Submitted:
20 October 2023
Posted:
23 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Genes and Proteins
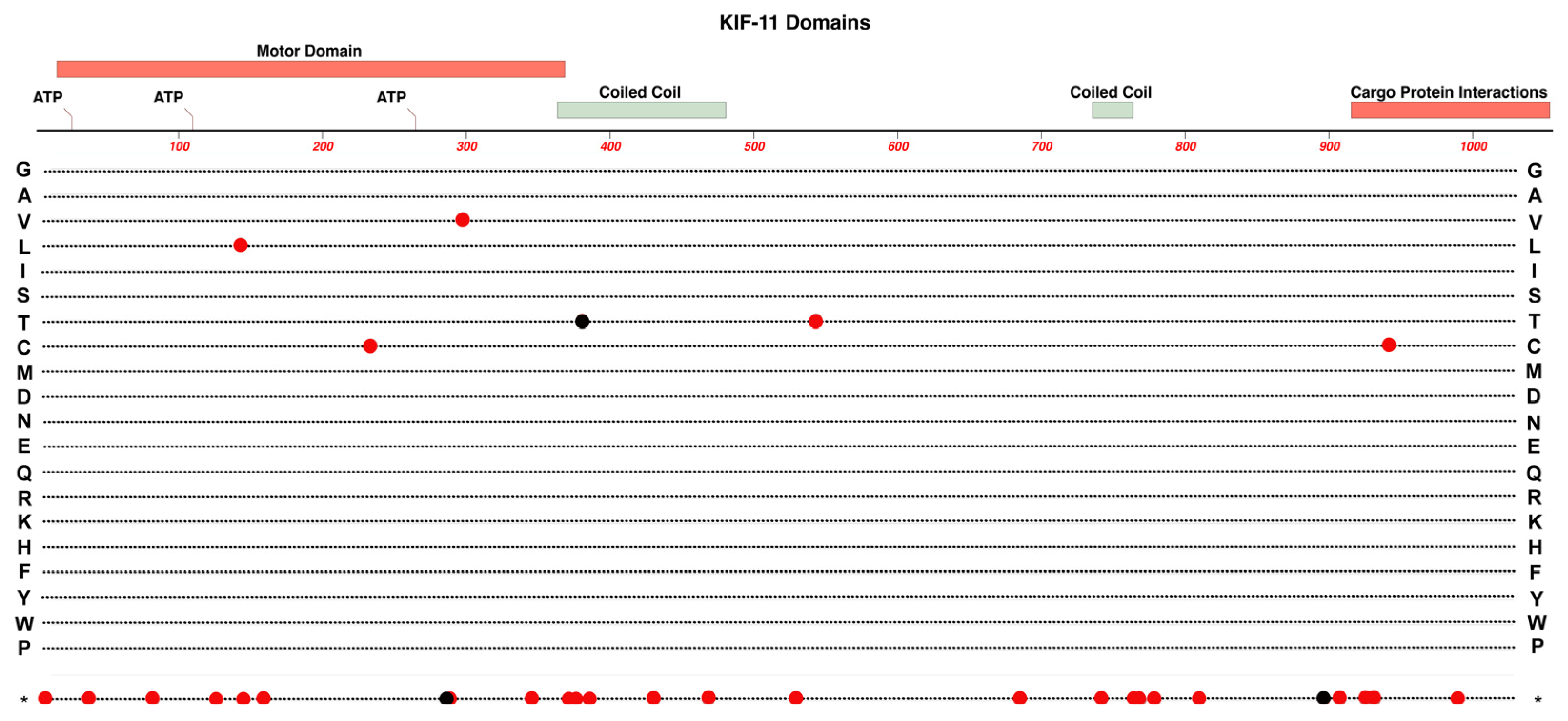
2.1. KIF11
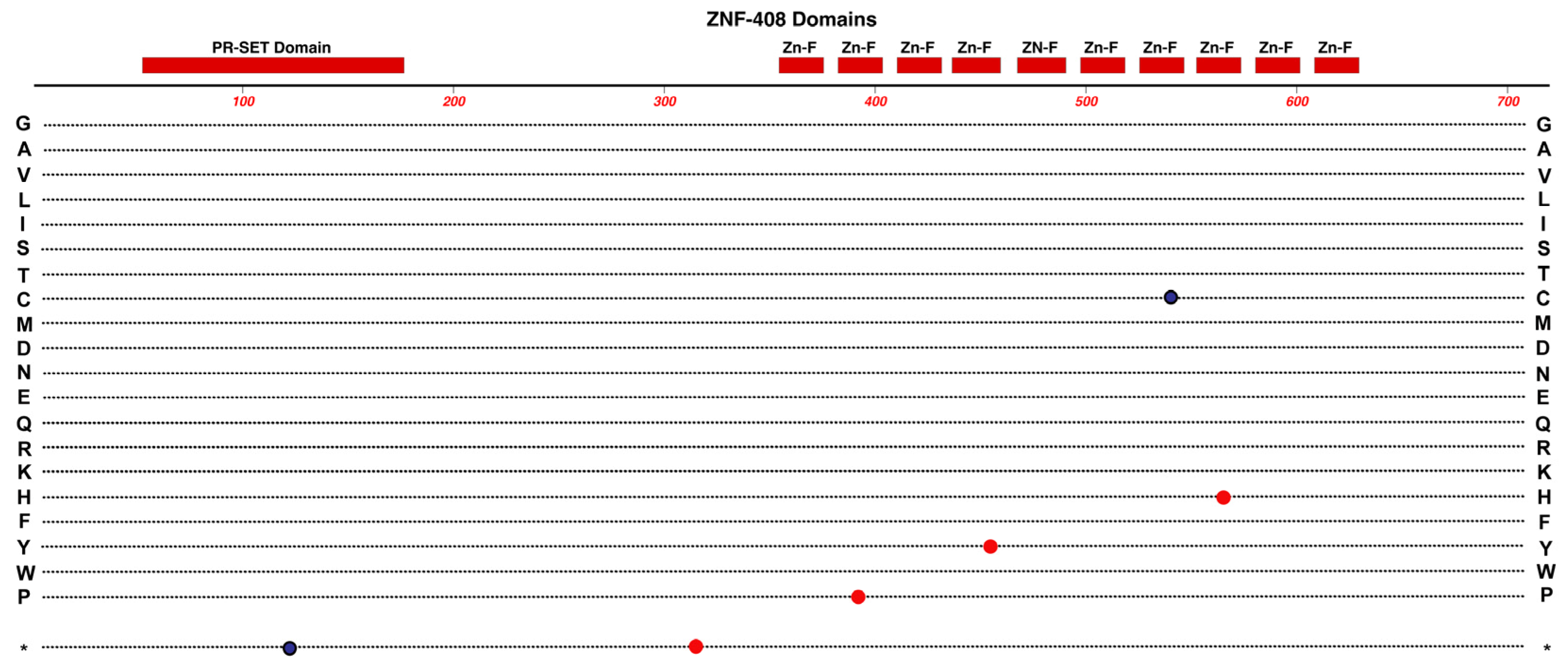
2.2. ZNF408
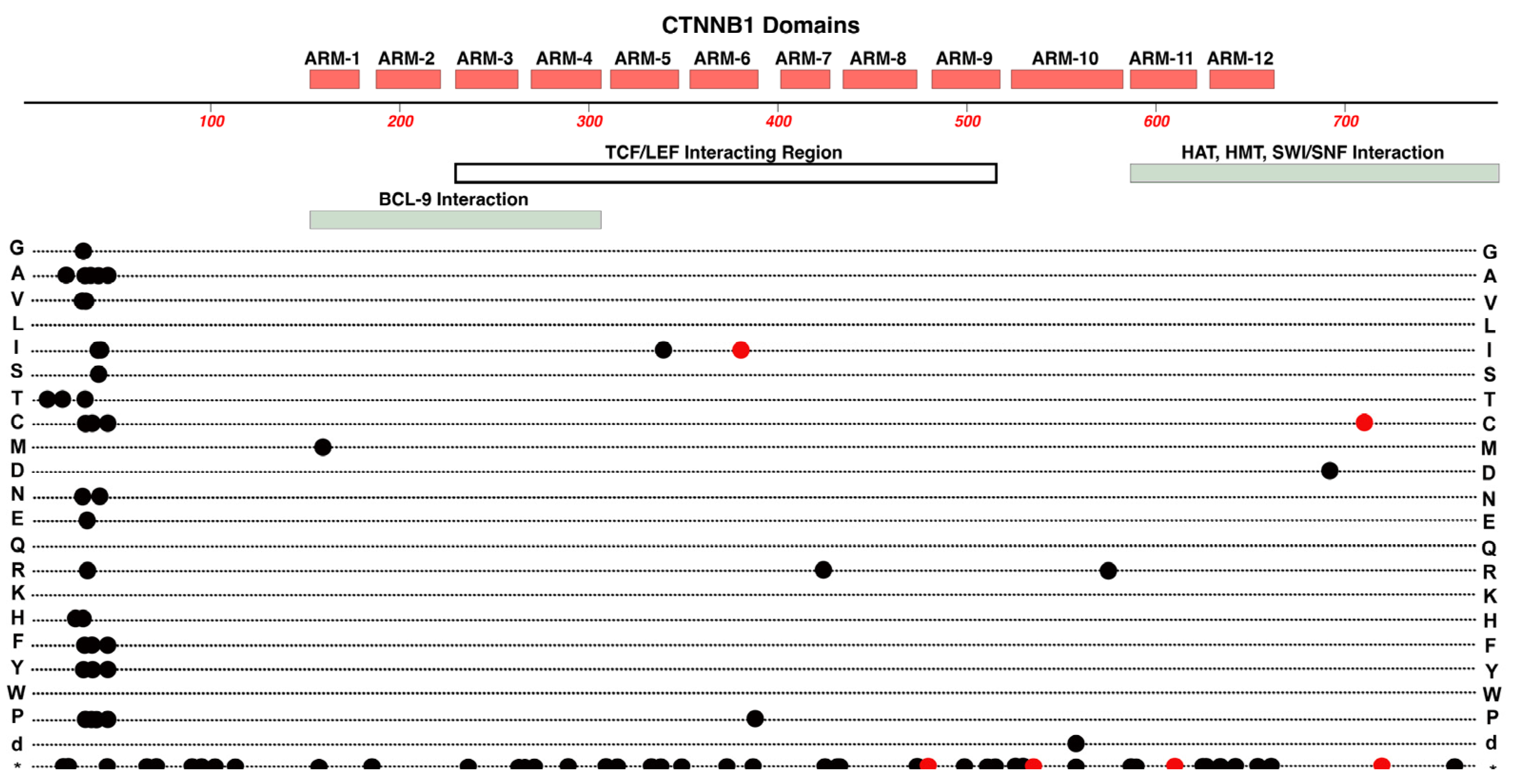
2.3. CTNNB1
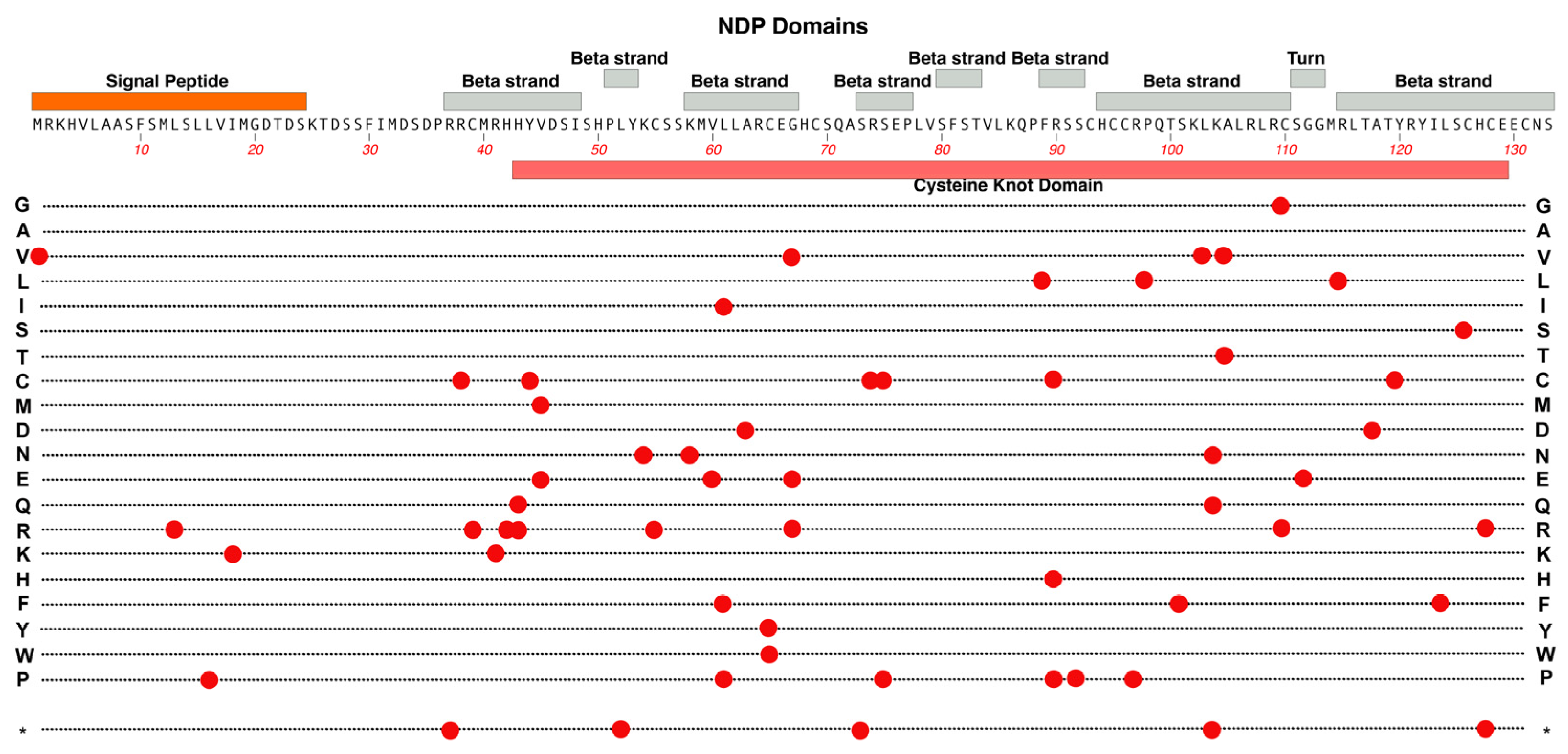
2.4. NDP
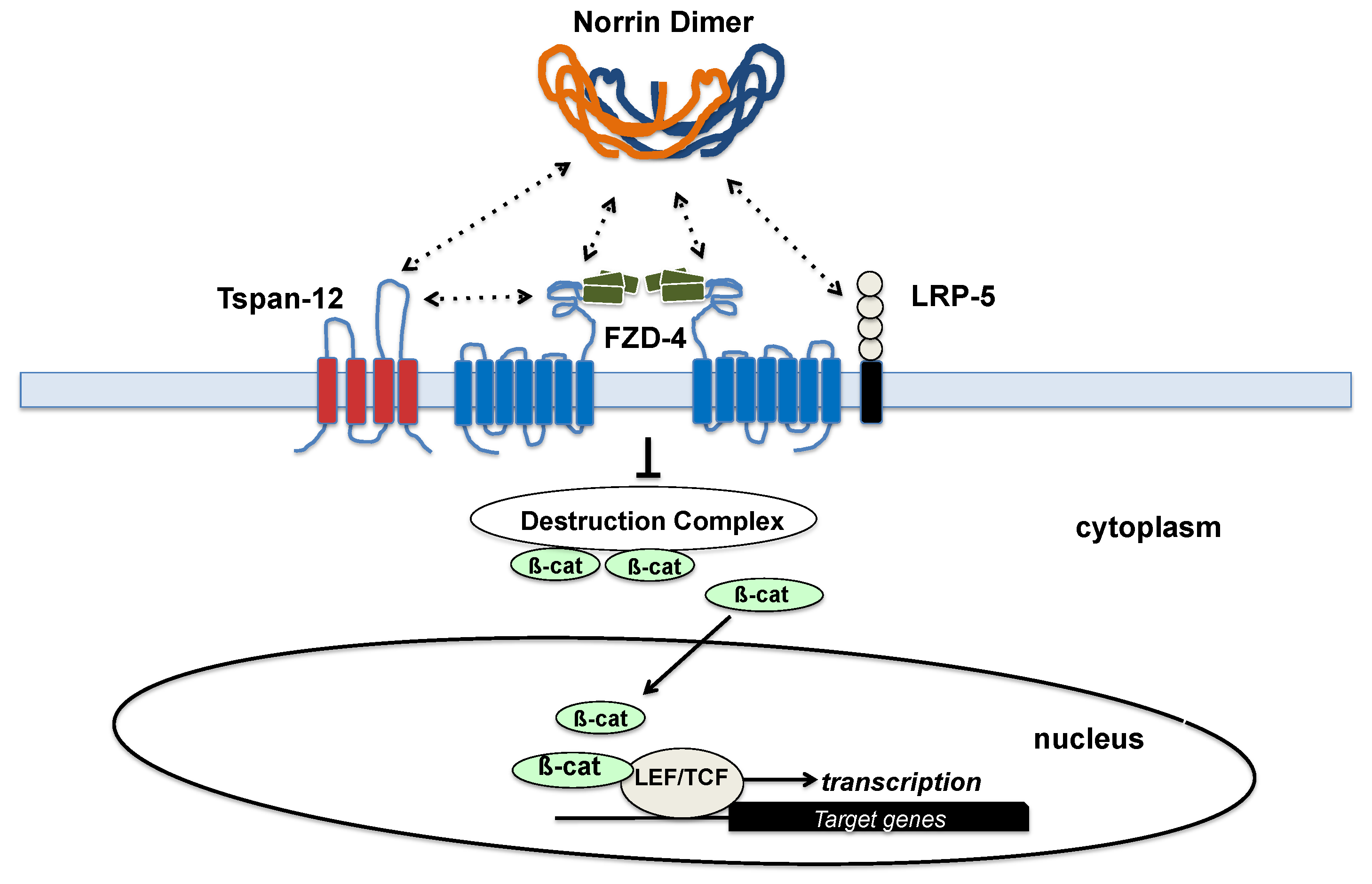
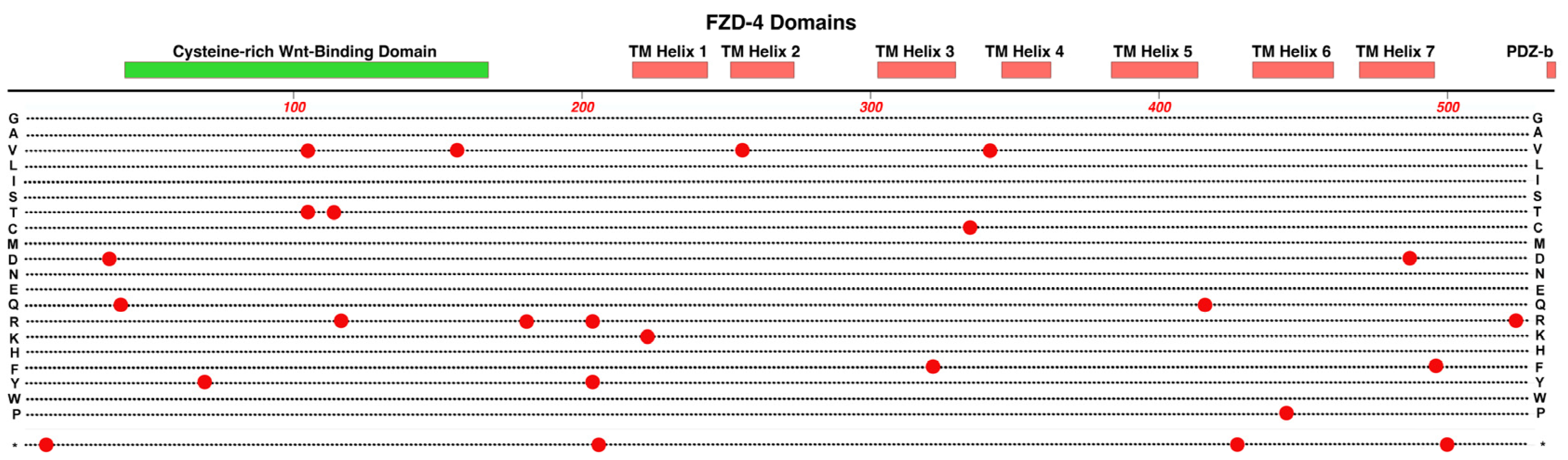
2.5. FZD4
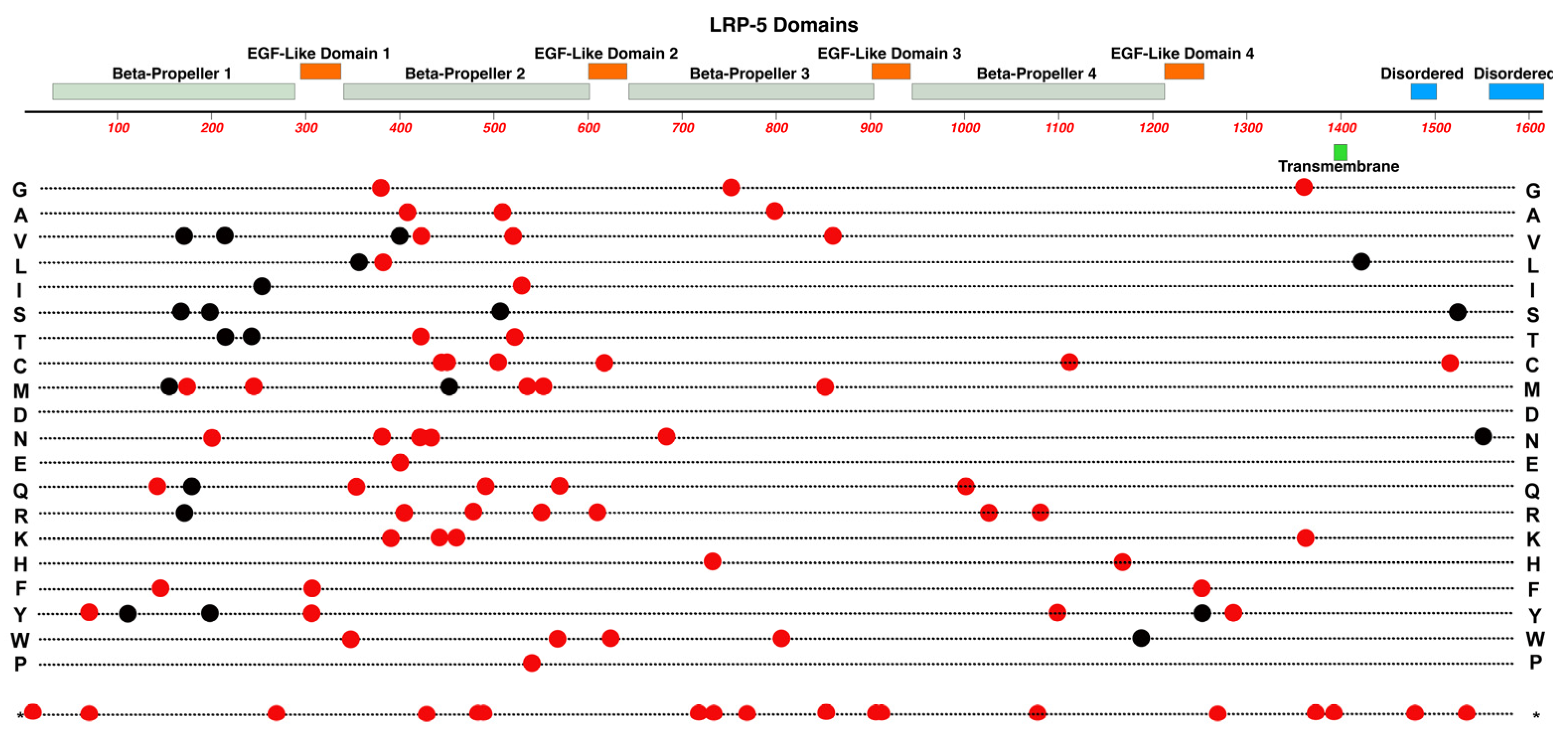
2.6. LRP5
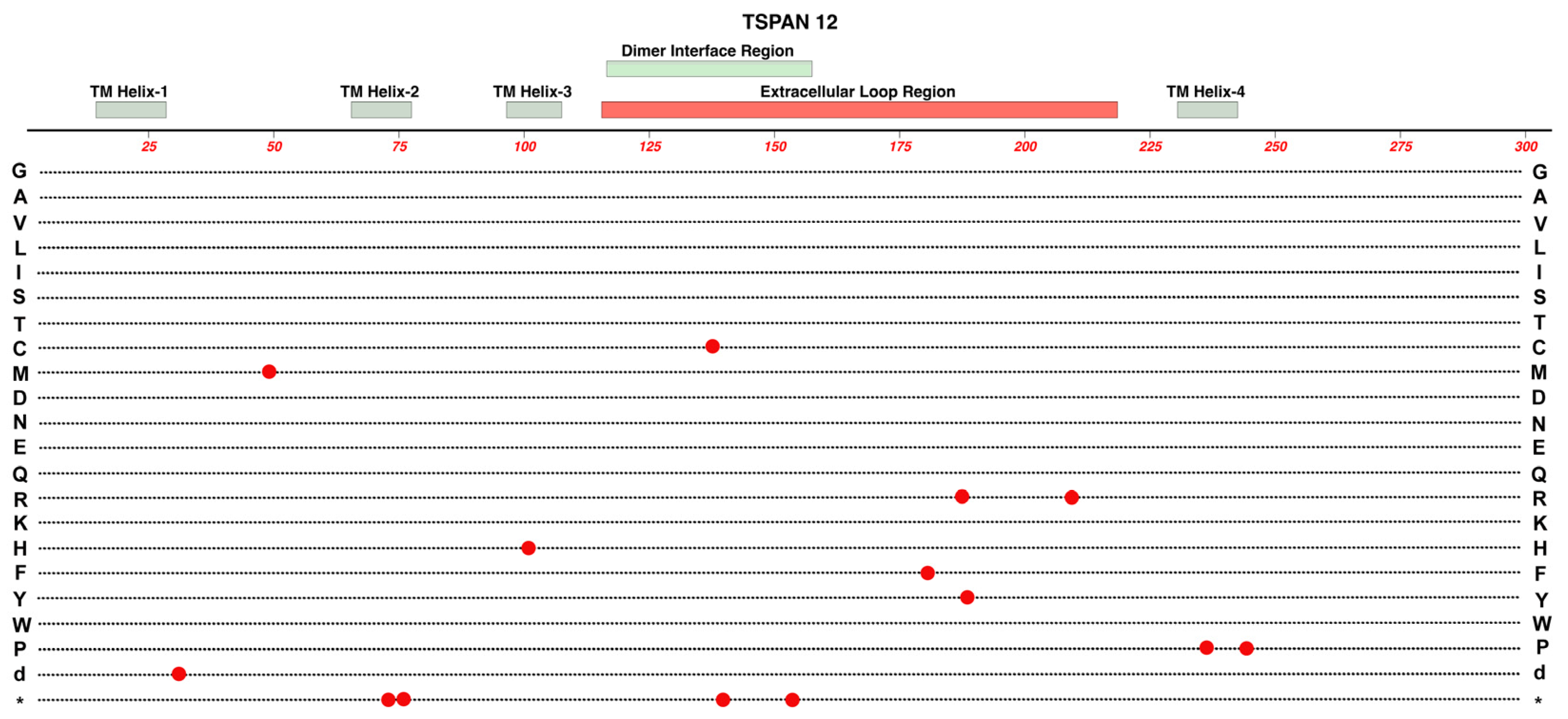
