Submitted:
18 September 2023
Posted:
20 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
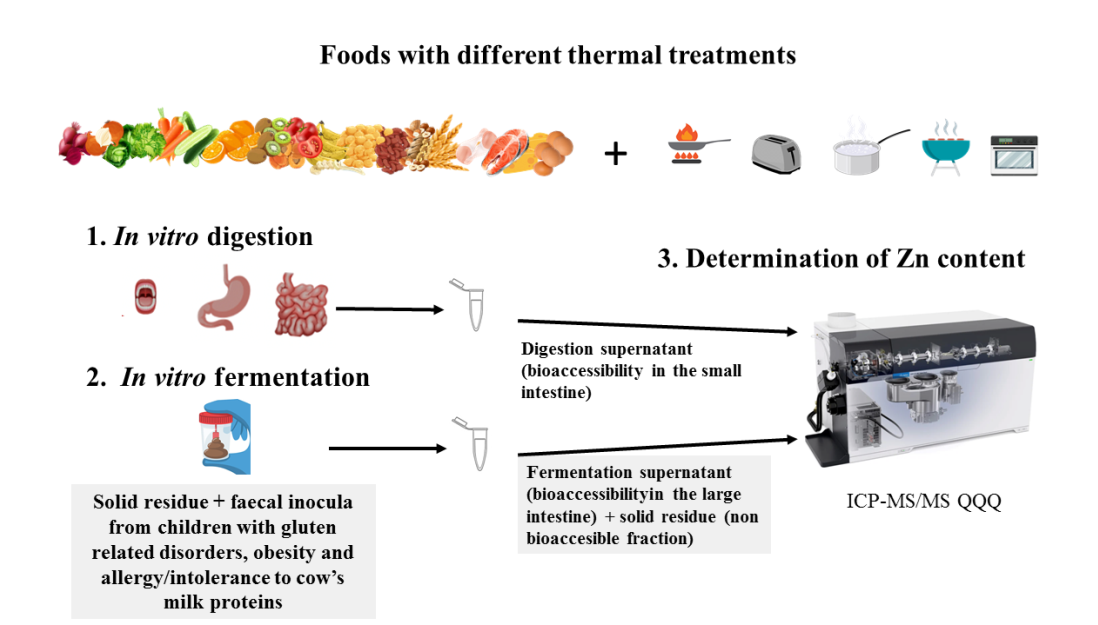
2. Materials and Methods
2.1. General reagents and equipment
2.2. Food samples and culinary techniques
2.3. Stool samples from GRD-CH, OB-CH and AICM-CH
2.4. In vitro digestion and fermentation method
2.5. Mineralisation procedure and analysis of Zn in fermentation liquids and solids
2.6. Statistical analysis
3. Results and discussion
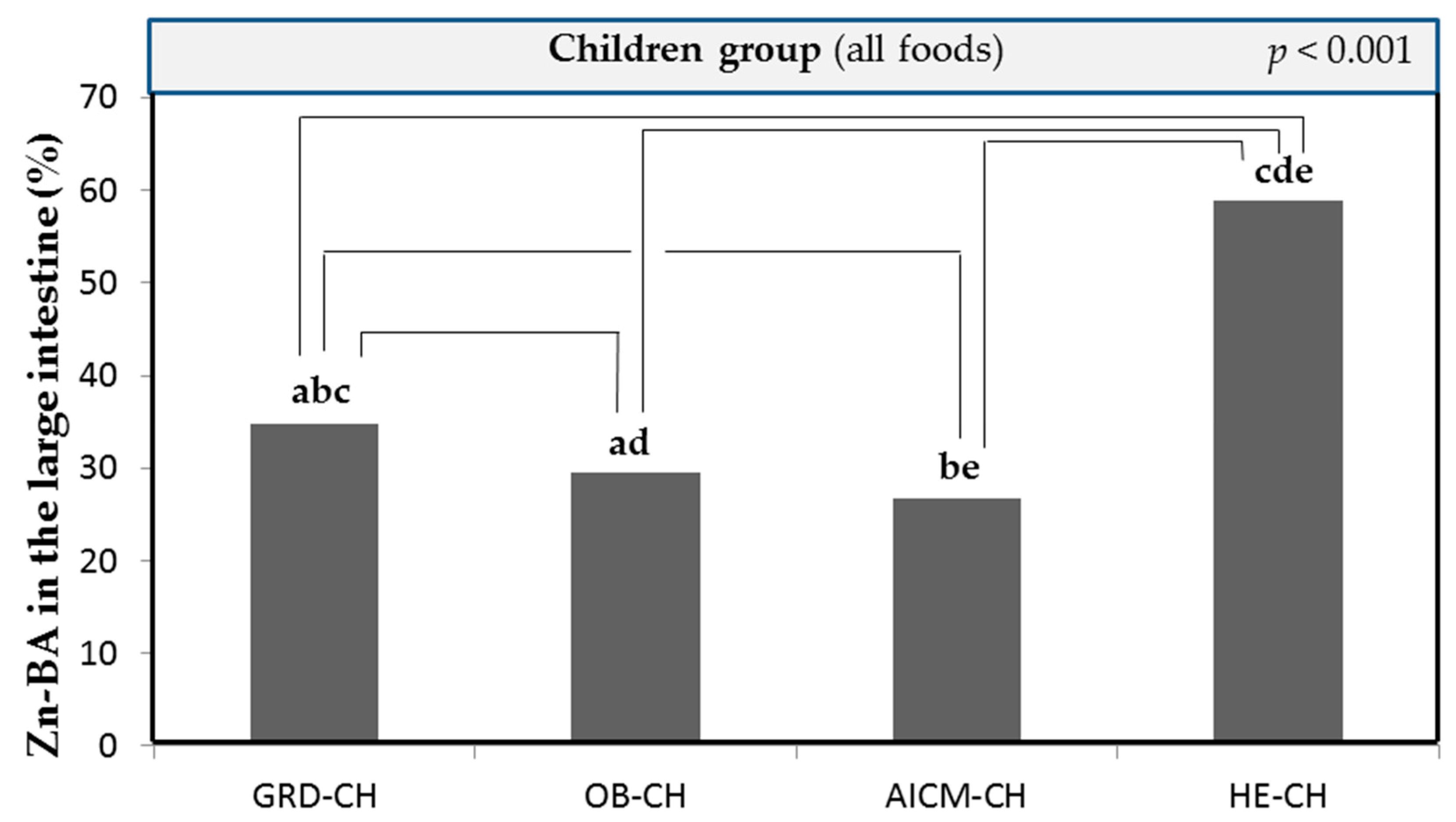
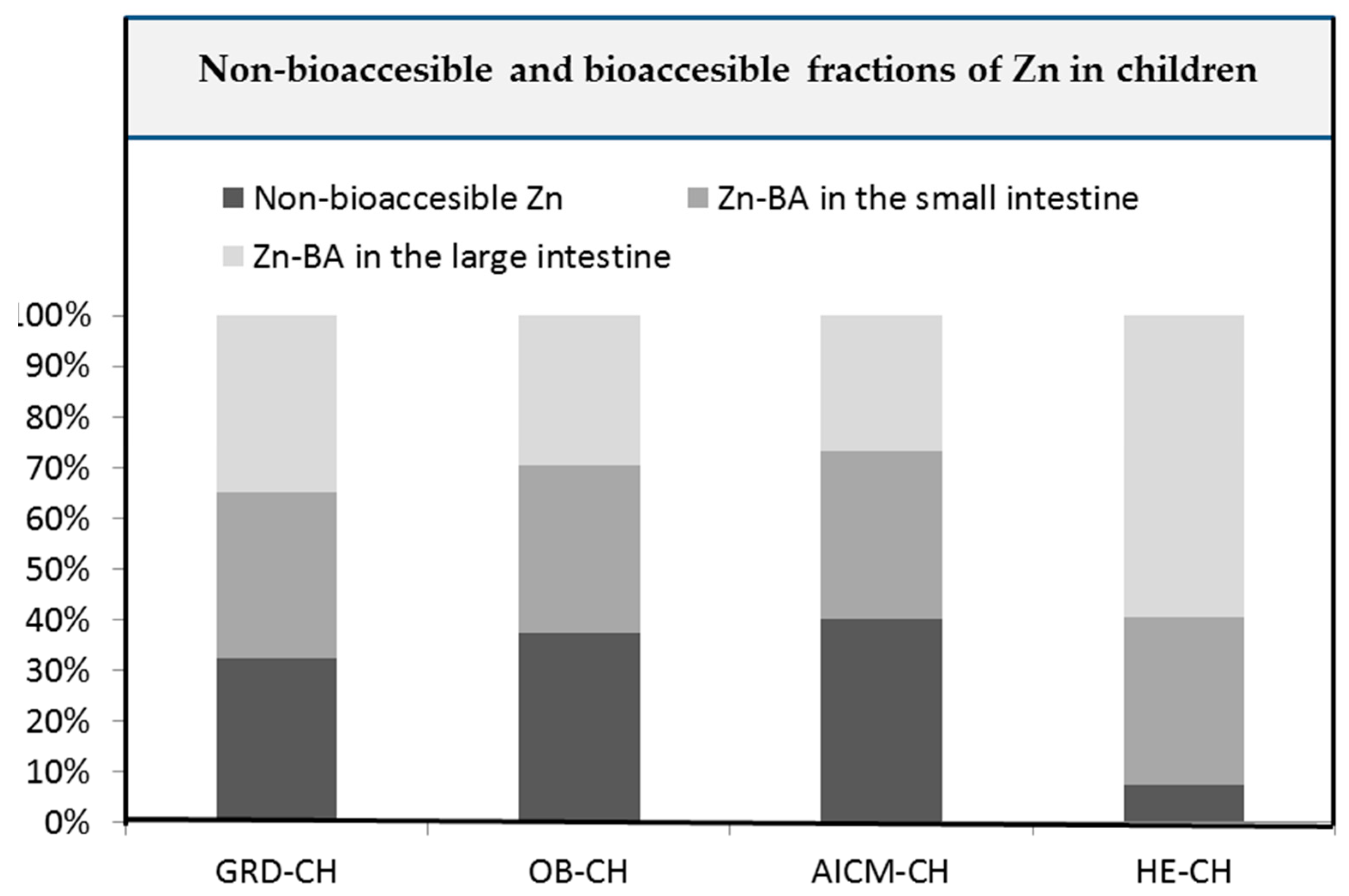
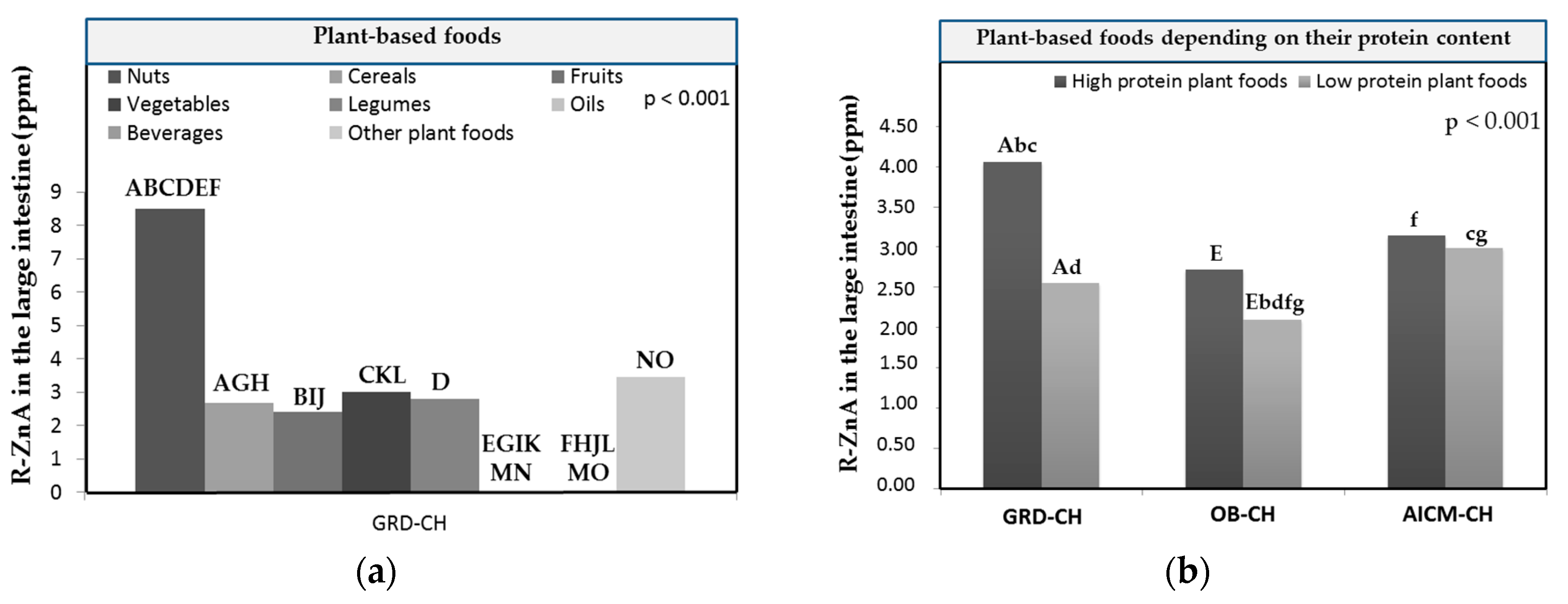
3.1. Zn-BA of foods in in children with gluten related disorders
3.2. Zn-BA of foods in children with obesity
3.3. Zn-BA of foods in in children with allergy/intolerance to cow`s milk proteins
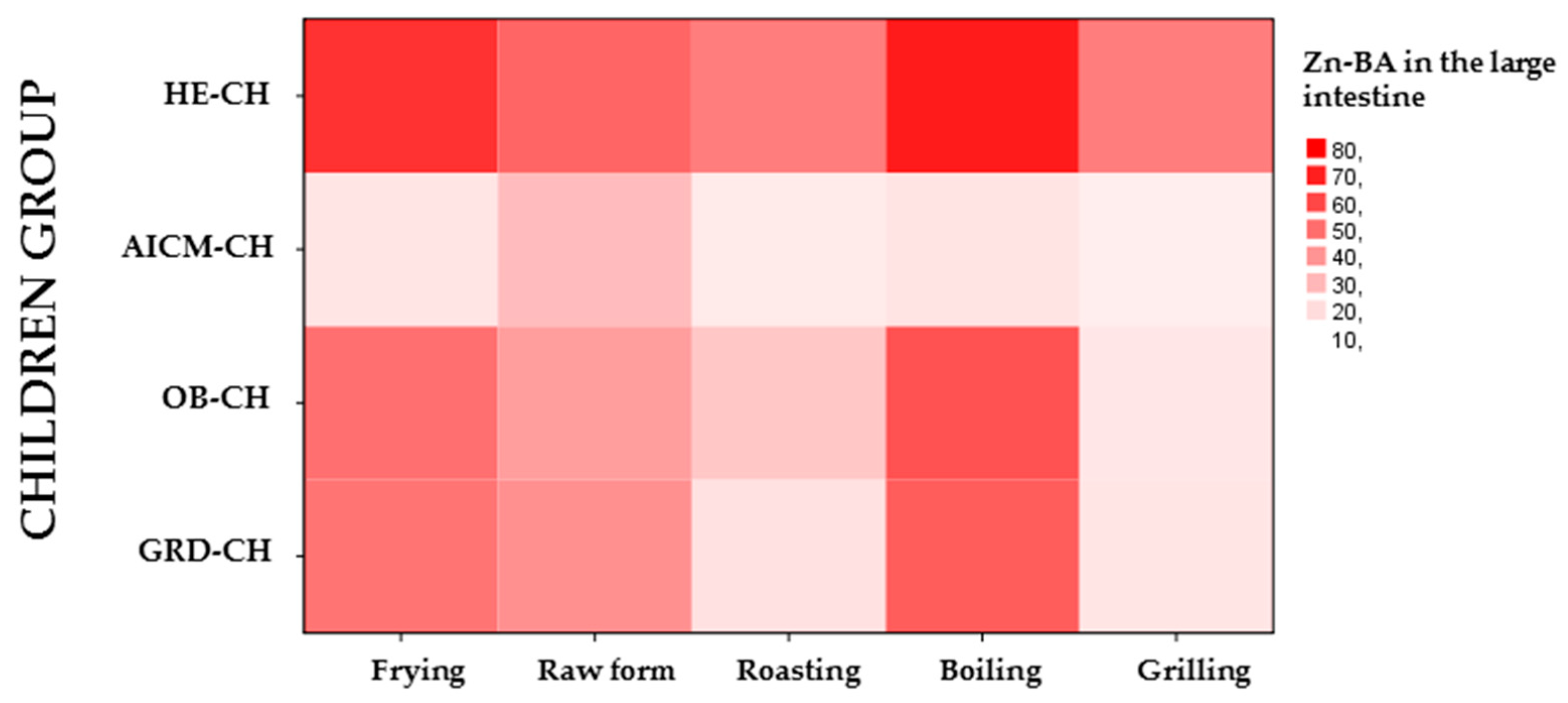
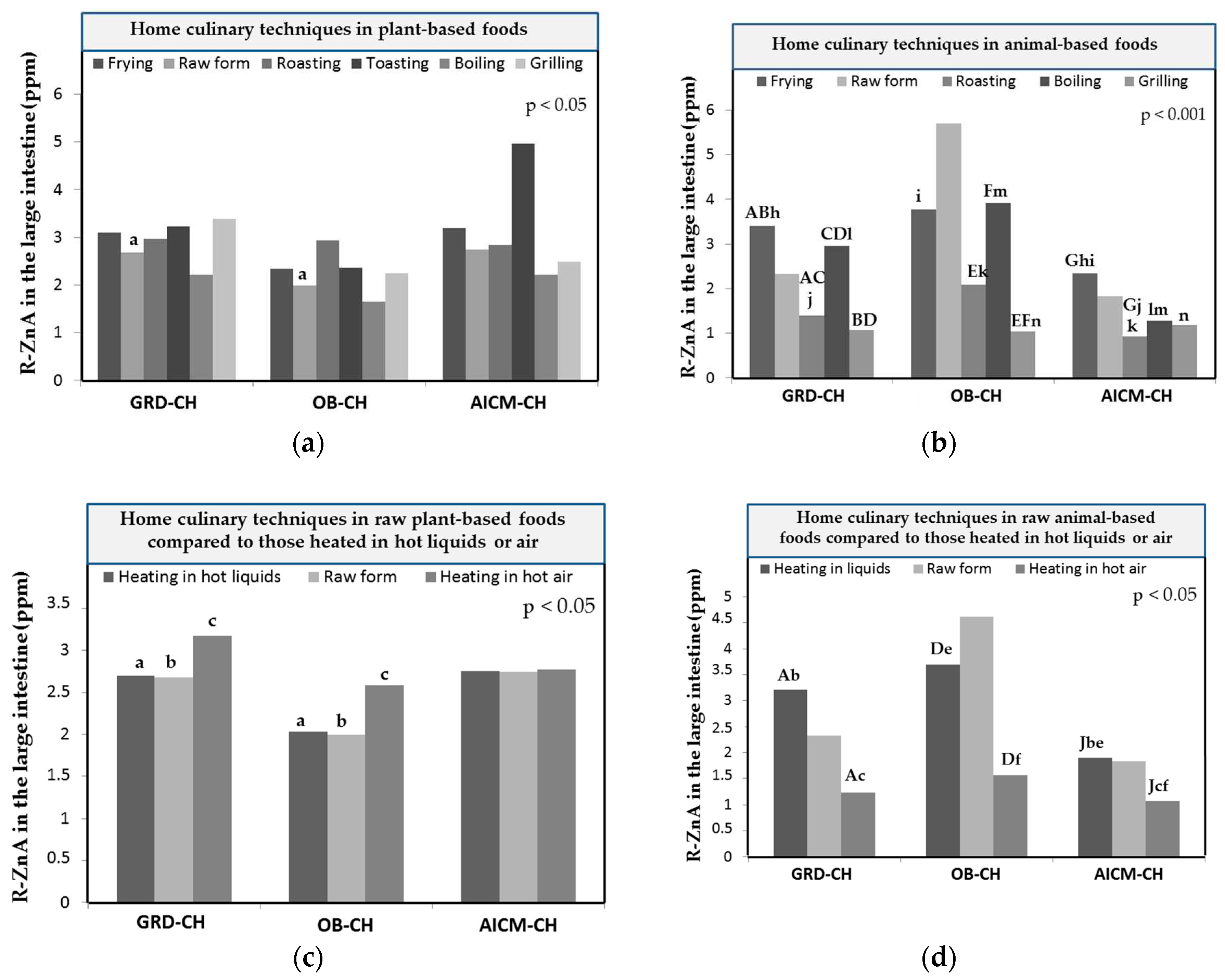
3.4. Zn-BA of foods depending on the home culinary techniques
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banaszak, M.; Górna, I.; Przysławski, J. Zinc and the Innovative Zinc-α2-Glycoprotein Adipokine Play an Important Role in Lipid Metabolism: A Critical Review. Nutrients 2021, 13, 2023. [Google Scholar] [CrossRef] [PubMed]
- Binaghia, M. J.; Dynera, L. M.; Beatriz Lopez, L. Bioaccesibilidad de minerales en alimentos elaborados con premezclas comerciales libres de gluten. Rev. Esp. Nutr. Hum. Diet. 2019, 23, 65–75. [Google Scholar] [CrossRef]
- Størsrud, S.; Hulthén, L.R.; Lenner, R.A. Beneficial effects of oats in the gluten-free diet of adults with special reference to nutrient status, symptoms and subjective experiences. Br. J. Nutr. 2003, 90, 101–107. [Google Scholar] [CrossRef]
- Ren, Z.; Pan, L.; Huang, Y.; Chen, H.; Liu, Y.; Liu, H.; Tu, X.; Liu, Y.; Li, B.; Dong, X.; et al. Gut microbiota-CRAMP axis shapes intestinal barrier function and immune responses in dietary gluten-induced enteropathy. EMBO Mol. Med. 2021, 13, e14059. [Google Scholar] [CrossRef]
- Rogaska, A.; Reguła, J.; Suliburska, J.; Krejpcio, Z. A Comparative Study of the Bioavailability of Fe, Cu and Zn from Gluten-Free Breads Enriched with Natural and Synthetic Additives. Foods 2020, 9, 1853. [Google Scholar] [CrossRef]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef]
- Soheilian-Khorzoghi, M.; Rezasoltani, S.; Moheb-Alian, A.; Yadegar, A.; Rostami-Nejad, M.; Azizmohammad-Looha, M.; Verma, A.K.; Haddadi, A.; Dabiri, H. Impact of Nutritional Profile on Gut Microbiota Diversity in Patients with Celiac Disease. Curr. Microbiol. 2022, 79, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Constante, M.; Libertucci, J.; Galipeau, H.J.; Szamosi, J.C.; Rueda, G.; Miranda, P.M.; Pinto-Sanchez, M.I.; Southward, C.M.; Rossi, L.; Fontes, M.E.; et al. Biogeographic Variation and Functional Pathways of the Gut Microbiota in Celiac Disease. Gastroenterology 2022, 163, 1351–1363. [Google Scholar] [CrossRef]
