Submitted:
18 September 2023
Posted:
20 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Patients and skin biopsies
Skin biopsies acquisition
RNA extraction and transcription assessment
Assessment of the mRNA expression of IL-1A, IL-1B, IL-1RN, IL-6, TGF-β1 and IFN-γ genes
Statistical analysis
3. Results
Patient characteristics
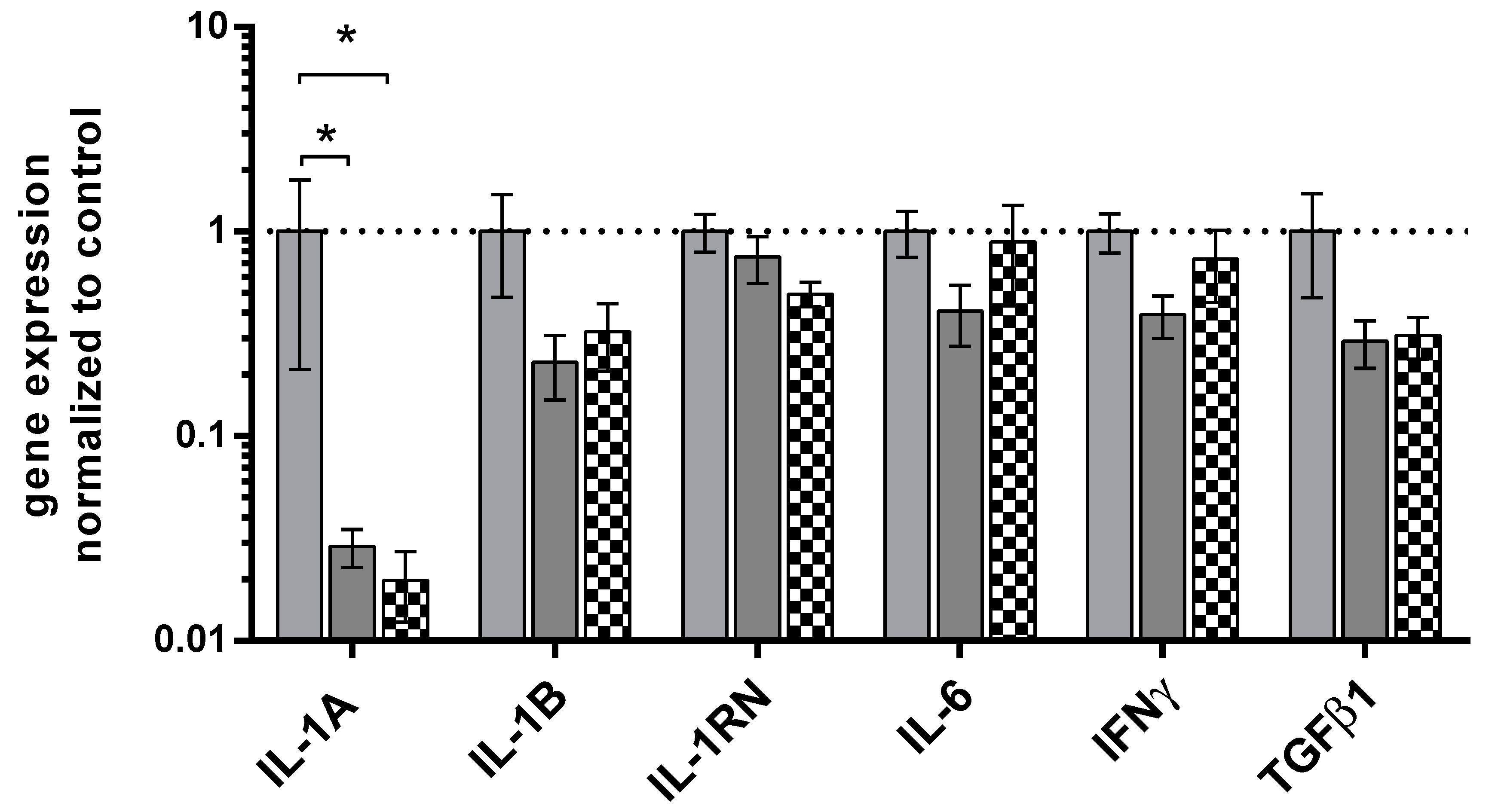
Expression of the cytokines’ gene in all groups of patients
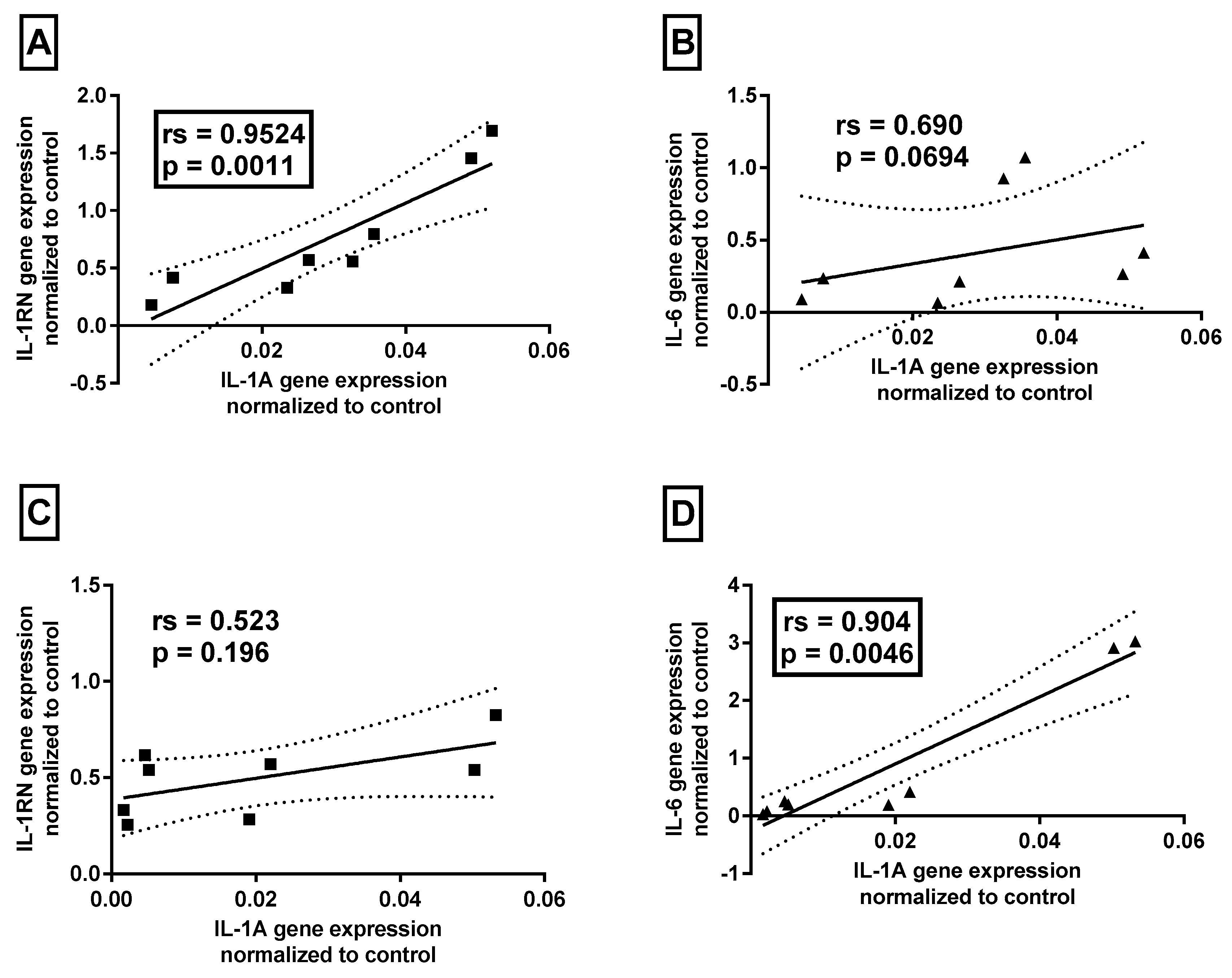
Association between cytokines’ mRNA levels and clinical data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dayal S, Sahu P. Zoon balanitis: A comprehensive review. Indian J Sex Transm Dis AIDS. 2016, 37, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Piaserico S, Orlando G, Linder MD, Cappozzo P, Zarian H, Iafrate M. A case-control study of risk factors associated with Zoon balanitis in men. J Eur Acad Dermatol Venereol. 2019, 33, 1591–1594. [Google Scholar] [CrossRef] [PubMed]
- Edwards SK, Bunker CB, Ziller F, van der Meijden WI. 2013 European guideline for the management of balanoposthitis. Int J STD AIDS. 2014, 25, 615–626. [Google Scholar] [CrossRef]
- Errichetti E, Lallas A, Di Stefani A, Apalla Z, Kyrgidis A, Lacarrubba F, et al. Accuracy of dermoscopy in distinguishing erythroplasia of Queyrat from common forms of chronic balanitis: results from a multicentric observational study. J Eur Acad Dermatol Venereol. 2019, 33, 966–972. [Google Scholar] [CrossRef]
- Jormanainen, A, Pasternack, C, Pasternack, R. A retrospective study of 129 patients with plasma cell balanitis. JEADV Clin Pract. 2023, 2, 293–299. [Google Scholar] [CrossRef]
- Niu X, Yang X, Wang F, Hou R, Yao L, Han Q, et al. A novel and effective therapy for Zoon balanitis: Topical crisaborole 2% ointment. J Eur Acad Dermatol Venereol. 2023, 37, e212–e214. [Google Scholar]
- Lee MA, Cohen PR. Zoon Balanitis Revisited: Report of Balanitis Circumscripta Plasmacellularis Resolving With Topical Mupirocin Ointment Monotherapy. J Drugs Dermatol. 2017, 16, 285–287. [Google Scholar]
