Submitted:
18 September 2023
Posted:
20 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
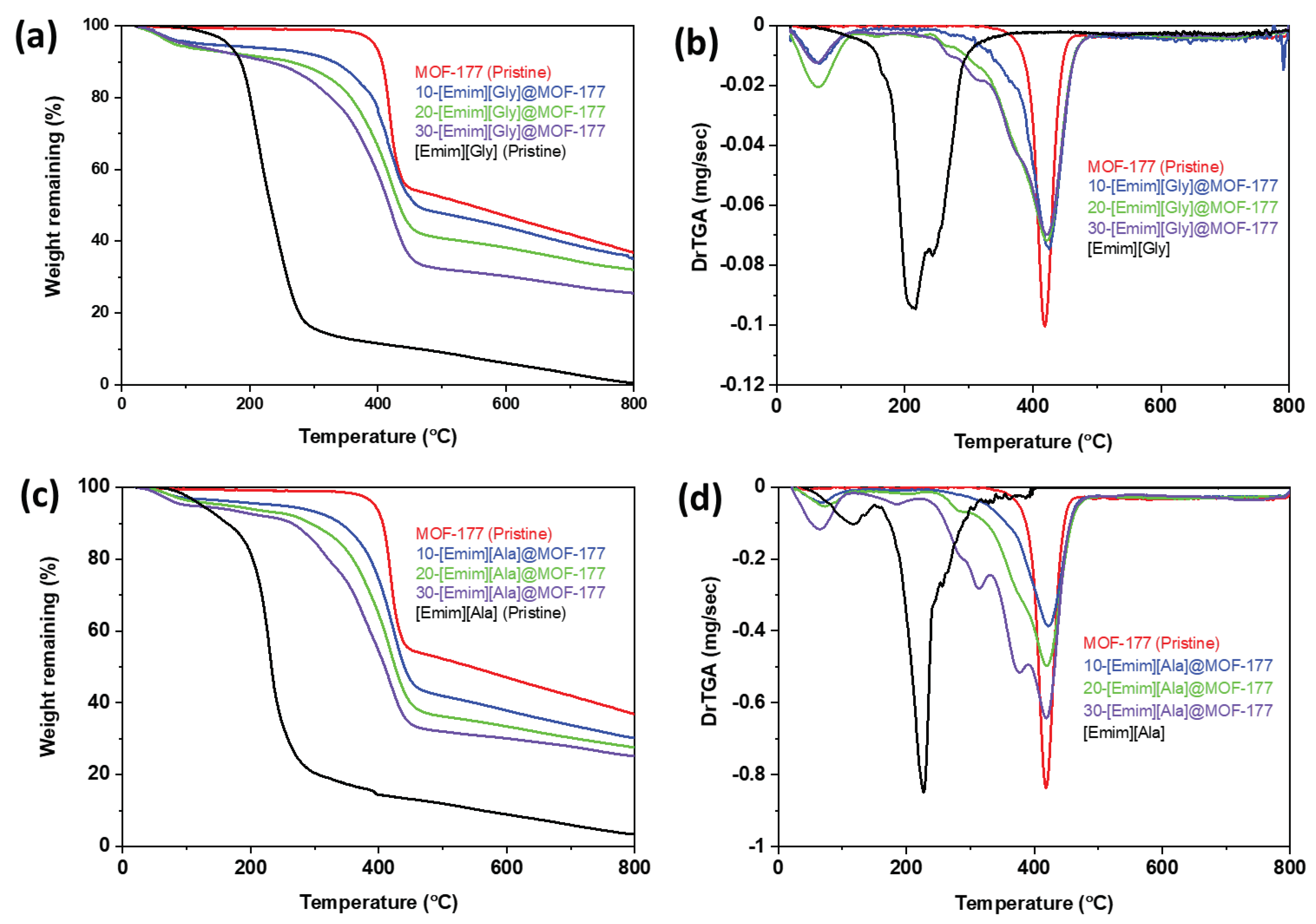
2.1. Characterizations of the AAILs-impregnated Sorbents
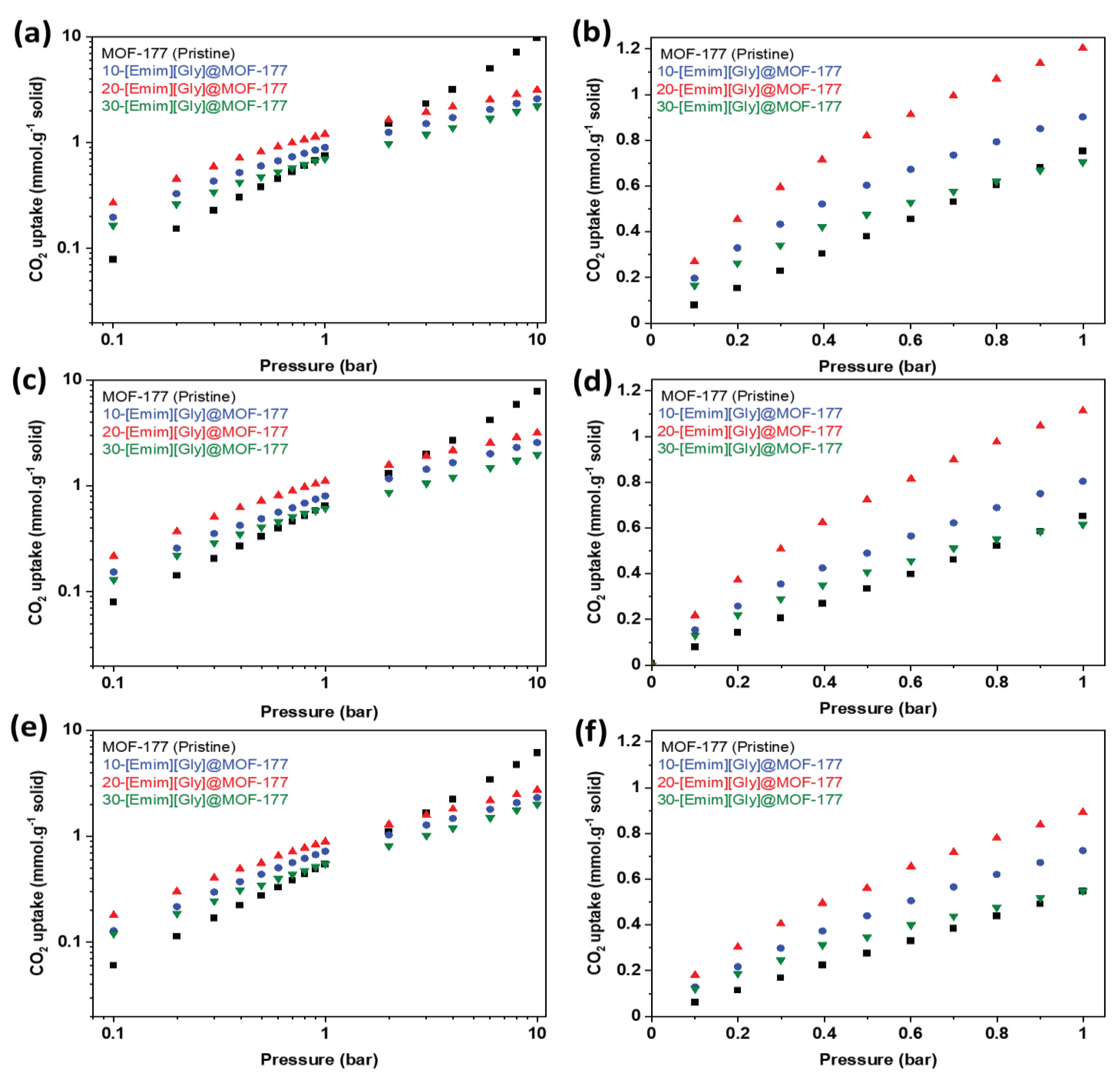
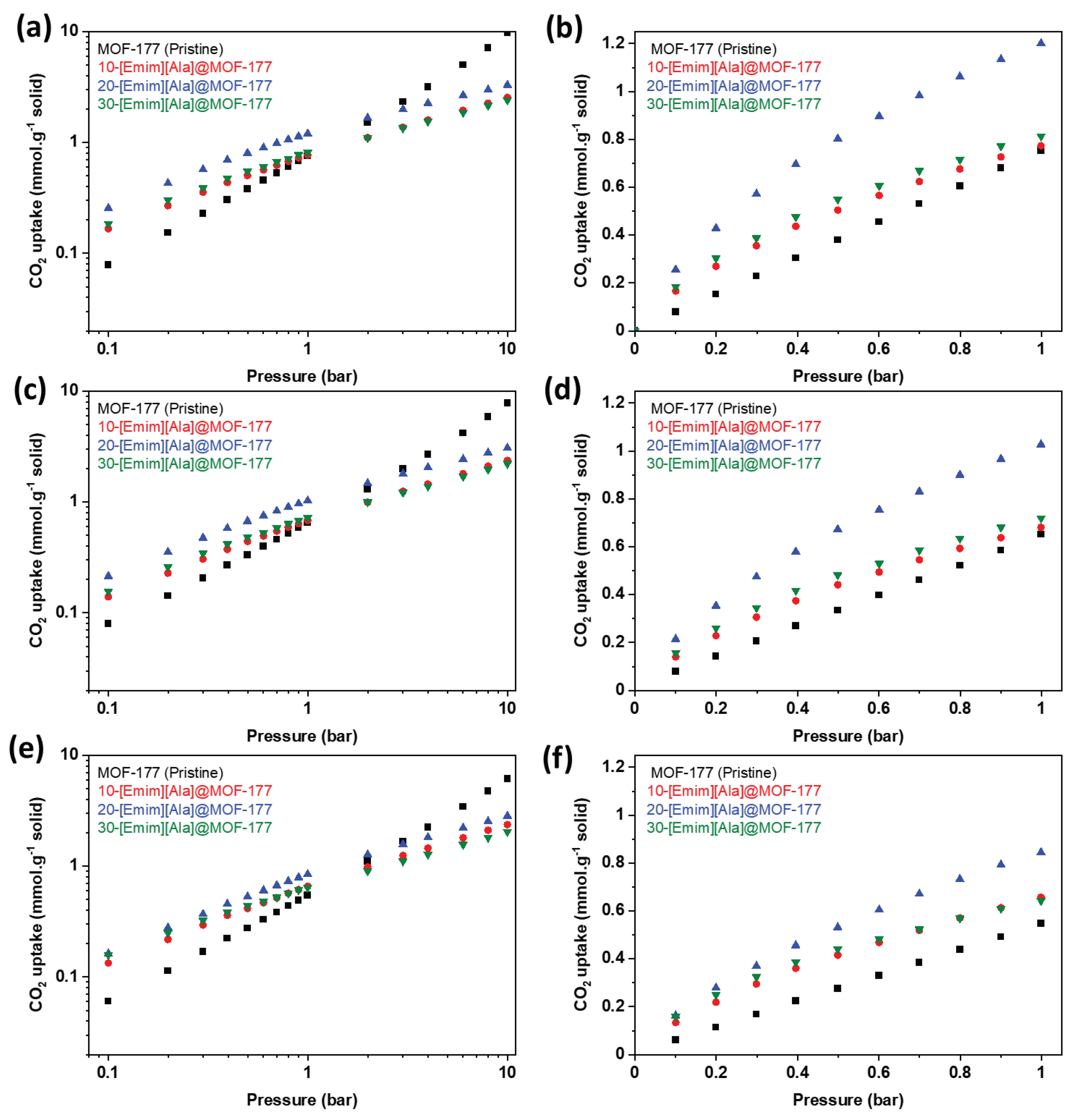
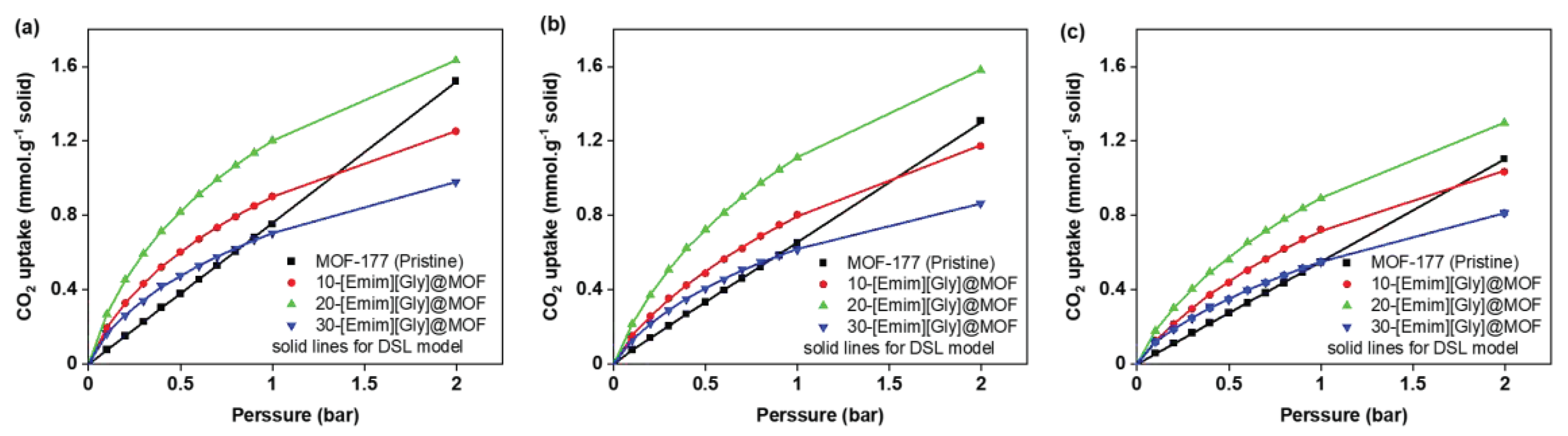
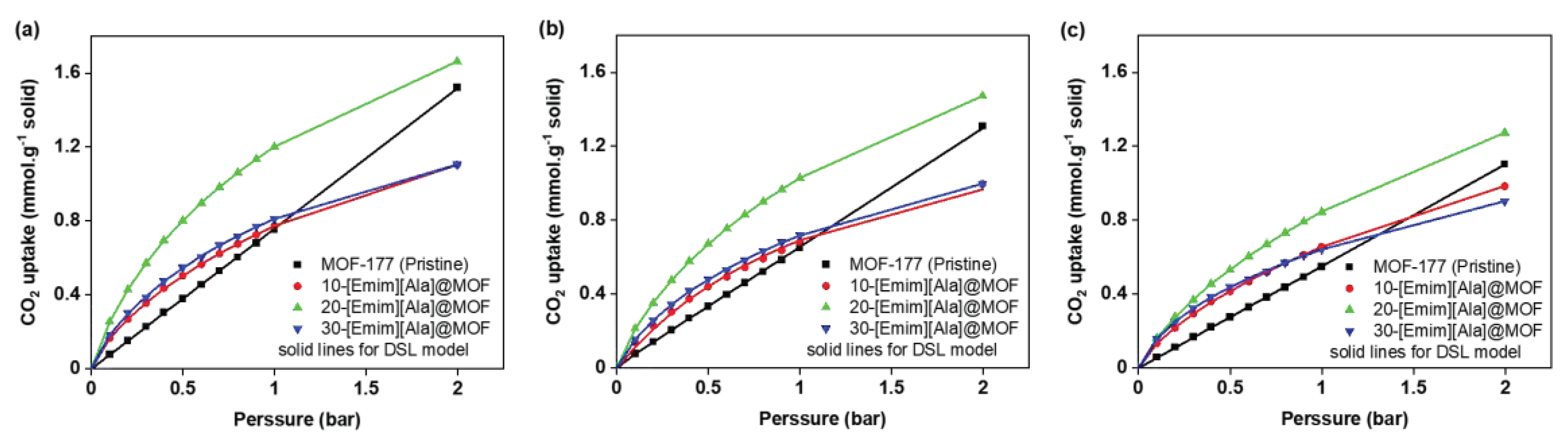
2.2. CO2 Adsorption Isotherms
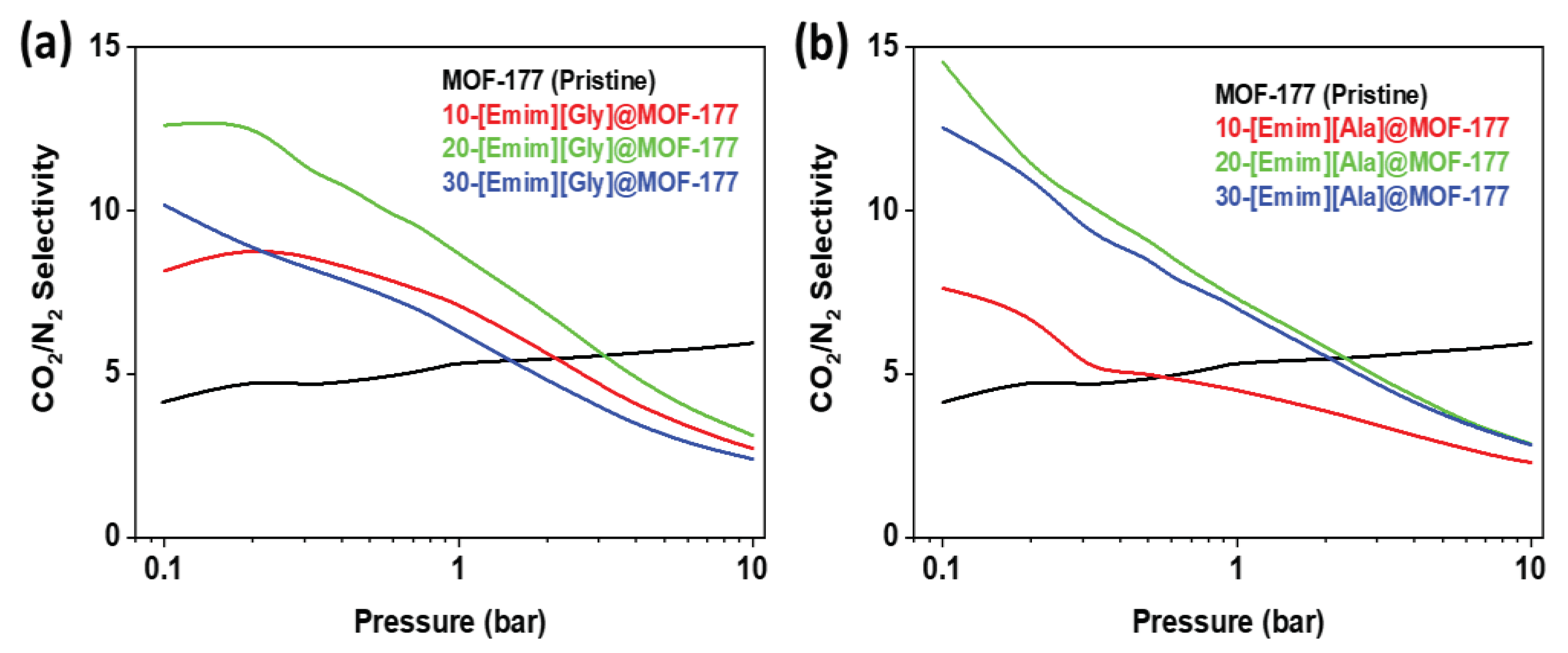
2.3. Selectivity for CO2/N2
2.4. Equilibrium Isotherm Modeling
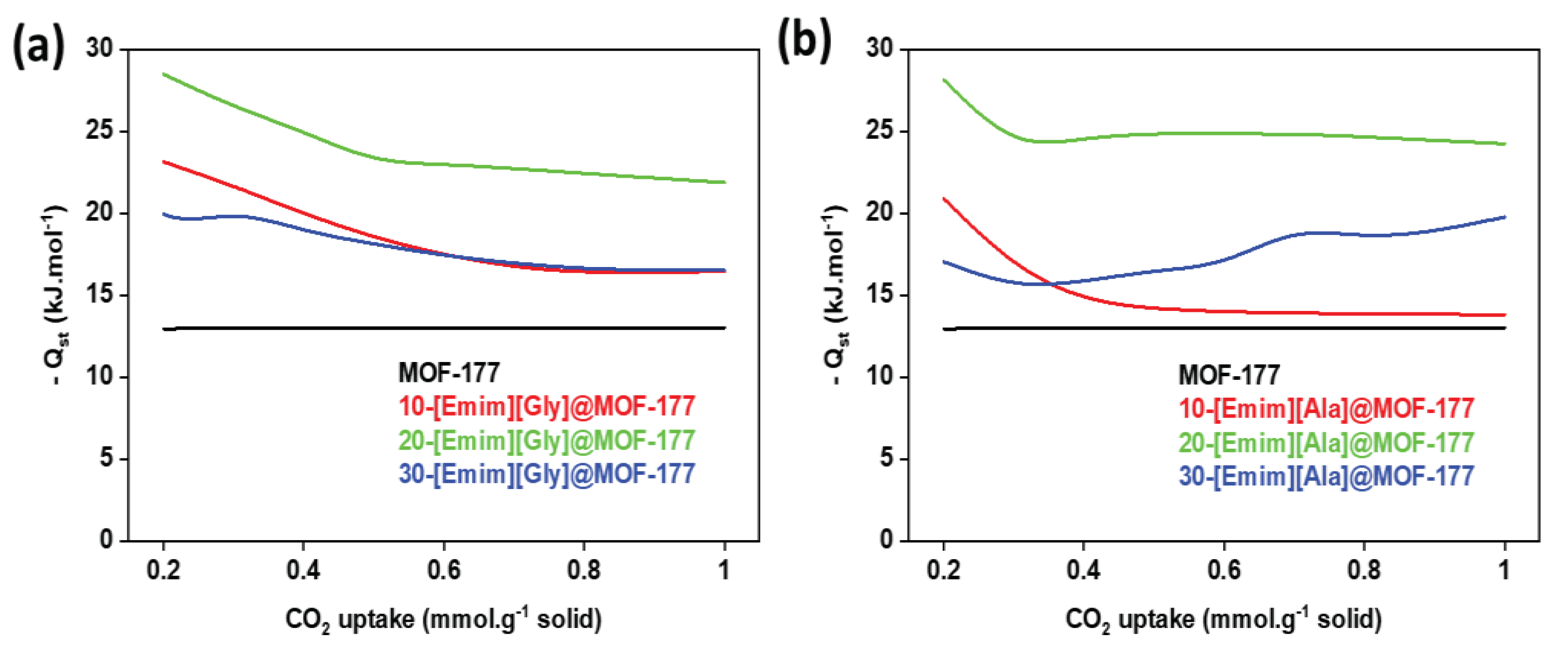
2.5. Isosteric heat of adsorption (Qst)
3. Materials and Methods
3.1. Materials
3.2. Preparation of AAIL@MOF-177 composite
3.3. Characterization
3.4. Adsorption Isotherms
3.5. Cyclic adsorption-desorption test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.H.; Long, J.R. Carbon Dioxide Capture in Metal-Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Kong, C.; Zhang, Q.; Chen, L. Metal-Organic Frameworks for Carbon Dioxide Capture and Methane Storage. Adv. Energy Mater. 2017, 7, 1601296. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Shah, B.B.; Zhao, D. CO2 Capture in Metal-Organic Framework Adsorbents: An Engineering Perspective. Adv. Sustain. Syst. 2018, 3, 1800080. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A Systematic Review on CO2 Capture with Ionic Liquids: Current Status and Future Prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Yu, J.; Xie, L.H.; Li, J.R.; Ma, Y.; Seminario, J.M.; Balbuena, P.B. CO2 Capture and Separations Using MOFs: Computational and Experimental Studies. Chem. Rev. 2017, 117, 9674–9754. [Google Scholar] [CrossRef]
- Belmabkhout, Y.; Guillerm, V.; Eddaoudi, M. Low Concentration CO2 Capture Using Physical Adsorbents: Are Metal-Organic Frameworks Becoming the New Benchmark Materials? Chem. Eng. J. 2016, 296, 386–397. [Google Scholar] [CrossRef]
- Yin, Z.; Wan, S.; Yang, J.; Kurmoo, M.; Zeng, M.H. Recent Advances in Post-Synthetic Modification of Metal–Organic Frameworks: New Types and Tandem Reactions. Coord. Chem. Rev. 2019, 378, 500–512. [Google Scholar] [CrossRef]
- Jasuja, H.; Walton, K.S. Experimental Study of CO2, CH4, and Water Vapor Adsorption on a Dimethyl-Functionalized UiO-66 Framework. J. Phys. Chem. C 2013, 117, 7062–7068. [Google Scholar] [CrossRef]
- Cmarik, G.E.; Kim, M.; Cohen, S.M.; Walton, K.S. Tuning the Adsorption Properties of Uio-66 via Ligand Functionalization. Langmuir 2012, 28, 15606–15613. [Google Scholar] [CrossRef]
- Shearer, G.C.; Vitillo, J.G.; Bordiga, S.; Svelle, S.; Olsbye, U.; Lillerud, K.P. Functionalizing the Defects: Postsynthetic Ligand Exchange in the Metal Organic Framework UiO-66. Chem. Mater. 2016, 28, 7190–7193. [Google Scholar] [CrossRef]