- Navarro-Alarcón, M.; Gil-Hernández, F.; Sánchez-González, C.; Llopis, J.; Villalón-Mir, M.; Olmedo, P.; Alarcón-Guijo, P.; Salagre, D.; Gaona, L.; Paredes, M.; et al. Melatonin Improves Levels of Zn and Cu in the Muscle of Diabetic Obese Rats. Pharmaceutics 2021, 13, 1535. [Google Scholar] [CrossRef] [PubMed]
- Noronha, N. Y.; Barato, M.; Sae-Lee, C.; de Souza Pinhel, M. A.; Watanabe, L. M.; Batista Pereira, V. A.; da Silva Rodrigues, G.; Araújo Morais, D.; Tavares de Sousa, W.; de Oliveira Souza, V. C.; Rodrigues Plaça, J.; Salgado, W.; Barbosa, F.; Plösch, T.; Barbosa Nonino, C. Novel zinc-related differentially methylated regions in leukocytes of women with and without obesity. Front. Nutr. 2022, 9, 785281. [CrossRef] [PubMed]
- Squizani, S.; Jantsch, J.; Rodrigues, F.d.S.; Braga, M.F.; Eller, S.; de Oliveira, T.F.; Silveira, A.K.; Moreira, J.C.F.; Giovenardi, M.; Porawski, M.; et al. Zinc Supplementation Partially Decreases the Harmful Effects of a Cafeteria Diet in Rats but Does Not Prevent Intestinal Dysbiosis. Nutrients 2022, 14, 3921. [Google Scholar] [CrossRef]
- Rios-Lugo, M.J.; Madrigal-Arellano, C.; Gaytán-Hernández, D.; Hernández-Mendoza, H.; Romero-Guzmán, E.T. Association of Serum Zinc Levels in Overweight and Obesity. Biol. Trace Element Res. 2020, 198, 51–57. [Google Scholar] [CrossRef]
- Costarelli, L.; Muti, E.; Malavolta, M.; Cipriano, C.; Giacconi, R.; Tesei, S.; Piacenza, F.; Pierpaoli, S.; Gasparini, N.; Faloia, E.; et al. Distinctive modulation of inflammatory and metabolic parameters in relation to zinc nutritional status in adult overweight/obese subjects. J. Nutr. Biochem. 2010, 21, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Aschner, M.; Lei, X.G.; Gritsenko, V.A.; Santamaria, A.; Alekseenko, S.I.; Prakash, N.T.; Chang, J.-S.; Sizova, E.A.; Chao, J.C.J.; et al. Gut Microbiota as a Mediator of Essential and Toxic Effects of Zinc in the Intestines and Other Tissues. Int. J. Mol. Sci. 2021, 22, 13074. [Google Scholar] [CrossRef] [PubMed]
- Gual-Grau, A.; Guirro, M.; Mayneris-Perxachs, J.; Arola, L.; Boqué, N. Impact of different hypercaloric diets on obesity features in rats: a metagenomics and metabolomics integrative approach. J. Nutr. Biochem. 2019, 71, 122–131. [Google Scholar] [CrossRef]
- Seppa, L.; Korpela, R.; Lönnerdal, B.; Metsäniitty, L.; Juntunen-Backman, K.; Klemola, T.; Paganus, A.; Vanto, T. A follow-up study of nutrient intake, nutritional status, and growth in infants with cow milk allergy fed either a soy formula or an extensively hydrolyzed whey formula. Am. J. Clin. Nutr. 2005, 82, 140–145. [Google Scholar] [CrossRef]
- Chandra, R.K. Food allergy and nutrition in early life: implications for later health. 59. [CrossRef]