2.7. TSPAN-12
2.8. Notable Recent Genes linked to FEVR Phenotypes
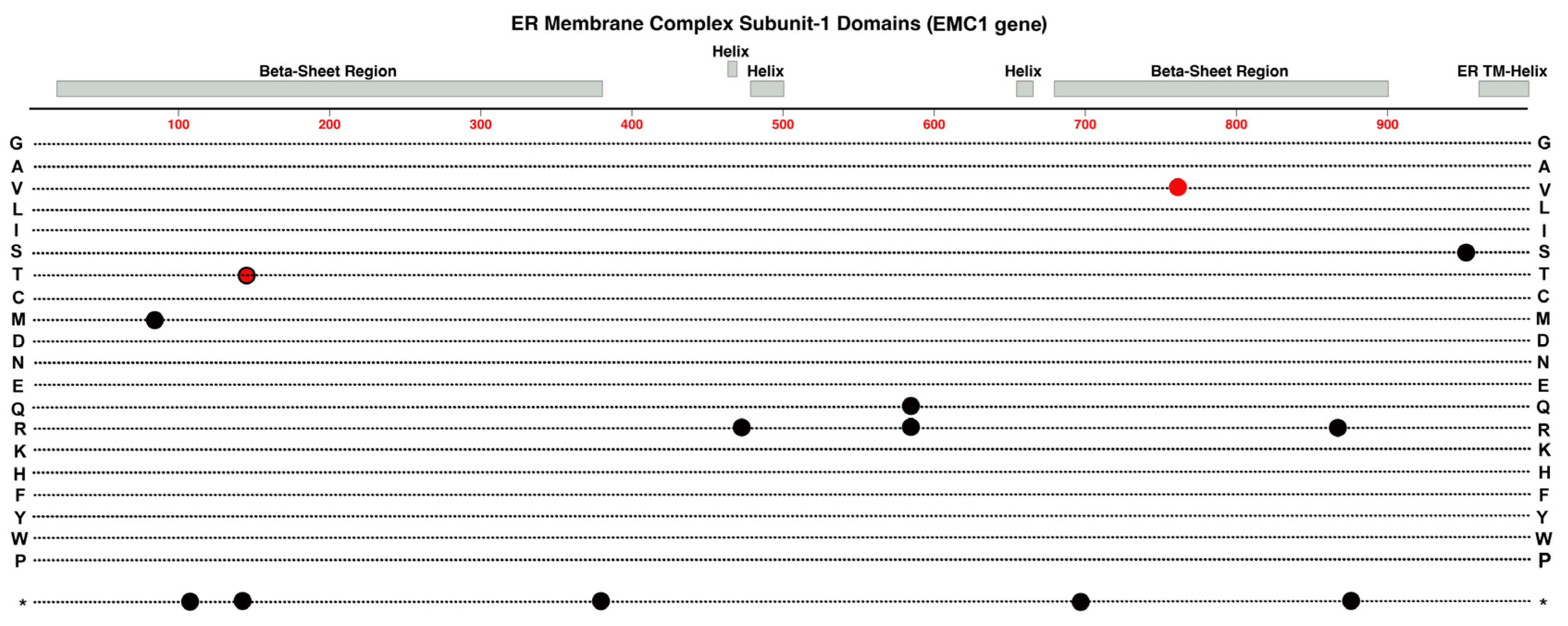
2.9. EMC-1
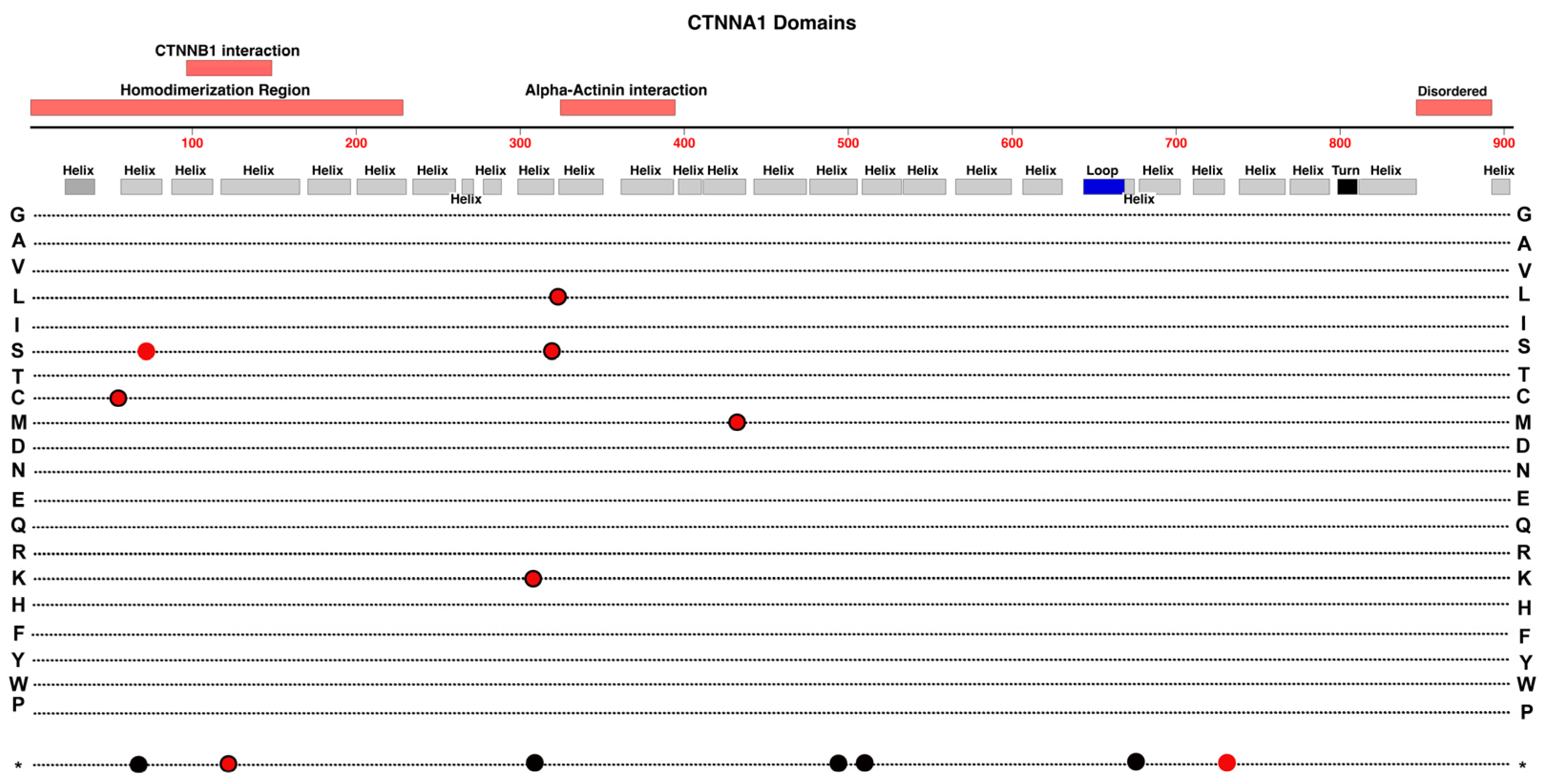
2.10. CTNNA1
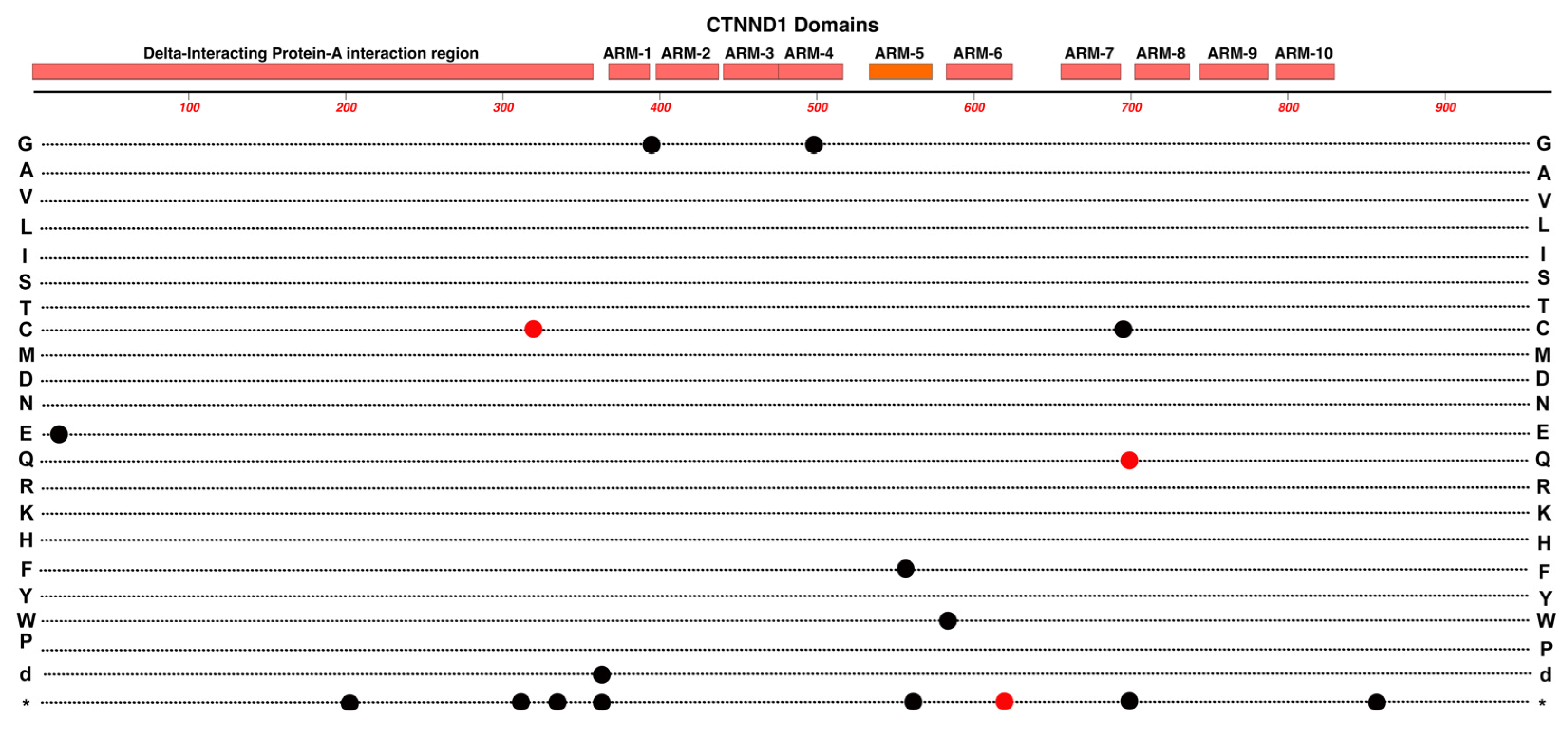
2.11. CTNND1
3. Conclusions
- As we survey the protein domain maps that we have presented of currently known pathogenic and likely-pathogenic variants; we can conclude that almost any functional subdomain of these proteins can be involved. This is not surprising because important