Submitted:
19 September 2023
Posted:
20 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Materials
2.2. Pretreatment of optical fiber
2.3. Preparation of Ag/Au nanocap arrays
2.4. Characterization
2.5. FDTD calculations
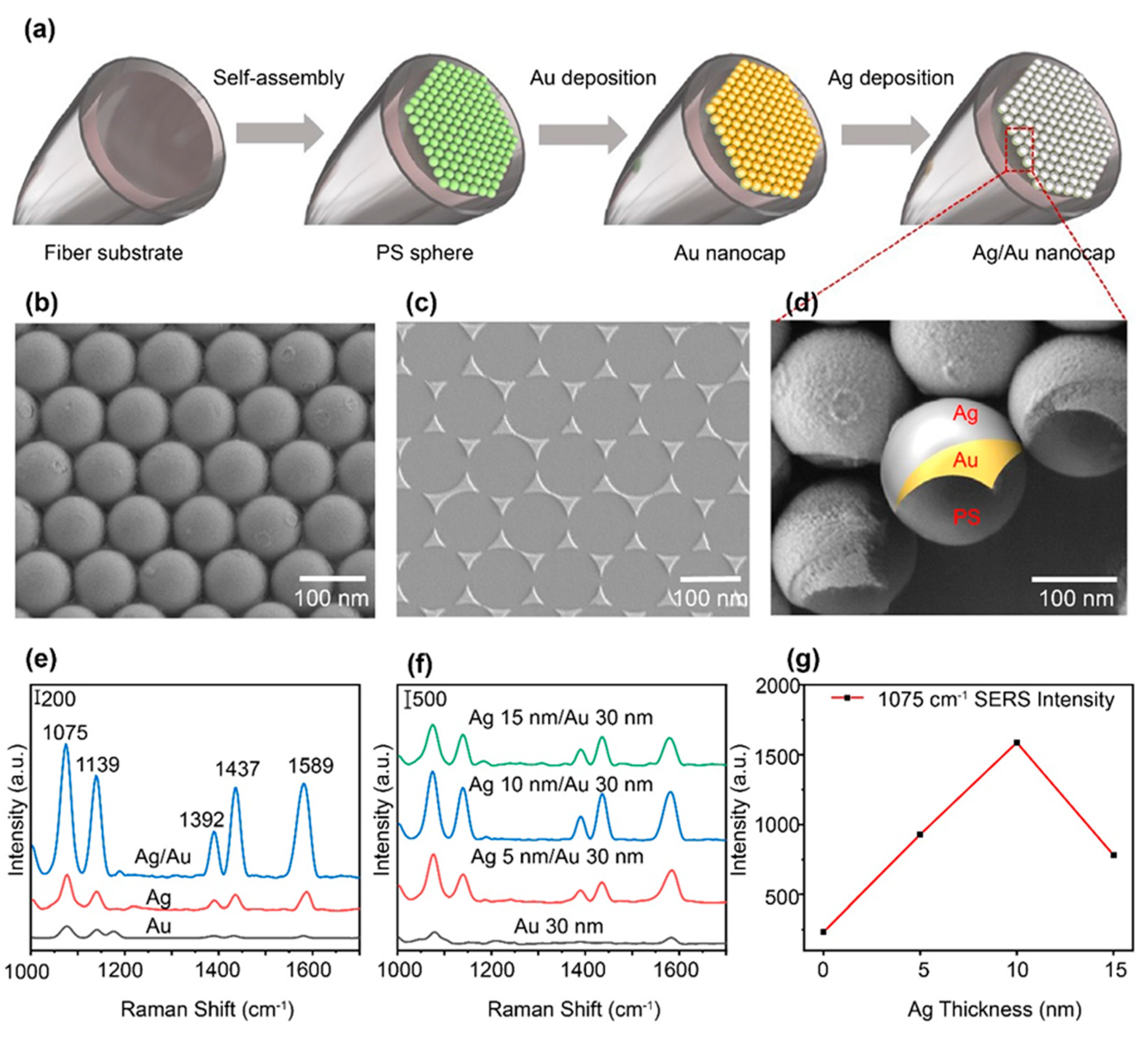
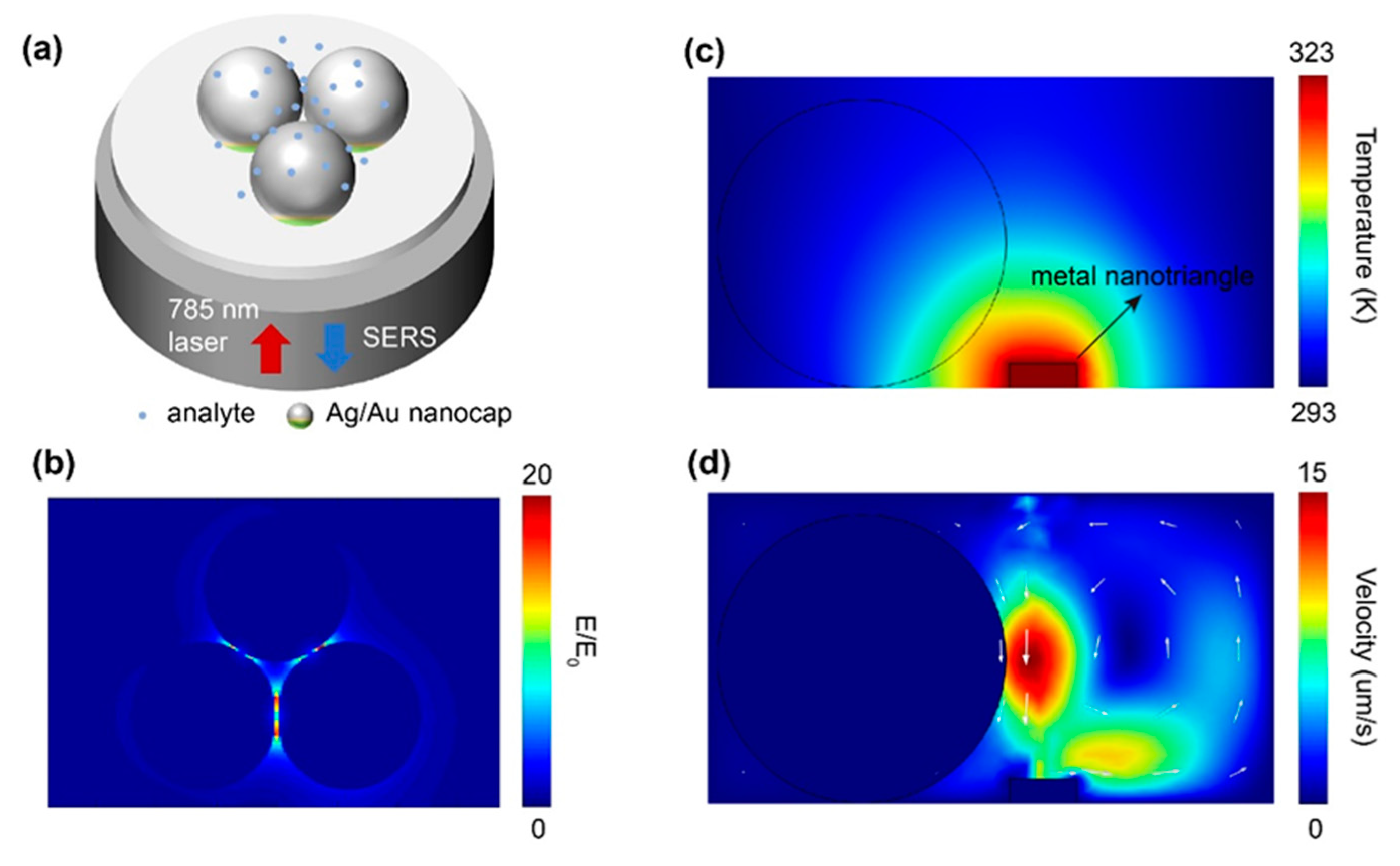
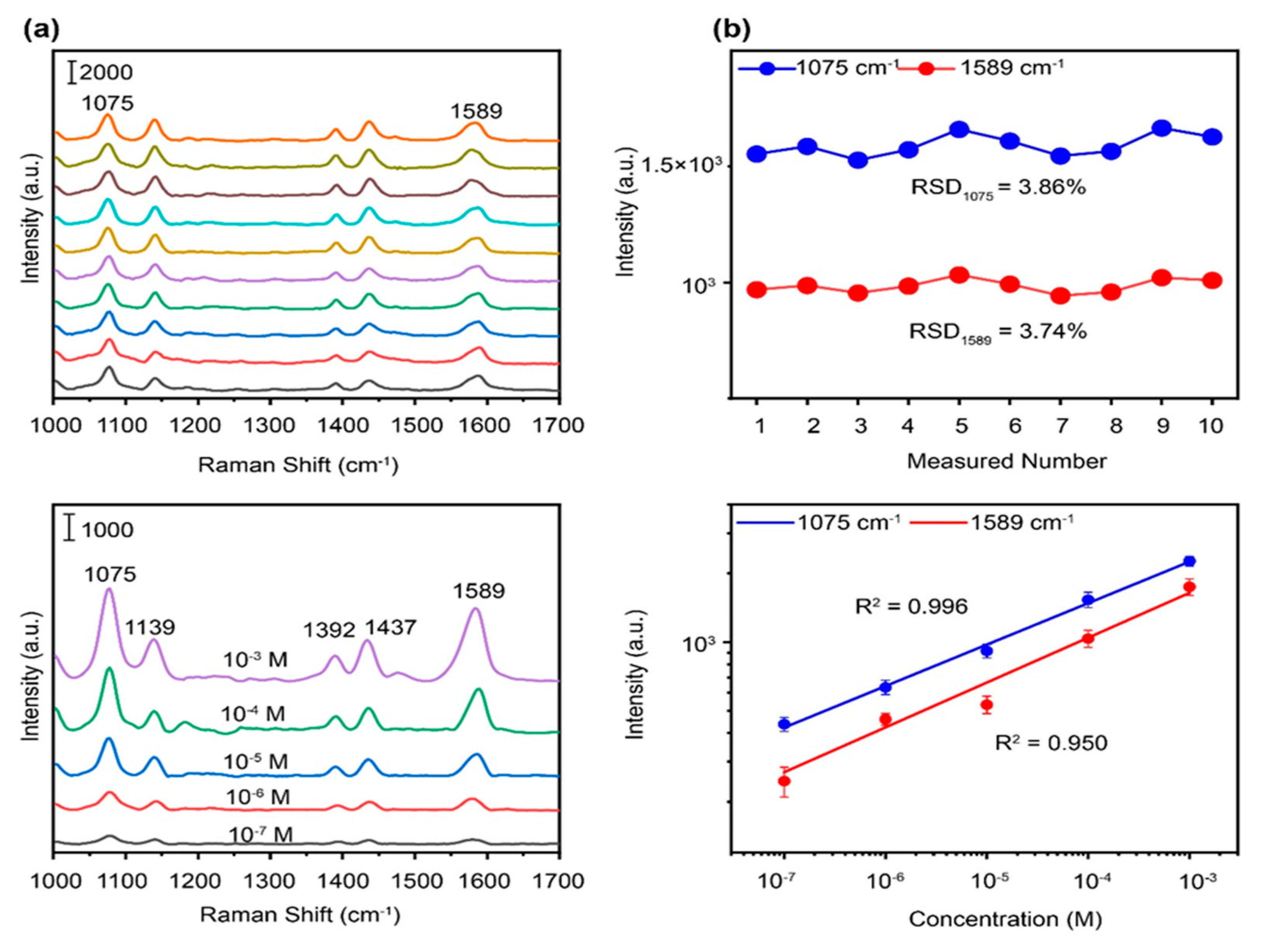
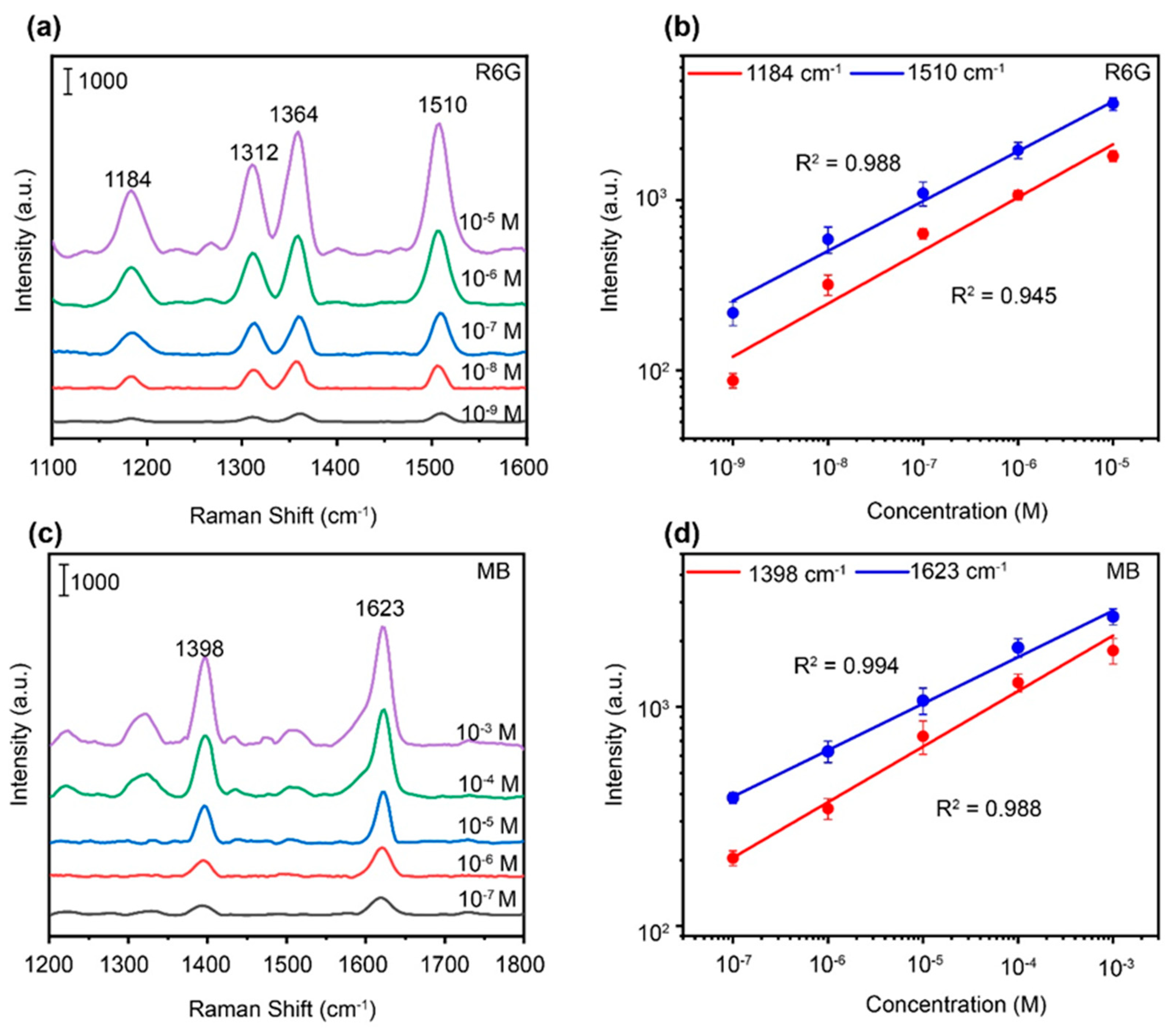
3. Results and discussion
4. Conclusions
Funding
CRediT authorship contribution statement
Declaration of Competing Interest
Data Availability
Acknowledgments
References
- Farrell, M.E.; Strobbia, P.; Pellegrino, P.M.; Cullum, B. Surface regeneration and signal increase in surface-enhanced Raman scattering substrates. Appl. Opt. 2017, 56, B198–B213. [Google Scholar] [CrossRef]
- Huang, H.; Wang, J.H.; Jin, W.; Li, P.; Chen, M.; Xie, H.H.; Yu, X.F.; Wang, H.; Dai, Z.; Xiao, X.; et al. Competitive reaction pathway for site-selective sonjugation of Raman dyes to hotspots on gold nanorods for greatly enhanced SERS performance. Small 2014, 10, 4012–4019. [Google Scholar] [CrossRef]
- Im, H.; Bantz, K.C.; Lee, S.H.; Johnson, T.W.; Haynes, C.L.; Oh, S.-H. Self-Assembled Plasmonic Nanoring Cavity Arrays for SERS and LSPR Biosensing. Adv. Mater. 