Submitted:
14 September 2023
Posted:
18 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Fig Latex inhibits the growth of HPV positive cervical cancer cell lines
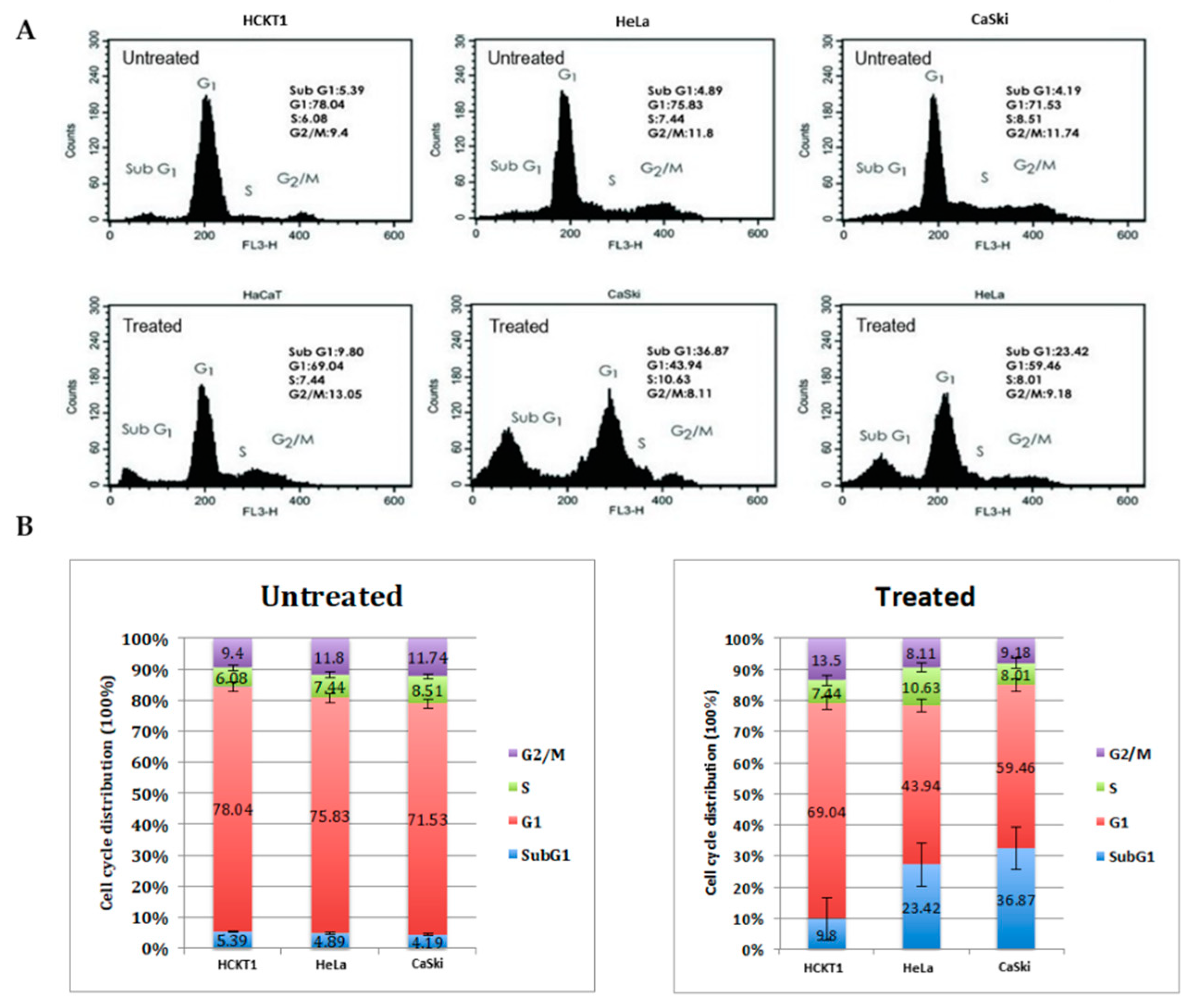
2.2. Fig latex induces cell cycle arrest at sub G1 in HPV positive cervical cancer cell lines
2.3. Transcriptomic profiling of different HPV positive cervical cancer cells upon whole fig latex treatment
2.4. Analysis of differential expressed genes in HPV positive cervical cancer cell lines upon fig latex treatment using KEGG pathway enrichment analysis.