- Welmer, D. S.; Beckler, M. D. Underlying immune mechanisms involved in cow’s milk-induced hypersensitivity reactions manifesting as atopic dermatitis. Cureus, 2022, 14, e27604. [Google Scholar]
- Yang, Y.; Li, X.; Yang, Y.; Shoaie, S.; Zhang, C.; Ji, B.; Wei, Y. Advances in the Relationships Between Cow’s Milk Protein Allergy and Gut Microbiota in Infants. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Bognanni, A.; Chu, M. K.; Firmino, R. T.; Arasi, S.; Waffenschmidtf, S.; Agarwal, A.; Dziechciarz, P.; Horvath, A.; Jebai, R.; Mihara, H.; Roldan, Y.; Said, M.; Shamir, R.; Bozzola, M.; Bahna, S.; Fiocchi, A.; Waserman, S.; Schünemann, H. J.; Brozek, J. L.; World allergy organization (WAO). Diagnosis and rationale for action against cow's milk allergy (DRACMA) guideline update – XIII – Oral immunotherapy for CMA – Systematic review. World Allergy Organ. J. 2022, 15, 100682. [Google Scholar] [CrossRef]
- Verduci, E.; Zuccotti, G.; Peroni, D. C. New insights in cow’s milk and allergy: Is the gut microbiota the missing link? Nutrients 2022, 14, 1631. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Stockmann, R.; Ng, K.; Ajlouni, S. Bioprocessing of Pea Protein can Enhance Fortified Fe But Reduce Zn In Vitro Bioaccessibility. J. Agric. Food Chem. 2022, 70, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- García-Conde, U.; Navarro-Alarcón, M.; Navajas-Porras, B.; Hinojosa-Nogueira, D.; Pérez-Burillo, S.; Pastoriza, S.; Navarro-Moreno, M.; Rufián-Henares, J. A. Total Zn amount and bioaccesible fractions of foods in the small and large intestine after in vitro digestion and fermentation with fecal material of healthy adults and children: influence of culinary techniques. Food Res. Intern. 2023, 169, 112817. [Google Scholar] [CrossRef]
- Navajas-Porras, B.; Pérez-Burillo, S.; Valverde-Moya. J.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.. Effect of Cooking Methods on the Antioxidant Capacity of Plant Foods Submitted to In Vitro Digestion–Fermentation. Antioxidants 2020, 9, 1312. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Rufián-Henares, J.; Pastoriza, S. Towards an improved global antioxidant response method (GAR+): Physiological-resembling in vitro digestion-fermentation method. Food Chem. 2018, 239, 1253–1262. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Molino, S.; Navajas-Porras, B.; Valverde-Moya. J.; Hinojosa-Nogueira, D.; López-Maldonado, A.; Pastoriza, S.; Rufián-Henares, J.. An in vitro batch fermentation protocol for studying the contribution of food to gut microbiota composition and functionality. Nat. Protoc. 2021, 16, 3186–3209. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Pastoriza, S.; Jiménez-Hernández, N.; D’auria, G.; Francino, M.P.; Rufián-Henares, J.A. Effect of Food Thermal Processing on the Composition of the Gut Microbiota. J. Agric. Food Chem. 2018, 66, 11500–11509. [Google Scholar] [CrossRef]
