Submitted:
15 September 2023
Posted:
19 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
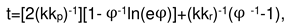
2. Results and Discussion
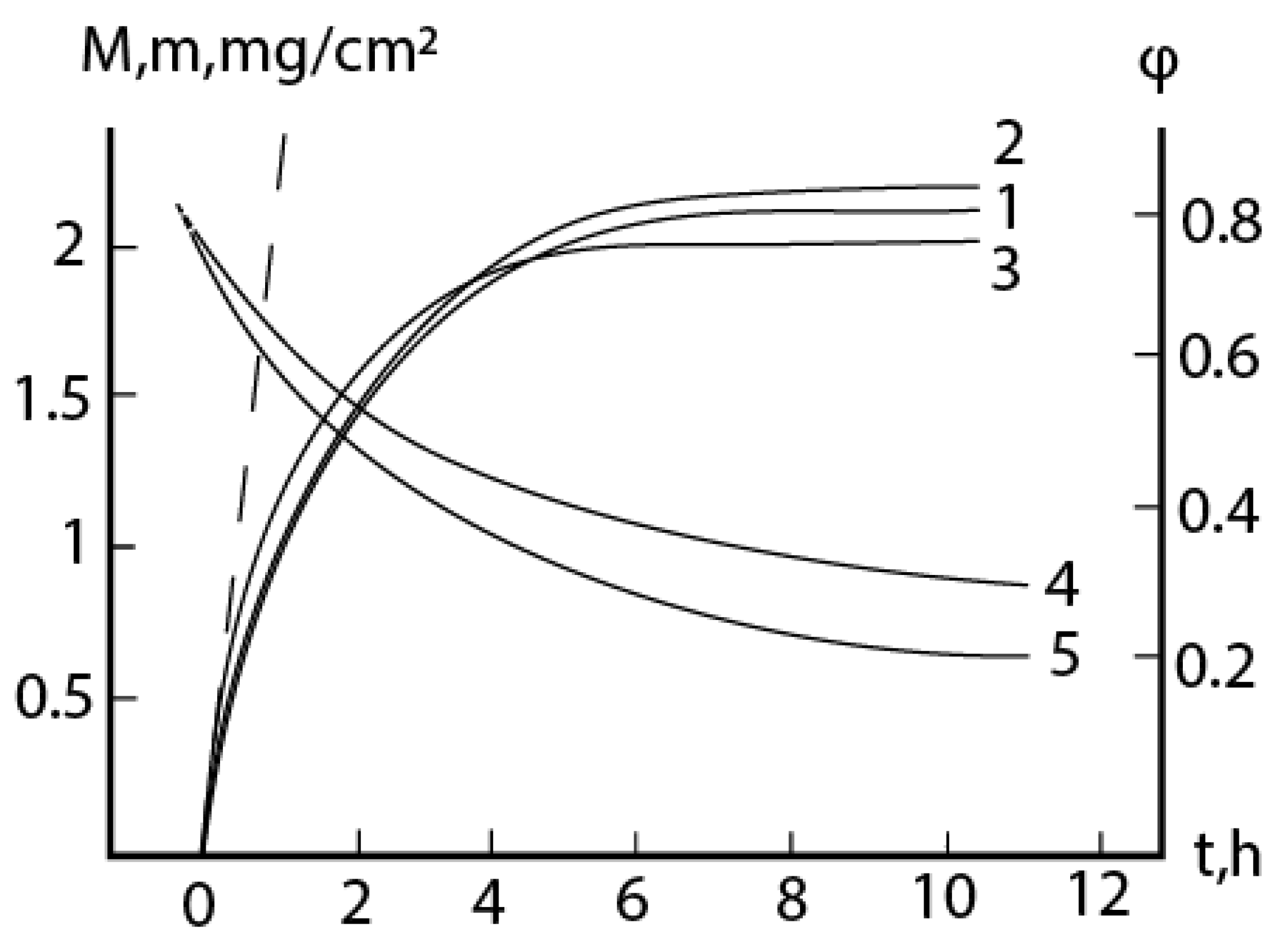
2.1. Oxidation of alloy Fe44Cr4Al0.25La at 1300oC
2.2. Oxidation of alloy Fe45Cr0.3La at 1300oC
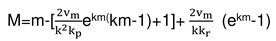
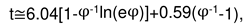
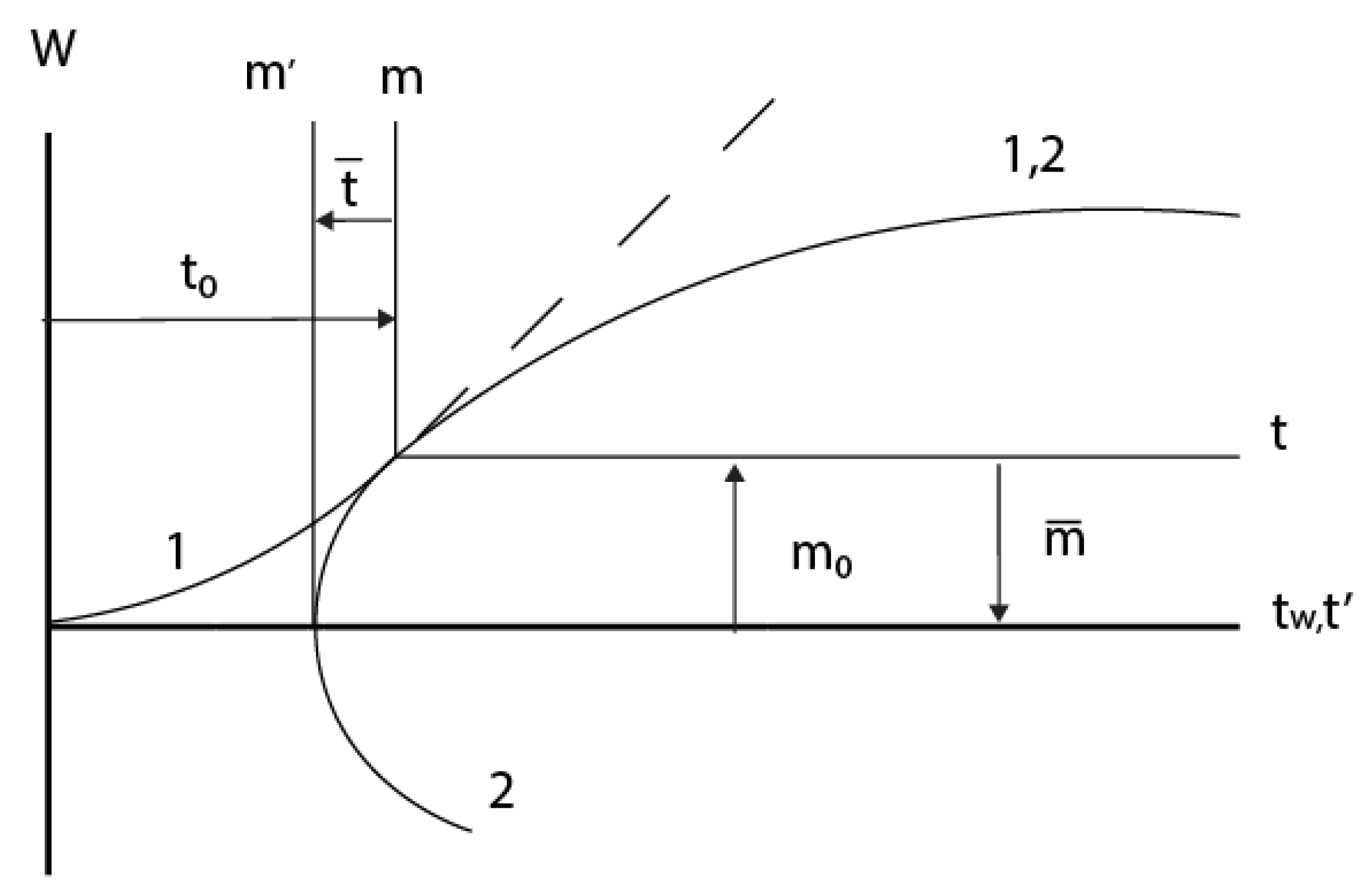
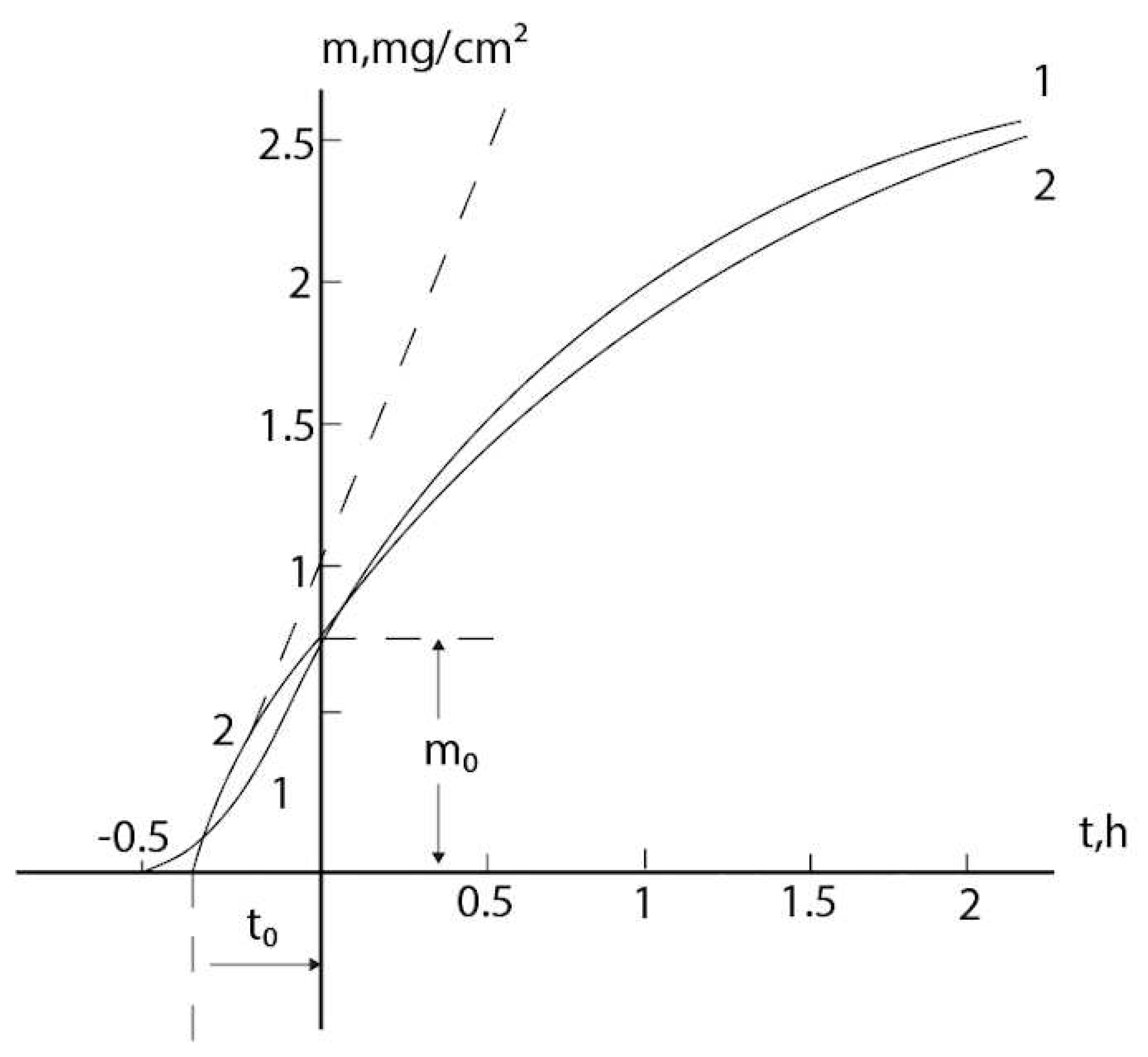
2.3. Consideration of initial non-isothermal heating during oxidation of alloys: Oxidation of alloy Fe45Cr0.32Y at 1400oC
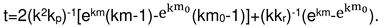
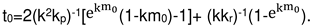
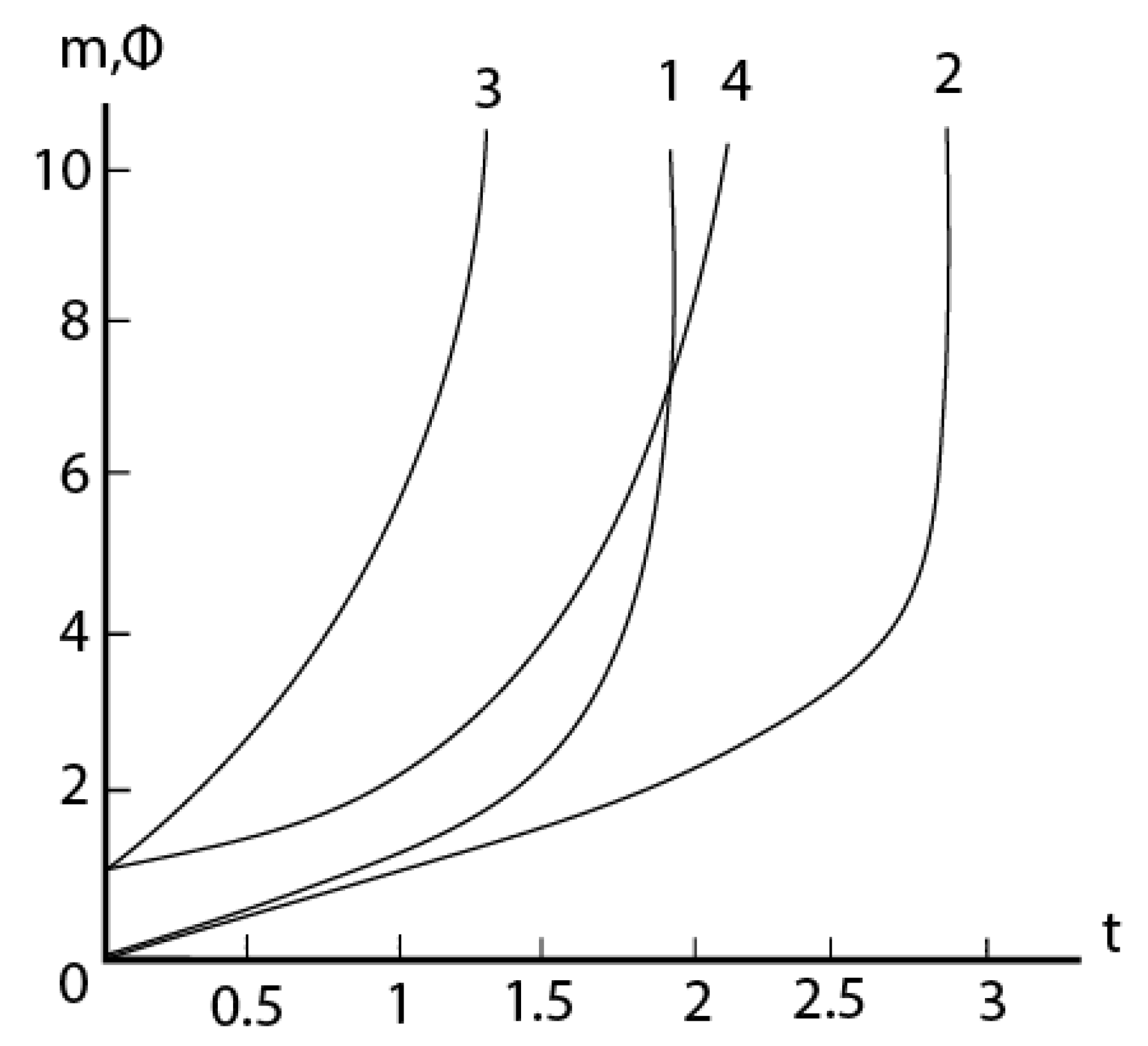