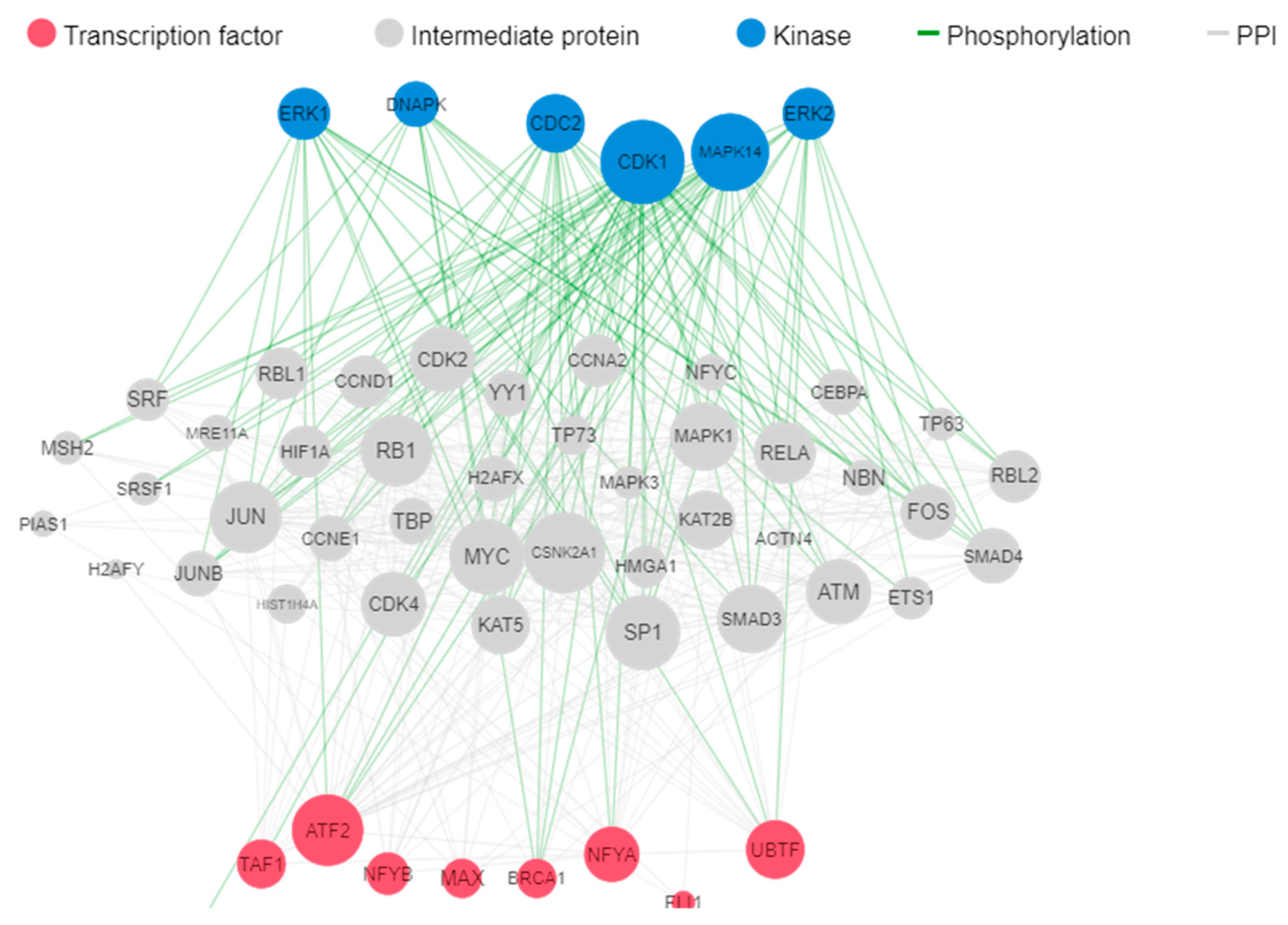
2.5. Analysis of differentially expressed genes by Kinase Enrichment and Chromatin Enrichment Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Collection and Purification of Whole Ficus Carica Latex
4.3. Cell Lines and Cell Culture Conditions
4.4. SRB Cell Viability Assay
4.5. Cell Cycle Analysis
4.6. RNA Preparation
4.7. RNA Sequencing (RNA Seq)
4.8. Bioinformatic Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- zur Hausen, H. Papillomaviruses and Cancer: From Basic Studies to Clinical Application. Nat Rev Cancer 2002, 2, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Maucort-Boulch, D.; Plummer, M.; Castle, P.E.; Demuth, F.; Safaeian, M.; Wheeler, C.M.; Schiffman, M. Predictors of Human Papillomavirus Persistence among Women with Equivocal or Mildly Abnormal Cytology. Int J Cancer 2010, 126, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, M.; Castle, P.E.; Jeronimo, J.; Rodriguez, A.C.; Wacholder, S. Human Papillomavirus and Cervical Cancer. The Lancet 2007, 370, 890–907. [Google Scholar] [CrossRef] [PubMed]
- Moody, C.A.; Laimins, L.A. Human Papillomavirus Oncoproteins: Pathways to Transformation. Nat Rev Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef]
- Münger, K.; Howley, P.M. Human Papillomavirus Immortalization and Transformation Functions. Virus Res 2002, 89, 213–228. [Google Scholar] [CrossRef]
- Crook, T.; Vousden, K.H.; Tidy, J.A. Degradation of P53 Can Be Targeted by HPV E6 Sequences Distinct from Those Required for P53 Binding and Trans-Activation. Cell 1991, 67, 547–556. [Google Scholar] [CrossRef]
- Scheffner, M.; Werness, B.A.; Huibregtse, J.M.; Levine, A.J.; Howley, P.M. The E6 Oncoprotein Encoded by Human Papillomavirus Types 16 and 18 Promotes the Degradation of P53. Cell 1990, 63, 1129–1136. [Google Scholar] [CrossRef]
- Shimada, M.; Yamashita, A.; Saito, M.; Ichino, M.; Kinjo, T.; Mizuki, N.; Klinman, D.M.; Okuda, K. The Human Papillomavirus E6 Protein Targets Apoptosis-Inducing Factor (AIF) for Degradation. Sci Rep 2020, 10, 14195. [Google Scholar] [CrossRef]
- Scheffner, M.; Huibregtse, J.M.; Vierstra, R.D.; Howley, P.M. The HPV-16 E6 and E6-AP Complex Functions as a Ubiquitin-Protein Ligase in the Ubiquitination of P53. Cell 1993, 75, 495–505. [Google Scholar] [CrossRef]
- Huibregtse, J.M.; Scheffner, M.; Howley, P.M. A Cellular Protein Mediates Association of P53 with the E6 Oncoprotein of Human Papillomavirus Types 16 or 18. EMBO J 1991, 10, 4129–4135. [Google Scholar] [CrossRef]
- Boyer, S.N.; Wazer, D.E.; Band, V. E7 Protein of Human Papilloma Virus-16 Induces Degradation of Retinoblastoma Protein through the Ubiquitin-Proteasome Pathway. Cancer Res 1996, 56, 4620–4624. [Google Scholar] [PubMed]