- Barera, G.; Beccio, S.; Proverbio, M.C.; Mora, S. Longitudinal changes in bone metabolism and bone mineral content in children with celiac disease during consumption of a gluten-free diet. Am. J. Clin. Nutr. 2004, 79, 148–154. [Google Scholar] [CrossRef]
- Bascuñán, K.A.; Vespa, M.C.; Araya, M. Celiac disease: understanding the gluten-free diet. Eur. J. Nutr. 2017, 56, 449–459. [Google Scholar] [CrossRef]
- Caruso, R.; Pallone, F.; Stasi, E.; Romeo, S.; Monteleone, G. Appropriate nutrient supplementation in celiac disease. Ann. Med. 2013, 45, 522–531. [Google Scholar] [CrossRef]
- Zafeiropoulou, K.; Nichols, B.; Mackinder, M.; Biskou, O.; Rizou, E.; Karanikolou, A.; Clark, C.; Buchanan, E.; Cardigan, T.; Duncan, H.; et al. Alterations in Intestinal Microbiota of Children With Celiac Disease at the Time of Diagnosis and on a Gluten-free Diet. Gastroenterology 2020, 159, 2039–2051. [Google Scholar] [CrossRef]
- Mudryj, A.N.; Waugh, A.K.; Slater, J.J.; Duerksen, D.R.; Bernstein, C.N.; Riediger, N.D. Nutritional implications of dietary gluten avoidance among Canadians: results from the 2015 Canadian Community Health Survey. Br. J. Nutr. 2020, 126, 738–746. [Google Scholar] [CrossRef]
- Punshon, T.; Jackson, B.P. Essential micronutrient and toxic trace element concentrations in gluten containing and gluten-free foods. Food Chem. 2018, 252, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Abdukhakimova, D.; Dossybayeva, K.; Poddighe, D. Fecal and Duodenal Microbiota in Pediatric Celiac Disease. Front. Pediatr. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Russo, E.; Renzi, D.; Baldi, S.; Nannini, G.; Lami, G.; Menicatti, M.; Pallecchi, M.; Bartolucci, G.; Niccolai, E.; Cerboneschi, M.; Smeazzetto, S.; Ramazzotti, M.; Amedei, A.; Calabrò, A. S. Characterization of the “gut microbiota-im munity axis” and microbial lipid metabolites in atrophic and potential celiac disease. Front. Microbiol. 2022, 13, 886008. [Google Scholar] [CrossRef]
- Velasco-Reynold, C.; Navarro-Alarcon, M.; . López-G de la Serrana, H.; Perez-Valero, V.; Lopez-Martinez, M. C. In vitro determination of zinc dialyzability from duplicate hospital meals: influence of other nutrients. Nutrition, 2008, 24, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.; Knez, M.; Uzan, A.; Stangoulis, J.C.R.; Glahn, R.P.; Koren, O.; Tako, E. Alterations in the Gut (Gallus gallus) Microbiota Following the Consumption of Zinc Biofortified Wheat (Triticum aestivum)-Based Diet. J. Agric. Food Chem. 2018, 66, 6291–6299. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A. V.; Aschne, M.; Tinkov, A. A. Zinc. Adv. Food Nutr. Res. 96, 251–310. [PubMed]
