Submitted:
19 September 2023
Posted:
20 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
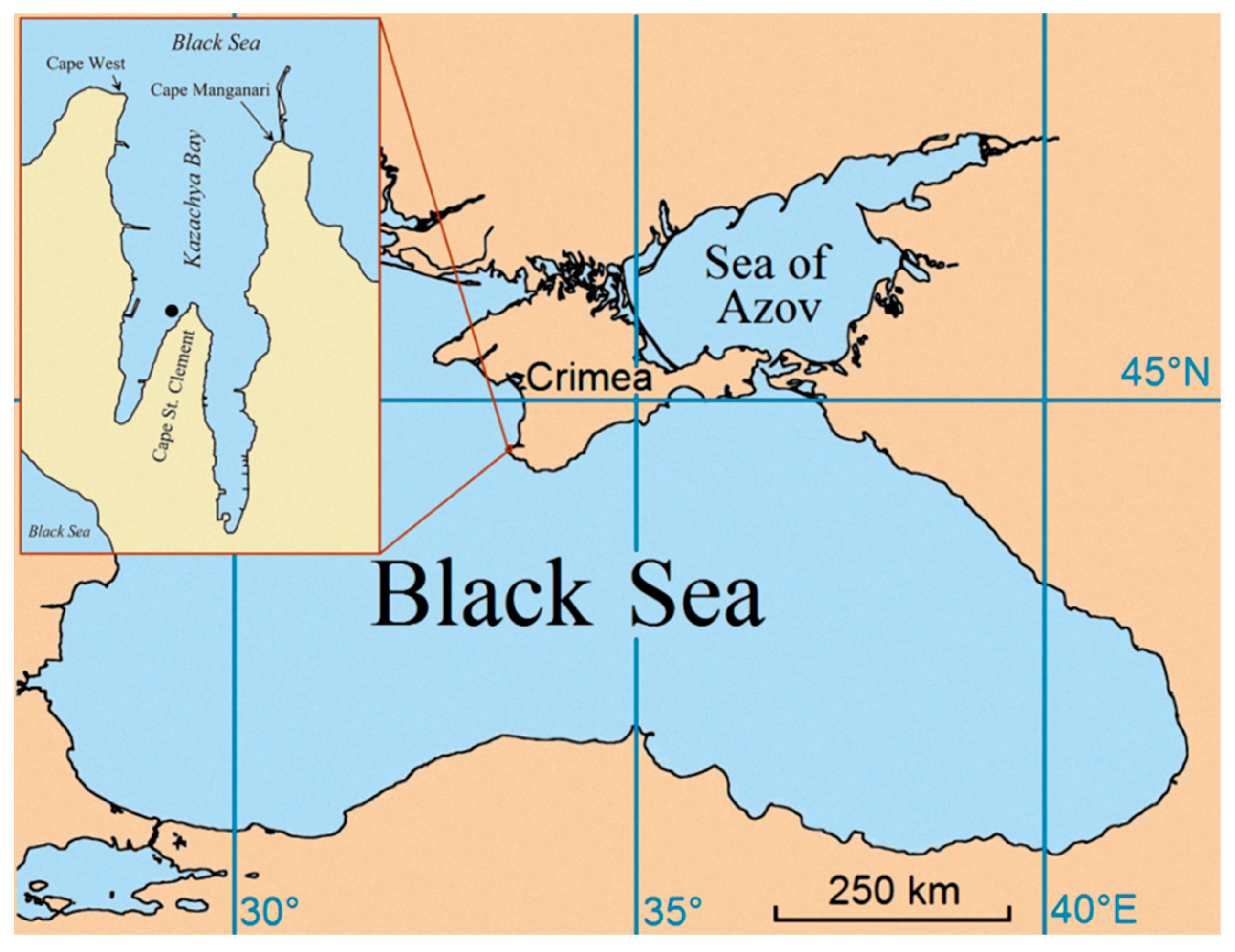
2. Materials and Methods
3. Results and discussion
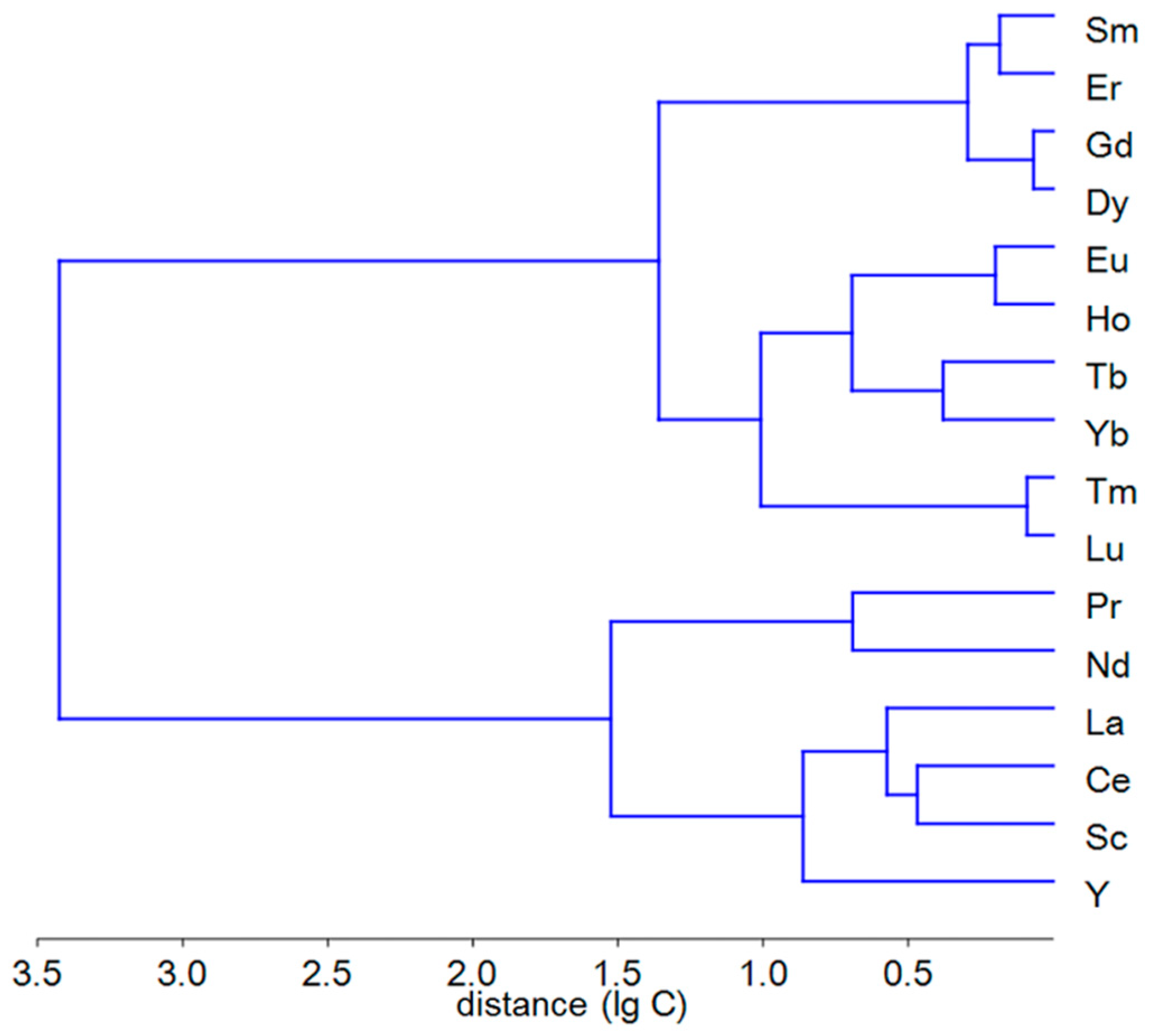
3.1. REE contents in the seagrass and sediments
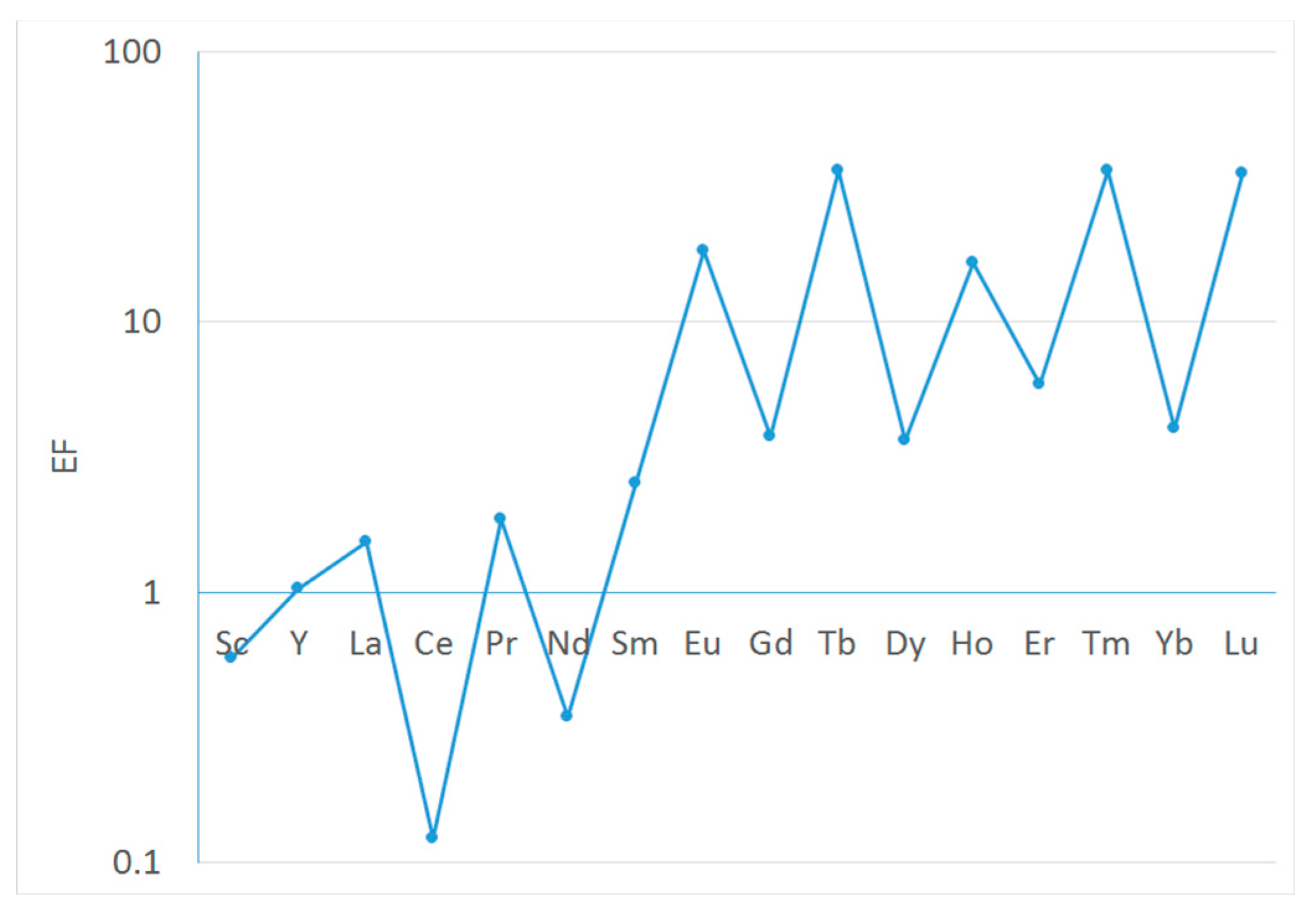
3.2. REE enrichment and anomalies in sediments
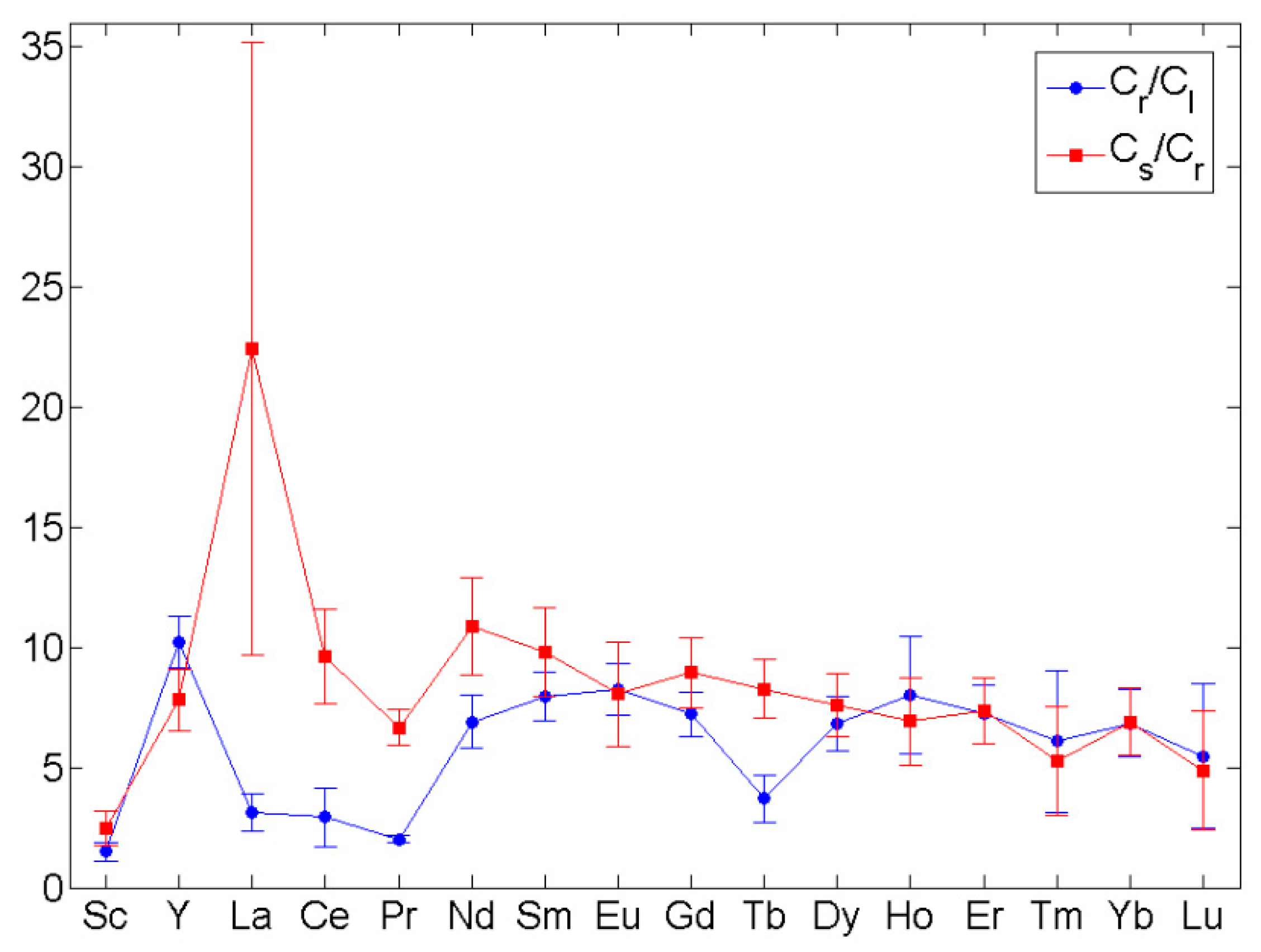
3.3. REE transfer and translocation in the seagrass
3.4. Seagrass wrack as a potential source of REE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damhus, T.; Hartshorn, R.M.; Hutton, A.T. Nomenclature of Inorganic Chemistry: IUPAC Recommendations; RSC Publishing: Cambridge, UK, 2005; p. 51. [Google Scholar]
