Submitted:
16 September 2023
Posted:
19 September 2023
You are already at the latest version
Abstract
Keywords:
I. Introduction
II. Materials and Methods
Cell Culture and Reagents
Plant Collection and Extraction
DPPH (α, α-diphenyl-β-picrylhydrazyl) Antioxidant Activity
In vitro Cytotoxicity Assay
Cell Aggregation Assay
Assay of Hemolysis and Anti-hemolytic activity
Scratch/ Wound-Healing Assay
Western Blotting Analysis
Spectroscopic determination of total phenolic content
Spectroscopic determination of total flavonoids content
Gas Chromatography/ Mass Spectrometry (GC/MS)
Statistical Analysis
III. Results
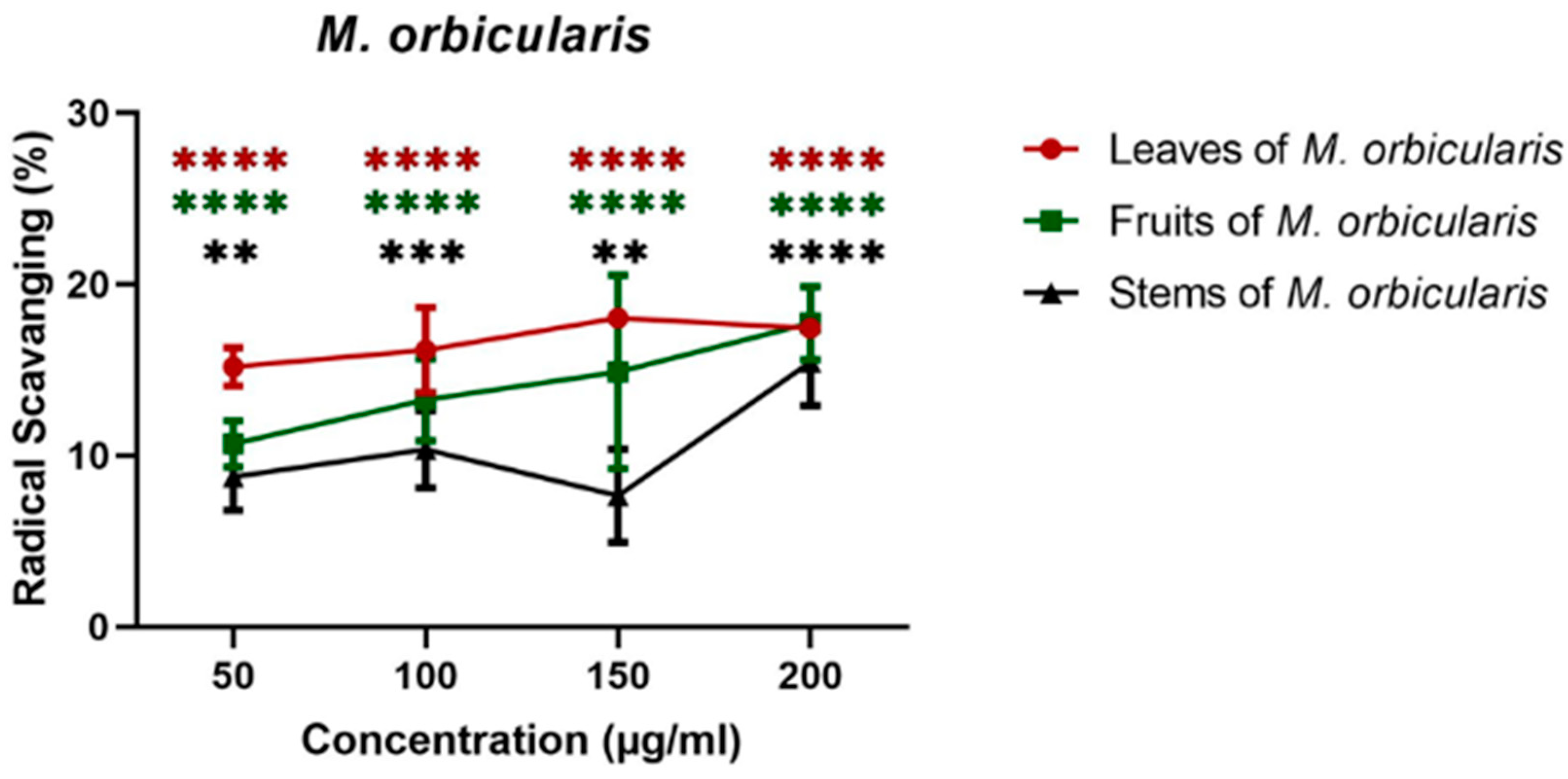
M. orbicularis plant ethanolic extracts exhibit remarkable antioxidant properties
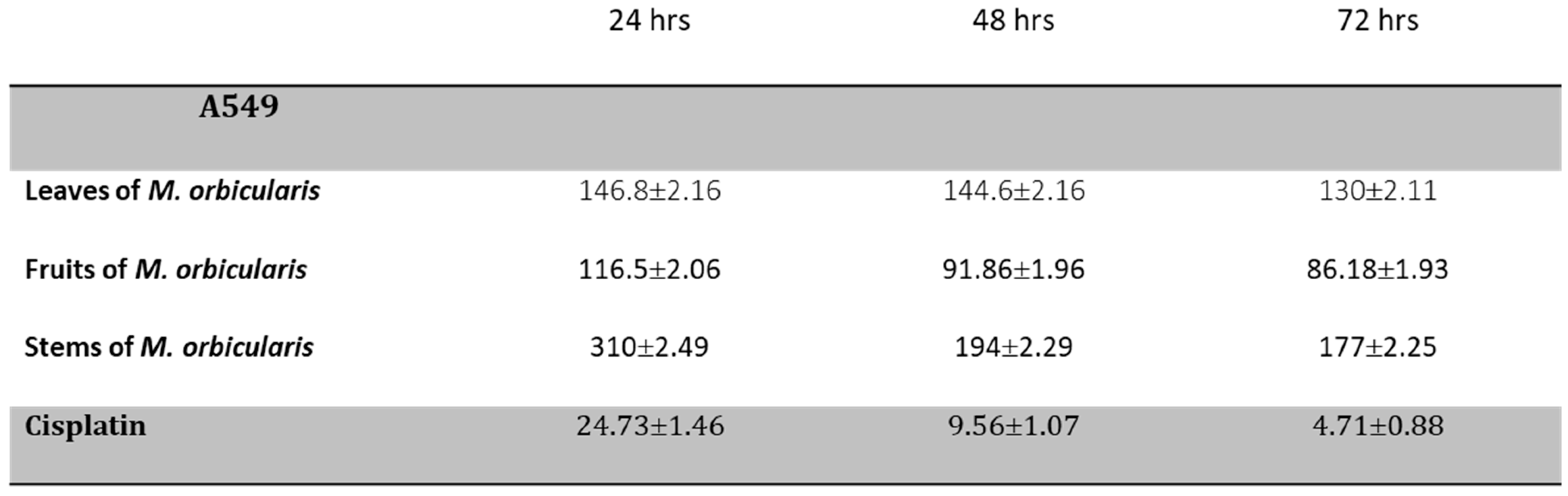
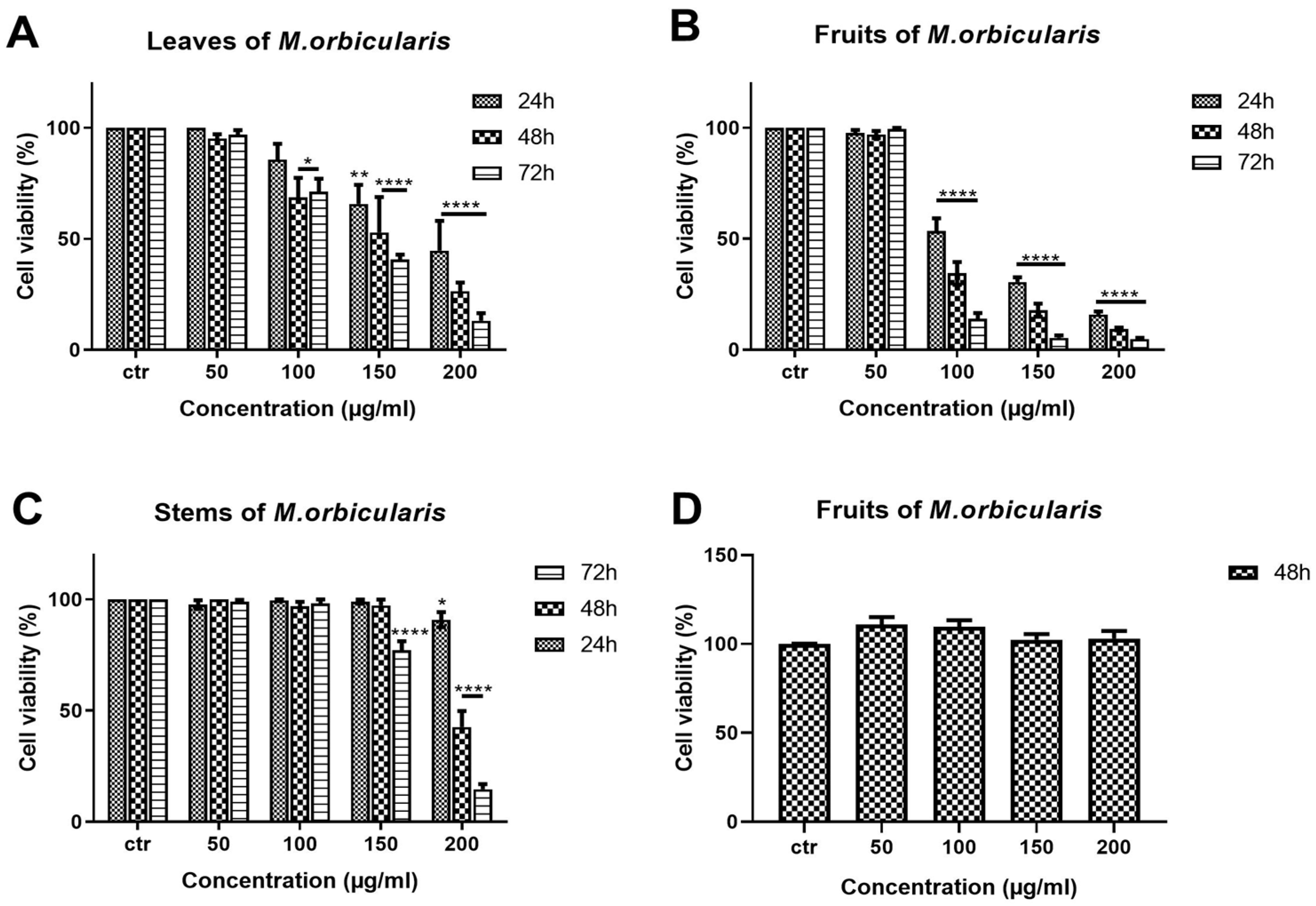
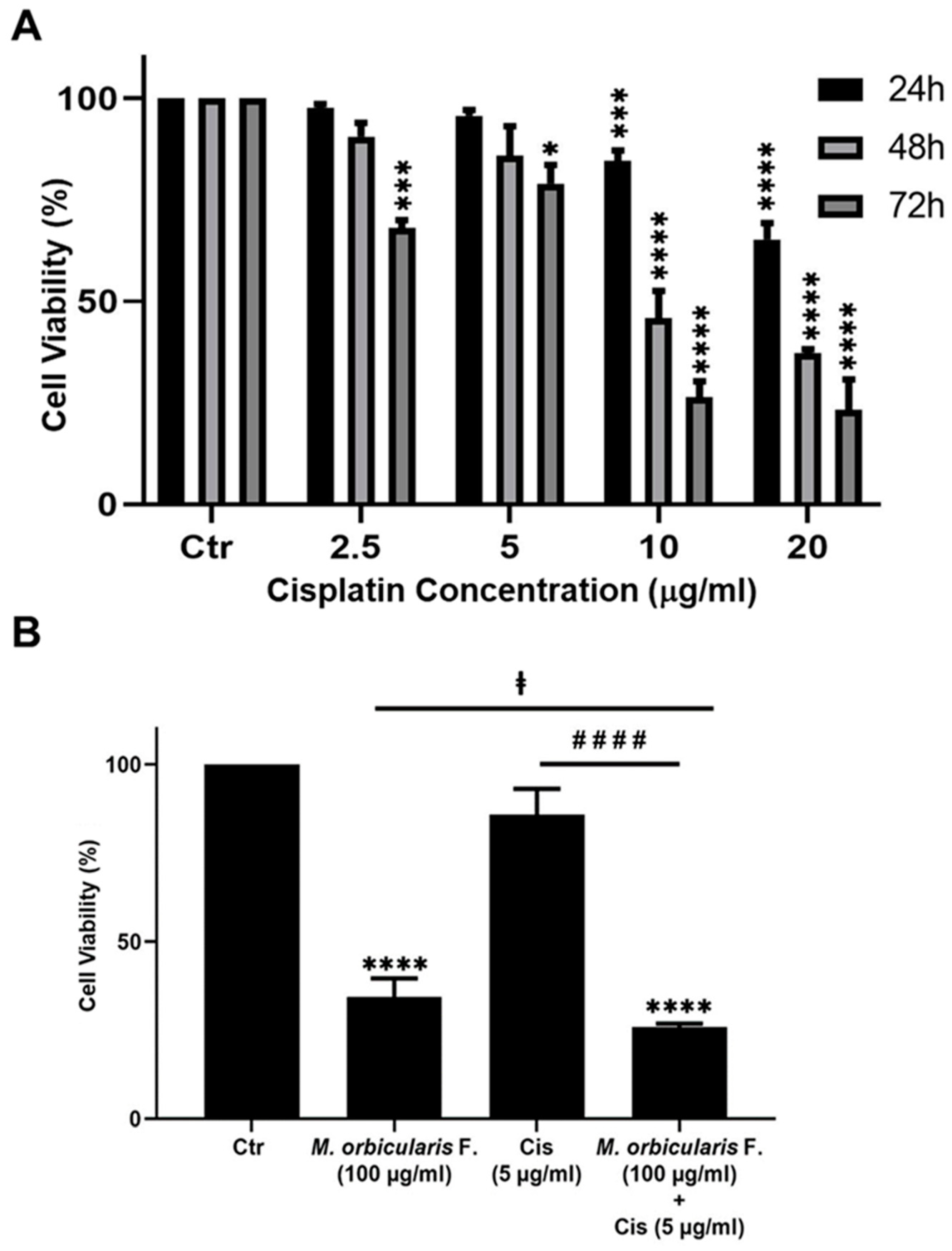
- Table 1. IC50 values (in μg/ml) of ethanolic extracts of parts of M. orbicularis used to treat A549 cells for 24, 48 and 72 hours. The IC50 values of cisplatin in A549 cells are also listed for comparison.
M. orbicularis fruit ethanolic extracts induce apoptosis of A549 lung adenocarcinoma cells
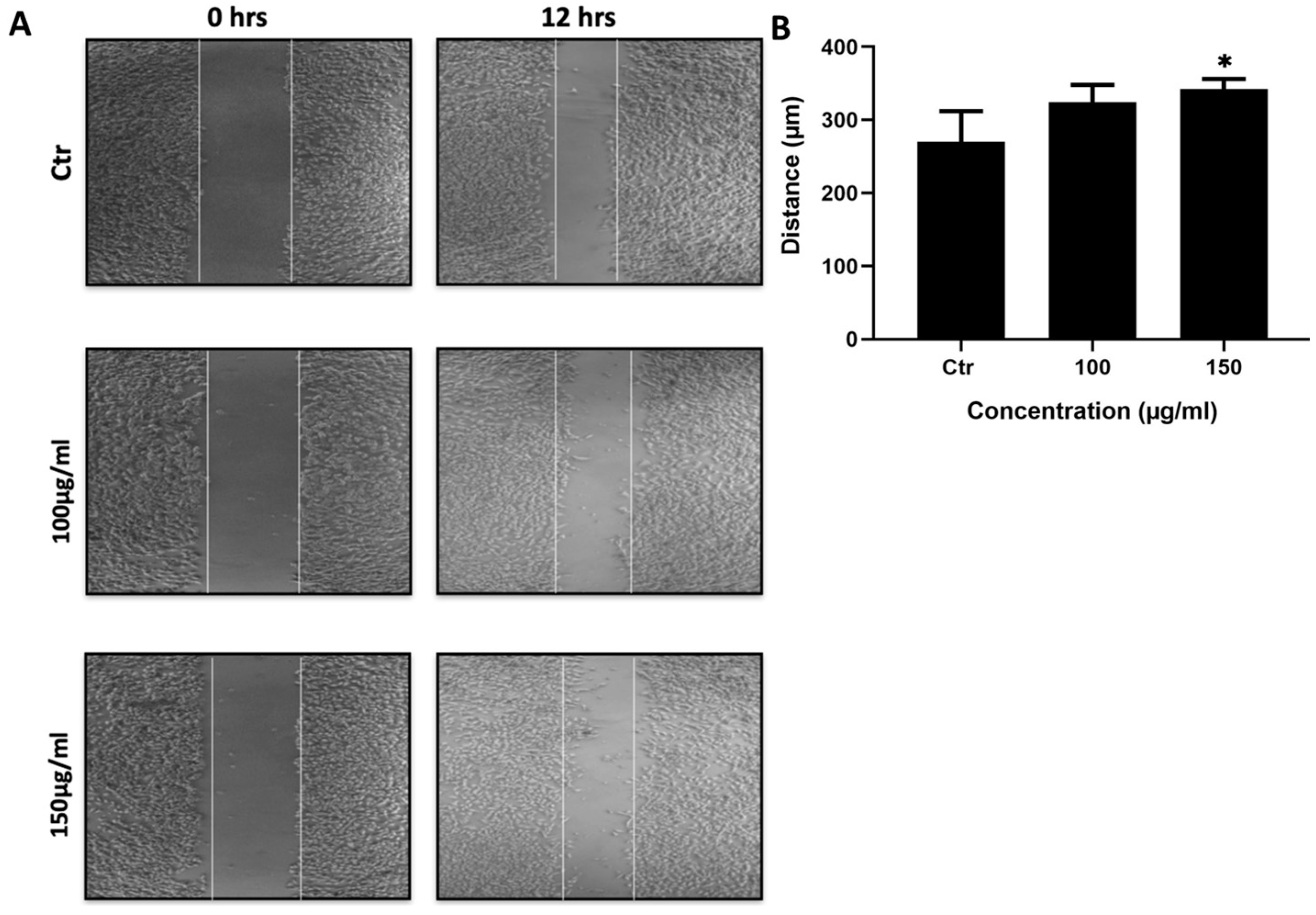
Ethanolic extracts of fruits of M. orbicularis reduce the migration of A549 lung adenocarcinoma cells
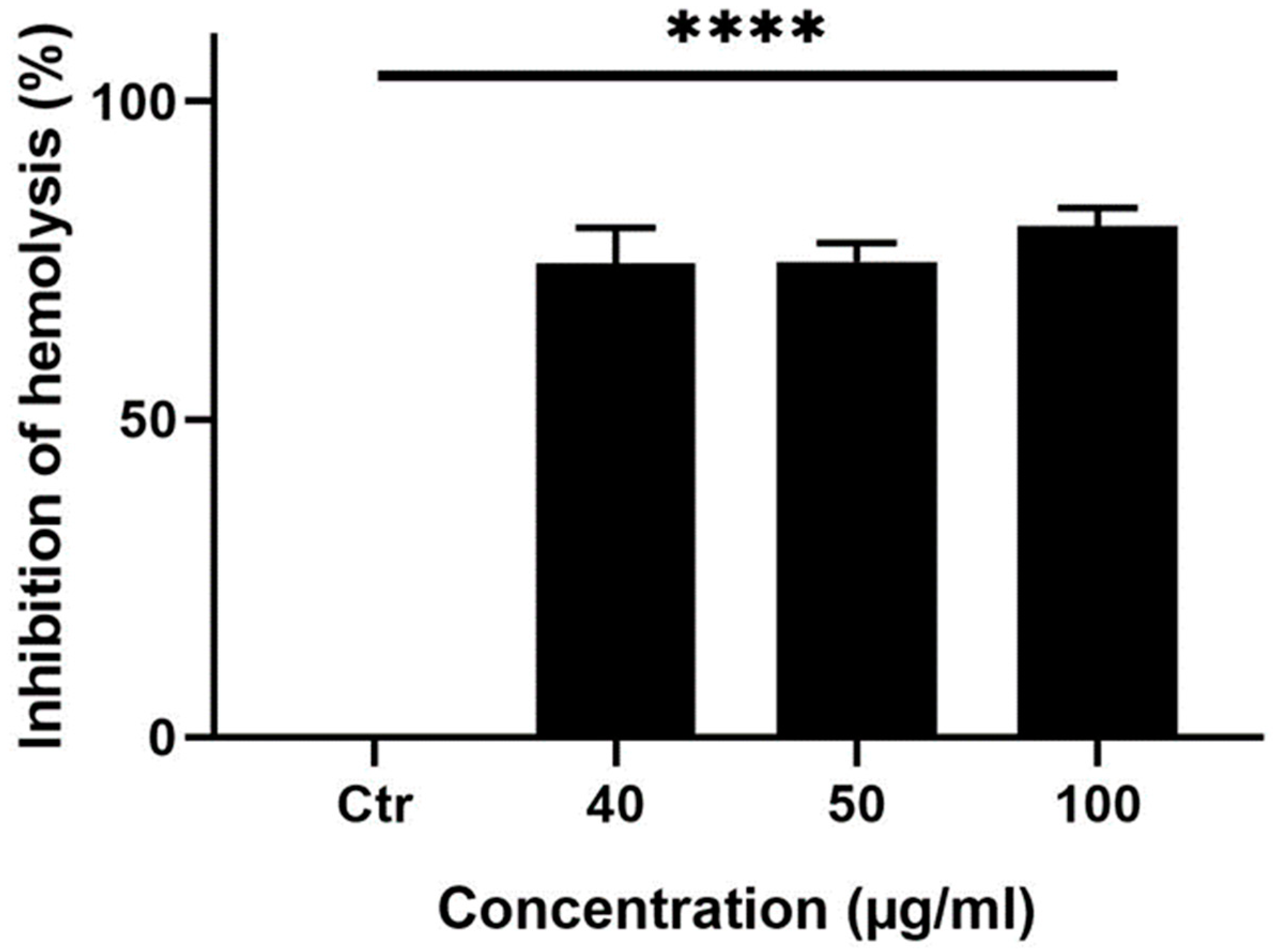
Ethanolic extracts of M. orbicularis fruits exhibit potent antihemolytic properties
Ethanolic extracts of fruits of M. orbicularis enhance cisplatin-induced cytotoxicity in A549 lung cancer cells
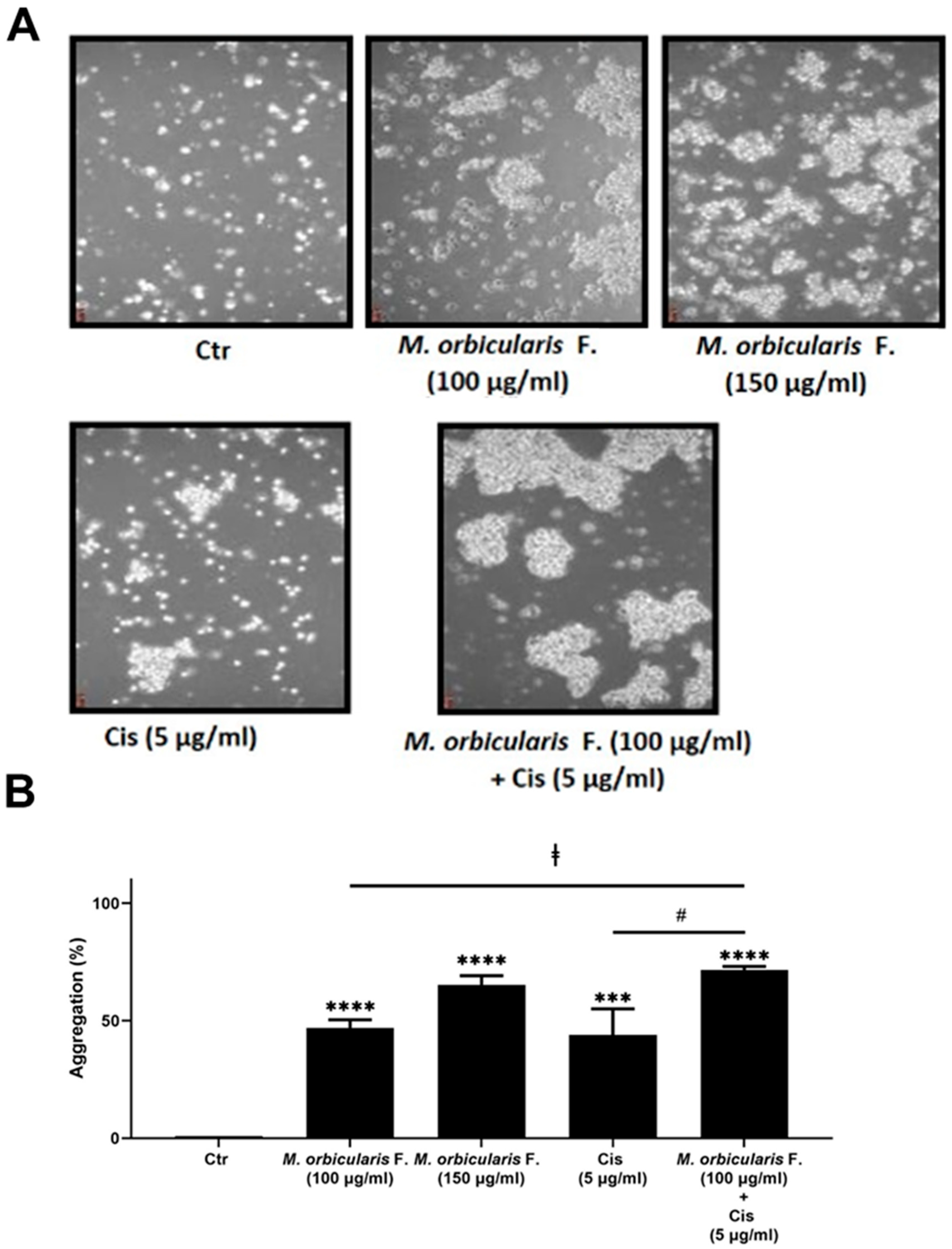
Ethanolic extracts of fruits of M. orbicularis augment cisplatin-induced aggregation of A549 lung cancer cells
Total polyphenols and flavonoids contents of M. orbicularis fruits ethanolic extracts
Identification of phytochemical composition of M. orbicularis fruits ethanolic extracts by GC/MS
IV. Discussion
Author Contributions
Funding
Acknowledgement
Conflict of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cancer Stat Facts: Lung and Bronchus Cancer. 2023.
