy. Out of the 288 surgically treated rectal cancer patients, 31 (11%) experienced local recurrence. Additionally, 11 patients who had surgery for rectal cancer in another hospital were included in the study, making a total of 42 patients (with an average age of 65.8 years, ranging from 37 to 86). For the purposes of our study 35 out of the 42 patients who were free from distant metastases at the time of their first surgical operation were examined (
Table 1).
In terms of primary tumor characteristics, 9 cases were T4 (25.7%), 18 cases were T3 (51.4%), 7 cases were T2 (20%), and 1 case was T1 (2.8%). The surgeries were performed using the open technique, with 21 patients undergoing anterior resection (60%), 9 patients undergoing perineal abdominal amputation according to Miles's technique (25.7%), 4 patients using Hartmann's surgical approach (11.4%), and 1 patient with TEM (Trans anal endoscopic microsurgery (2.8%). R0 resection was achieved in 31 cases (88.5%), while R1-R2 resection was done in 4 cases (11.5%). Neoadjuvant chemoradiation therapy was performed in 6 patients (17.1%), and 20 patients (57.1%) underwent adjuvant chemoradiation therapy.
Regarding the site of recurrence, 13 patients developed an anastomotic recurrence (37.1%), 12 had central-pelvic recurrences (31.4%), 9 had presacral recurrences (25.7%), and 2 had perineal recurrences (5.7%). Commonly infiltrated organs included the ureters, bladder, sacral and pelvic bones, with no documented involvement of the sciatic nerve or iliac vessels.
The disease-free survival interval between the diagnosis of recurrence and the previous surgery for the primary tumor was 13.4 months, ranging from 3 to 51 months. Abdominal pain was the primary symptom in 27 cases (77.1%), a palpable mass in 16 cases (45.7%), and an increase in tumor markers (CEA) was the initial sign in 12 patients (34.3%).
In 7 patients (20%), surgery was not feasible due to widespread lung and liver metastases (4 cases) or invasion of the sacral or pelvic bones. Palliative surgical therapy (ileum/colostomy) was performed in 5 cases (14.3%), while curative surgical therapy was carried out in the remaining 23 cases (65.7%) (
Table 2).
Out of these 23 patients, 7 underwent a new recto-colic resection (all of whom had previously undergone RAR), 10 underwent excision of the recurrence (5 of whom had a previous RAR, 4 had an abdominoperineal amputation, and 1 had a Hartmann intervention), and 6 had abdominoperineal amputation according to Miles (4 with a previous RAR, 1 with a Hartmann intervention, and 1 with a TEM). All procedures were performed using an open technique.
In 18 cases (64.2%), the resection was radical (R0), with a 2-year follow-up survival rate averaging 29.2 months, ranging from 9 to 85 months after the diagnosis of relapse. In the remaining cases (R1-R2), recurrence progression was observed in the following months, ranging from 2 to 13 months, with an average of 5 months.
Regarding integrated therapy, 23 patients (78.5%) underwent adjuvant chemotherapy and radiotherapy. Mortality was 7.1%, and morbidity was 42.8%, primarily due to anastomotic dehiscence (6 cases).
4. Discussion
The management of pelvic metastases from colorectal cancer is complex. Even the classification of these metastases in the literature lacks unanimous consensus, with numerous classifications such as the Mayo Clinic classification
12 (based on the site of fixation and symptomatology), the Memorial Sloan Kettering classification
13(
Figure 1), and the Royal Marsden Hospital classification
14 (based on the degree of invasion of one or more of the seven pelvic compartments described in pre-operative MRI).
The treatment of recurrence of rectal carcinoma is multimodal. The location and extent of the recurrence, together with an evaluation of previous treatment, should be considered when seeking guidance for the choice of the appropriate treatment strategy to adopt.
In many of the studies in literature, about 40-50% of patients with local recurrence are considered candidates for surgical exploration, though only 30-40% undergo R0 resection15.