2.4. Formal kinetics of growth of scale with the increase of the reaction area
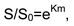


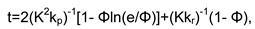
Conclusions
Conflicts of Interest
References
- Sharp, W.H. High temperature alloys for the gas turbine - The state of the art. SAE Transactions 1966, 74, 323–332. [Google Scholar]
- Klopp, W.D. Recent developments in chromium and chromium alloys. JOM 1969, 21, 23–32. [Google Scholar] [CrossRef]
- Bell, S.; Sarvghad, M.; Ong, T.-C.; et al. Corrosion of iron–nickel–chromium alloys in high temperature carbonate salt under argon atmosphere. Solar Energy Materials and Solar Cells 2023, 256, 112317. [Google Scholar] [CrossRef]
- Ma, K.; Blackburn, T.; Magnussen, J.P.; Kerbstadt, M. Chromium-based bcc-superalloys strengthened by iron supplements. Acta Materialia 2023, 257, 119183. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, W.; Zhang, D.; et al. Influence of rare-earth element doping on interface and mechanical properties of WC particles reinforced steel matrix composites. Mater. Res.Express 2021, 8, 036512. [Google Scholar] [CrossRef]
- Othman, N.K.; Jalar, A.; Young, D. Effects of lanthanum on Fe-25Cr alloys under cyclic oxidation. Adv. Materials Res. 2010, 97, 1212–1215. [Google Scholar] [CrossRef]
- Shi, Z.; Chao, W.; Rao, L.; et al. Effects of Ce doping on mechanical properties of M7C3 carbides in hypereutectic Fe–Cr–C hardfacing alloy. Alloys and Comp. 2021, 850, 156656. [Google Scholar] [CrossRef]