- Mutyala, S.; Yakout, S.M.; Ibrahim, S.S.; Jonnalagadda, M.; Mitta, H. Enhancement of CO2 Capture and Separation of CO2/N2 Using Post-Synthetic Modified MIL-100(Fe). New J. Chem. 2019, 43, 9725–9731. [Google Scholar] [CrossRef]
- Su, X.; Bromberg, L.; Martis, V.; Simeon, F.; Huq, A.; Hatton, T.A. Postsynthetic Functionalization of Mg-MOF-74 with Tetraethylenepentamine: Structural Characterization and Enhanced CO2 Adsorption. ACS Appl. Mater. Interfaces 2017, 9, 11299–11306. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yan, Q.; Kong, C.; Chen, L. Polyethyleneimine Incorporated Metal-Organic Frameworks Adsorbent for Highly Selective CO2 Capture. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Watanabe, T.; Bae, T.H.; Sholl, D.S.; Jones, C.W. Modification of the Mg/DOBDC MOF with Amines to Enhance CO 2 Adsorption from Ultradilute Gases. J. Phys. Chem. Lett. 2012, 3, 1136–1141. [Google Scholar] [CrossRef]
- Fujie, K.; Kitagawa, H. Ionic Liquid Transported into Metal-Organic Frameworks. Coord. Chem. Rev. 2016, 307, 382–390. [Google Scholar] [CrossRef]
- Adil, K.; Bhatt, P.M.; Belmabkhout, Y.; Abtab, S.M.T.; Jiang, H.; Assen, A.H.; Mallick, A.; Cadiau, A.; Aqil, J.; Eddaoudi, M. Valuing Metal–Organic Frameworks for Postcombustion Carbon Capture: A Benchmark Study for Evaluating Physical Adsorbents. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Kulak, H.; Polat, H.M.; Kavak, S.; Keskin, S.; Uzun, A. Improving CO2 Separation Performance of MIL-53(Al) by Incorporating 1-n-Butyl-3-Methylimidazolium Methyl Sulfate. Energy Technol. 2019. [Google Scholar] [CrossRef]
- Kinik, F.P.; Altintas, C.; Balci, V.; Koyuturk, B.; Uzun, A.; Keskin, S. [BMIM][PF6] Incorporation Doubles CO2 Selectivity of ZIF-8: Elucidation of Interactions and Their Consequences on Performance. ACS Appl. Mater. Interfaces 2016, 8, 30992–31005. [Google Scholar] [CrossRef]
- Koyuturk, B.; Altintas, C.; Kinik, F.P.; Keskin, S.; Uzun, A. Improving Gas Separation Performance of ZIF-8 by [BMIM][BF4] Incorporation: Interactions and Their Consequences on Performance. J. Phys. Chem. C 2017, 121, 10370–10381. [Google Scholar] [CrossRef]
- Bates, E.D.; Mayton, R.D.; Ntai, I.; Davis, J.H. CO2 Capture by a Task-Specific Ionic Liquid. J. Am. Chem. Soc. 2002, 124, 926–927. [Google Scholar] [CrossRef]
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room Temperature Ionic Liquids from 20 Natural Amino Acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef] [PubMed]
- Sistla, Y.S.; Khanna, A. CO2 Absorption Studies in Amino Acid-Anion Based Ionic Liquids. Chem. Eng. J. 2015, 273, 268–276. [Google Scholar] [CrossRef]