Whatever the type of resection, it should always be performed by removing the recurrence and the affected adjacent organs or structures en-bloc. Careful patient selection is essential to exclude formal contraindications to surgery (
Table 3).
In the pre-Total Mesorectal Excision (TME) era, recurrences were often confined to the residual mesorectum or anastomotic sites. Today, recurrences can occur in any pelvic compartment, often necessitating extensive surgery.
The surgical approach typically involved a median navel-pubic laparotomy, preserving the lower epigastric vessels for potential use in a rectus abdominis muscle flaps. Several intraoperative scenarios could arise with this approach16:
- -
Non-fixed recurrence located at the anastomotic site or in the central compartment of the pelvis or in the perineal wound. In these cases, radical resection can be achieved by "limited" surgery: extensive local excision or rescue AAP or, if feasible, rectocolic resection.
- -
Anterior pelvic recurrence. If the genitourinary tract is involved, an en-bloc resection is recommended. A limited involvement of structures, such as the bladder dome or the posterior wall of the vagina, can be managed by partial excision with negative margins. Conversely, relapses with invasion of the bladder trigon or prostate in males and the uterine cervix in females usually require total pelvic exenteration. The mortality rate of this operation is still high (2-14%), as is the morbidity rate (33-75%) 17.
- -
Lateral pelvic recurrence. These relapses have a worse prognosis because it is extremely difficult to obtain tumour-free resection margins (< 19%)18; sometimes these are impossible due to the involvement of larger vessels, the sciatic nerve, or pelvic bones.
- -
Dorsal recurrence. When the recurrence is localised in the dorsal compartment, an abdominal sacral resection can be performed. This operation is facilitated by a position of the patient in the ventral decubitus or "jack-knife" position Although numerous studies in literature demonstrate the feasibility and safety of sacral resections below the S2/S3 junction, the risk/benefit ratio of such interventions is still under discussion, since they expose the patient to a high risk of neurological lesions (high sacrectomies) and uncontrollable venous bleeding (
Table 4).
Moreover, it is not always easy to distinguish intraoperative bone infiltration from fibrous adhesions, as made evident by a recent series of 29 cases of recurrence reported by the Memorial-Sloan Kettering, where only 38% had real bone infiltration, while 68% regarded adhesions.
E) Extended recurrences. The indication for pelvic exenteration combined with secretory surgery is a dependent institution. To date, the highest number of cases of exenteration with sacrectomy by institution is that of Solomon and Milne of the Royal Prince Alfred Hospital in Sydney, with 100 patients operated, with an R0 resection rate of 72%, with an average survival-rate of 45 months and 5 years for 38%, complications in 74%, neurological complications (39%) in high sacrectomies17,19.
In recent years, laparoscopic and robotic approaches have become accepted methods for treating primary colorectal cancer. However, there is limited literature on follow-up studies regarding the safety and feasibility of laparoscopic surgery for the treatment of locoregional pelvic recurrence
21,22(
Table 5).
The authors of these studies note that the minimally invasive approach offers the advantage of improved visibility, making dissection in a complex anatomical area like the pelvis, with altered anatomical planes, more precise. However, it's worth mentioning that these procedures often have longer operating times and demand a higher level of surgical expertise. Additionally, laparoscopic techniques tend to result in reduced blood loss, quicker restoration of intestinal function, and, from an oncological perspective, can achieve comparable rates of R0 resections23-25.
Ultimately, this approach should not be used for extended multi-visceral resections but limited to salvaging treatment of isolated central recurrences or of those closer to the lateral pelvic lymph nodes.