protein-interaction functions or structural functions exist throughout their entire amino-acid sequences. There are little, if any, non-essential regions in the ten proteins reviewed. This would suggest that there may be many novel variants awaiting discovery in the human population, especially for more recently linked genes such as CTNNA1.
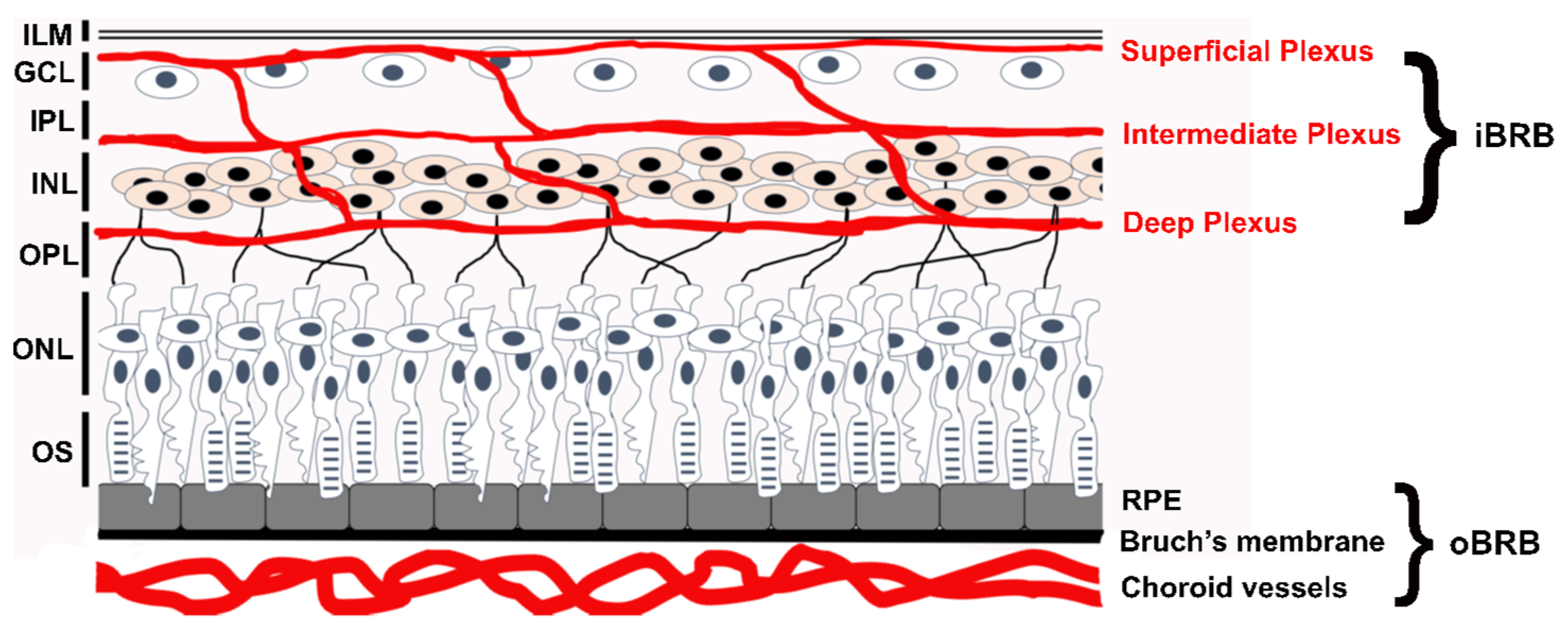
- FEVR can involve the disruption of any one of several different functions in endothelial cells, not just those related directly to the Norrin Wnt-signaling pathway. This is established by disease-causing variants in ZNF408, KIF11 and EMC1. However, what all these genes and their protein products have in common is that they are particularly important for critical retinal endothelial cell functions. These include correct membrane insertion of transmembrane proteins in the ER, regulation of adherens and tight junctions, cell growth, cell proliferation, migration of endothelial cells during formation of the retinal vasculature, and maturation of a high-barrier character endothelium. It is possible, and expected, that we may continue to discover novel FEVR-linked genes and good candidates would be any gene that is particularly enriched in retinal endothelial cells versus other endothelial cells. However more broadly expressed genes with roles in any of the above noted functions may be good candidates as well. That may include nine other genes for subunits of the Endoplasmic Reticulum Membrane Complex.