- Castor, S.B.; Hedrick, J.B. Rare earth elements. Industrial minerals and rocks 2006, 7, 769–792. [Google Scholar]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Du, X.; Graedel, T.E. Global in-use stocks of the rare earth elements: a first estimate. Environ. Sci. Technol. 2011, 45, 4096–4101. [Google Scholar] [CrossRef] [PubMed]
- Sinharoy, A.; Lens, P. Environmental technologies to treat rare earth element pollution: Principles and Engineering; IWA Publishing: London, 2022. [Google Scholar] [CrossRef]
- Brown, P.H.; Rathjen, A.H.; Graham, R.D.; Tribe, D.E. Rare earth elements in biological systems. In Handbook on the physics and chemistry of rare earths; Gschneidner, K.A., Eyring, L., Eds.; Elsevier Science Publishers: Amsterdam, 1990; Volume 13, pp. 423–452. [Google Scholar] [CrossRef]
- Tyler, G. Rare earth elements in soil and plant systems: a review. Plant Soil 2004, 267, 191–206. [Google Scholar] [CrossRef]
- Loell, M.; Reiher, W.; Felix-Henningsen, P. Contents and bioavailability of rare earth elements in agricultural soils in Hesse (Germany). J. Plant Nutr. Soil Sci. 2011, 174, 644–654. [Google Scholar] [CrossRef]
- Das, N.; Das, D. Recovery of rare earth metals through biosorption: an overview. J. Rare Earths 2013, 31, 933–943. [Google Scholar] [CrossRef]