2013, 25, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Lucotti, A.; Pesapane, A.; Zerbi, G. Use of a Geometry Optimized Fiber-Optic Surface-Enhanced Raman Scattering Sensor in Trace Detection. Appl. Spectrosc. 2007, 61, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lu, Y.; Zhang, L.; Xu, X.; Li, B.; Zhao, X.; Yan, X.; Wang, C.; Sun, P.; Liu, X.; et al. Flexible and wearable glove-based SERS sensor for rapid sampling and sensitive detection of controlled drugs. Sensors Actuators B: Chem. 2023, 386. [Google Scholar] [CrossRef]
- Fateixa, S.; Nogueira, H.I.S.; Trindade, T. Hybrid nanostructures for SERS: materials development and chemical detection. Phys. Chem. Chem. Phys. 2015, 17, 21046–21071. [Google Scholar] [CrossRef] [PubMed]
- Purwidyantri, A.; El-Mekki, I.; Lai, C.-S. Tunable Plasmonic SERS “Hotspots” on Au-Film Over Nanosphere by Rapid Thermal Annealing. IEEE Trans. Nanotechnol. 2017, 16, 551–559. [Google Scholar] [CrossRef]
- Yun, B.J.; Koh, W.-G. Highly-sensitive SERS-based immunoassay platform prepared on silver nanoparticle-decorated electrospun polymeric fibers. J. Ind. Eng. Chem. 2020, 82, 341–348. [Google Scholar] [CrossRef]
- Lucotti, A.; Zerbi, G. Fiber-optic SERS sensor with optimized geometry. Sensors Actuators B: Chem. 2007, 121, 356–364. [Google Scholar] [CrossRef]