- Rossi, A.; Sacco, P.C.; Sgambato, A.; Casaluce, F.; Santabarbara, G.; Palazzolo, G.; Maione, P.; Gridelli, C. Optimal drugs for second-line treatment of patients with small-cell lung cancer. Expert Opin Pharmacother 2016, 17, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Ismaila, N.; Hann, C.L.; Malhotra, N.; Movsas, B.; Norris, K.; Pietanza, M.C.; Ramalingam, S.S.; Turrisi, A.T., 3rd; Giaccone, G. Treatment of Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. J Clin Oncol 2015, 33, 4106–4111. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Almquist, D.; Mosalpuria, K.; Ganti, A.K. Multimodality Therapy for Limited-Stage Small-Cell Lung Cancer. J Oncol Pract 2016, 12, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.Y.; Lee, B.; Kim, K.I.; Lee, B.J. Herbal medicine on cancer-related fatigue of lung cancer survivors: Protocol for a systematic review. Medicine (Baltimore) 2020, 99, e18968. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, M.; Kapulnik, Y.; Koltai, H. Plant derived substances with anti-cancer activity: from folklore to practice. Front Plant Sci 2015, 6, 799. [Google Scholar] [CrossRef]
- Buyel, J.F. Plants as sources of natural and recombinant anti-cancer agents. Biotechnol Adv 2018, 36, 506–520. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J Ethnopharmacol 2005, 100, 72–79. [Google Scholar] [CrossRef]
- Shaik, B.B.; Katari, N.K.; Jonnalagadda, S.B. Role of Natural Products in Developing Novel Anticancer Agents: A Perspective. Chem Biodivers 2022, 19, e202200535. [Google Scholar] [CrossRef]
- Konkimalla, V.B.; Efferth, T. Evidence-based Chinese medicine for cancer therapy. J Ethnopharmacol 2008, 116, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.J.; Meng, M.; Liu, Y.; Su, T.; Kwan, H.Y. Medicinal herbs and bioactive compounds overcome the drug resistance to epidermal growth factor receptor inhibitors in non-small cell lung cancer. Oncol Lett 2021, 22, 646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, S.; Li, C.; Li, T.; Huang, Y. Remodeling tumor microenvironment with natural products to overcome drug resistance. Front Immunol 2022, 13, 1051998. [Google Scholar] [CrossRef] [PubMed]
- Nisar, S.; Masoodi, T.; Prabhu, K.S.; Kuttikrishnan, S.; Zarif, L.; Khatoon, S.; Ali, S.; Uddin, S.; Akil, A.A.; Singh, M. , et al. Natural products as chemo-radiation therapy sensitizers in cancers. Biomed Pharmacother 2022, 154, 113610. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jeong, D.; Song, M.; Kim, B. Recent Advances in Anti-Metastatic Approaches of Herbal Medicines in 5 Major Cancers: From Traditional Medicine to Modern Drug Discovery. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Malik, J.A.; Ahmed, S.; Kameshwar, V.A.; Alanazi, J.; Alamri, A.; Ahemad, N. Can Natural Products Targeting EMT Serve as the Future Anticancer Therapeutics? Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Zitouna, N.; Marghali, S.; Gharbi, M.; Haddioui, A.; Trifi-Farah, N. Sequence divergence of microsatellites for phylogeographic assessment of Moroccan Medicago species. Genet Mol Res 2014, 13, 1548–1562. [Google Scholar] [CrossRef]
- Morshedi, Z.; Assadi, M.; Small, E.; Dehshiri, M.M.; Mehregan, I. Systematic Studies on Populations of Medicago orbicularis (L.) Bartal: Molecular, Morphological and Ecological Characterizations. Journal of Genetic Resources 2022, 8, 178–187. [Google Scholar] [CrossRef]
- Karam, N.; Choueiry, Z.; Al-Beyrouthy, J.; Shehadeh, A.; Chalak, L.; Yazbek, M. Phenotypic diversity of Medicago crop wild relatives growing in Lebanon. Genetic Resources and Crop Evolution 2023, 70, 1487–1499. [Google Scholar] [CrossRef]
- Bora, K.S.; Sharma, A. Phytochemical and pharmacological potential of Medicago sativa: a review. Pharm Biol 2011, 49, 211–220. [Google Scholar] [CrossRef]
- Khan, M.I.; Asad, S.; Zaman, G.; Rehman, H.; Rehman, S.; Iqbal, A.; Ullah, A.; Ullah, I.; Ali, S. Antioxidant And Cytotoxic Activities Of Crude Methanolic Extract Of Medicago Polymorpha. IOSR Journal of Pharmacy 2013, 3, 32–37. [Google Scholar]
- Usman, M.; Khan, W.R.; Yousaf, N.; Akram, S.; Murtaza, G.; Kudus, K.A.; Ditta, A.; Rosli, Z.; Rajpar, M.N.; Nazre, M. Exploring the Phytochemicals and Anti-Cancer Potential of the Members of Fabaceae Family: A Comprehensive Review. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front Pharmacol 2019, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Anifowose, S.O.; Alqahtani, W.S.N.; Al-Dahmash, B.A.; Sasse, F.; Jalouli, M.; Aboul-Soud, M.A.M.; Badjah-Hadj-Ahmed, A.Y.; Elnakady, Y.A. Efforts in Bioprospecting Research: A Survey of Novel Anticancer Phytochemicals Reported in the Last Decade. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed Pharmacother 2022, 146, 112442. [Google Scholar] [CrossRef] [PubMed]