Pelvic recurrence of rectal cancer has significantly decreased in recent times thanks to the implementation of total mesorectal excision (TME) for rectal cancer and the integration of surgery with neoadjuvant chemoradiotherapy treatments. Most of these recurrences occur within 2 years of surgery, and without treatment, patients face an unfavorable prognosis with a substantial reduction in their quality of life, mainly due to severe pain. Non-surgical therapies like radiotherapy or chemoradiation therapy can provide some palliative relief, albeit with limited benefits and notable side effects. Surgical treatment offers the best chance of survival when performed with curative intent. Achieving an R0 resection is now possible for up to 50% of patients, provided careful selection criteria are met26.
Limited surgery is a feasible option for isolated anastomotic and central peri-anastomotic recurrences, which have a relatively favorable prognosis with a 5-year survival rate of up to 65%. However, for ventral, dorsal, or lateral recurrences involving pelvic viscera or neighboring bone structures like the sacrum, a more extensive and en bloc resection is necessary. These highly complex and destructive operations should be performed in specialized high-volume centers by experienced multidisciplinary teams, including oncological surgeons, neurosurgeons, orthopedists, plastic surgeons, and other specialists. The goal of these procedures is to achieve an R0 resection, which can provide an average 5-year survival rate of up to 44%27.
In our case study, the rate of R0 resection was 65.7%, consistent with similar experiences reported in the literature (
Table 2). The surgical interventions predominantly focused on favorable sites of recurrence, resulting in acceptable rates of mortality and morbidity.
While minimally invasive surgery offers advantages such as improved visualization and more precise dissection, it may not be suitable for extensive multivisceral resections that can only be achieved with open surgery. Multidisciplinary therapy is essential not only in managing primary rectal cancer but also in addressing its recurrence. Integrating radiotherapy, chemotherapy, and surgery increases resectability rates and yields promising outcomes in terms of recurrence percentages.management of cancer of the primary rectum but also to its recurrence.
5. Conclusions
Local recurrence of rectal cancer remains a significant complication, leading to poor survival and quality of life if left untreated. Advanced disease stage and non-radical surgery of the primary tumor are key risk factors. Early diagnosis is crucial, achievable through effective follow-up, especially during the initial 2-3 years post-surgery. Upon recurrence diagnosis, patients should receive comprehensive care from a multidisciplinary oncology team to determine the most suitable treatment. Rescue surgery is technically feasible and justifiable for specific patient groups. An aggressive multimodal approach can provide disease-free survival rates of up to 30% in carefully selected cases. Surgical resection demonstrates a more favorable cost-to-benefit ratio compared to non-surgical treatments. Refinements in diagnostic and staging methods, surgical techniques, chemoradiation therapy, and the availability of multidisciplinary teams hold promise for further improving surgical outcomes.
Author Contributions
P.I., S.I., C.D.I. and M.M. contributed to manuscript writing and editing and data collection; A.P., D.C. and S.S contributed to data analysis; L.I. and M.C. contributed to conceptualization and supervision; all authors have read and approved the final manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Local Institutional Review Board deemed the study exempt from review.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- “I numeri del cancro in Italia 2022- Aiom; accessible at: “https://www.aiom.it/i-numeri-del-cancro-in-italia”, visited on 20/08/2023.