- Muhammad, N.; Man, Z.B.; Bustam, M.A.; Mutalib, M.I.A.; Wilfred, C.D.; Rafiq, S. Synthesis and Thermophysical Properties of Low Viscosity Amino Acid-Based Ionic Liquids. J. Chem. Eng. Data 2011, 56, 3157–3162. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Dong, K.; Zhang, Y.; Shen, Y.; Lv, X. Supported Absorption of CO2 by Tetrabutylphosphonium Amino Acid Ionic Liquids. Chem. - A Eur. J. 2006, 12, 4021–4026. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Akhmedov, N.G.; Duan, Y.; Luebke, D.; Hopkinson, D.; Li, B. Amino Acid-Functionalized Ionic Liquid Solid Sorbents for Post-Combustion Carbon Capture. ACS Appl. Mater. Interfaces 2013, 5, 8670–8677. [Google Scholar] [CrossRef] [PubMed]
- Uehara, Y.; Karami, D.; Mahinpey, N. CO2 Adsorption Using Amino Acid Ionic Liquid-Impregnated Mesoporous Silica Sorbents with Different Textural Properties. Microporous Mesoporous Mater. 2019, 278, 378–386. [Google Scholar] [CrossRef]
- Uehara, Y.; Karami, D.; Mahinpey, N. Amino Acid Ionic Liquid-Modified Mesoporous Silica Sorbents with Remaining Surfactant for CO 2 Capture. Adsorption 2019, 1, 703–716. [Google Scholar] [CrossRef]
- Philip, F.A.; Henni, A. Enhancement of Post-Combustion CO2 Capture Capacity by Incorporation of Task-Specific Ionic Liquid into ZIF-8. Microporous Mesoporous Mater. 2021, 111580. [Google Scholar] [CrossRef]
