Submitted:
18 September 2023
Posted:
20 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
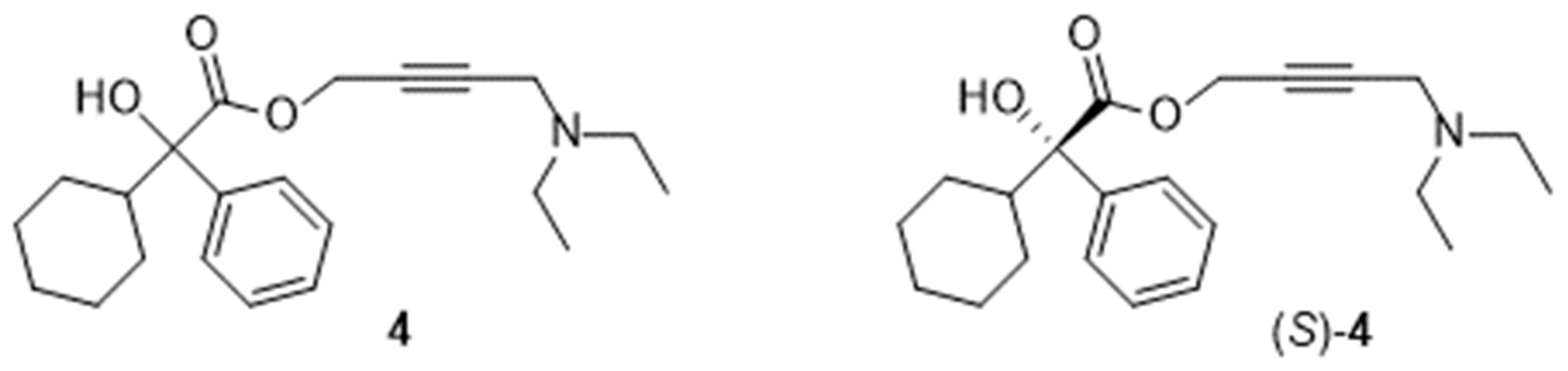
2. Chemical Names
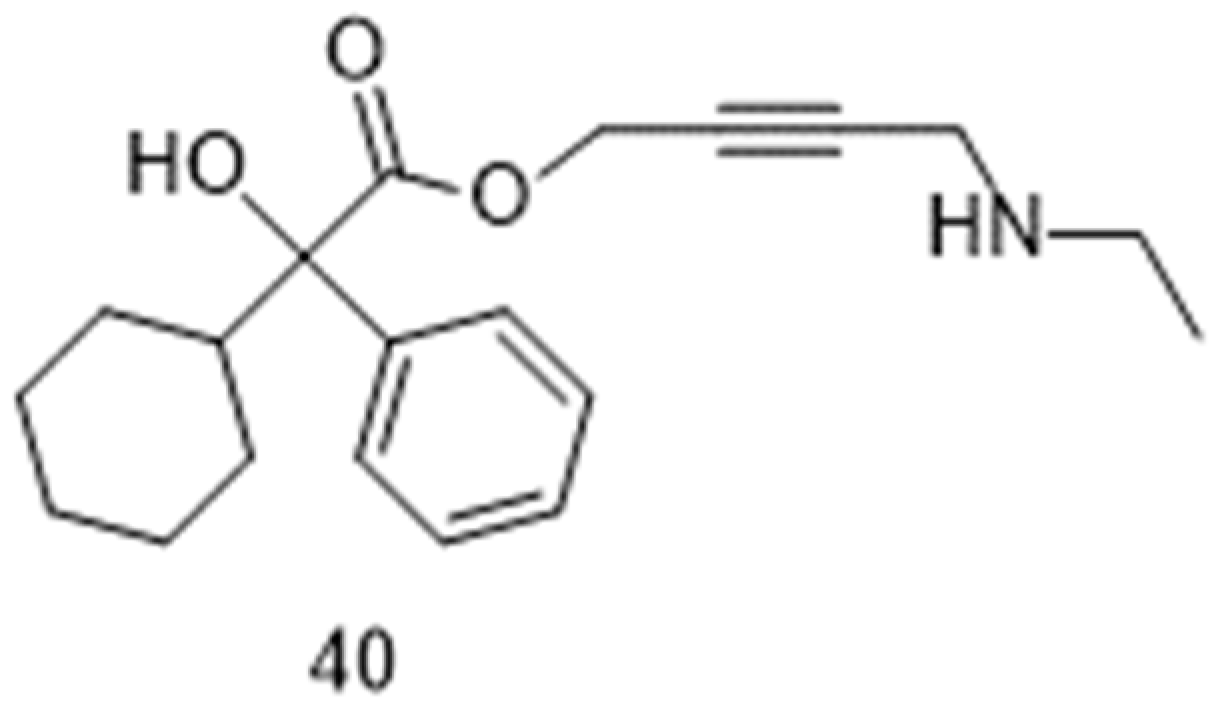
- of glycolic acid: 4-diethylamino-2-butynyl phenylcyclohexylglycolate,
- or of phenylacetic acid: 4-diethylamino-2-butynyl 2-cyclohexyl-2-hydroxy-2-phenylacetate.
3. Syntheses
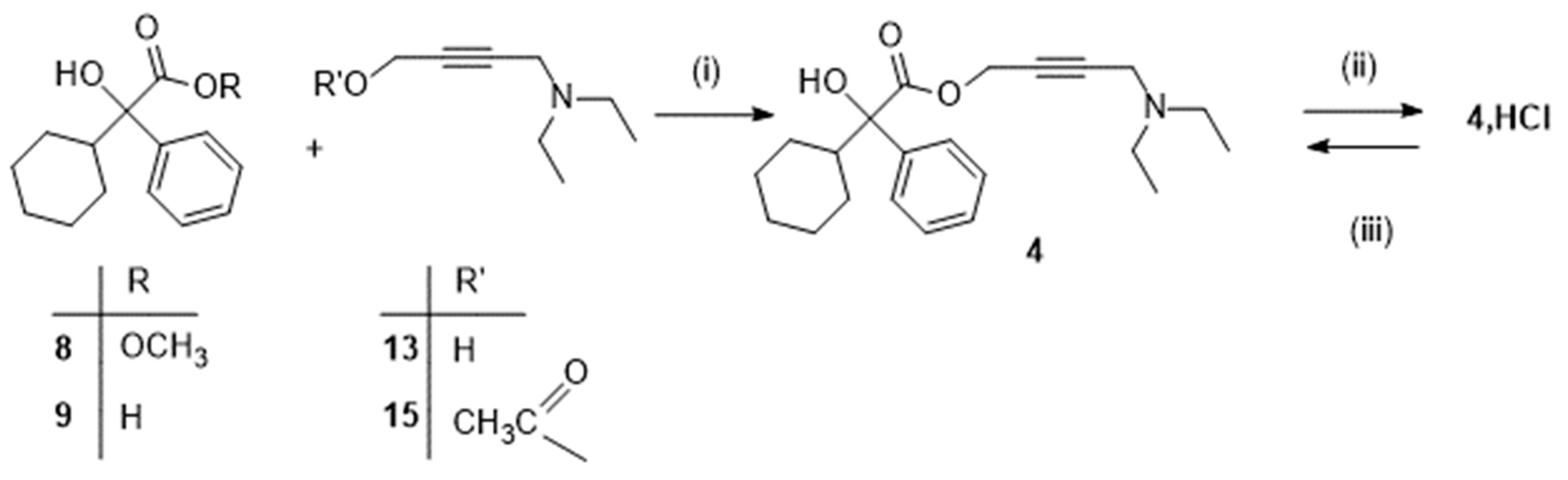
3.1. Syntheses of the Racemic
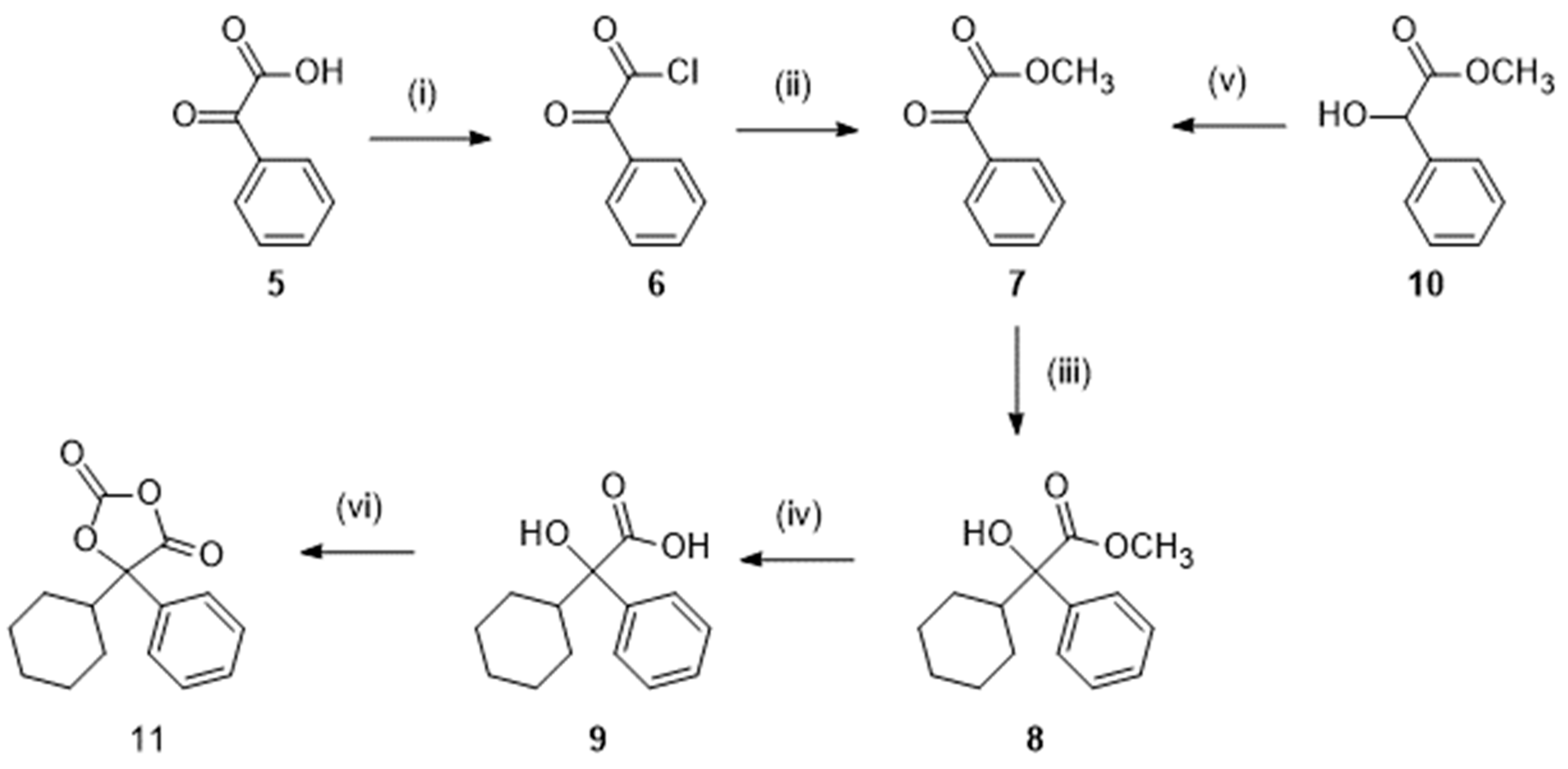
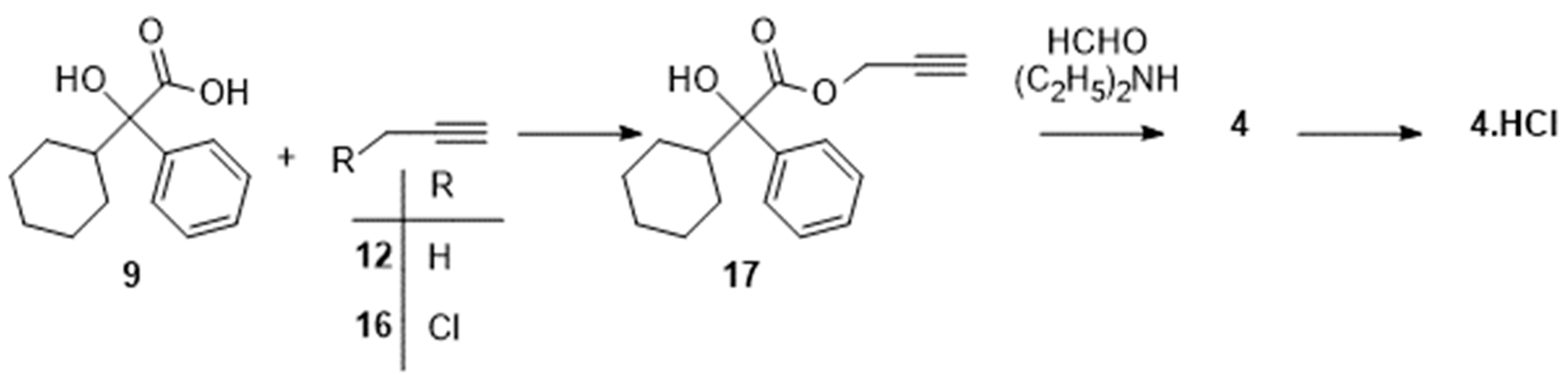
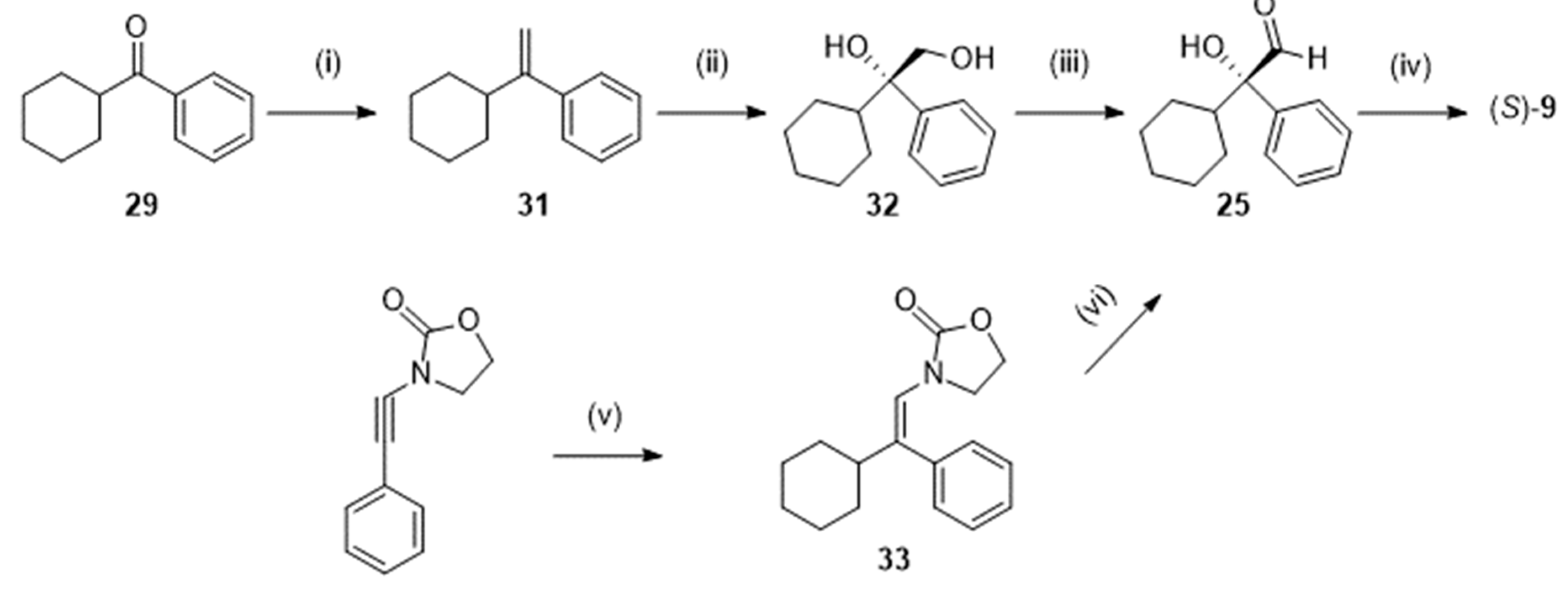
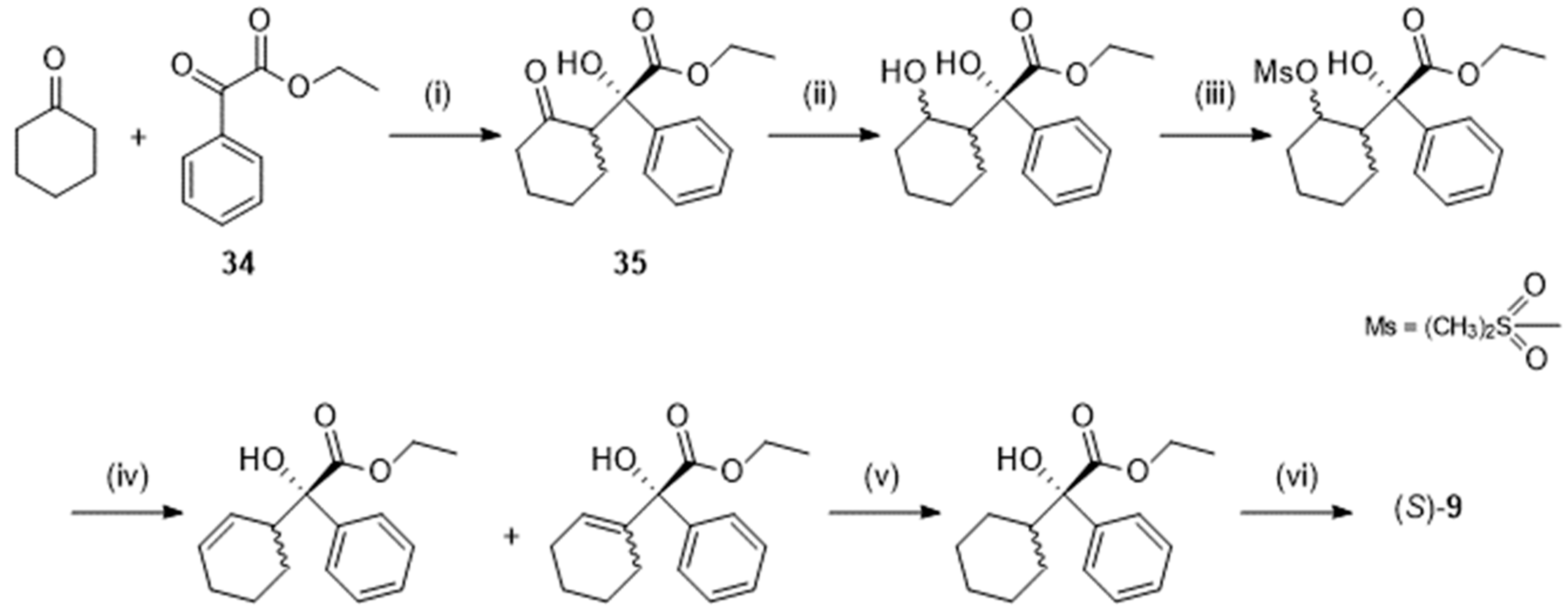