- Stamou K, Gouvas N, Pechlivanides G, Xynos E. Primary curative surgery and preemptive or adjuvant hyperthermic peritoneal chemotherapy in colorectal cancer patients at high risk to develop peritoneal carcinomatosis. A systematic review. J BUON 2018, 23, 1249–1261. [Google Scholar]
- Gunawardene A, Desmond B, Shekouh A, Larsen P, Dennett E. Disease recurrence following surgery for colorectal cancer: five-year follow-up. N Z Med J. 2018, 131, 51–58. [Google Scholar]
- Maslekar S, Sharma A, Macdonald A, Gunn J, Monson JR, Hartley JE. Mesorectal grades predict recurrences after curative resection for rectal cancer. Dis Colon Rectum. 2007, 50, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Rödel, C., Grabenbauer, G. G., Matzel, K. E., Schick, C., Fietkau, R., Papadopoulos, T., Martus, P., Ho-henberger, W., & Sauer, R. Extensive surgery after high-dose preoperative chemoradiotherapy for locally advanced recurrent rectal cancer. Diseases of the colon and rectum 2000, 43, 312–319. [Google Scholar] [CrossRef]
- Melton, G. B., Paty, P. B., Boland, P. J., Healey, J. H., Savatta, S. G., Casas-Ganem, J. E., Guillem, J. G., Weiser, M. R., Cohen, A. M., Minsky, B. D., Wong, W. D., & Temple, L. K. Sacral resection for recurrent rectal cancer: analysis of morbidity and treatment results. Diseases of the colon and rectum 2006, 49, 1099–1107. [Google Scholar] [CrossRef]
- Nagasaki, T., Akiyoshi, T., Ueno, M., Fukunaga, Y., Nagayama, S., Fujimoto, Y., Konishi, T., & Yamaguchi, T. Laparoscopic salvage surgery for locally recurrent rectal cancer. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract 2014, 18, 1319–1326. [Google Scholar] [CrossRef]
- Beets-Tan, R. G., Beets, G. L., Vliegen, R. F., Kessels, A. G., Van Boven, H., De Bruine, A., von Meyenfeldt, M. F., Baeten, C. G., & van Engelshoven, J. M. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet (London, England) 2001, 357, 497–504. [Google Scholar] [CrossRef]
- Umpleby, H. C., Ranson, D. L., & Williamson, R. C. Peculiarities of mucinous colorectal carcinoma. The British journal of surgery 1985, 72, 715–718. [Google Scholar] [CrossRef]
- Moore, H. G., Shoup, M., Riedel, E., Minsky, B. D., Alektiar, K. M., Ercolani, M., Paty, P. B., Wong, W. D., & Guillem, J. G. Colorectal cancer pelvic recurrences: determinants of resectability. Diseases of the colon and rectum 2004, 47, 1599–1606. [Google Scholar] [CrossRef]
- Vignali A, Elmore U, Milone M, Rosati R. Transanal total mesorectal excision (TaTME): current status and future perspectives. Updates Surg. 2019, 71, 29–37. [Google Scholar] [CrossRef]
- PelvEx Collaborative. Surgical and Survival Outcomes Following Pelvic Exenteration for Locally Advanced Primary Rectal Cancer: Results From an International Collaboration. Annals of surgery 2019, 269, 315–321. [Google Scholar] [CrossRef]
- Moore HG, Shoup M, Riedel E, Minsky BD, Alektiar KM, Ercolani Met al. Colorectal cancer pelvic recurrences: determinants of resectability. Dis Colon Rectum 2004, 47, 1599–1606. [Google Scholar] [CrossRef]
- Georgiou PA, Tekkis PP, Constantinides VA, Patel U, Goldin RD, Darzi AW et al. Diagnostic accuracy and value of magnetic 12 | BJS Open, 2021, Vol. 00, No. 0 resonance imaging (MRI) in planning exenterative pelvic surgery for advanced colorectal cancer. Eur J Surg Oncol 2013, 49, 72–81. [Google Scholar]
- Bakx, R., Visser, O., Josso, J., Meijer, S., Slors, J. F., & van Lanschot, J. J. Management of recurrent rectal cancer: a population-based study in greater Amsterdam. World journal of gastroenterology 2008, 14, 6018–6023. [Google Scholar] [CrossRef]