- Sinai, T.; Goldberg, M. R.; Nachshon, L.; Amitzur-Levy, R.; Yichie, T.; Katz, Y.; Monsonego-Ornan, E.; Elizur, A. Reduced final height and inadequate nutritional intake in cow's milk-allergic young adults. J. Allergy Clin. Immunol. Pract. 2019, 7, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.; Koker, C.; Dziubak, R.; Godwin, H.; Domínguez-Ortega, G. ; Shah. N. Dietary elimination of children with food protein induced gastrointestinal allergy – micronutrient adequacy with and without a hypoallergenic formula? Clin. Translat. Allerg. 2014, 4, 31. [Google Scholar] [CrossRef]
- Kvammen, J.A.; Thomassen, R.A.; Eskerud, M.B.; Rugtveit, J.; Henriksen, C. Micronutrient Status and Nutritional Intake in 0- to 2-Year-old Children Consuming a Cows’ Milk Exclusion Diet. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 831–837. [Google Scholar] [CrossRef]
- Maslin, K.; Oliver, E.M.; Scally, K.S.; Atkinson, J.; Foote, K.; Venter, C.; Roberts, G.; Grimshaw, K.E.C. Nutritional adequacy of a cows’ milk exclusion diet in infancy. Clin. Transl. Allergy 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Canani, R. B.; De Filippis, F.; Nocerino, R. , Paparo, L. ; Di Scala, C.; Cosenza, L.; Della Gatta, G.; Cal;ignano, A.; De Caro, C.; Laiola, M.; Gilbert, J.; Ercolin, D. Gut microbiota composition and butyrate production in children afected by non-IgE-mediated cow’s milk allergy. Sci. Rep. 2018, 8, 12500. [Google Scholar]
- Gwala, S.; Kyomugasho, C.; Wainaina, I.; Rousseau, S.; Hendrickx, M.; Grauwet, T. Ageing, dehulling and cooking of Bambara groundnuts: consequences for mineral retention and in vitro bioaccessibility. Food Funct. 2020, 11, 2509–2521. [Google Scholar] [CrossRef]
- Bridide, P.; Viva de Toledo, N. M.; López-Nicolás, R.; Ros, G.; Frontela Sasetac, C.; Vieira de Carvalho, R. Fe and Zn in vitro bioavailability in relation to antinutritional factors in biofortified beans subjected to different processes. Food Funct. 2019, 10, 4802–4810. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.D.; Russo, P.; Rufián-Henares, J. .; Hinojosa-Nogueira, D.; Pérez-Burillo, S.; de la Cueva, S.P.; Rohn, S.; Fatouros, A.; Douros, K.; González-Vigil, V.; et al. The Stance4Health Project: Evaluating a Smart Personalised Nutrition Service for Gut Microbiota Modulation in Normal- and Overweight Adults and Children with Obesity, Gluten-Related Disorders or Allergy/Intolerance to Cow’s Milk. Foods 2022, 11, 1480. [Google Scholar] [CrossRef]
- Maldonado-Mateus, L. Y.; Perez-Burillo, S.; Lerma-Aguilera, A.; Hinojosa-Nogueira, D.; Ruíz-Pérez, S.; Gosalbes, M. J.; Francino, P.; Rufián-Henares, J. A.; Pastoriza de la Cueva, S. Effect of roasting conditions on cocoa bioactivity and gut microbiota modulation, Food Funct. 2021, 12, 9680-9692.