- Jones, D.L.; Münger, K. Interactions of the Human Papillomavirus E7 Protein with Cell Cycle Regulators. Semin Cancer Biol 1996, 7, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.L.; Stremlau, M.; He, X.; Basile, J.R.; Münger, K. Degradation of the Retinoblastoma Tumor Suppressor by the Human Papillomavirus Type 16 E7 Oncoprotein Is Important for Functional Inactivation and Is Separable from Proteasomal Degradation of E7. J Virol 2001, 75, 7583–7591. [Google Scholar] [CrossRef]
- Davies-Oliveira, J.C.; Smith, M.A.; Grover, S.; Canfell, K.; Crosbie, E.J. Eliminating Cervical Cancer: Progress and Challenges for High-Income Countries. Clin Oncol 2021, 33, 550–559. [Google Scholar] [CrossRef]
- Pal, A.; Kundu, R. Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy. Front Microbiol 2020, 10. [Google Scholar] [CrossRef]
- Burmeister, C.A.; Khan, S.F.; Schäfer, G.; Mbatani, N.; Adams, T.; Moodley, J.; Prince, S. Cervical Cancer Therapies: Current Challenges and Future Perspectives. Tumour Virus Res 2022, 13, 200238. [Google Scholar] [CrossRef] [PubMed]
- George, I.A.; Chauhan, R.; Dhawale, R.E.; Iyer, R.; Limaye, S.; Sankaranarayanan, R.; Venkataramanan, R.; Kumar, P. Insights into Therapy Resistance in Cervical Cancer. Advances in Cancer Biology - Metastasis 2022, 6, 100074. [Google Scholar] [CrossRef]
- Tewari, K.S.; Sill, M.W.; Long, H.J.; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; et al. Improved Survival with Bevacizumab in Advanced Cervical Cancer. New England Journal of Medicine 2014, 370, 734–743. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Last 25 Years. J Nat Prod 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. New Horizons for Old Drugs and Drug Leads. J Nat Prod 2014, 77, 703–723. [Google Scholar] [CrossRef]
- Kubczak, M.; Szustka, A.; Rogalińska, M. Molecular Targets of Natural Compounds with Anti-Cancer Properties. Int J Mol Sci 2021, 22, 13659. [Google Scholar] [CrossRef]
- Soltana, H.; Pinon, A.; Limami, Y.; Zaid, Y.; Khalki, L.; Zaid, N.; Salah, D.; Sabitaliyevich, U.Y.; Simon, A.; Liagre, B.; et al. Antitumoral Activity of Ficus Carica L. on Colorectal Cancer Cell Lines. Cell Mol Biol 2019, 65, 6–11. [Google Scholar] [CrossRef]
- Hashemi SA, A.S.G.M.A.M.Y.Y.D.AA. The Effect of Fig Tree Latex (Ficus Carica) on Stomach Cancer Line. Iran Red Crescent Medical Journal 2011, 13, 272–275. [Google Scholar]