- Wu, Z.; Wen, G.; Han, Y. Grain growth and oxidation resistance of Fe-Cr-Al electrothermal alloy doped with yttrium. Ann. Chimie – Sci. des Matériaux 2020, 44, 29–36. [Google Scholar] [CrossRef]
- Liu, Y.; Chabane, D.; El Kedim, O. First principles investigation of the substitutional doping of rare-earth elements and Co in La4MgNi19 phase. Energy Stor. 2023, 67, 107638. [Google Scholar] [CrossRef]
- Qin, Y.; Yuan, J.; Zhuang, Y.; et al. Study on effect of high-entropy alloy binder on microstructure and properties of WC cemented carbide doped with rare earth oxide. Coatings 2023, 13, 273. [Google Scholar] [CrossRef]
- Milyutin, V.A.; Birčáková, Z.; Fáberová, M.; Bures, R. Effect of rare-earth doping on dynamic magnetic properties of FeGa alloy. Materials Sci. Forum 2023, 1081, 149–154. [Google Scholar] [CrossRef]
- Wang, R.; Tian, X.; Yao, Z. Influence of rare earth element terbium doping on microstructure and magnetostrictive properties of Fe81Al19 alloy. Rare Earth 2020, 40, 451–456. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, K.; Tan, C.; Guo, E. Influence of doping Tb on the mechanical properties and martensitic transformation of Ni-Mn-Sn magnetic shape memory alloys. Crystals 2018, 8, 247. [Google Scholar] [CrossRef]
- Evans, U.R. An introduction to metallic corrosion. London 1981, 302.