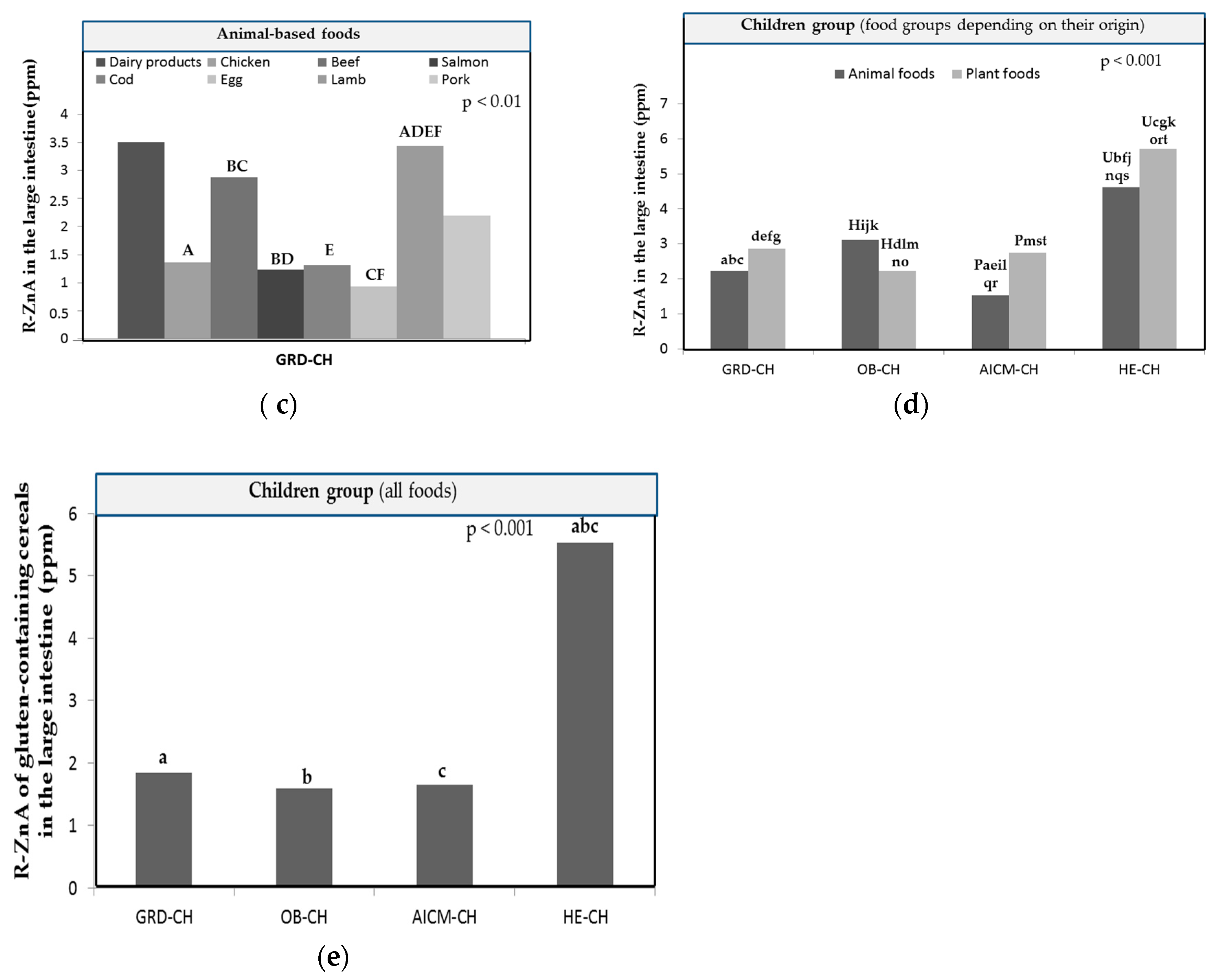
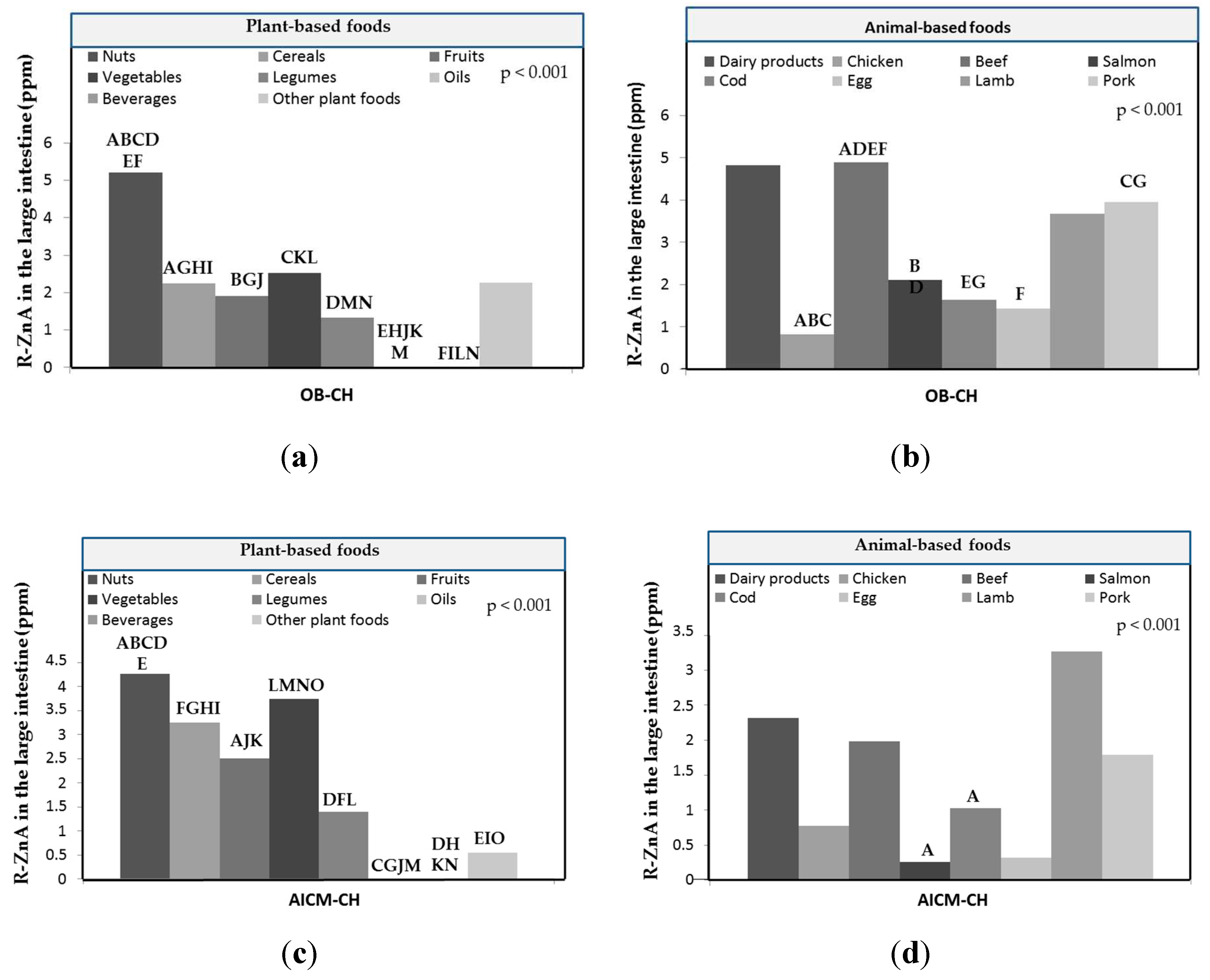
| Food group | Zn (μg/g, ppm) | Zn-BA in GRD-CH (%) |
Zn-BA in OB-CH (%) |
Zn-BA in AICM-CH (%) |
Zn-BA in HE-CH* (%) |
|---|---|---|---|---|---|
| Plant-based foods | |||||
| Nuts | 13.1 ± 6.98 | 52.7 ± 31.4a | 39.7 ± 28.2ab | 18.8 ± 29.9c | 66.6 ± 25.3bc |
| Cereals | 6.63 ± 3.67 | 33.5 ± 28.6a | 33.8 ± 28.8b | 34.3 ± 36.6c | 56.4 ± 27.3abc |
| Fruits | 8.51 ± 5.37 | 36.7 ± 28.7ab | 27.8 ± 32.1ac | 27.3 ± 29.5d | 48.2 ± 31.2bcd |
| Vegetables | 5.99 ± 3.45 | 36.9 ± 26.0ab | 29.5 ± 29.3acd | 34.8 ± 29.3ce | 62.5 ± 20.8bde |
| Legumes | 2.25 ± 2.13 | 41.6 ± 35.3a | 22.4 ± 29.2b | 18.9 ± 30.6c | 57.8 ± 26.7abc |
| Oils | 1.94 ± 0.89 | 0.46 ± 0.58 | 0.21 ± 0.28 | 20.2 ± 33.9 | 1.29 ± 1.60 |
| Beverages | 7.05 ± 9.18 | 0.35 ± 0.17 | 0.23 ± 0.26 | 1.32 ± 5.58 | 0.64 ± 0.63 |
| Others | 7.32 ± 5.83 | 38.2 ± 30.4 | 24.2 ± 30.1 | 16.6 ± 26.5 | 39.9 ± 39.5 |
| Animal-based foods | |||||
| Dairy products | 7.87 ± 5.75 | 37.1 ± 22.1a | 30.8 ± 33.2 | 25.6 ± 34.2ab | 46.9 ± 37.8b |
| Chicken | 5.52 ± 3.08 | 48.6 ± 38.8a | 31.5 ± 38.4b | 15.7 ± 30.0c | 62.0 ± 28.8abc |
| Beef | 13.6 ± 4.60 | 30.7 ± 23.4a | 42.5 ± 24.3b | 16.2 ± 20.6bc | 45.8 ± 24.6ac |
| Salmon | 6.14 ± 3.04 | 31.8 ± 25.0ab | 42.7 ± 26.7cd | 9.36 ± 20.3ace | 55.7 ± 25.6bde |
| Cod | 5.60 ± 3.47 | 37.6 ± 34.1a | 43.5 ± 30.7 | 16.2 ± 24.6b | 58.6 ± 32.5ab |
| Egg | 8.97 ± 4.37 | 14.5 ± 12.6ab | 20.6 ± 19.4c | 36.1 ± 27.1acd | 40.4 ± 27.6bd |
| Lamb | 6.28 ± 4.03 | 45.2 ± 25.8 | 41.5 ± 32.9 | 12.4 ± 29.3 | 31.2 ± 32.4 |
| Pork | 6.67 ± 3.10 | 37.4 ± 31.7 | 49.2 ± 27.4 | 9.2 ± 23.7 | 42.7 ± 32.4 |
| Cereal by-product | Zn (μg/g, ppm) | Zn-BA in GRD-CH (%) |
Zn-BA in OB-CH (%) |
Zn-BA in AICM-CH (%) |
Zn-BA in HE-CH* (%) |
|---|---|---|---|---|---|
| Bread | 7.33 ± 3.54 | 33.6 ± 27.3 | 30.4 ± 23.5a | 25.8 ± 30.8b | 61.2 ± 21.5ab |