- Sparks, D.L. Toxic metals in the environment: the role of surfaces. Elements 2005, 1, 193–197. [Google Scholar] [CrossRef]
- Reddy, V.M.; Babu, K.S.; Balaram, V.; Satyanarayanan, M. Assessment of the effects of municipal sewage, immersed idols and boating on the heavy metal and other elemental pollution of surface water of the eutrophic Hussainsagar lake (Hyderabad, India). Environ. Monit. Assess. 2012, 184, 1991–2000. [Google Scholar] [CrossRef]
- Wei, B.; Li, Y.; Li, H.; Yu, J.; Ye, B.; Liang, T. Rare earth elements in human hair from a mining area of China. Ecotoxicol. Environ. Saf. 2013, 96, 118–123. [Google Scholar] [CrossRef]
- Ali, S.H. Social and environmental impact of the rare earth industries. Resources 2014, 3, 123–134. [Google Scholar] [CrossRef]
- Vysetti, B. Current advances in the miniaturization of analytical instruments—Applications in cosmochemistry, geochemistry, exploration, and environmental sciences. Spectroscopy 2016, 31, 40–44. [Google Scholar] [CrossRef]
- RIVM Report 601501011: Maximum permissible concentrations and negligible concentrations for rare earth elements (REEs); Sneller F.E.C.; Kalf D.F.; Weltje L.; Van Wezel A.P., Eds.; Bilthoven, Netherlands, 2000; 60 p. (National Institute of Public Health and Environmental Protection RIVM).