- Xie, Z.; Tao, J.; Lu, Y.; Lin, K.; Yan, J.; Wang, P.; Ming, H. Polymer optical fiber SERS sensor with gold nanorods. Opt. Commun. 2009, 282, 439–442. [Google Scholar] [CrossRef]
- Yuan, H.; Ji, W.; Chu, S.; Liu, Q.; Qian, S.; Guang, J.; Wang, J.; Han, X.; Masson, J.-F.; Peng, W. Mercaptopyridine-Functionalized Gold Nanoparticles for Fiber-Optic Surface Plasmon Resonance Hg2+ Sensing. ACS Sensors 2019, 4, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guang, J.; Liu, C.; Bi, S.; Liu, Q.; Li, P.; Zhang, N.; Chen, S.; Yuan, H.; Zhou, D.; Peng, W. Simple and low-cost plasmonic fiber-optic probe as SERS and biosensing platform. Adv. Opt. Mater. 2019, 7, 1900337. [Google Scholar] [CrossRef]
- Bharadwaj, R.; Mukherji, S. Gold nanoparticle coated U-bend fiber optic probe for localized surface plasmon resonance based detection of explosive vapours. Sens. Actuators B Chem. 2014, 192, 804–811. [Google Scholar] [CrossRef]
- Zheng, Z.; Shi, M.; Xu, Y.; Liu, S.; Zhong, H.; Shou, Q.; Huang, J.; Luan, T.; Li, Z.; Xing, X. Light-Induced Dynamic Assembly of Gold Nanoparticles in a Lab-on-Fiber Platform for Surface-Enhanced Raman Scattering Detection. ACS Appl. Nano Mater. 2022, 5, 8005–8011. [Google Scholar] [CrossRef]
- Liu, S.; Zhong, H.; Li, Z.; Xu, Y.; Hu, X.; Zheng, Z.; Liu, L.; Chen, P.; Cai, X.; Jiang, X.; et al. Photothermal microfluidic-assisted self-cleaning effect for a highly reusable SERS sensor. Opt. Lett. 2021, 46, 4714–4717. [Google Scholar] [CrossRef]