3.1.1. Preparation of methyl 2-cyclohexyl-2-hydroxy-2-phenylacetate (8) and the corresponding acid (9)
3.1.2. Preparation of derivatives of 2-propyn-1-ol
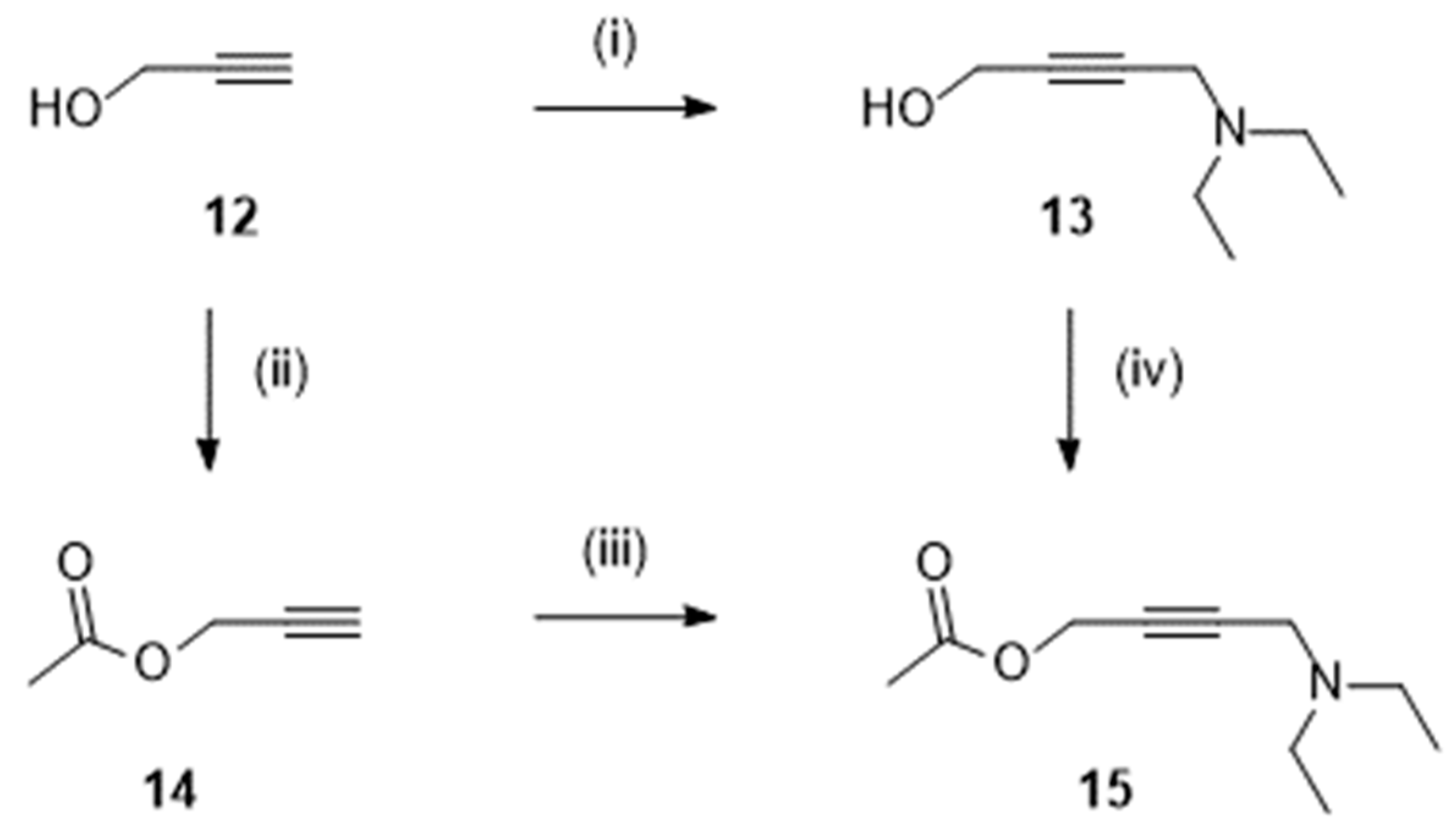
3.1.3. Final step
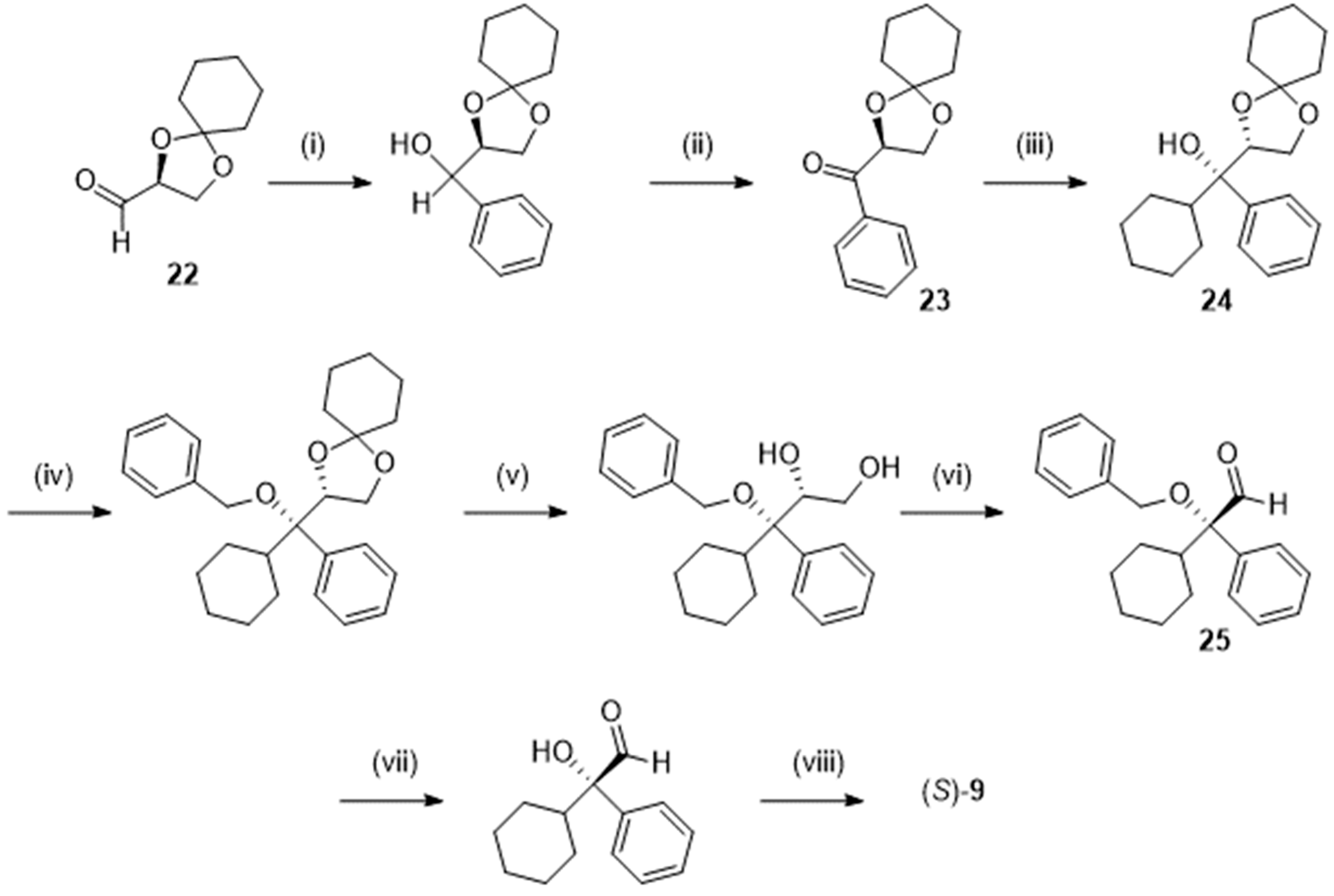
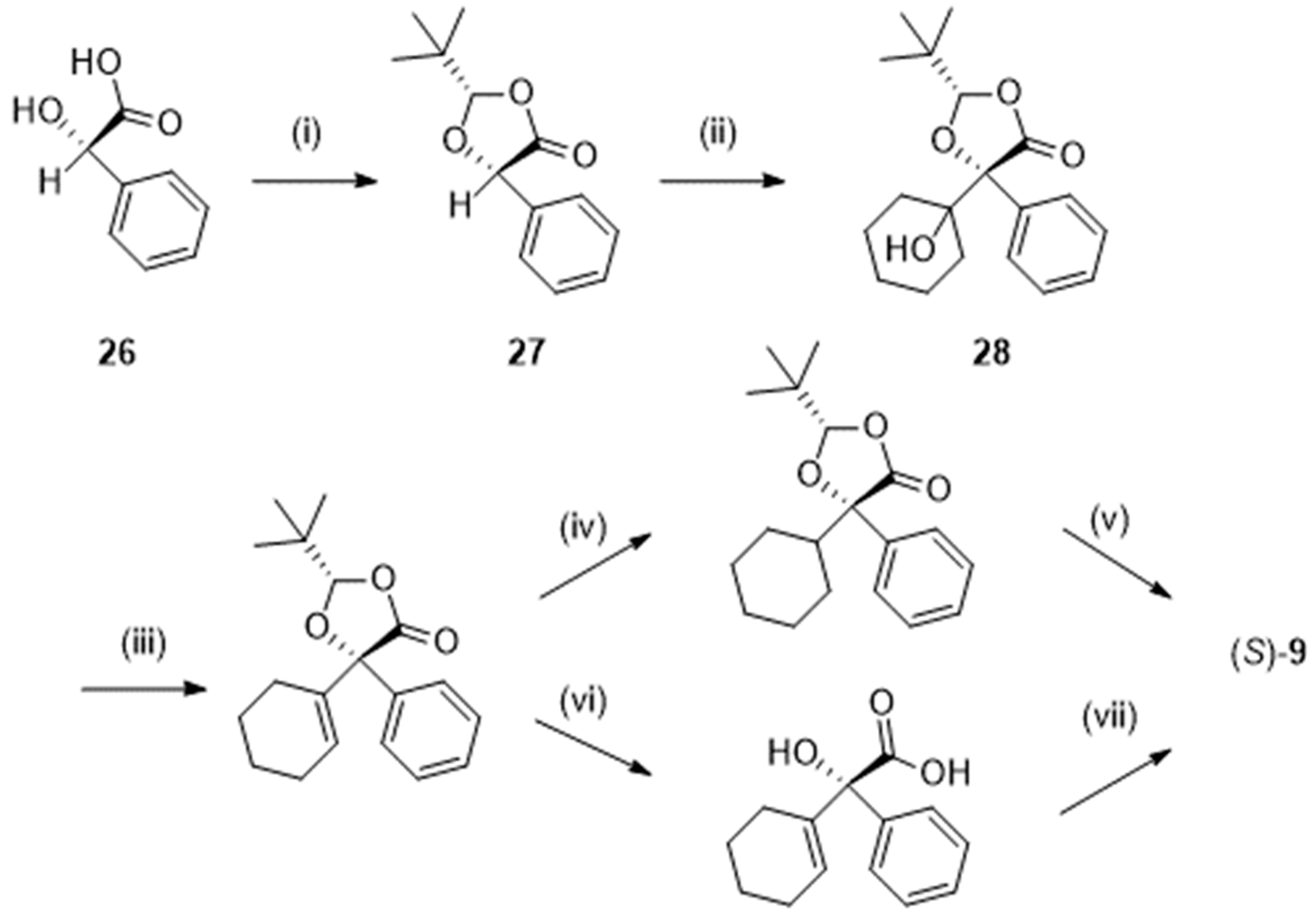
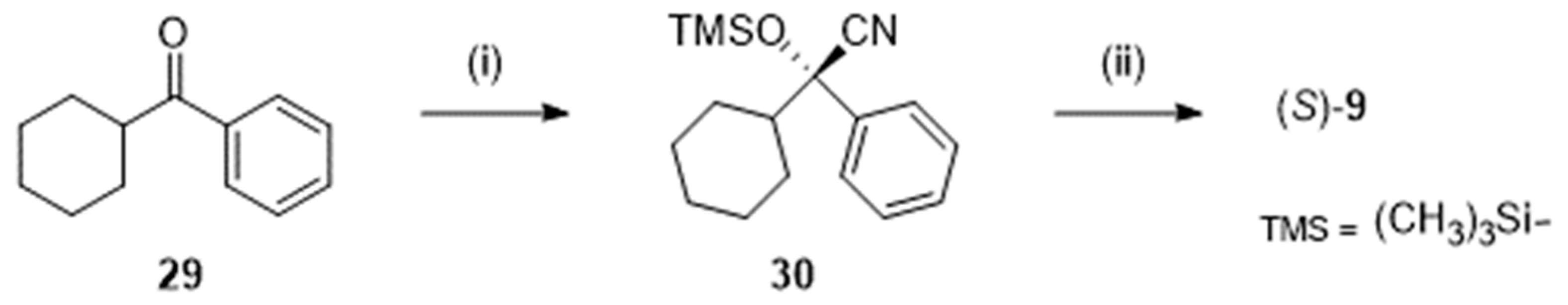
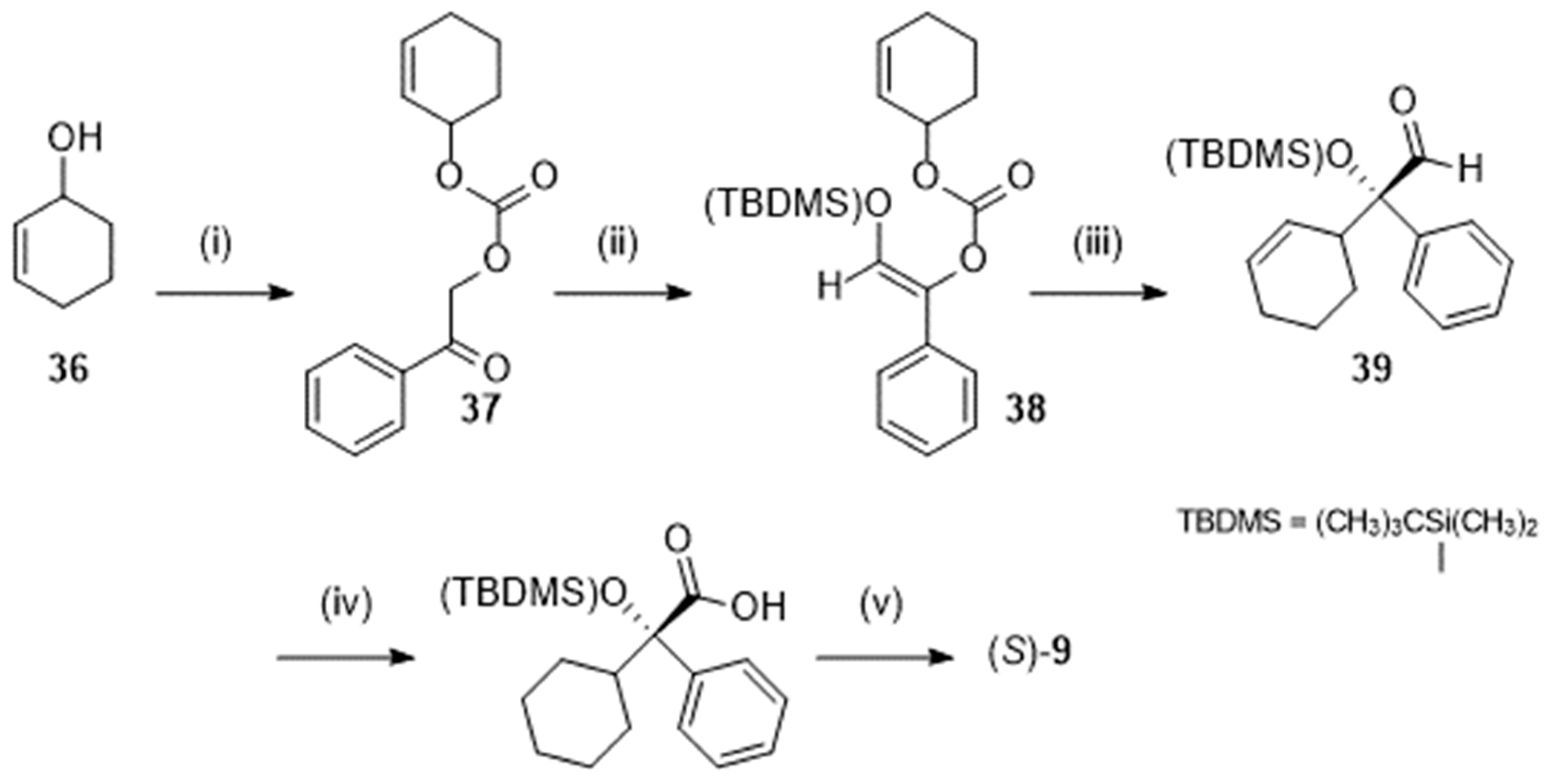
3.2. Enantioselective Syntheses
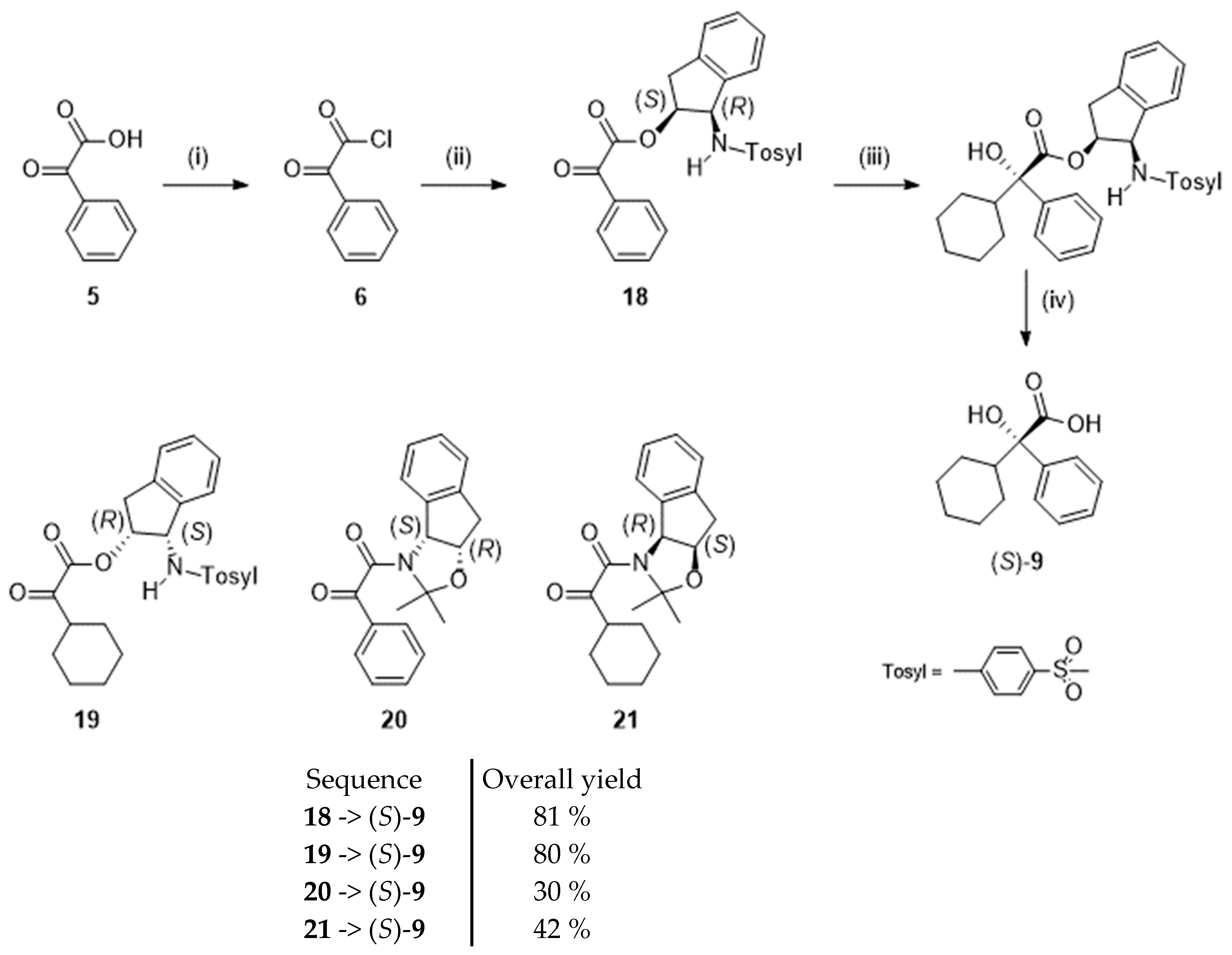
3.3. Resolution of the Racemic
4. Mode of action and metabolism
5. Formulations and Brand Names
6. Repositioning Opportunities
6.1. Hyperhidrosis
6.2. Hot Flashes (vasomotor symptoms)
6.3. Obstructive Sleep Apnea
7. Conclusion