- Mesmar, J.; Abdallah, R.; Hamade, K.; Baydoun, S.; Al-Thani, N.; Shaito, A.; Maresca, M.; Badran, A.; Baydoun, E. Ethanolic extract of Origanum syriacum L. leaves exhibits potent anti-breast cancer potential and robust antioxidant properties. Front Pharmacol 2022, 13, 994025. [Google Scholar] [CrossRef] [PubMed]
- AlKahlout, A.; Fardoun, M.; Mesmar, J.; Abdallah, R.; Badran, A.; Nasser, S.A.; Baydoun, S.; Kobeissy, F.; Shaito, A.; Iratni, R.; et al. Origanum syriacum L. Attenuates the Malignant Phenotype of MDA-MB231 Breast Cancer Cells. Front Oncol 2022, 12, 922196. [Google Scholar] [CrossRef]
- Imran Khan, M. Antioxidant And Cytotoxic Activities Of Crude Methanolic Extract Of Medicago Polymorpha. IOSR Journal of Pharmacy (IOSRPHR) 2013, 03, 32–37. [Google Scholar] [CrossRef]
- Shaito, A.; Thuan, D.T.B.; Phu, H.T.; Nguyen, T.H.D.; Hasan, H.; Halabi, S.; Abdelhady, S.; Nasrallah, G.K.; Eid, A.H.; Pintus, G. Herbal Medicine for Cardiovascular Diseases: Efficacy, Mechanisms, and Safety. Front Pharmacol 2020, 11, 422. [Google Scholar] [CrossRef]
- Alsamri, H.; Athamneh, K.; Pintus, G.; Eid, A.H.; Iratni, R. Pharmacological and Antioxidant Activities of Rhus coriaria L. (Sumac). Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef]
- Visnevschi-Necrasov, T.; Barreira, J.C.; Cunha, S.C.; Pereira, G.; Nunes, E.; Oliveira, M.B. Advances in isoflavone profile characterisation using matrix solid-phase dispersion coupled to HPLC/DAD in Medicago species. Phytochem Anal 2015, 26, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Güleç, M. , Erarslan, Z. B. & Kültür, Ş.. The Medicinal Plants Traditionally Used Against Cardiovascular Diseases In Türkiye. International Journal of Traditional and Complementary Medicine Research 2023, 4, 81–96. [Google Scholar] [CrossRef]
- NASREDDINE, S.; MCHEIK, M.; KHALIL, M.; El-Rashed, Z.; DAHER, A.; KHALIFE, A. The antioxidant, antibacterial, antihemolytic and epithelial ovarian cancer antiproliferative activities of the lebanese plant salvia libanotica. Asian Journal of Science and Technology 2018, 09, 8695–8703. [Google Scholar]
- Malagoli, D. A full-length protocol to test hemolytic activity of palytoxin on human erythrocytes. Invertebrate Survival Journal 2007, 4, 92–94. [Google Scholar]