- The multiple allele knockout studies by Junge et al. (2009) in mice suggested the possibility that more severe FEVR-like phenotypes could result from combinations of two or more different alleles that have a minimal impact alone [65]. Our group recently surveyed a cohort of FEVR patients and immediate relatives to confirm that the incidence of protein-altering variants in two or three different FEVR-linked genes was substantially greater than in the general population [53]. Thus, it is possible that combined mild-alleles might result in more severe phenotypes, but we have not yet described clear examples of this in FEVR. To explore such possibilities for this relatively rare condition it will be helpful to apply genetic testing methods that survey as many genes as possible and to form global research collaborations to investigate genotypes and phenotypes from varied populations.
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Fruttiger, M. Development of the Mouse Retinal Vasculature: Angiogenesis versus Vasculogenesis. Invest Ophthalmol Vis Sci 2002, 43, 522–527. [Google Scholar]
- Zuercher, J.; Fritzsche, M.; Feil, S.; Mohn, L.; Berger, W. Norrin Stimulates Cell Proliferation in the Superficial Retinal Vascular Plexus and Is Pivotal for the Recruitment of Mural Cells. Hum Mol Genet 2012, 21, 2619–2630. [Google Scholar] [CrossRef]
- Selvam, S.; Kumar, T.; Fruttiger, M. Retinal Vasculature Development in Health and Disease. Prog Retin Eye Res 2018, 63, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, C.C.; Mitton, K.P.; Dailey, W.; Massoll, C.; Roumayah, K.; Guzman, E.; Tarabishy, N.; Cheng, M.; Drenser, K.A. Effects of Anti-VEGF Treatment on the Recovery of the Developing Retina Following Oxygen-Induced Retinopathy. Invest Ophthalmol Vis Sci 2014, 55, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Dailey, W.A.; Drenser, K.A.; Wong, S.C.; Cheng, M.; Vercellone, J.; Roumayah, K.K.; Feeney, E. V.; Deshpande, M.; Guzman, A.E.; Trese, M.; et al. Ocular Coherence Tomography Image Data of the Retinal Laminar Structure in a Mouse Model of Oxygen-Induced Retinopathy. Data Brief 2017, 15, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Dailey, W.A.; Drenser, K.A.; Wong, S.C.; Cheng, M.; Vercellone, J.; Roumayah, K.K.; Feeney, E. V.; Deshpande, M.; Guzman, A.E.; Trese, M.; et al. Norrin Treatment Improves Ganglion Cell Survival in an Oxygen-Induced Retinopathy Model of Retinal Ischemia. Exp Eye Res 2017, 164, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Fresta, C.G.; Fidilio, A.; Caruso, G.; Caraci, F.; Giblin, F.J.; Leggio, G.M.; Salomone, S.; Drago, F.; Bucolo, C. A New Human Blood–Retinal Barrier Model Based on Endothelial Cells, Pericytes, and Astrocytes. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Glenney, J.R.; Soppet, D. Sequence and Expression of Caveolin, a Protein Component of Caveolae Plasma Membrane Domains Phosphorylated on Tyrosine in Rous Sarcoma Virus-Transformed Fibroblasts. Proceedings of the National Academy of Sciences 1992, 89, 10517–10521. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.W.; Antonetti, D.A.; Barber, A.J.; Lanoue, K.F.; Levison, S.W.; Penn, T.; Retina, S.; Group, R. Diabetic Retinopathy: More Than Meets the Eye; 2002; Vol. 47. [Google Scholar]
- Feng, Y.; Venema, V.J.; Venema, R.C.; Tsai, N.; Caldwell, R.B. VEGF Induces Nuclear Translocation of Flk-1/KDR, Endothelial Nitric Oxide Synthase, and Caveolin-1 in Vascular Endothelial Cells. Biochem Biophys Res Commun 1999, 256, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-C.C.W.-C.; Drenser, K.; Trese, M.; Capone, A.; Dailey, W.; Capone Jr., A.; Dailey, W.; Capone Jr., A.; Dailey, W. Retinal Phenotype-Genotype Correlation of Pediatric Patients Expressing Mutations in the Norrie Disease Gene. Archives of ophthalmology 2007, 125, 225–230. [Google Scholar] [CrossRef]
- Drenser, K.A.; Trese, M.T. Familial Exudative Vitreoretinopathy and Osteoporosis-Pseudoglioma Syndrome Caused by a Mutation in the LRP5 Gene. Arch Ophthalmol 2007, 125, 431–432. [Google Scholar] [CrossRef]
- Drenser, K.A.; Dailey, W.; Capone, A.; Trese, M.T. Genetic Evaluation to Establish the Diagnosis of X-Linked Familial Exudative Vitreoretinopathy. Ophthalmic Genet 2006, 27, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Criswick, V.G.; Schepens, C.L. Familial Exudative Vitreoretinopathy. Am J Ophthalmol 1969, 68, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Ranchod, T.M.; Ho, L.Y.; Drenser, K.A.; Capone, A.; Trese, M.T. Clinical Presentation of Familial Exudative Vitreoretinopathy. Ophthalmology 2011, 118, 2070–2075. [Google Scholar] [CrossRef] [PubMed]
- Scruggs, B.A.; Reding, M.Q.; Schimmenti, L.A. NDP-Related Retinopathies. GeneReviews® 2022. [Google Scholar]
- Cicerone, A.P.; Dailey, W.; Sun, M.; Santos, A.; Jeong, D.; Jones, L.; Koustas, K.; Drekh, M.; Schmitz, K.; Haque, N.; et al. A Survey of Multigenic Protein-Altering Variant Frequency in Familial Exudative Vitreo-Retinopathy (FEVR) Patients by Targeted Sequencing of Seven FEVR-Linked Genes. Genes (Basel) 2022, 13. [Google Scholar] [CrossRef]
- Dixon, M.W.; Stem, M.S.; Schuette, J.L.; Keegan, C.E.; Besirli, C.G. CTNNB1 Mutation Associated with Familial Exudative Vitreoretinopathy (FEVR) Phenotype. Ophthalmic Genet 2016, 37, 468–470. [Google Scholar] [CrossRef]
- Li, J.K.; Fei, P.; Li, Y.; Huang, Q.J.; Zhang, Q.; Zhang, X.; Rao, Y.Q.; Li, J.; Zhao, P. Identification of Novel KIF11 Mutations in Patients with Familial Exudative Vitreoretinopathy and a Phenotypic Analysis. Sci Rep 2016, 6. [Google Scholar] [CrossRef]
- Collin, R.W.J.; Nikopoulos, K.; Dona, M.; Gilissen, C.; Hoischen, A.; Boonstra, F.N.; Poulter, J.A.; Kondo, H.; Berger, W.; Toomes, C.; et al. ZNF408 Is Mutated in Familial Exudative Vitreoretinopathy and Is Crucial for the Development of Zebrafish Retinal Vasculature. Proc Natl Acad Sci U S A 2013, 110, 9856–9861. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, S.; Huang, L.; Zhao, R.; Dai, E.; Jiang, X.; He, Y.; Lu, J.; Peng, L.; Liu, W.; et al. CTNND1 Variants Cause Familial Exudative Vitreoretinopathy through the Wnt/Cadherin Axis. JCI Insight 2022, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yang, M.; Zhao, P.; Li, S.; Zhang, L.; Huang, L.; Huang, Y.; Fei, P.; Yang, Y.; Zhang, S.; et al. Catenin α 1 Mutations Cause Familial Exudative Vitreoretinopathy by Overactivating Norrin/β-Catenin Signaling. Journal of Clinical Investigation 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, M.; Zhao, R.; Peng, L.; Liu, W.; Jiang, X.; He, Y.; Dai, E.; Zhang, L.; Yang, Y.; et al. Defective EMC1 Drives Abnormal Retinal Angiogenesis via Wnt/β-Catenin Signaling and May Be Associated with the Pathogenesis of Familial Exudative Vitreoretinopathy. Genes Dis 2023, 10, 2572–2585. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.; Martin, M.J.; Orchard, S.; Magrane, M.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; Bye-A-Jee, H.; Cukura, A.; et al. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res 2023, 51, D523–D531. [Google Scholar] [CrossRef]