Funding
Conflicts of Interest
References
- Ashburn, T.T.; Thor, K.B. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Patrono, C. Aspirin as an antiplatelet drug N. Engl. J. Med. 1994, 330, 1287–1294. [Google Scholar] [CrossRef]
- Patrono, C.; Rocca, B. Aspirin at 120: Retiring, recombining, or repurposing? Res Pract Thromb Haemost. 2021, 5, e12516. [Google Scholar] [CrossRef]
- Goldstein, I.; Burnett, A.L.; Rosen, R.C.; Park, P.W.; Stecher, V.J. The serendipitous story of sildenafil: An unexpected oral therapy for erectile dysfunction Sex. Med. Rev. 2019, 7, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Burgos, M.; Losada-Garcia, A.; Cruz-Hernández, C.D.; Cortés-Ramı́rez, S.A.; Camacho-Arroyo, I.; Gonzalez-Covarrubias, V.; Morales-Pacheco, M.; Trujillo-Bornios, S.I.; Rodrı́guez-Dorantes, M. New approaches in oncology for repositioning drugs: The case of PDE5 inhibitor sildenafil Front. Oncol. 2021, 11, 627229. [Google Scholar] [CrossRef]
- Vargesson, N.; Stephens, T. Thalidomide: History, withdrawal, renaissance, and safety concerns Expert Opin. Drug Saf. 2021, 20, 1455–1457. [Google Scholar] [CrossRef]
- Bauzon, J.; Lee, G.; Cummings, J. Repurposed agents in the Alzheimer’s disease drug development pipeline Alzheimer's Res. Ther. 2020, 12, 98. [Google Scholar] [CrossRef]
- Anderson, S.D. Repurposing drugs as inhaled therapies in asthma Adv. Drug Deliv. Rev. 2018, 113, 19–33. [Google Scholar] [CrossRef]
- Vanden Eynde, J.J. COVID-19: Failure of the DisCoVeRy clinical trial, and now–New hopes? Pharmaceuticals 2021, 14, 664. [Google Scholar] [CrossRef]
- Rosenberg, R.; Abaluck, B.; Thein, S. Combination of atomoxetine with the novel antimuscarinic aroxybutynin improves mild to moderate OSA. J. Clin. Sleep Med. 2022, 18, 2837–2844. [Google Scholar] [CrossRef]
- Esoxybutynin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Esoxybutynin (accessed on 17 September 2023).