- James, O.; Alewo, I.M. In vitro Antihemolytic Activity of Gymnema Sylvestre Extracts Against Hydrogen Peroxide (H2O2) Induced Haemolysis in Human Erythrocytes. American Journal of Phytomedicine and Clinical Therapeutics 2014, 2, 861–869. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American journal of Enology and Viticulture 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of food and drug analysis 2002, 10. [Google Scholar]
- Katarina, Š.; Živković, J.; Zdunić , G.; Gođevac, D.; Đorđević, B.; Dojčinović, B.; Đorđević, N. Phenolic and mineral profiles of four Balkan indigenous apple cultivars monitored at two different maturity stages. Journal of food composition and analysis 2014, v. 35, pp. 101-111-2014 v.2035 no.2012. [CrossRef]
- Rolim, P.M.; Fidelis, G.P.; Padilha, C.E.A.; Santos, E.S.; Rocha, H.A.O.; Macedo, G.R. Phenolic profile and antioxidant activity from peels and seeds of melon (Cucumis melo L. var. reticulatus) and their antiproliferative effect in cancer cells. Brazilian Journal of Medical and Biological Research 2018, 51. [Google Scholar] [CrossRef]
- Li, J.; Yuan, J. Caspases in apoptosis and beyond. Oncogene 2008, 27, 6194–6206. [Google Scholar] [CrossRef]
- Cory, S.; Roberts, A.W.; Colman, P.M.; Adams, J.M. Targeting BCL-2-like Proteins to Kill Cancer Cells. Trends Cancer 2016, 2, 443–460. [Google Scholar] [CrossRef]
- Tseng, C.Y.; Lin, C.H.; Wu, L.Y.; Wang, J.S.; Chung, M.C.; Chang, J.F.; Chao, M.W. Potential Combinational Anti-Cancer Therapy in Non-Small Cell Lung Cancer with Traditional Chinese Medicine Sun-Bai-Pi Extract and Cisplatin. PloS one 2016, 11, e0155469. [Google Scholar] [CrossRef] [PubMed]
- Omairi, I.; Kobeissy, F.; Nasreddine, S. Anti-Oxidant, Anti-Hemolytic Effects of Crataegus aronia Leaves and Its Anti- Proliferative Effect Enhance Cisplatin Cytotoxicity in A549 Human Lung Cancer Cell Line. Asian Pacific journal of cancer prevention : APJCP 2020, 21, 2993–3003. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, U.; Casadei, C.; Bergamini, A.; Attademo, L.; Cormio, G.; Lorusso, D.; Pignata, S.; Mangili, G. Therapeutic challenges for cisplatin-resistant ovarian germ cell tumors. Cancers 2019, 11, 1584. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Matsuo, K.; Ueoka, H.; Kiura, K.; Tabata, M.; Tanimoto, M. Meta-analysis of randomized clinical trials comparing cisplatin to carboplatin in patients with advanced non–small-cell lung cancer. Journal of Clinical Oncology 2004, 22, 3852–3859. [Google Scholar] [CrossRef] [PubMed]