- Liu, G.; Cai, W.; Kong, L.; Duan, G.; Li, Y.; Wang, J.; Cheng, Z. Trace detection of cyanide based on SERS effect of Ag nanoplate-built hollow microsphere arrays. J. Hazard. Mater. 2013, 248, 435–441. [Google Scholar] [CrossRef]
- Liu, G.; Li, X.; Wang, W.; Zhou, F.; Duan, G.; Li, Y.; Xu, Z.; Cai, W. Gold Binary-Structured Arrays Based on Monolayer Colloidal Crystals and Their Optical Properties. Small 2014, 10, 2374–2381. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Dai, X.; Stogin, B.B.; Wong, T.-S. Ultrasensitive surface-enhanced Raman scattering detection in common fluids. Proc. Natl. Acad. Sci. 2016, 113, 268–273. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Duan, G.; Wang, J.; Changhao, L.; Cai, W. Tunable Surface Plasmon Resonance and Strong SERS Performances of Au Opening-Nanoshell Ordered Arrays. ACS Appl. Mater. Interfaces 2012, 4, 1–5. [Google Scholar] [CrossRef]
- Zhu, Y.; Dluhy, R.A.; Zhao, Y. Development of silver nanorod array based fiber optic probes for SERS detection. Sensors Actuators B: Chem. 2011, 157, 42–50. [Google Scholar] [CrossRef]