- Pai, N.R.; Dubhashi, D.S. Design, synthesis and evaluation of substituted benzeneacetic acid ester derivatives as potential antiinflammatory and analgesic agents. Int. J. Pharmtech Res. 2010, 2, 443–452. [Google Scholar]
- Gant, T.G.; Sarshar, S. Substituted phenylcyclohexylglycolates. US patent 2009/0247628A1 (2009).
- Vandenbossche, C.P.; de Croos, P.; Singh, S.P.; Bakale, R.P.; Wagler, T.R. Formation of (S)-5-cyclohexyl-5-phenyl-1,3-dioxolane-2,4-dione: A key intermediate in the synthesis of (S)-oxybutynin hydrochloride. Org. Process Res. Dev. 2010, 14, 921–925. [Google Scholar] [CrossRef]
- Ray, P.C.; Sethi, M.; Mahajan, S.; Tyagi, O.D. Crystalline oxybutynin and process for preparing the same. PCT Int. Appl. WO 2009/122429 A2 (2009).
- Campbel, K.N.; Majewski, R.F. Substituted aminobutynyl acetates. US patent 3,76,019 (1965).
- Scigel, R.; Smrz, R. Process for preparing oxybutynin. Czech Republic patent CZ 296056 B6 ( 2005.
- Molnar, D; Johnston, S. Preparation of polymorphic forms of (R)-oxybutynin hydrochloride. PCT Int. Appl. WO 2021226020 A1 (2021).
- Johnston, S.; Molnar, D. Solid forms of (R)-oxybutynin d-malate. PCT Int. Appl. WO 2022235726 A1 ( 2022.
- Yang, P.; Yuan, H.; Zhou, M. Preparation method of oxybutynin hydrochloride from α-cyclohexyl-α-hydroxyphenylacetic acid via esterification, Mannich reaction and salifying. Chinese patent CN-114805100-A (2022).
- Czarnik, A.W. Deuterium-enriched oxybutynin. US patent 2008/02992.19 A1 (2008).
- Li, F.; Jiang, W.; Czarnik, A.W.; Li, W. Combinatorial synthesis of deuterium-enriched (S)-oxybutynin. Mol. Divers. 2016, 20, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Bakale, R.P.; Lopez, J.L.; McConville, F.X.; Vandenbossche, C.P.; Senanayake, C.H. Carbonate intermediates useful in the preparation of optically active cyclohexylphenylglycolate esters. US patent 5,973, 182 1999. [Google Scholar]
- Senanayake, C.H.; Fang, K.; Grover, P.; Bakale, R.P.; Vandenbossche, C.P.; Wald, S.A. Tetrahedron Lett.1999, 40, 819-822. [CrossRef]
- Roy, S.; Sharma, A.; Chattopadhyaya, N.; Chattopadhyay, S. An efficient asymmetric synthesis of (S)-2-cyclohexyl-2-phenylglycolic acid, the acid segment of oxybutynin. Tetrahedron Lett. 2006, 47, 7067–7069. [Google Scholar] [CrossRef]
- Grover, P.T.; Bhongle, N.N.; Wald, S.A.; Senanayake, C.H. Chiral mandelic acid template provides a highly practical solution for (S)-oxybutynin synthesis. J. Org. Chem. 2000, 65, 6283–6287. [Google Scholar] [CrossRef]
- Masumoto, S.; Suzuki, M.; Kanaia, M.; Shibasakia, M. A practical synthesis of (S)-oxybutynin. Tetrahedron Lett. 2002, 43, 8647–8651. [Google Scholar] [CrossRef]
- Recuero, V.; Ferrero, M.; Gotor-Fernandez, V.; Brieva, R.; Gotor, V. Enzymatic resolution of hindered cyanohydrins, key precursors of muscarinic receptor antagonists. Tetrahedron: Asymmetry 2007, 18, 994–1002. [Google Scholar] [CrossRef]
- Gupta, P.; Fernandes, R.A.; Kumar, P. An asymmetric dihydroxylation route to (S)-oxybutynin. Tetrahedron Lett. 2003, 44, 4231–4232. [Google Scholar] [CrossRef]
- Gourdet, B.; Lam, H.W. Catalytic asymmetric dihydroxylation of enamides and application to the total synthesis of (+)-tanikolide. Angew. Chem. Int. Ed. 2010, 49, 8733–8737. [Google Scholar] [CrossRef]
- Tokuda, O.; Kano, T.; Gao, W.-G.; Ikemoto, T.; Maruoka, K. A practical synthesis of (S)-2-cyclohexyl-2-phenylglycolic acid via organocatalytic asymmetric construction of a tetrasubstituted carbon center. Org. Lett. 2005, 7, 5103–5105. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Xu, J.; Reichle, M. Enantioselective synthesis of α-tertiary hydroxyaldehydes by palladium-catalyzed asymmetric allylic alkylation of enolates. J. Am. Chem. Soc. 2007, 129, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Sitadevi, P.; Rao, P.L.K.M. Development and validation of a method for the enantioseparation of oxybutynin hydrochloride by HPTLC. Anal. Chem.: Indian J. 2010, 9, 378–383. [Google Scholar]
- Walker, T.A. The chiral separation of oxybutynin enantiomers using an ovomucoid column. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 841–853. [Google Scholar] [CrossRef]
- Miyamoto, E.; Demizu, Y.; Murata, Y.; Yamada, Y.; Kawashima, S.; Kontani, H.; Sakai, T. High-performance liquid chromatographic preparation of oxybutynin enantiomers on a chiral stationary phase. J. Chromatogr. A 1993, 653, 135–137. [Google Scholar] [CrossRef]
- Zin, L.C.; Silva, C.F.; Guimaraes, L.; Borges, K.B.; Nascimento, C.S. Jr. Enantioselective separation of oxybutynin: a theoretical and experimental investigation. Quim. Nova 2022, 45, 263–267. [Google Scholar] [CrossRef]
- Cai, L.; Xue, M.; Lun, J.; Li, S.; Yu, J.; Guo, X. Enantioseparation and molecular modeling study of eight psychoactive drugs on a coated polysaccharide-based chiral stationary phase. Electrophoresis 2020, 41, 2092–2101. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, G. Hydroxypropyl-β-cyclodextrin encapsulated stationary phase based on silica monolith particles for enantioseparation in liquid chromatography. J. Sep. Sci. 2021, 44, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Song, P.; Wen, X.; Deng, M.; Wang, J.; Guo, X. Chiral separation of 12 pairs of enantiomers by capillary electrophoresis using heptakis-(2,3-diacetyl-6-sulfato)-β-cyclodextrin as the chiral selector and the elucidation of the chiral recognition mechanism by computational methods. J. Sep. Sci. 2017, 40, 2999–3007. [Google Scholar] [CrossRef]
- Tang, K.; Cai, J.; Yang, C.; Liu, Y.; Zhang, P.; Liu, Y. Kinetic study on reactive extraction for chiral separation of oxybutynin enantiomers. Sep. Purif. Technol. 2012, 92, 30–35. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, G.; Tang, K.; Yang, W.; Sui, G.; Zhou, C. Enantiomeric separation of oxybutynin by recycling high-speed counter-current chromatography with hydroxypropyl-β-cyclodextrin as chiral selector. J. Sep. Sci. 2014, 37, 3443–3450. [Google Scholar] [CrossRef] [PubMed]
- Dmochowski, R. Improving the tolerability of anticholinergic agents in the treatment of overactive bladder. Drug Saf. 2005, 28, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Ito, Y.; Nishijima, S.; Kadekawa, K.; Sugaya, K. Basic and clinical aspects of antimuscarinic agents used to treat overactive bladder. Pharmacol. Ther. 2018, 189, 130–148. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.; Andersson, K.-E.; Buccafusco, J.J.; Chapple, C.; de Groat, W.C.; Fryer, A.D.; Kay, G.; Laties, A.; Nathanson, N.M.; Pasricha, P.J.; Wein, A.J. Muscarinic receptors: Their distribution and function in body systems, and the implications for treating overactive bladder. Br. J. Pharmacol. 2006, 148, 565–578. [Google Scholar] [CrossRef]
- Welk, B.; Richardson, K.; Panicker, J.N. The cognitive effect of anticholinergics for patients with overactive bladder. Nat. Rev. Urol. 2021, 18, 686–700. [Google Scholar] [CrossRef]