- Ghanbari, A.; Le Gresley, A.; Naughton, D.; Kuhnert, N.; Sirbu, D.; Ashrafi, G.H. Biological Activities of Ficus Carica Latex for Potential Therapeutics in Human Papillomavirus (HPV) Related Cervical Cancers. Sci Rep 2019, 9, 1013. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.-K.; Park, J.-S. The Role of HPV E6 and E7 Oncoproteins in HPV-Associated Cervical Carcinogenesis. Cancer Res Treat 2005, 37, 319. [Google Scholar] [CrossRef] [PubMed]
- Cosper, P.F.; McNair, C.; González, I.; Wong, N.; Knudsen, K.E.; Chen, J.J.; Markovina, S.; Schwarz, J.K.; Grigsby, P.W.; Wang, X. Decreased Local Immune Response and Retained HPV Gene Expression during Chemoradiotherapy Are Associated with Treatment Resistance and Death from Cervical Cancer. Int J Cancer 2020, 146, 2047–2058. [Google Scholar] [CrossRef]
- Behm-Ansmant, I.; Kashima, I.; Rehwinkel, J.; Saulière, J.; Wittkopp, N.; Izaurralde, E. MRNA Quality Control: An Ancient Machinery Recognizes and Degrades MRNAs with Nonsense Codons. FEBS Lett 2007, 581, 2845–2853. [Google Scholar] [CrossRef]
- Nicholson, P.; Yepiskoposyan, H.; Metze, S.; Zamudio Orozco, R.; Kleinschmidt, N.; Mühlemann, O. Nonsense-Mediated MRNA Decay in Human Cells: Mechanistic Insights, Functions beyond Quality Control and the Double-Life of NMD Factors. Cellular and Molecular Life Sciences 2010, 67, 677–700. [Google Scholar] [CrossRef]
- Durand, S.; Lykke-Andersen, J. SnapShot: Nonsense-Mediated MRNA Decay. Cell 2011, 145, 324–324.e2. [Google Scholar] [CrossRef]
- Poulsen, S.L.; Hansen, R.K.; Wagner, S.A.; van Cuijk, L.; van Belle, G.J.; Streicher, W.; Wikström, M.; Choudhary, C.; Houtsmuller, A.B.; Marteijn, J.A.; et al. RNF111/Arkadia Is a SUMO-Targeted Ubiquitin Ligase That Facilitates the DNA Damage Response. Journal of Cell Biology 2013, 201, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Kadaja, M.; Isok-Paas, H.; Laos, T.; Ustav, E.; Ustav, M. Mechanism of Genomic Instability in Cells Infected with the High-Risk Human Papillomaviruses. PLoS Pathog 2009, 5, e1000397. [Google Scholar] [CrossRef] [PubMed]
- Hengstermann, A.; Linares, L.K.; Ciechanover, A.; Whitaker, N.J.; Scheffner, M. Complete Switch from Mdm2 to Human Papillomavirus E6-Mediated Degradation of P53 in Cervical Cancer Cells. Proceedings of the National Academy of Sciences 2001, 98, 1218–1223. [Google Scholar] [CrossRef]
- Prochasson, L.; Jalinot, P.; Mocquet, V. The Complex Relationship between HTLV-1 and Nonsense-Mediated MRNA Decay (NMD). Pathogens 2020, 9, 287. [Google Scholar] [CrossRef]
- Leon, K.; Ott, M. An ‘Arms Race’ between the Nonsense-Mediated MRNA Decay Pathway and Viral Infections. Semin Cell Dev Biol 2021, 111, 101–107. [Google Scholar] [CrossRef]