- Furukawa, H.; Miller, M.A.; Yaghi, O.M. Independent Verification of the Saturation Hydrogen Uptake in MOF-177 and Establishment of a Benchmark for Hydrogen Adsorption in Metal-Organic Frameworks. J. Mater. Chem. 2007, 17, 3197–3204. [Google Scholar] [CrossRef]
- Mohamedali, M.; Henni, A.; Ibrahim, H. Investigation of CO2 Capture Using Acetate-Based Ionic Liquids Incorporated into Exceptionally Porous Metal–Organic Frameworks. Adsorption 2019, 25, 675–692. [Google Scholar] [CrossRef]
- Saha, D.; Deng, S. Structural Stability of Metal Organic Framework MOF-177. J. Phys. Chem. Lett. 2010, 1, 73–78. [Google Scholar] [CrossRef]
- Li, Y.; Yang, R.T. Gas Adsorption and Storage in Metal-Organic Framework MOF-177. Langmuir 2007, 23, 12937–12944. [Google Scholar] [CrossRef]
- Santos, K.M.C.; Santos, R.J.O.; De Araújo Alves, M.M.; De Conto, J.F.; Borges, G.R.; Dariva, C.; Egues, S.M.; Santana, C.C.; Franceschi, E. Effect of High Pressure CO2 Sorption on the Stability of Metalorganic Framework MOF-177 at Different Temperatures. J. Solid State Chem. 2019, 269, 320–327. [Google Scholar] [CrossRef]
- Saha, D.; Wei, Z.; Deng, S. Equilibrium, Kinetics and Enthalpy of Hydrogen Adsorption in MOF-177. Int. J. Hydrogen Energy 2008, 33, 7479–7488. [Google Scholar] [CrossRef]
- Ren, J.; Wu, L.; Li, B.G. Preparation and CO2 Sorption/Desorption of N -(3-Aminopropyl)Aminoethyl Tributylphosphonium Amino Acid Salt Ionic Liquids Supported into Porous Silica Particles. Ind. Eng. Chem. Res. 2012, 51, 7901–7909. [Google Scholar] [CrossRef]