- Welk, B.; Etaby, K.; McArthur, E.; Chou, Q. The risk of delirium and falls or fractures with the use of overactive bladder anticholinergic medications. Neuroulol. Urodyn. 2022, 41, 348–356. [Google Scholar] [CrossRef]
- Nishtala, P.S.; Chyou, T.-Y. Risk of delirium associated with antimuscarinics in older adults: A case-time-control study. Pharmacoepidemiol. Drug Saf. 2022, 31, 883–891. [Google Scholar] [CrossRef]
- Kennelly, M.J. A comparative review of oxybutynin chloride formulations: Pharmacokinetics and therapeutic efficacy in overactive bladder. Rev Urol. 2010, 12, 12–19. [Google Scholar] [CrossRef]
- Vozmediano-Chicharro, R.; Blasco Hernández, P.; Madurga-Patuel, B. Insights into the management of overactive bladder with transdermal oxybutynin: A practical review. Res. Rep. Urol. 2020, 12, 321–330. [Google Scholar] [CrossRef]
- Dmochowski, R.R. (S)-oxybutynin (Sepracor). Curr. Opin. Investig. Drugs, 2002, 3, 1508–11. [Google Scholar]
- Kachur, J.F.; Peterson, J.S.; Carter, J.P.; Rzeszotarski, W.J.; Hanson, R.C.; Noronha-Blob, L. R and S enantiomers of oxybutynin: Pharmacological effects in guinea pig bladder and intestine. J. Pharmacol. Exp. Ther. 1988, 247, 867–872. [Google Scholar] [PubMed]
- Noronha-Blob, L.; Kachur, J.F. Enantiomers of oxybutynin: In vitro pharmacological characterization at M1, M2 and M3 muscarinic receptors and in vivo effects on urinary bladder contraction, mydriasis and salivary secretion in guinea pigs. J. Pharmacol. Exp. Ther. 1991, 256, 562–567. [Google Scholar] [PubMed]
- Starkman, J.S.; Dmochowski, R. Management of overactive bladder with transdermal oxybutynin. Rev. Urol. 2006, 8, 93–103. [Google Scholar] [PubMed]
- Kretschmar, M.; Suleiman, A.A.; Krause, P.; Albrecht, U.; Stein, R.; Rubenwolf, P.; Fuhr, U.; Taubert, M. A population pharmacokinetic model of (R)- and (S-) oxybutynin and its active metabolites after oral and intravesical administration to healthy volunteers. J. Clin. Pharmacol. 2021, 21, 961–971. [Google Scholar] [CrossRef]
- Ditropan, XL. Available online: https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/DITROPAN+XL-pi.pdf (accessed on 17 September 2023).
- Oxybutynin. Available on line: https://go.drugbank.com/drugs/DB01062 (accessed on 17 September 2023).
- Oxytrol. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021351s005lbl.pdf (accessed on 17 September 2023).
- Kentera (previously Oxybutynin Nicobrand). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/kentera-previously-oxybutynin-nicobrand (accessed on 17 September 2023).
- Gelnique. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/022204s010lbl.pdf (accessed on 17 September 2023).
- Anturol. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202513s000lbl.pdf (accessed on 17 September 2023).
- Sheng, Y.; Zhang, S.; Ling, J.; Hu, C.; Zhang, Z.; Lv, H. Oxybutynin nanosuspension gel for enhanced transdermal treatment for overactive bladder syndrome. Pharm. Dev. Technol. 2022, 27, 459–468. [Google Scholar] [CrossRef]
- Cohn, J.A.; Brown, E.T.; Reynolds, W.S.; Kaufman, M.R.; Milam, D.F.; Dmochowski, R.R. An update on the use of transdermal oxybutynin in the management of overactive bladder disorder. Ther. Adv. Urol. 2016, 8, 83–90. [Google Scholar] [CrossRef]
- Sathyan, G.; Chancellor, M.B.; Gupta, S.K. Effect of OROS® controlled-release delivery on the pharmacokinetics and pharmacodynamics of oxybutynin chloride. Br. J. Clin. Pharmacol., 2001, 52, 409–417. [Google Scholar] [CrossRef]
- Winkler, H.A.; Sand, P.K. Treatment of detrusor instability with oxybutynin rectal suppositories. Int. Urogynecol. J. Pelvic Floor Dysfunct. 1998, 9, 100–102. [Google Scholar] [CrossRef]
- Bedse, A. : Mahajan, H.; Dhamane, S. Formulation of oxybutynin chloride microparticle-loaded suppositories: in vitro characterization and in vivo pharmacokinetic study. Future J. Pharm. Sci. 2022, 8, 22. [Google Scholar] [CrossRef]
- Brendler, C.B.; Radebaugh, L.C.; Mohler, J.L. Topical oxybutynin chloride for relaxation of dysfunctional bladders. J. Urol., 1989, 141, 1350–1352. [Google Scholar] [CrossRef]
- Fraser, M.O.; Lavelle, J.P.; Sacks, M.S.; Chancellor, M.B. The future of bladder control—Intravesical drug delivery, a pinch of pepper, and gene therapy. Rev Urol. 2002, 4, 1–11. [Google Scholar] [PubMed]
- Moon, H.S. Research on novel intravesical drug delivery devices. Int. Neurourol. J. 2016, 20, 89–90. [Google Scholar] [CrossRef] [PubMed]
- Kuehbacher, A.; Zeppek, C.; Thielicke, W. Syringe sterilization method. PCT Int. Appl. WO 2022223174 A1 ( 2022.
- Gordon, A.; Malchi, N.; Nasser, T.; Touitou, D. Compositions and methods for drug instillation into the urinary bladder. PCT Int. Appl. WO 2023062638 A1 ( 2023.
- Levin, R.M.; Whitbeck, C.; Borow, A.; Burden, O.; Leggett, R.E. Effectiveness of vaginally administered oxybutynin on rabbit bladder function. Urology, 2003, 61, 1273–1277. [Google Scholar] [CrossRef]
- Tugcu-Demiröz, F.; Acartürka, F.; Erdogan, D. Development of long-acting bioadhesive vaginal gels of oxybutynin: Formulation, in vitro and in vivo evaluations. Int. J. Pharm. 2013, 457, 25–39. [Google Scholar] [CrossRef]
- Schröder, A.; Levin, R.M.; Kogan, B.A.; Das, A.K.; Kay, F.; Mahashabde, A. Absorption of oxybutynin from vaginal inserts: Drug blood levels and the response of the rabbit bladder. Urology, 2000, 56, 1063–1067. [Google Scholar] [CrossRef]
- Woolfson, A.D.; Malcolm, R.K.; Gallagher, R.J. Design of a silicone reservoir intravaginal ring for the delivery of oxybutynin. J. Control. Release, 2003, 91, 465–476. [Google Scholar] [CrossRef]
- Gittelman, M.; Weiss, H.; Seidman, L. A phase 2, randomized, double-blind, efficacy and safety study of oxybutynin vaginal ring for alleviation of overactive bladder symptoms in women. J. Urol., 2014, 191, 1014–21. [Google Scholar] [CrossRef]
- de Laat, W.; Pagan, L.; Malcolm, R.K.; Wiegerinck, M.; Nickolson, V.; Huisman, B.; Stuurman, R.; van Esdonk, M.; Klarenbeek, N. First-in-human study to assess the pharmacokinetics, tolerability, and safety of single-dose oxybutynin hydrochloride administered via a microprocessor-controlled intravaginal ring. Drug Deliv. 2023, 30, 2180113. [Google Scholar] [CrossRef]
- Palugan, L.; Cerea, M.; Cirilli, M.; Moutaharrik, S.; Maroni, A.; Zema, L.; Melocchi, A.; Uboldi, M.; Filippin, I.; Foppoli, A.; Gazzaniga, A. Intravesical drug delivery approaches for improved therapy of urinary bladder diseases. Int. J. Pharm.: X 2021, 3, 100100. [Google Scholar] [CrossRef] [PubMed]
- Srikrishna, S.; Cardozo, L. The vagina as a route for drug delivery: A review. Int. Urogynecol. J., 2013, 24, 537–543. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine - Clinical Trials. Available online: https://clinicaltrials.gov/ct2/home (accessed on 17 September 2023).