- Roé E, Dalmau J, Peramiquel L, Pérez M, López-Lozano HE, Alomar A. Plasma cell balanitis of zoon treated with topical tacrolimus 0.1%: report of three cases. J Eur Acad Dermatol Venereol. 2007, 21, 284–285. [Google Scholar] [CrossRef]
- Kumar B, Narang T, Dass Radotra B, Gupta S. Plasma cell balanitis: clinicopathologic study of 112 cases and treatment modalities. J Cutan Med Surg. 2006, 10, 11–15. [Google Scholar] [CrossRef]
- Wierzbicki PM, Czajkowski M, Kotulak-Chrząszcz A, Bukowicz J, Dzieciuch K, Sokołowska-Wojdyło M, et al. Altered mRNA Expression of NFKB1 and NFKB2 Genes in Penile Lichen Sclerosus, Penile Cancer and Zoon Balanitis. J Clin Med. 2022, 11, 7254. [Google Scholar] [CrossRef]
- Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicki PM, Klacz J, Kotulak-Chrzaszcz A, Wronska A, Stanislawowski M, Rybarczyk A, et al. Prognostic significance of VHL, HIF1A, HIF2A, VEGFA and p53 expression in patients with clear-cell renal cell carcinoma treated with sunitinib as first-line treatment. Int J Oncol. 2019, 55, 371–390. [Google Scholar]
- Schlotter YM, Veenhof EZ, Brinkhof B, Rutten VP, Spee B, Willemse T, et al. A GeNorm algorithm-based selection of reference genes for quantitative real-time PCR in skin biopsies of healthy dogs and dogs with atopic dermatitis. Vet Immunol Immunopathol. 2009, 129, 115–118. [Google Scholar] [CrossRef]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Malik A, Kanneganti TD. Function and regulation of IL-1α in inflammatory diseases and cancer. Immunol Rev. 2018, 281, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Kumari N, Dwarakanath BS, Das A, Bhatt AN. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef] [PubMed]
- Mojic M, Takeda K, Hayakawa Y. The Dark Side of IFN-γ: Its Role in Promoting Cancer Immunoevasion. Int J Mol Sci. 2017, 19, E89. [Google Scholar] [CrossRef]