- Gurkan, B.E.; De La Fuente, J.C.; Mindrup, E.M.; Ficke, L.E.; Goodrich, B.F.; Price, E.A.; Schneider, W.F.; Brennecke, J.F. Equimolar CO2 Absorption by Anion-Functionalized Ionic Liquids. J. Am. Chem. Soc. 2010, 132, 2116–2117. [Google Scholar] [CrossRef]
- Wang, X.; Akhmedov, N.G.; Duan, Y.; Luebke, D.; Li, B. Immobilization of Amino Acid Ionic Liquids into Nanoporous Microspheres as Robust Sorbents for CO2 Capture. J. Mater. Chem. A 2013, 1, 2978–2982. [Google Scholar] [CrossRef]
- Ferreira, T.J.; Ribeiro, R.P.P.L.; Mota, J.P.B.; Rebelo, L.P.N.; Esperança, J.M.S.S.; Esteves, I.A.A.C. Ionic Liquid-Impregnated Metal-Organic Frameworks for CO2/CH4 Separation. ACS Appl. Nano Mater. 2019, 2, 7933–7950. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Mohamedali, M.; Ibrahim, H.; Henni, A. Incorporation of Acetate-Based Ionic Liquids into a Zeolitic Imidazolate Framework (ZIF-8) as Efficient Sorbents for Carbon Dioxide Capture. Chem. Eng. J. 2018, 334, 817–828. [Google Scholar] [CrossRef]

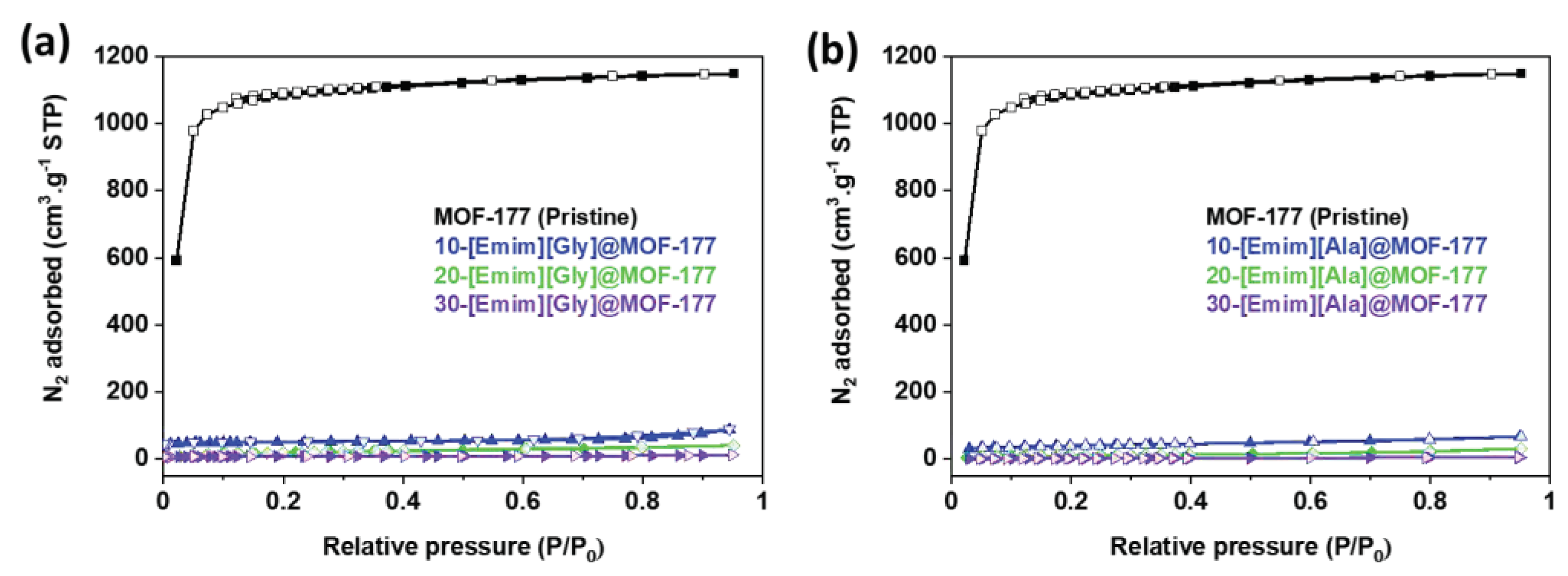

| Samples | SBET (m2·g-1) |
SLangmuir (m2·g-1) |
Pore Volume (cm3·g-1) |
|---|---|---|---|
| MOF-177 | 4172 | 4962 | 1.78 |
| 10-[Emim][Gly]@MOF-177 | 187 | 250 | 0.13 |
| 20-[Emim][Gly]@MOF-177 | 74 | 147 | 0.06 |
| 30-[Emim][Gly]@MOF-177 | 27 | 41 | 0.02 |