- Suzuki, K., Dozois, R. R., Devine, R. M., Nelson, H., Weaver, A. L., Gunderson, L. L., & Ilstrup, D. M. Curative reoperations for locally recurrent rectal cancer. Diseases of the colon and rectum 1996, 39, 730–736. [Google Scholar] [CrossRef]
- Moriya, Y., Akasu, T., Fujita, S., & Yamamoto, S. Total pelvic exenteration with distal sacrectomy for fixed recurrent rectal cancer in the pelvis. Diseases of the colon and rectum 2004, 47, 2047–2054. [Google Scholar] [CrossRef]
- Ferenschild, F. T., Vermaas, M., Verhoef, C., Dwarkasing, R. S., Eggermont, A. M., & de Wilt, J. H. Abdominosacral resection for locally advanced and recurrent rectal cancer. The British journal of surgery 2009, 96, 1341–1347. [Google Scholar] [CrossRef]
- Weber, K. L., Nelson, H., Gunderson, L. L., & Sim, F. H. Sacropelvic resection for recurrent anorectal cancer. A multidisciplinary approach. Clinical orthopaedics and related research 2000, (372), 231–240. [Google Scholar] [CrossRef]
- van Hooft JE, van Halsema EE, Vanbiervliet G, Beets-Tan RG, DeWitt JM, Donnellan F, Dumonceau JM, Glynne-Jones RG, Hassan C, Jiménez-Perez J, Meisner S, Muthusamy VR, Parker MC, Regimbeau JM, Sabbagh C, Sagar J, Tanis PJ, Vandervoort J, Webster GJ, Manes G, Barthet MA, Repici A; European Society of Gastrointestinal Endoscopy (ESGE). Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Gastrointest Endosc. 2014, 80, 747. [Google Scholar] [CrossRef]
- Izzo, L., Impara, L., Pugliese, F., Mansour, M. A., De Felice, F., De Dominicis, C., De Santis, A., De Felice, C., Gabriele, R., Basso, L., Di Cello, P., Caputo, M., & Izzo, P. Preoperative staging of resectability of colon cancer using virtual colonoscopy: correlation with surgical results. Our experience. Annali italiani di chirurgia 2015, 86, 432–436. [Google Scholar]
- Lee, D. J., Sagar, P. M., Sadadcharam, G., & Tan, K. Y. Advances in surgical management for locally recurrent rectal cancer: How far have we come? World journal of gastroenterology 2017, 23, 4170–4180. [Google Scholar] [CrossRef]
- Lu, A. G., Wang, M. L., Hu, W. G., Li, J. W., Zang, L., Mao, Z. H., Dong, F., Feng, B., Ma, J. J., Zong, Y. P., & Zheng, M. H. Zhonghua wai ke za zhi [Chinese journal of surgery] 2006, 44, 597–599.
- Mcall JL, Cox MR, Wattchow DA. Analysis of local recurrence rate after surgery for rectal cancer. Int J Colorect Dis 1995, 10, 126–132. [Google Scholar] [CrossRef]
- Kim, S. H., Neve, R. S., & Joh, Y. G. Multimedia article. Relaparoscopy for salvage surgery in anastomotic recurrence of rectal cancer: feasible and safe. Diseases of the colon and rectum 2008, 51, 1712–1713. [Google Scholar] [CrossRef]
- Park, S. Y., Choi, G. S., Jun, S. H., Park, J. S., & Kim, H. J. Laparoscopic salvage surgery for recurrent and metachronous colorectal cancer: 15 years' experience in a single center. Surgical endoscopy 2011, 25, 3551–3558. [Google Scholar] [CrossRef]
- Barbaro, B., Leccisotti, L., Vecchio, F. M., Di Matteo, M., Serra, T., Salsano, M., Poscia, A., Coco, C., Persiani, R., Alfieri, S., Gambacorta, M. A., Valentini, V., Giordano, A., & Bonomo, L. The potential predictive value of MRI and PET-CT in mucinous and nonmucinous rectal cancer to identify patients at high risk of metastatic disease. The British journal of radiology 2017, 90, 20150836. [Google Scholar] [CrossRef]
- Platt, E., Dovell, G., & Smolarek, S. Systematic review of outcomes following pelvic exenteration for the treatment of primary and recurrent locally advanced rectal cancer. Techniques in coloproctology 2018, 22, 835–845. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).