- van Gent, M.; Reich, A.; Velu, S.E.; Gack, M.U. Nonsense-Mediated Decay Controls the Reactivation of the Oncogenic Herpesviruses EBV and KSHV. PLoS Biol 2021, 19, e3001097. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.T.; Laimins, L.A. Human Papillomavirus Oncoproteins E6 and E7 Independently Abrogate the Mitotic Spindle Checkpoint. J Virol 1998, 72, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Nor Rashid, N.; Yusof, R.; Watson, R.J. Disruption of Repressive P130–DREAM Complexes by Human Papillomavirus 16 E6/E7 Oncoproteins Is Required for Cell-Cycle Progression in Cervical Cancer Cells. Journal of General Virology 2011, 92, 2620–2627. [Google Scholar] [CrossRef]
- Thomas, M.; Laura, R.; Hepner, K.; Guccione, E.; Sawyers, C.; Lasky, L.; Banks, L. Oncogenic Human Papillomavirus E6 Proteins Target the MAGI-2 and MAGI-3 Proteins for Degradation. Oncogene 2002, 21, 5088–5096. [Google Scholar] [CrossRef] [PubMed]
- Kruiswijk, F.; Labuschagne, C.F.; Vousden, K.H. P53 in Survival, Death and Metabolic Health: A Lifeguard with a Licence to Kill. Nat Rev Mol Cell Biol 2015, 16, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Vousden, K.H.; Prives, C. Blinded by the Light: The Growing Complexity of P53. Cell 2009, 137, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of P53 in Tumor Initiation and Progression. Cold Spring Harb Perspect Med 2016, 6, a026104. [Google Scholar] [CrossRef] [PubMed]
- Scheffner, M.; Werness, B.A.; Huibregtse, J.M.; Levine, A.J.; Howley, P.M. The E6 Oncoprotein Encoded by Human Papillomavirus Types 16 and 18 Promotes the Degradation of P53. Cell 1990, 63, 1129–1136. [Google Scholar] [CrossRef]
- Wang, Q.; Bode, A.M.; Zhang, T. Targeting CDK1 in Cancer: Mechanisms and Implications. NPJ Precis Oncol 2023, 7, 58. [Google Scholar] [CrossRef]
- Baker, S.J.; Poulikakos, P.I.; Irie, H.Y.; Parekh, S.; Reddy, E.P. CDK4: A Master Regulator of the Cell Cycle and Its Role in Cancer. Genes Cancer 2022, 13, 21–45. [Google Scholar] [CrossRef]
- Yang, W.; Feng, Q.; Ma, H.; Lei, D.; Zheng, P. NF-YA Promotes the Cell Proliferation and Tumorigenic Properties by Transcriptional Activation of SOX2 in Cervical Cancer. J Cell Mol Med 2020, 24, 12464–12475. [Google Scholar] [CrossRef]
- Gurtner, A.; Manni, I.; Piaggio, G. NF-Y in Cancer: Impact on Cell Transformation of a Gene Essential for Proliferation. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms 2017, 1860, 604–616. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, H.; Zhong, A.; Yu, T. Expression and Prognosis of CyclinA and CDK2 in Patients with Advanced Cervical Cancer after Chemotherapy. Cell Mol Biol (Noisy-le-grand) 2020, 66, 85–91. [Google Scholar] [CrossRef]