- Leber, M.F.; Efferth, T. Molecular principles of cancer invasion and metastasis. International journal of oncology 2009, 34, 881–895. [Google Scholar] [PubMed]
- Larzabal, L.; El-Nikhely, N.; Redrado, M.; Seeger, W.; Savai, R.; Calvo, A. Differential effects of drugs targeting cancer stem cell (CSC) and non-CSC populations on lung primary tumors and metastasis. PloS one 2013, 8, e79798. [Google Scholar] [CrossRef] [PubMed]
- Maubant, S.; Cruet-Hennequart, S.; Poulain, L.; Carreiras, F.; Sichel, F.; Luis, J.; Staedel, C.; Gauduchon, P. Altered adhesion properties and alpha v integrin expression in a cisplatin-resistant human ovarian carcinoma cell line. International journal of cancer 2002, 97, 186–194. [Google Scholar] [CrossRef]
- Pareek, A.; Pant, M.; Gupta, M.M.; Kashania, P.; Ratan, Y.; Jain, V.; Pareek, A.; Chuturgoon, A.A. Moringa oleifera: An Updated Comprehensive Review of Its Pharmacological Activities, Ethnomedicinal, Phytopharmaceutical Formulation, Clinical, Phytochemical, and Toxicological Aspects. International Journal of Molecular Sciences 2023, 24, 2098. [Google Scholar] [CrossRef]
- Seglab, F.; Hamia, C.; Khacheba, I.; Djeridane, A.; Yousfi, M. High in vitro antioxidant capacities of Algerian Cleome arabica leaves’ extracts. Phytothérapie 2021, 19, 16–24. [Google Scholar] [CrossRef]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: a narrative review. Transl Cancer Res 2020, 9, 7619–7631. [Google Scholar] [CrossRef]
- Winslow, L.C.; Kroll, D.J. Herbs as medicines. Archives of internal medicine 1998, 158, 2192–2199. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.W.; Leung, Y.; Chan, C. Herbal medicine in the treatment of cancer. Current Medicinal Chemistry-Anti-Cancer Agents 2002, 2, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Vong, C.T.; Chen, H.; Gao, Y.; Lyu, P.; Qiu, L.; Zhao, M.; Liu, Q.; Cheng, Z.; Zou, J. , et al. Naturally occurring anti-cancer compounds: shining from Chinese herbal medicine. Chinese Medicine 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Suffness, M.P., J.M. Assays related to cancer drug discovery. In Methods in plant biochemistry: assays for bioactivity, Hostettmann, K., Ed. Academic Press: London, 1991; Vol. 6, pp. 71-133.