| 10-[Emim][Ala]@MOF-177 | 152 | 226 | 0.10 |
| 20-[Emim][Ala]@MOF-177 | 39 | 89 | 0.05 |
| 30-[Emim][Ala]@MOF-177 | 9.3 | 29 | 0.01 |
| Model Parameters | 10-[Emim][Gly]@ MOF-177 | 20-[Emim][Gly]@MOF-177 | 30-[Emim][Gly]@MOF-177 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 °C | 40 °C | 50 °C | 30 °C | 40 °C | 50 °C | 30 °C | 40 °C | 50 °C | |
| NA | 2.019 | 2.350 | 1.921 | 0.436 | 0.292 | 0.165 | 1.590 | 0.097 | 0.087 |
| bA | 0.514 | 0.426 | 0.562 | 5.117 | 4.698 | 9.030 | 0.508 | 10.678 | 18.396 |
| NB | 0.247 | 0.098 | 0.024 | 2.371 | 2.694 | 2.444 | 0.190 | 1.405 | 1.628 |
| bB | 6.692 | 15.04 | 10000 | 0.545 | 0.479 | 0.439 | 8.468 | 0.609 | 0.404 |
| R2 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| Model Parameters | 10-[Emim][Ala]@ MOF-177 | 20-[Emim][Ala]@MOF-177 | 30-[Emim][Ala]@MOF-177 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 °C | 40 °C | 50 °C | 30 °C | 40 °C | 50 °C | 30 °C | 40 °C | 50 °C | |
| NA | 1.960 | 0.000 | 0.151 | 0.306 | 0.230 | 2.791 | 1.698 | 1.602 | 0.246 |
| bA | 0.501 | 0.000 | 7.765 | 6.003 | 7.147 | 0.326 | 0.697 | 0.539 | 7.046 |
| NB | 0.129 | 1.600 | 2.182 | 2.607 | 2.618 | 0.185 | 0.122 | 0.180 | 1.599 |
| bB | 12.735 | 0.763 | 0.315 | 0.564 | 0.462 | 6.700 | 15.549 | 6.987 | 0.365 |
| R2 | 1.000 | 0.999 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