- Nakhutsrishvili, I.; Mikadze, G. On high-temperature oxidation of alloy FeCr(La): Reduction of the effective diffusion area. Bull. Georg. Acad. Sci. 2023, 17, 57–63. [Google Scholar]
- Mikadze, O.; Kandelaki, A.; Nakhutsrishvili, I. On the kinetics of scale growth with the change of the effective area of diffusion. Bull. Georgian Acad. Sci. 2011, 5, 73–75. [Google Scholar]
- Nakhutsrishvili, I.; Tkeshelashvili, O.; Chanishvili, A. Mathematical model of thermogravimetric curves of FeCrAl(La) alloy. Technical Sci. & Technologies 2016, 5, 35–37. [Google Scholar]
- Xi, S.; An, G.; Zhang, X.; et al. Corrosion resistance of FeCrAl coatings on Mo–La alloys. Chinese Mech. Eng. Soc. 2023, 41, 90–96. [Google Scholar]
- Wang, I.; Wang, B.; Luo, L.; et al. Effects of process atmosphere on additively manufactured FeCrAl oxide dispersion strengthened steel: Printability, microstructure and tensile properties. Materials Sci. and Eng. A 2023, 882, 145438. [Google Scholar] [CrossRef]
- Wang, I.; Binbin Wang, B.; Luo, L.; et al. Effects of process atmosphere on additively manufactured FeCrAl oxide dispersion strengthened steel: Printability, microstructure and tensile properties. Materials Sci. and Eng. A 2023, 882, 145438. [Google Scholar] [CrossRef]
- Hoelzer, D.T.; Heidel, D.; Yamamoto, Y.; Massey, C.P. High-Temperature creep behavior of thin-walled FeCrAl alloy tubes. Oak Ridge National Lab. 2023, ORNL/LTR-2023/2888.
- Jiang, H.; Zhao, X.; Wang, D.; et al. Effects of Y2O3 addition on the microstructure and static lead-bismuth eutectic thermal corrosion behaviors of FeCrAlTiC-xY2O3lLaser clade coatings. Coatings 2022, 12, 1759. [Google Scholar] [CrossRef]
- Saito, Y. Effect of rare earth elements on the high-temperature oxidation of heat-resisting alloys. In: Selected topics in high-temperature chemistry 1989, Amsterdam-Oxford-New-York-Tokyo: Elsevier, 227.
- Ishii, K.; Kohno, M.; Ishikawa, S.; Satoh, S. Effect of rare-earth elements on high-temperature oxidation resistance of Fe-20Cr-5Al alloy foils. Materials Transact. 2011, 38, 787–792. [Google Scholar] [CrossRef]
- Hou, P.Y. The reactive element effect – Past, present and future. Materials Sci. Forum 2011, 696, 39–44. [Google Scholar] [CrossRef]
- Ozawa, M.; Araki, K.-I. Effect of La modification on the stability of coating alumina layer on FeCrAl alloy substrate. Surf. and Coatings Technology 2015, 271, 80–86. [Google Scholar] [CrossRef]
- He, Y.; Liu, J.H.; Han, Z.B.; et al. Effect of La on mechanical Properties of FeCrAl stainlees steel under high temperature. Contin. Casting 2015, 40, 1–9. [Google Scholar]
- Nakatsuka, A.; Ohtaka, O.; Arima, H.; et al. Cubic phase of single-crystal LaAlO3 perovskite synthesized at 4.5 GPa and 1273 K. Acta Crystallog. 2005, E61, i148–i150. [Google Scholar]
- Boudali, A.; Saadaoui, F.; Zemouli, M. Recalculate structural, elastic, electronic and thermal properties in LaAlO3 rhombohedral perovskites. Adv. in Materials Phys. and Chem. 2013, 3, 146–152. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, W.; He, Y. Modeling of oxidation kinetics of Y-doped Fe-Cr-Al alloys. Oxidat. Metals 2000, 53, 341–350. [Google Scholar] [CrossRef]