- Gao, D.; Yang, X.; Teng, P.; Kong, D.; Liu, Z.; Yang, J.; Luo, M.; Li, Z.; Wen, X.; Yuan, L.; et al. On-line SERS detection of adenine in DNA based on the optofluidic in-fiber integrated GO/PDDA/Ag NPs. Sensors Actuators B: Chem. 2021, 332, 129517. [Google Scholar] [CrossRef]
- Huang, R.; Lian, S.; Li, J.; Feng, Y.; Bai, S.; Wu, T.; Ruan, M.; Wu, P.; Li, X.; Cai, S.; et al. High-sensitivity and throughput optical fiber SERS probes based on laser-induced fractional reaction method. Results Phys. 2023, 48. [Google Scholar] [CrossRef]
- Yoo, H.-W.; Jung, J.-M.; Lee, S.-K.; Jung, H.-T. The fabrication of highly ordered silver nanodot patterns by platinum assisted nanoimprint lithography. Nanotechnology 2011, 22, 095304. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Gao, W.; Chen, L.; Chen, S.; Wei, M.; Gao, M.; Wang, C.; Zhang, Y.; Yang, J. Au/Ag bimetal nanogap arrays with tunable morphologies for surface-enhanced Raman scattering. RSC Adv. 2014, 5, 7454–7460. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, Y.; Hang, L.; Li, H.; Liu, G.; Zhang, X.; Lyu, X.; Cai, W.; Li, Y. Periodic Porous Alloyed Au–Ag Nanosphere Arrays and Their Highly Sensitive SERS Performance with Good Reproducibility and High Density of Hotspots. ACS Appl. Mater. Interfaces 2018, 10, 9792–9801. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xiao, X.; Wang, P.; Ji, L.; Yang, C. Fiber surface enhanced Raman scattering sensor based on patterned biphasic gold–silver nanoalloys. Chem. Phys. Lett. 2012, 553, 51–54. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, C.; Gao, W.; Han, Z.; Liu, T.; Li, C.; Wang, Z.; He, E.; Zheng, H. Ag-Au alloy nanoparticles: Synthesis and in situ monitoring SERS of plasmonic catalysis. Sensors Actuators B: Chem. 2016, 231, 609–614. [Google Scholar] [CrossRef]
- Ran, Y.; Strobbia, P.; Cupil-Garcia, V.; Vo-Dinh, T. Fiber-optrode SERS probes using plasmonic silver-coated gold nanostars. Sensors Actuators B: Chem. 2019, 287, 95–101. [Google Scholar] [CrossRef]
- Retsch, M.; Zhou, Z.; Rivera, S.; Kappl, M.; Zhao, X.S.; Jonas, U.; Li, Q. Fabrication of Large-Area, Transferable colloidal monolayers utilizing self-assembly at the air/water interface. Macromol. Chem. Phys. 2009, 210, 230–241. [Google Scholar] [CrossRef]
- Yu, J.; Yan, Q.; Shen, D. Co-Self-Assembly of Binary Colloidal Crystals at the Air−Water Interface. ACS Appl. Mater. Interfaces 2010, 2, 1922–1926. [Google Scholar] [CrossRef]
- Quero, G.; Zito, G.; Managò, S.; Galeotti, F.; Pisco, M.; De Luca, A.C.; Cusano, A. Nanosphere Lithography on Fiber: Towards Engineered Lab-On-Fiber SERS Optrodes. Sensors 2018, 18, 680. [Google Scholar] [CrossRef]