- Nawrocki, S.; Cha, J. The etiology, diagnosis, and management of hyperhidrosis: A comprehensive review. J. Am. Acad. Dermatol. 2019, 81, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Gregoriou, S.; Sidiropoulou, P.; Kontochristopoulos, G.; Rigopoulos, D. Clin. Cosmet. Investig. Dermatol. 2019, 12, 733–738. [CrossRef] [PubMed]
- Wohlrab, J.; Bechara, F.G.; Schick, C.; Naumann, M. Hyperhidrosis: A central nervous dysfunction of sweat secretion. Dermatol. Ther. 2023; 13, 453–463. [Google Scholar]
- Wong. N.S.; Adlam, T.M.; Potts, G.E.; Farshchian, M. Hyperhidrosis: A review of recent advances in treatment with topical anticholinergics. Dermatol. Ther. 2022, 12, 2705–2714. [Google Scholar] [CrossRef]
- Wolosker, N.; Milanez de Campos, J.R.; Kauffman, P.; Neves, S.; Munia, M.A.; BiscegliJatene, F.; Puech-Leão, P. The use of oxybutynin for treating axillary hyperhidrosis. J. Vasc. Surg. 2011, 25, 1057–1062. [Google Scholar] [CrossRef]
- Wolosker, N.; Milanez de Campos, J.R.; Kauffman, P.; Puech-Leão, P. A randomized placebo-controlled trial of oxybutynin for the initial treatment of palmar and axillary hyperhidrosis. J. Vasc. Surg. 2012, 55, 1696–1700. [Google Scholar] [CrossRef]
- Wolosker, N.; Kauffman, P.; de Campos, J.R.M.; Faustino, C.B.; da Silva, M.F.A.; Teivelis, M.P.; Puech-Leão, P. Long-term results of the treatment of primary hyperhidrosis with oxybutynin: follow-up of 1,658 cases. Int. J. Dermatol. 2020, 59, 709–715 doiorg/101111/ijd14872. [Google Scholar] [CrossRef]
- El-Samahy, M.; Mouffokes, A.; Badawy, M.M.; Amro, S.; Fayad, T.; Abdelwahab, O.A. Safety and efficacy of oxybutynin in patients with hyperhidrosis: systematic review and meta-analysis of randomized controlled trials. Arch. Dermatol. Res. 2023; Ahead of Print. [Google Scholar] [CrossRef]
- Oxybutynin as treatment for hyperhidrosis. Available online: https://duradry.com/blogs/hyperhidrosis/oxybutynin-for-the-treatment-of-hyperhidrosis (accessed on 17 September 2023).
- Oxybutynine/Ditropan contre la transpiration/hyperhidrose. Available online: https://observatoire-hyperhidrose.fr/2020/05/11/effet-oxybutynine-hyperhidrose/#google_vignette (accessed on 17 September 2023).
- Hyperhidrosis. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/indicatieteksten/hyperhidrosis (accessed on 17 September 2023).
- Ravnikar, V. Physiology and treatment of hot flushes. Obstet. Gynecol. 1990, 75, 3S–8S. [Google Scholar] [CrossRef]
- Kronenberg, F. Hot flashes: Phenomenology, quality of life, and search for treatment options. Exp. Gerontol. 1994, 29, 319–336. [Google Scholar] [CrossRef]
- Bansal, R.; Aggarwal, N. Menopausal hot flashes: A concise review. J. Mid-life Health 2019, 10, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Faubion, S.S.; Loprinzi, C.L.; Ruddy, K.J. Management of hormone deprivation symptoms after cancer. Mayo Clin. Proc. 2016, 91, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Loprinzi, C.L.; Deville, C. Oxybutynin for hot flashes due to androgen deprivation in men. N. Engl. J. Med. 2018, 378, 1745–1746. [Google Scholar] [CrossRef]
- Leon-Ferre, R.A.; Majithia, N.; Loprinzi, C.L. Management of hot flashes in women with breast cancer receiving ovarian function suppression. Cancer Treat. Rev. 2017, 52, 82–90. [Google Scholar] [CrossRef]
- LaGuardia, K.D. Treatment of hot flashes using muscarinic receptor antagonists. US patent US 2007/028 1998 A1.
- Simon, J.A.; LaGuardia, K.D. Extended-release oxybutynin relieves vasomotor symptoms in healthy postmenopausal women. 55th Annual Clinical Meeting of the American College of Obstetricians and Gynecologists, May 5-9, 2007, San Diego (CA, USA). [CrossRef]
- Simon, J.A.; Gaines, T.; LaGuardia, K.D. Extended-release oxybutynin therapy for vasomotor symptoms in women: a randomized clinical trial. Menopause 2016, 23, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Leon-Ferre, R.A.; Novotny, P.J.; Wolfe, E.G.; Faubion, S.S.; Ruddy, K.J.; Flora, D.; Dakhil, C.S.R.; Rowland, K.M.; Graham, M.L.; Le-Lindqwister, N.; Smith, T.J.; Loprinzi, C.L. Oxybutynin vs placebo for hot flashes in women with or without breast cancer: A randomized, double-blind clinical trial (ACCRU SC-1603). JNCI Cancer Spectr. 2020, 4, pkz088. [Google Scholar] [CrossRef] [PubMed]
- Le manuel MSD – Menopause. Available online: https://www.msdmanuals.com/fr/professional/gyn%C3%A9cologie-et-obst%C3%A9trique/m%C3%A9nopause/m%C3%A9nopause (accessed on 17 September 2023).
- Kaiser Permanente - Nonhormonal prescription medications for menopause symptoms. Available online: https://mydoctor.kaiserpermanente.org/ncal/article/nonhormonal-prescription-medications-for-menopause-symptoms-2209311 (accessed on 17 September 2023).
- HealthCentral - What to know about menopause medications. Available on line: https://www.healthcentral.com/condition/menopause/menopause-drugs-medications (accessed on 17 September 2023).
- Chan, E.; Steenland, H.W.; Liu, H.; Horner, R.L. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am. J. Respir. Crit. Care Med. 2006, 174, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Grace, K.P.; Hughes, S.W.; Horner, R.L. Identification of the mechanism mediating genioglossus muscle suppression in REM sleep. Am. J. Respir. Crit. Care Med. 2013, 187, 311–319. [Google Scholar] [CrossRef]
- Lim, R.; Carberry, J.C.; Wellman, A.; Grunstein, R.; Eckert, D.J. Reboxetine and hyoscine butylbromide improve upper airway function during nonrapid eye movement and suppress rapid eye movement sleep in healthy individuals. Sleep, 2019, 42, zsy261 doiorg/101093/sleep/zsy261. [Google Scholar] [CrossRef]
- Taranto-Montemurro, L.; Messineo, L.; Sands, S.A.; Azarbarzin, A.; Marques, M.; Edwards, B.A.; Eckert, D.J.; White, D.P.; Wellman, A. The Combination of Atomoxetine and Oxybutynin Greatly Reduces Obstructive Sleep Apnea Severity: A Randomized, Placebo-Controlled, Double-Blind Crossover Trial. Am. J. Respir. Crit. Care Med. 2019, 199, 1267–1276. [Google Scholar] [CrossRef]
- Taranto-Montemurro, L.; Pho, H.; White, D.P. Development of a combination of noradrenergic and antimuscarinic drugs for the treatment of obstructive sleep apnea: Challenges and progress. Front. Sleep, 2023, 2, 1148282. [Google Scholar] [CrossRef]
- Schweitzer, P.K.; Maynard, J.P.; Wylie, P.E.; Emsellem, H.A.; Sands, S.A. Efficacy of atomoxetine plus oxybutynin in the treatment of obstructive sleep apnea with moderate pharyngeal collapsibility. Sleep Breath. 2023, 27, 495–503. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