- Cauci S, Buligan C, Rocchi F, Salvador I, Xodo L, Stinco G. Interleukin 1 receptor antagonist gene variable number of tandem repeats polymorphism and cutaneous melanoma. Oncol Lett. 2019, 18, 5759–5768. [Google Scholar]
- Lichtman MK, Otero-Vinas M, Falanga V. Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis. Wound Repair Regen. 2016, 24, 215–222. [Google Scholar] [CrossRef]
- Moreno-Arias GA, Camps-Fresneda A, Llaberia C, Palou-Almerich J. Plasma cell balanitis treated with tacrolimus 0.1%. Br J Dermatol. 2005, 153, 1204–1206. [Google Scholar] [CrossRef]
- Bunker, CB. Zoon balanitis-does it exist. J Eur Acad Dermatol Venereol. 2020, 34, e116–e117. [Google Scholar] [CrossRef] [PubMed]
- Khalil S, Donthi D, Gru AA. Cutaneous reactive B-cell lymphoid proliferations. J Cutan Pathol. 2022, 49, 898–916. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski M, Wierzbicki P, Kotulak-Chrząszcz A, Czajkowska K, Bolcewicz M, Kłącz J, et al. The role of occlusion and micro-incontinence in the pathogenesis of penile lichen sclerosus: an observational study of pro-inflammatory cytokines' gene expression. Int Urol Nephrol. 2022, 54, 763–772. [Google Scholar] [CrossRef]
- Pyrillou K, Burzynski LC, Clarke MCH. Alternative Pathways of IL-1 Activation, and Its Role in Health and Disease. Front Immunol. 2020, 11, 613170. [Google Scholar] [CrossRef]
- Burzynski LC, Humphry M, Pyrillou K, Wiggins KA, Chan JNE, Figg N, et al. The Coagulation and Immune Systems Are Directly Linked through the Activation of Interleukin-1α by Thrombin. Immunity 2019, 50, 1033–1042. [Google Scholar] [CrossRef]
- Cavalli G, Colafrancesco S, Emmi G, Imazio M, Lopalco G, Maggio MC, et al. Interleukin 1α: a comprehensive review on the role of IL-1α in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun Rev. 2021, 20, 102763. [Google Scholar] [CrossRef]