- Badini, C.; Laurella, F. Oxidation of FeCrAl alloy: Influence of temperature and atmosphere on scale growth rate and mechanism. Surface and Coatings Technol. 2001, 135, 291–298. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Gesmundo, F.; Hou, P.Y.; Niu, Y. Criteria for the formation of protective Al2O3 scales on Fe-10Al and FeCrAl alloys. Corrosion Sci. 2006, 48, 741–765. [Google Scholar] [CrossRef]
- Young, D.J.; Niewalak, L.; Wessel, E.; et al. Oxidation kinetics of Y-doped FeCrAl alloys in low and high PO2 gases. Materials and Corros. 2010, 61, 838–844. [Google Scholar] [CrossRef]
- Othman, N.K.; Zhang, J.; Young, D.J. Water vapour effects on Fe-Cr alloy oxidation. Oxidat. Metals 2010, 73, 337–352. [Google Scholar] [CrossRef]
- Tallman, D.J.; Anasori, B.; Barsoum, M.W. A critical review of the oxidation of Ti2AlC, Ti3AlC2 and Cr2AlC in air. Materials Res. Lett. 2013, 1, 115–125. [Google Scholar] [CrossRef]
- Hellström, K.; Israelsson, N.; Mortazavi, N.; et al. Oxidation of a dispersion-strengthened powder metallurgical FeCrAl alloy in the presence of O2 at 1,100oC: the influence of water vapour. Oxidat. Metals 2015, 83, 533–558. [Google Scholar] [CrossRef]
- Quadakkers, W.J.; Wessel, E.; Kochubey, V.; et al. Growth rates of alumina scales on Fe-Cr-Al alloys. Oxidat. Metals 2004, 61, 17–37. [Google Scholar] [CrossRef]
- Chegroune, R.; Salhi, E.; Grisci, A.; et al. High-temperature corrosion of dilute chromium-lanthanum alloys. Oxidat. Metals 2008, 70, 331–350. [Google Scholar] [CrossRef]
- Taniguchi, S.; Andoh, A. Improvement in the oxidation resistance of an Al-deposited Fe–Cr–Al foil by preoxidation. Oxidat. Metals 2022, 58, 545–562. [Google Scholar] [CrossRef]
- Wolff, M.; EIorio, L.; Rumpf, T. Oxidation and corrosion behaviour of Fe–Cr and Fe–Cr–Al alloys with minor alloying additions. Materials Sci. Eng. A 1998, 241, 264–276. [Google Scholar] [CrossRef]
- Pint, B.A. Optimization of reactive-element additions to improve oxidation performance of alumina-forming alloys. Am. Ceramic. Soc. 2003, 86, 686–695. [Google Scholar] [CrossRef]
- du Prees, S.P.; van Kaam, T.P.M.; Ringdalen, E.; et al. An Overview of currently applied ferrochrome production processes and their waste management practices. Minerals 2023, 13, 809. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Q.; Wang, X.; et al. Non-isothermal aging: A heat treatment method that simultaneously improves the mechanical properties and corrosion resistance of ultra-high strength Al-Zn-Mg-Cu alloy. Alloys and Comp. 2020, 845, 156286. [Google Scholar] [CrossRef]
- Sharma, P.; Kainth, S.; Singh, K.; et al. Investigating non-isothermal oxidation kinetics of a non-stoichiometrically synthesized Ti3AlC2 MAX phase. Alloys and Comp. 2023, 959, 170488. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H.; Zeng, T.; et al. Effect of non-isothermal aging on the mechanical properties and corrosion resistance of 2A12 aluminum alloy. Materials 2023, 16, 3921. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, P.; Mi, G.; Li, P.; et al. Non-isothermal oxidation behaviors and mechanisms of Ti-Al intermetallic compounds. Materials 2019, 12, 2114. [Google Scholar] [CrossRef] [PubMed]
- Nakhutsrishvili, I.; Mikadze, G.; Mikadze, O. Mutual connection of unisothermal initial heating with isothermal oxidation kinetics of heat resistant chromium alloys. Proc. Georgian Acad. Sci., chem. ser. 2007, 33, 60–63. [Google Scholar]