- Chen, J.; Pang, S.; He, L.; Nugen, S.R. Highly sensitive and selective detection of nitrite ions using Fe3O4@SiO2/Au magnetic nanoparticles by surface-enhanced Raman spectroscopy. Biosens. Bioelectron. 2016, 85, 726–733. [Google Scholar] [CrossRef]
- Xing, L.; Xiahou, Y.; Zhang, X.; Du, W.; Zhang, P.; Xia, H. Large-Area Monolayer Films of Hexagonal Close-Packed Au@Ag Nanoparticles as Substrates for SERS-Based Quantitative Determination. ACS Appl. Mater. Interfaces 2022, 14, 13480–13489. [Google Scholar] [CrossRef]
- Yang, C.; Liang, P.; Tang, L.; Zhou, Y.; Cao, Y.; Wu, Y.; Zhang, D.; Dong, Q.; Huang, J.; He, P. Synergistic effects of semiconductor substrate and noble metal nano-particles on SERS effect both theoretical and experimental aspects. Appl. Surf. Sci. 2018, 436, 367–372. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Lin, L.; Peng, X.; Wang, M.; Scarabelli, L.; Mao, Z.; Liz-Marzán, L.M.; Becker, M.F.; Zheng, Y. Light-Directed Reversible Assembly of Plasmonic Nanoparticles Using Plasmon-Enhanced Thermophoresis. ACS Nano 2016, 10, 9659–9668. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Li, J.; Wu, L.; Wu, C.; Chen, Y. Dynamic and Reversible Accumulation of Plasmonic Core-Satellite Nanostructures in a Light-Induced Temperature Gradient for In Situ SERS Detection. Part. Part. Syst. Charact. 2018, 35. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