- Chipuk, J.E.; Kuwana, T.; Bouchier-Hayes, L.; Droin, N.M.; Newmeyer, D.D.; Schuler, M.; Green, D.R. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 2004, 303, 1010–1014. [Google Scholar] [CrossRef]
- Wolf, B.B.; Green, D.R. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem 1999, 274, 20049–20052. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol 2013, 14, 32. [Google Scholar] [CrossRef]
- Fernandes-Alnemri, T.; Litwack, G.; Alnemri, E.S. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem 1994, 269, 30761–30764. [Google Scholar] [CrossRef] [PubMed]
- Widłak, P. The DFF40/CAD endonuclease and its role in apoptosis. Acta Biochim Pol 2000, 47, 1037–1044. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Desnoyers, S.; Ottaviano, Y.; Davidson, N.E.; Poirier, G.G. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res 1993, 53, 3976–3985. [Google Scholar]
- Chaitanya, G.V.; Steven, A.J.; Babu, P.P. PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Commun Signal 2010, 8, 31. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ 2018, 25, 27–36. [Google Scholar] [CrossRef]
- Mayer, B.; Bartolmäs, T.; Yürek, S.; Salama, A. Variability of Findings in Drug-Induced Immune Haemolytic Anaemia: Experience over 20 Years in a Single Centre. Transfus Med Hemother 2015, 42, 333–339. [Google Scholar] [CrossRef]
- Talmadge, J.E.; Fidler, I.J. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 2010, 70, 5649–5669. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Settleman, J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Lim, C.T. Tumor dissemination: an EMT affair. Cancer Cell 2013, 23, 272–273. [Google Scholar] [CrossRef] [PubMed]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell migration. Compr Physiol 2012, 2, 2369–2392. [Google Scholar] [CrossRef] [PubMed]
- El-Hajjar, L.; Jalaleddine, N.; Shaito, A.; Zibara, K.; Kazan, J.M.; El-Saghir, J.; El-Sabban, M. Bevacizumab induces inflammation in MDA-MB-231 breast cancer cell line and in a mouse model. Cell Signal 2019, 53, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Abduljauwad, S.N.; Ahmed, H.U. Enhancing cancer cell adhesion with clay nanoparticles for countering metastasis. Sci Rep 2019, 9, 5935. [Google Scholar] [CrossRef] [PubMed]