- Kashina, A.S.; Baskin, R.J.; Cole, D.G.; Wedaman, K.P.; Saxton, W.M.; Scholey, J.M. A Bipolar Kinesin. Nature 1996 379:6562 1996, 379, 270–272. [Google Scholar] [CrossRef]
- Jones, G.E.; Ostergaard, P.; Moore, A.T.; Connell, F.C.; Williams, D.; Quarrell, O.; Brady, A.F.; Spier, I.; Hazan, F.; Moldovan, O.; et al. Microcephaly with or without Chorioretinopathy, Lymphoedema, or Mental Retardation (MCLMR): Review of Phenotype Associated with KIF11 Mutations. Eur J Hum Genet 2014, 22, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Mirzaa, G.M.; Enyedi, L.; Parsons, G.; Collins, S.; Medne, L.; Adams, C.; Ward, T.; Davitt, B.; Bicknese, A.; Zackai, E.; et al. Congenital Microcephaly and Chorioretinopathy Due to de Novo Heterozygous KIF11 Mutations: Five Novel Mutations and Review of the Literature. Am J Med Genet A 2014, 164, 2879–2886. [Google Scholar] [CrossRef]
- Wang, Y.; Smallwood, P.M.; Williams, J.; Nathans, J. A Mouse Model for Kinesin Family Member 11 (Kif11)-Associated Familial Exudative Vitreoretinopathy. Hum Mol Genet 2020, 29, 1121–1131. [Google Scholar] [CrossRef]
- Di Zazzo, E.; De Rosa, C.; Abbondanza, C.; Moncharmont, B. PRDM Proteins: Molecular Mechanisms in Signal Transduction and Transcriptional Regulation. Biology (Basel) 2013, 2, 107–141. [Google Scholar] [CrossRef]
- Cassandri, M.; Smirnov, A.; Novelli, F.; Pitolli, C.; Agostini, M.; Malewicz, M.; Melino, G.; Raschellà, G. Zinc-Finger Proteins in Health and Disease. Cell Death Discov 2017, 3. [Google Scholar] [CrossRef]
- Avila-Fernandez, A.; Perez-Carro, R.; Corton, M.; Lopez-Molina, M.I.; Campello, L.; Garanto, A.; Fernandez-Sanchez, L.; Duijkers, L.; Lopez-Martinez, M.A.; Riveiro-Alvarez, R.; et al. Whole-Exome Sequencing Reveals ZNF408 as a New Gene Associated with Autosomal Recessive Retinitis Pigmentosa with Vitreal Alterations. Hum Mol Genet 2015, 24, 4037–4048. [Google Scholar] [CrossRef] [PubMed]
- Karjosukarso, D.W.; van Gestel, S.H.C.; Qu, J.; Kouwenhoven, E.N.; Duijkers, L.; Garanto, A.; Zhou, H.; Collin, R.W.J. An FEVR-Associated Mutation in ZNF408 Alters the Expression of Genes Involved in the Development of Vasculature. Hum Mol Genet 2018, 27, 3519–3527. [Google Scholar] [CrossRef]
- Xu, W.; Kimelman, D. Mechanistic Insights from Structural Studies of Beta-Catenin and Its Binding Partners. J Cell Sci 2007, 120, 3337–3344. [Google Scholar] [CrossRef]
- Huber, A.H.; Weis, W.I. The Structure of the Beta-Catenin/E-Cadherin Complex and the Molecular Basis of Diverse Ligand Recognition by Beta-Catenin. Cell 2001, 105, 391–402. [Google Scholar] [CrossRef]
- Xu, W.; Kimelman, D. Mechanistic Insights from Structural Studies of β-Catenin and Its Binding Partners. J Cell Sci 2007, 120, 3337–3344. [Google Scholar] [CrossRef]
- Taylor, R.L.; Soriano, C.S.; Williams, S.; Dzulova, D.; Ashworth, J.; Hall, G.; Gale, T.; Lloyd, I.C.; Inglehearn, C.F.; Toomes, C.; et al. Bi-Allelic Mutation of CTNNB1 Causes a Severe Form of Syndromic Microphthalmia, Persistent Foetal Vasculature and Vitreoretinal Dysplasia. Orphanet J Rare Dis 2022, 17, 1–8. [Google Scholar] [CrossRef]
- Ohlmann, A.; Tamm, E.R. Norrin: Molecular and Functional Properties of an Angiogenic and Neuroprotective Growth Factor. Prog Retin Eye Res 2012, 31, 243–257. [Google Scholar] [CrossRef]
- Zeilbeck, L.F.; Müller, B.B.; Leopold, S.A.; Senturk, B.; Langmann, T.; Tamm, E.R.; Ohlmann, A. Norrin Mediates Angiogenic Properties via the Induction of Insulin-like Growth Factor-1. Exp Eye Res 2016, 145, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Shastry, B.S.; Hejtmancik, J.F.; Trese, M.T. Identification of Novel Missense Mutations in the Norrie Disease Gene Associated with One X-Linked and Four Sporadic Cases of Familial Exudative Vitreoretinopathy. Hum Mutat 1997, 9, 396–401. [Google Scholar] [CrossRef]
- Ke, J.; Harikumar, K.G.; Erice, C.; Chen, C.; Gu, X.; Wang, L.; Parker, N.; Cheng, Z.; Xu, W.; Williams, B.O.; et al. Structure and Function of Norrin in Assembly and Activation of a Frizzled 4-Lrp5/6 Complex. Genes Dev 2013, 27, 2305–2319. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, Y.; Dabdoub, A.; Smallwood, P.M.; Williams, J.; Woods, C.; Kelley, M.W.; Jiang, L.; Tasman, W.; Zhang, K.; et al. Vascular Development in the Retina and Inner Ear: Control by Norrin and Frizzled-4, a High-Affinity Ligand-Receptor Pair. Cell 2004, 116, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-C.; Drenser, K.; Trese, M.; Capone, A.; Dailey, W. Retinal Phenotype-Genotype Correlation of Pediatric Patients Expressing Mutations in the Norrie Disease Gene; 2007. [CrossRef]
- Xiao, H.; Tong, Y.; Zhu, Y.; Peng, M. Familial Exudative Vitreoretinopathy-Related Disease-Causing Genes and Norrin/ β -Catenin Signal Pathway: Structure, Function, and Mutation Spectrums. J Ophthalmol 2019, 2019. [Google Scholar] [CrossRef]
- Wang, Y.; Cho, C.; Williams, J.; Smallwood, P.M.; Zhang, C.; Junge, H.J.; Nathans, J. Interplay of the Norrin and Wnt7a/Wnt7b Signaling Systems in Blood-Brain Barrier and Blood-Retina Barrier Development and Maintenance. Proc Natl Acad Sci U S A 2018, 115, E11827–E11836. [Google Scholar] [CrossRef]
- Ye, X.; Wang, Y.; Nathans, J. The Norrin/Frizzled4 Signaling Pathway in Retinal Vascular Development and Disease. Trends Mol Med 2010, 16, 417–425. [Google Scholar] [CrossRef]
- Ohlmann, A.; Scholz, M.; Goldwich, A.; Chauhan, B.K.; Hudl, K.; Ohlmann, A. V; Zrenner, E.; Berger, W.; Cvekl, A.; Seeliger, M.W.; et al. Ectopic Norrin Induces Growth of Ocular Capillaries and Restores Normal Retinal Angiogenesis in Norrie Disease Mutant Mice. J Neurosci 2005, 25, 1701–1710. [Google Scholar] [CrossRef]
- Díaz-Coránguez, M.; Lin, C.-M.; Liebner, S.; Antonetti, D.A. Norrin Restores Blood-Retinal Barrier Properties after Vascular Endothelial Growth Factor-Induced Permeability. J Biol Chem 2020, 295, 4647–4660. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, C.C.; Chen, Y.-H.; Dailey, W.; Cheng, M.; Drenser, K.A. Retinal Vascular Rescue of Oxygen-Induced Retinopathy in Mice by Norrin. Investigative Opthalmology & Visual Science 2013, 54, 222. [Google Scholar] [CrossRef]
- Bang, I.; Kim, H.R.; Beaven, A.H.; Kim, J.; Ko, S.B.; Lee, G.R.; Lee, H.; Im, W.; Seok, C.; Chung, K.Y.; et al. Biophysical and Functional Characterization of Norrin Signaling through Frizzled4. Proc Natl Acad Sci U S A 2018, 115, 8787–8792. [Google Scholar] [CrossRef]
- Paes, K.T.; Wang, E.; Henze, K.; Vogel, P.; Read, R.; Suwanichkul, A.; Kirkpatrick, L.L.; Potter, D.; Newhouse, M.M.; Rice, D.S. Frizzled 4 Is Required for Retinal Angiogenesis and Maintenance of the Blood-Retina Barrier. Invest Ophthalmol Vis Sci 2011, 52, 6452–6461. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Hayashi, H.; Oshima, K.; Tahira, T.; Hayashi, K. Frizzled 4 Gene (FZD4) Mutations in Patients with Familial Exudative Vitreoretinopathy with Variable Expressivity. British Journal of Ophthalmology 2003, 87, 1291–1295. [Google Scholar] [CrossRef]