- Pillis, M.F.; Correa, O.V.; Ramanathan, L.V. V. High-temperature corrosion behavior of yttrium dioxide coated Fe-20Cr Alloy. Materials Res. 2016, 19, 611–617. [Google Scholar] [CrossRef]
- Fontana, S.; Vuksa, M.; et al. On the effect of surface treatment to improve oxidation resistance and conductivity of metallic interconnects for SOFC in operating conditions. Mater. Sci. Forum 2008, 595/598, 753–762. [Google Scholar] [CrossRef]
- Sayto, Y.; Ӧnay, B.; Maruyama, T. The Reactive Element Effect (REE) in oxidation of alloys. Phys. IV Fr. 1993, 3, C217–C230. [Google Scholar] [CrossRef]
- Molin, S.; Persson, A.H.; et al. Effective yttrium based coating for steel interconnects of solid oxide cells: Corrosion evaluation in steam-hydrogen atmosphere. power sources. 2019, 440, 26814. [Google Scholar] [CrossRef]
- Yoneda, S.; Hayashi, S.; Ukai, S. The transition from transient oxide to protective Al2O3 scale on Fe–Cr–Al alloys during heating to 1000°C. Oxidat. Met. 2018, 89, 81–97. [Google Scholar] [CrossRef]
- Pillis, M.F.; Ramanathan, L.V. Effect of pre-oxidation on high temperature sulfidation behavior of FeCr and FeCrAl alloys. Materials Res. 2004, 7, 97–102. [Google Scholar] [CrossRef]
- Gheno, T.; Monceau, D.; Young, D.J. Kinetics of breakaway oxidation of Fe–Cr and Fe–Cr–Ni alloys in dry and wet carbon dioxide. Corrosion Science 2013, 77, 246–256. [Google Scholar] [CrossRef]
- Wright, I.G.; Peraldt, R. Oxidation-limited lifetime of ODS-FeCrAl alloys: Observations on influence of surface shape. High Temp. Corrosion of Materials 2023, 99, 183–200. [Google Scholar] [CrossRef]
- Ma, S.; Ding, Q.; Wei, X.; et al. The effects of alloying elements Cr, Al, and Si on oxidation behaviors of Ni-based superalloys. Materials 2022, 15, 7352. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, B.; Westerlund, A. Oxidation comparison of alumina-forming and chromia-forming commercial alloys at 1100 and 1200°C. Oxidat. Met. 2017, 88, 315–326. [Google Scholar] [CrossRef]
- Cheng, C.; Li, X.; Le, Q.; et al. Effect of REs (Y, Nd) addition on high temperature oxidation kinetics, oxide layer characteristic and activation energy of AZ80 alloy. Magnesium and Alloys 2020, 8, 1281–1295. [Google Scholar] [CrossRef]
- Zemlyanov, D.; Klötzer, B.; Gabasch, H.; et al. Kinetics of palladium oxidation in the mbar pressure range: Ambient pressure XPS study. Topics Catal. 2013, 56, 885–895. [Google Scholar] [CrossRef]
- Gasparini, C.; Chater, R.J.; Horlait, D.; et al. Zirconium carbide oxidation: Kinetics and oxygen diffusion through the intermediate layer Amer. Ceramic Soc. 2018, 101, 2638–2652. [Google Scholar] [CrossRef]
- Lo, K.-C.; Chang, Y.-J.; Murakami, H.; et al. An oxidation resistant refractory high entropy alloy protected by CrTaO4-based oxide. Sci. Pep. 2019, 9, 7266. [Google Scholar] [CrossRef]
- Motta, A.T.; Gomes da Silva, M.J.; Yilmazbayhan, A.; et al. Microstructural characterization of oxides formed on model Zr alloys using synchrotron radiation. ASTM Intern. 2008, 5, JAI101257. [Google Scholar] [CrossRef]
- Labbe, J.-C.; Duchez, F.; Billy, M. Nitruration du germanium pulvérulent par l'ammoniac. Compt. Rend. Acad. Sci. Paris 1971, 273, 1750–1753. [Google Scholar]
- Brown, M.E. The Prout-Tompkins rate equation in solid-state kinetics. Thermochim. Acta 1997, 300, 93–106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