- Jalaleddine, N.; El-Hajjar, L.; Dakik, H.; Shaito, A.; Saliba, J.; Safi, R.; Zibara, K.; El-Sabban, M. Pannexin1 Is Associated with Enhanced Epithelial-To-Mesenchymal Transition in Human Patient Breast Cancer Tissues and in Breast Cancer Cell Lines. Cancers (Basel) 2019, 11. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, X.; Xie, L.; Deng, M.; Chen, H.; Song, J.; Long, J.; Li, X.; Luo, J. Lupeol and its derivatives as anticancer and anti-inflammatory agents: Molecular mechanisms and therapeutic efficacy. Pharmacological research 2021, 164, 105373. [Google Scholar] [CrossRef]
- Sharma, N.; Palia, P.; Chaudhary, A.; Verma, K.; Kumar, I. A review on pharmacological activities of lupeol and its triterpene derivatives. Journal of Drug Delivery and Therapeutics 2020, 10, 325–332. [Google Scholar] [CrossRef]

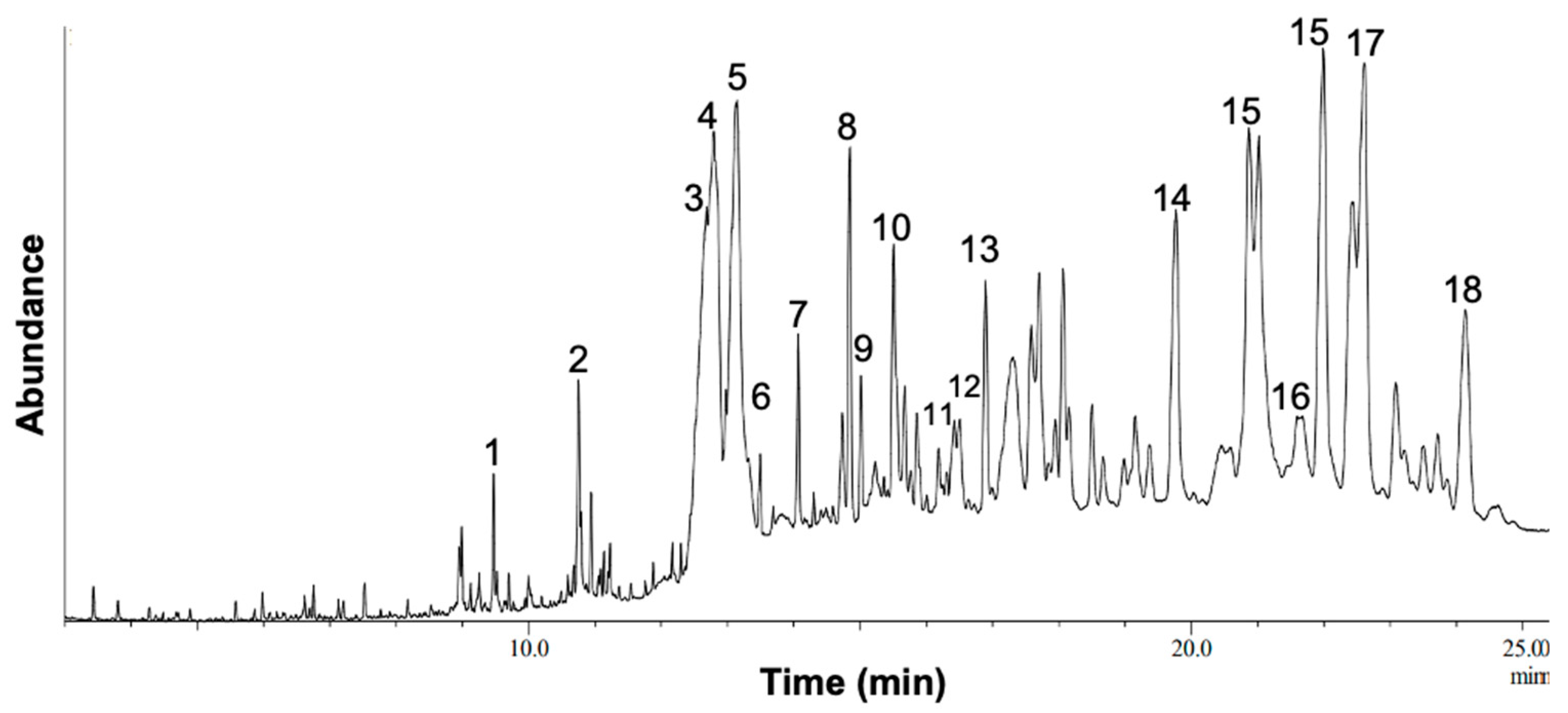
| S.N0 | Name of the Compound | CompoundNature | RT(min) | Molecular Formula |
MW |
|---|---|---|---|---|---|
| 1 | 7-Hexadecenal, (Z)- | Unsaturated aldehyde | 9.530 | C16H30O | 238 |
| 2 | Octadecanamide, N-(2-hydroxyethyl)- | Fatty acid | 10.95 | C20H41NO2 | 327 |
| 3 | Lup-20(29)-en-3-ol, acetate, (3.beta.) | Triterpenes | 12.690 | C32H52O2 | 468 |
| 4 | 6-methoxy-2-methyltetrahydroquinoline | Quinolines | 12.88 | C32H50N6O2 | 550 |
| 5 | 12-Oleanen-3-yl acetate, (3.alpha.) - | Triterpenoids | 13.19 | C32H52O2 | 468 |
| 6 | Trismethoxyresveratrol, trans- | Polyphenol resveratrol | 13.49 | C17H18O3 | 270 |
| 7 | Linoleic acid ethyl ester | Fatty acid | 14.03 | C20H36O2 | 308 |
| 8 | Lup-20(29)-en-3-one | Triterpenoid | 14.86 | C30H48O | 424 |
| 9 | 4-Nitrophenyl laurate | 4-Nitrophenyl esters | 15.15 | C18H27NO4 | 321 |
| 10 | Lupeol, trifluoroacetate | Triterpene alcohol | 15.69 | C32H49F3O2 | 522 |
| 11 | N-Methyl-pseudotomatidine diacetate | Alkaloids | 16.74 | C32H51NO4 | 513 |
| 12 | Pyridine, 1-acetyl-1,2,3,4-tetrahydro-5-(2-piperidinyl)- | Piperidine alkaloid. | 16.91 | C12H20N2O | 208 |
| 13 | Meristic acid | Fatty acid | 17.19 | C14H28O2 | 228 |
| 14 | Oxirane | Rrganic compound | 19.74 | C18H36O | 268 |
| 15 | gamma-Sitosterol | Sterol | 20.91 | C29H50O | 414 |
| 16 | beta-Amyrone | Sesquiterpene | 21.62 | C30H48O | 424 |
| 17 | Lupeol | Triterpene alcohol | 22.89 | C30H50O | 426 |
| 18 | Dihydroniloticin diacetate | Flavonoids | 24.35 | C34H54O5 | 542 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