| Bread whole meal | 9.08 ± 3.31 | 31.9 ± 18.9a | 29.7 ± 24.1b | 34.1 ± 32.1 | 61.4 ± 20.4ab |
| Penne | 2.89 ± 0.41 | 8.4 ± 12.6 | 20.5 ± 35.5 | 26.4 ± 45.4 | 45.2 ± 12.2 |
| Whole penne | 7.43 ± 1.98 | 29.4 ±27.9 | 50.6 ± 14.8 | 34.7 ± 30.0 | 85.6 ± 12.4 |
| Rice | 7.55 ± 2.86 | 3.37 ± 2.57 | 2.73 ± 1.24 | 3.33 ± 5.78 | 27.0 ± 9.73 |
| Whole rice | 3.44 ± 0.41 | 6.73 ± 6.00 | 19.8 ± 34.2 | 34.8 ± 46.7 | 63.7 ± 1.34 |
| Biscuits | 4.07 ± 2.11 | 58.3 ± 27.1 | 55.9 ± 20.7 | 30.5 ± 52.5 | 73.9 ± 11.0 |
| Biscuits whole grain | 3.93 ± 2.58 | 50.5 ± 44.8 | 41.8 ± 36.3 | 51.2 ± 43.7 | 63.0 ± 21.3 |
| Wholemeal breakfast cereals | 7.46 ± 6.03 | 66.1 ± 23.7 | 45.1 ± 38.6 | 60.1 ± 51.9 | 85.5 ± 8.17 |
| Breakfast cereals | 6.93 ± 3.14 | 49.9 ± 36.1 | 57.2 ± 46.6 | 59.6 ±51.4 | 77.9 ± 2.56 |
| Food group | Zn (μg/g, ppm) | Zn-BA in GRD-CH (%) |
Zn-BA in OB-CH (%) |
Zn-BA in AICM-CH (%) |
Zn-BA in HE-CH* (%) |
|---|---|---|---|---|---|
| Vegetables | |||||
| Zucchini | 6.55 ± 3.67 | 39.3 ± 18.6a | 30.7 ± 28.9 | 53.1 ± 32.3 | 60.9 ± 19.5a |
| Capsicum | 6.30 ± 3.77 | 29.9 ± 18.9a | 32.3 ± 29.3b | 23.3 ± 31.1c | 70.7 ± 11.5abc |
| Carrot | 6.52 ± 4.98 | 41.9 ± 22.2a | 33.8 ± 34.3b | 43.5 ± 36.2 | 67.5 ± 17.7ab |
| Potatoe | 8.84 ± 4.26 | 3.00 ± 3.96ab | 7.82 ± 11.4cd | 29.3 ± 23.1ac | 39.0 ± 18.7bd |
| Sweet potatoe | 11.5 ± 6.76 | 33.4 ± 21.2a | 27.4 ± 24.4b | 33.0 ± 26.2c | 62.6 ± 12.6abc |
| Eggplant | 11.3 ± 5.27 | 37.7 ± 30.9a | 28.0 ± 32.1b | 41.4 ± 29.1c | 72.0 ± 10.3abc |
| Onion | 8.95 ± 6.78 | 46.6 ± 24.4a | 29.7 ± 33.7b | 29.6 ± 31.5c | 80.4 ± 8.05abc |
| Cauliflower | 8.84 ± 5.99 | 72.8 ± 13.6a | 43.7 ± 38.2b | 40.7 ± 35.9ac | 73.0 ± 19.0bc |
| Spinach | 9-00 ± 3.69 | 46.7 ± 18.2a | 31.0 ± 30.0b | 33.9 ± 25.5c | 66.8 ± 9.91abc |
| Garlic | 9.15 ± 4.29 | 21.5 ± 21.5a | 21.7 ± 23.0b | 33.0 ± 25.1 | 50.4 ± 14.2ab |
| Tomatoe | 8.09 ± 6.13 | 18.0 ± 16.2a | 20.7 ± 19.8b | 33.3 ± 26.2 | 52.0 ± 18.5ab |