- Spulber S, Bartfai T, Schultzberg M. IL-1/IL-1ra balance in the brain revisited - evidence from transgenic mouse models. Brain Behav Immun. 2009, 23, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Volarevic V, Al-Qahtani A, Arsenijevic N, Pajovic S, Lukic ML. Interleukin-1 receptor antagonist (IL-1Ra) and IL-1Ra producing mesenchymal stem cells as modulators of diabetogenesis. Autoimmunity 2010, 43, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Cahill CM, Rogers JT. Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J Biol Chem. 2008, 283, 25900–25912. [Google Scholar] [CrossRef]
- Kwan Tat S, Padrines M, Théoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004, 15, 49–60. [Google Scholar] [CrossRef]
| Gene name | Primers’ sequences | qPCR reaction conditions | qPCR reaction content |
|---|---|---|---|
| IL-1α | 5’-TAGGTCAGCACCTTTTAGCTTC 5’-GTATCTCAGGCATCTCCTTCAG |
95°C, 3 min; 45x (95°C, 5 sec; 59°C, 10 sec; 72°C, 10 sec; 75 °C, 10 sec – sample reading) Melting curve: 95°C, 15 sec; 60°C, 1 min; 60°C → 95°C reading every 0.3°C |
5 µl AmplifyMe NoRox SybrGreen (with SybrGreen fluorophore) ( Blirt, Poland), 200 nM each primer, Σ 10 µl |
| IL-1β | 5’- CCTTAGGGTAGTGCTAAGAGGA 5’- TACAGACACTGCTACTTCTTGC |
||
| IL-1RN | 5’- GGCACTTGGAGACTTGTATGAA 5’- GAGCTGAAGTCACAGGAAGTAG |
||
| IL-6 | 5’- CACTCACCTCTTCAGAACGAAT 5’- AGGCAAGTCTCCTCATTGAATC |
||
| INF- γ | 5’- TGGAAAGAGGAGAGTGACAGAA 5’- TATTGCTTTGCGTTGGACATTC |
||
| TNF-β | 5’- GAGCTGTACCAGAAATACAGCA 5’- AACTCCGGTGACATCAAAAGAT |
||
| GUSB | 5’ – ATGCAGGTGATGGAAGAAGTGGTG 5’ - AGAGTTGCTCACAAAGGTCACAGG |
| Group | N | Age [y]: mean ± SD; median (range) | P (all groups) | BMI: mean ± SD | P* (all groups) | CRP [mg/dL]: mean ± SD | P *(all groups) |
|---|---|---|---|---|---|---|---|
| Zoon Balanitis | 4 | 59.25 ± 25.00; 65.0 (24-83) | 25.75 ± 3.13 | 1.83 ± 0.79 | |||
| Control | 13 | 31.38 ± 14.80; 24.0 (21-65) | 0.0011 | 28.21 ± 6.96 | Ns (0.86) | 1.76 ± 2.85 | Ns (0.11) |
| *Student t test used since Shapiro-Wilk normality test was passed, otherwise Mann-Whitney U test. | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