- ZHANG, W.; LIU, H.T. MAPK Signal Pathways in the Regulation of Cell Proliferation in Mammalian Cells. Cell Res 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Abba, M.C.; Laguens, R.M.; Dulout, F.N.; Golijow, C.D. The C-Myc Activation in Cervical Carcinomas and HPV 16 Infections. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 2004, 557, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, T.; Xu, G.; Liu, H.; Ren, C.; Xie, W.; Wang, M. Cyclin-Dependent Kinase 2 Promotes Tumor Proliferation and Induces Radio Resistance in Glioblastoma. Transl Oncol 2016, 9, 548–556. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK Signalling Pathway and Tumorigenesis (Review). Exp Ther Med 2020. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lei, R.; Ding, S.-W.; Zhu, S. Skewer: A Fast and Accurate Adapter Trimmer for next-Generation Sequencing Paired-End Reads. BMC Bioinformatics 2014, 15, 182. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol 2014, 15, 550. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database Hallmark Gene Set Collection. Cell Syst 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proceedings of the National Academy of Sciences 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A Comprehensive Gene Set Enrichment Analysis Web Server 2016 Update. Nucleic Acids Res 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and Collaborative HTML5 Gene List Enrichment Analysis Tool. BMC Bioinformatics 2013, 14, 128. [Google Scholar] [CrossRef]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr Protoc 2021, 1. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.J.B.; Kuleshov, M.V.; Schilder, B.M.; Torre, D.; Duffy, M.E.; Keenan, A.B.; Lachmann, A.; Feldmann, A.S.; Gundersen, G.W.; Silverstein, M.C.; et al. EXpression2Kinases (X2K) Web: Linking Expression Signatures to Upstream Cell Signaling Networks. Nucleic Acids Res 2018, 46, W171–W179. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; Xu, H.; Gordonov, S.; Lim, M.P.; Perkins, M.H.; Ma’ayan, A. Expression2Kinases: MRNA Profiling Linked to Multiple Upstream Regulatory Layers. Bioinformatics 2012, 28, 105–111. [Google Scholar] [CrossRef] [PubMed]

| Description | Number of Overlap Genes | p-value | FDR q-value | Overlap genes |
|---|---|---|---|---|
| Nonsense-Mediated Decay (NMD) | 7 | 1.45E-07 | 2.21E-04 | RPS27A,RNF111,RPS6,RPL27,RPL37,RPL39,UPF2,SMG6 |
| Cell Cycle | 12 | 5.91E-06 | 3.02E-03 | PCNA,POLD3,PRIM1,ORC2,RAD1,YWHAQ,CDC14A,CENPK,CEP72,MZT1,DIDO1 |
| Transcriptional Regulation by TP53 | 8 | 3.42E-05 | 5.82E-03 | RPS27ARAD1,YWHAQ, RPS27A,PCNA,COX7A2L,NDRG1,PIP4K2B |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