- Dailey, W.A.; Gryc, W.; Garg, P.G.; Drenser, K.A. Frizzled-4 Variations Associated with Retinopathy and Intrauterine Growth Retardation. Ophthalmology 2015, 122, 1917–1923. [Google Scholar] [CrossRef]
- Cicerone, A.P.; Dailey, W.; Sun, M.; Santos, A.; Jeong, D.; Jones, L.; Koustas, K.; Drekh, M.; Schmitz, K.; Haque, N.; et al. A Survey of Multigenic Protein-Altering Variant Frequency in Familial Exudative Vitreo-Retinopathy (FEVR) Patients by Targeted Sequencing of Seven FEVR-Linked Genes. Genes (Basel) 2022, 13, 495. [Google Scholar] [CrossRef]
- He, X.; Semenov, M.; Tamai, K.; Zeng, X. LDL Receptor-Related Proteins 5 and 6 in Wnt/Beta-Catenin Signaling: Arrows Point the Way. Development 2004, 131, 1663–1677. [Google Scholar] [CrossRef]
- Xia, C.; Yablonka-Reuveni, Z.; Gong, X. LRP5 Is Required for Vascular Development in Deeper Layers of the Retina. PLoS One 2010, 5, e11676. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Li, Q.; Amiry-Moghaddam, M.; Hokama, M.; Sardi, S.H.; Nagao, M.; Warman, M.L.; Olsen, B.R. Critical Endothelial Regulation by LRP5 during Retinal Vascular Development. PLoS One 2016, 11, e0152833. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Semenov, M.; Tamai, K.; Zeng, X. LDL Receptor-Related Proteins 5 and 6 in Wnt/β-Catenin Signaling: Arrows Point the Way. Development 2004, 131, 1663–1677. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Hayashi, H.; Oshima, K.; Tahira, T.; Hayashi, K.; Kondo, H. Complexity of the Genotype-Phenotype Correlation in Familial Exudative Vitreoretinopathy with Mutations Im the LRP5 and/or FZD4 Genes. Hum Mutat 2005, 26, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Seigneuret, M.; Delaguillaumie, A.; Lagaudrière-Gesbert, C.; Conjeaud, H. Structure of the Tetraspanin Main Extracellular Domain: A PARTIALLY CONSERVED FOLD WITH A STRUCTURALLY VARIABLE DOMAIN INSERTION. Journal of Biological Chemistry 2001, 276, 40055–40064. [Google Scholar] [CrossRef]
- Poulter, J.A.; Ali, M.; Gilmour, D.F.; Rice, A.; Kondo, H.; Hayashi, K.; Mackey, D.A.; Kearns, L.S.; Ruddle, J.B.; Craig, J.E.; et al. Mutations in TSPAN12 Cause Autosomal-Dominant Familial Exudative Vitreoretinopathy. The American Journal of Human Genetics 2010, 86, 248–253. [Google Scholar] [CrossRef]
- Zhang, C.; Lai, M.B.; Pedler, M.G.; Johnson, V.; Adams, R.H.; Petrash, J.M.; Chen, Z.; Junge, H.J. Endothelial Cell–Specific Inactivation of TSPAN12 (Tetraspanin 12) Reveals Pathological Consequences of Barrier Defects in an Otherwise Intact Vasculature. Arterioscler Thromb Vasc Biol 2018, 38. [Google Scholar] [CrossRef]
- Charrin, S.; Jouannet, S.; Boucheix, C.; Rubinstein, E. Tetraspanins at a Glance. J Cell Sci 2014, 127, 3641–3648. [Google Scholar] [CrossRef]
- Mohd Khair, S.Z.N.; Ismail, A.S.; Embong, Z.; Mohamed Yusoff, A.A. Detection of FZD4, LRP5 and TSPAN12 Genes Variants in Malay Premature Babies with Retinopathy of Prematurity. J Ophthalmic Vis Res 2019, 14, 171–178. [Google Scholar] [CrossRef]
- Nikopoulos, K.; Gilissen, C.; Hoischen, A.; Erik van Nouhuys, C.; Boonstra, F.N.; Blokland, E.A.W.; Arts, P.; Wieskamp, N.; Strom, T.M.; Ayuso, C.; et al. Next-Generation Sequencing of a 40 Mb Linkage Interval Reveals TSPAN12 Mutations in Patients with Familial Exudative Vitreoretinopathy. Am J Hum Genet 2010, 86, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Junge, H.J.; Yang, S.; Burton, J.B.; Paes, K.; Shu, X.; French, D.M.; Costa, M.; Rice, D.S.; Ye, W. TSPAN12 Regulates Retinal Vascular Development by Promoting Norrin- but Not Wnt-Induced FZD4/β-Catenin Signaling. Cell 2009, 139, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lai, M.B.; Pedler, M.G.; Johnson, V.; Adams, R.H.; Petrash, J.M.; Chen, Z.; Junge, H.J. Endothelial Cell–Specific Inactivation of TSPAN12 (Tetraspanin 12) Reveals Pathological Consequences of Barrier Defects in an Otherwise Intact Vasculature. Arterioscler Thromb Vasc Biol 2018, 38, 2691–2705. [Google Scholar] [CrossRef] [PubMed]
- Harel, T.; Yesil, G.; Bayram, Y.; Coban-Akdemir, Z.; Charng, W.L.; Karaca, E.; Al Asmari, A.; Eldomery, M.K.; Hunter, J. V.; Jhangiani, S.N.; et al. Monoallelic and Biallelic Variants in EMC1 Identified in Individuals with Global Developmental Delay, Hypotonia, Scoliosis, and Cerebellar Atrophy. Am J Hum Genet 2016, 98, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Hong, S.; Indra, I.; Sergeeva, A.P.; Troyanovsky, R.B.; Shapiro, L.; Honig, B.; Troyanovsky, S.M. α-Catenin-Mediated Cadherin Clustering Couples Cadherin and Actin Dynamics. J Cell Biol 2015, 210, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Majewski, I.J.; Kluijt, I.; Cats, A.; Scerri, T.S.; de Jong, D.; Kluin, R.J.C.; Hansford, S.; Hogervorst, F.B.L.; Bosma, A.J.; Hofland, I.; et al. An α-E-Catenin (CTNNA1) Mutation in Hereditary Diffuse Gastric Cancer. J Pathol 2013, 229, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Piao, H.-L.; Yuan, Y.; Wang, M.; Sun, Y.; Liang, H.; Ma, L. α-Catenin Acts as a Tumour Suppressor in E-Cadherin-Negative Basal-like Breast Cancer by Inhibiting NF-ΚB Signalling. Nat Cell Biol 2014, 16, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Saksens, N.T.M.; Krebs, M.P.; Schoenmaker-Koller, F.E.; Hicks, W.; Yu, M.; Shi, L.; Rowe, L.; Collin, G.B.; Charette, J.R.; Letteboer, S.J.; et al. Mutations in CTNNA1 Cause Butterfly-Shaped Pigment Dystrophy and Perturbed Retinal Pigment Epithelium Integrity. Nat Genet 2016, 48, 144–151. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021 596:7873 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Alharatani, R.; Ververi, A.; Beleza-Meireles, A.; Ji, W.; Mis, E.; Patterson, Q.T.; Griffin, J.N.; Bhujel, N.; Chang, C.A.; Dixit, A.; et al. Novel Truncating Mutations in CTNND1 Cause a Dominant Craniofacial and Cardiac Syndrome. Hum Mol Genet 2020, 29, 1900. [Google Scholar] [CrossRef]
- Ishiyama, N.; Lee, S.H.; Liu, S.; Li, G.Y.; Smith, M.J.; Reichardt, L.F.; Ikura, M. Dynamic and Static Interactions between P120 Catenin and E-Cadherin Regulate the Stability of Cell-Cell Adhesion. Cell 2010, 141, 117–128. [Google Scholar] [CrossRef]
- Anastasiadis, P.Z.; Moon, S.Y.; Thoreson, M.A.; Mariner, D.J.; Crawford, H.C.; Zheng, Y.; Reynolds, A.B. Inhibition of RhoA by P120 Catenin. Nature Cell Biology 2000 2:9 2000, 2, 637–644. [Google Scholar] [CrossRef]
- Park, J. Il; Kim, S.W.; Lyons, J.P.; Ji, H.; Nguyen, T.T.; Cho, K.; Barton, M.C.; Deroo, T.; Vleminckx, K.; McCrea, P.D. Kaiso/P120-Catenin and TCF/β-Catenin Complexes Coordinately Regulate Canonical Wnt Gene Targets. Dev Cell 2005, 8, 843–854. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