| Cabbage | 7.86 ± 5.77 | 39.3 ± 25.6ab | 34.6 ± 28.3c | 9.36 ± 19.5ad | 64.0 ± 19.3bcd |
| Fruits | |||||
| Apple | 10.8 ± 4.65 | 57.1 ± 30.2 | 46.4 ± 38.5 | 42.6 ± 28.3a | 80.3 ± 7.76a |
| Banana | 7.57 ± 5.29 | 24.9 ± 23.2a | 25.5 ± 32.8b | 34.1 ± 32.2a | 58.3 ± 25.9b |
| Orange | 7.88 ± 4.67 | 25.9 ± 21.4a | 16.4 ± 27.9b | 35.8 ± 27.0c | 64.5 ± 15.9abc |
| Grapes | 9.95 ± 9.40 | 36.3 ± 26.0 | 26.0 ± 29.4 | 30.0 ± 26.3 | 50.6 ± 24.8 |
| Plum | 4.28 ± 1.82 | 35.2 ± 26.6a | 27.0 ± 28.1 | 5.60 ± 10.1ab | 41.5 ± 19.3b |
| Peach | 4.79 ± 2.26 | 31.2 ± 29.4a | 17.6 ± 25.1 | 13.9 ± 25.7b | 60.8 ± 8.11ab |
| Home culinary technique | Zn (μg/g, ppm) | Mean Zn-BA values ± SD in GRD-CH | Mean Zn-BA values ± SD in OB-CH | Mean Zn-BA values ± SD in AICM-CH | Mean Zn-BA values ± SD in HE-CH* |
|---|---|---|---|---|---|
| Plant-based foods | |||||
| Frying | 9.56 ± 6.04 | 25.7 ± 26.8a | 23.9 ± 28.6b | 36.8 ± 29.9c | 59.1 ± 23.6abc |
| Raw form | 6.66 ± 5.68 | 36.1 ± 29.7a | 28.3 ± 30.8ab | 25.9 ± 31.7c | 53.3 ± 31.9bc |
| Roasting | 9.28 ± 6.53 | 36.2 ± 29.4c | 28.8 ± 31.6b | 26.7 ± 29.6d | 64.1 ± 20.4bcd |
| Toasting | 6.23 ± 2.24 | 55.2 ± 21.6b | 47.1 ± 25.7a | 41.6 ± 35.9 | 74.0 ± 5.22ab |
| Boiling | 6.81 ± 4.69 | 33.5 ± 27.8b | 25.5 ± 28.3a | 32.6 ± 32.8c | 62.0 ± 20.3abc |
| Grilling | 7.36 ± 4.66 | 38.4 ± 27.7b | 27.5 ± 28.6a | 24.9 ± 27.6c | 60.1 ± 19.3abc |
| Animal-based foods | |||||
| Frying | 6.66 ± 4.53 | 48.5 ± 27.6cd | 49.5 ± 32.9ab | 17.4 ± 23.2ace | 65.8 ± 23.6bde |
| Raw form | 6.36 ± 6.25 | 40.7 ± 33.1 | 37.2± 33.1 | 28.7 ± 36.6a | 52.2 ± 32.2a |
| Roasting | 7.09 ± 3.66 | 18.4 ± 16.2b | 25.6 ± 17.1a | 16.0 ± 24.9abc | 46.0 ± 17.4c |
| Boiling | 5.79 ± 4.58 | 54.8 ± 32.8b | 57.4 ± 29.6a | 18.0 ± 30.0abc | 71.0 ± 21.0c |
| Grilling | 6.77 ± 3.90 | 17.5 ± 17.1bc | 17.2 ± 17.9a | 15.2 ± 28.3bd | 46.0 ± 17.9acd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