- Al-Rimawi, F.; Kanan, K.; Qutob, M. Analysis of different rare metals, rare earth elements, and other common metals in groundwater of South West Bank/Palestine by ICP/MS-Data and health aspects. J. Environ. Protect. 2013, 4, 1157–1164. [Google Scholar] [CrossRef]
- Neira, P.; Romero-Freire, A.; Basallote, M.D.; Qiu, H.; Cobelo-García, A.; Canovas, C.R. Review of the concentration, bioaccumulation, and effects of lanthanides in marine systems. Front. Mar. Sci. 2022, 9, 920405. [Google Scholar] [CrossRef]
- Khan, A.M.; Bakar, N.K.A.; Bakar, A.F.A.; Ashraf, M.A. Chemical speciation and bioavailability of rare earth elements (REEs) in the ecosystem: a review. Environ. Sci. Pollut. Res. 2017, 24, 22764–22789. [Google Scholar] [CrossRef]
- Merschel, G.; Bau, M. Rare earth elements in the aragonitic shell of freshwater mussel corbicula fluminea and the bioavailability of anthropogenic lanthanum, samarium and gadolinium in river water. Sci. Total Environ. 2015, 533, 91–101. [Google Scholar] [CrossRef]
- Armand, R.; Cherubini, C.; Tuduri, J.; Pastore, N.; Pourret, O. Rare earth elements in French stream waters - revisiting the geochemical continental cycle using FOREGS dataset. J. Geochem. Explor. 2015, 157, 132–142. [Google Scholar] [CrossRef]
- Schijf, J.; de Baar, H.J.W. Rare earth element exchange through the Bosporus: The black Sea as a net source of REEs to the Mediterranean Sea. Geochim. Cosmochim. Acta 1995, 59, 3503–3509. [Google Scholar] [CrossRef]
- den Hartog, C. Seagrasses and seagrass ecosystem, an appraisal of the research approach. Aquat. Bot. 1979, 7, 105–117. [Google Scholar] [CrossRef]
- Schlacher-Hoenlinger, M.A.; Schlacher, T.A. Differential accumulation patterns of heavy metals among the dominant macrophytes of a Mediterranean seagrass meadow. Chemosphere 1998, 37, 1511–1519. [Google Scholar] [CrossRef]
- Govers, L.L.; Lamers, L.P.; Bouma, T.J.; Eygensteyn, J.; de Brouwer, J.H.; Hendriks, A.J.; Huijbers, C.M.; van Katwijk, M.M. Seagrasses as indicators for coastal trace metal pollution: a global meta-analysis serving as a benchmark, and a Caribbean case study. Environ. Pollut. 2014, 195, 210–217. [Google Scholar] [CrossRef]
- Deniz, F. Bioremediation potential of waste biomaterials originating from coastal Zostera marina L. meadows for polluted aqueous media with industrial effluents. Prog. Biophys. Mol. Biol. 2019, 145, 78–84. [Google Scholar] [CrossRef]
- Lee, G.; Suonan, Z.; Kim, S.H.; Hwang, D.-W.; Lee, K.-S. Heavy metal accumulation and phytoremediation potential by transplants of the seagrass Zostera marina in the polluted bay systems. Mar. Pollut. Bull. 2019, 149, 110509. [Google Scholar] [CrossRef]
- Serrano, O.; Lavery, P.S.; Bongiovanni, J.; Duarte, C.M. Impact of seagrass establishment, industrialization and coastal infrastructure on seagrass biogeochemical sinks. Mar. Environ. Res. 2020, 160, 104990. [Google Scholar] [CrossRef]
- Serrano, R.; Gras, L.; Giménez-Casalduero, F.; del-Pilar-Ruso, Y.; Grindlay, G.; Mora, J. The role of Cymodocea nodosa on the dynamics of trace elements in different marine environmental compartments at the Mar Menor Lagoon (Spain). Mar. Pollut. Bull. 2019, 141, 52–60. [Google Scholar] [CrossRef]
- Sena, I.C.M.; Souza, L.A.; Patire, V.F.; Arias-Ortiz, A.; Creed, J.C.; Cruz, I.; Hatje, V. Environmental settings of seagrass meadows control rare earth element distribution and transfer from soil to plant compartments. Sci. Total Environ. 2022, 843, 157095. [Google Scholar] [CrossRef]
- Chemnitzer, R. Strategies for achieving the lowest possible detection limits in ICP-MS. Spectroscopy 2019, 34, 12–16. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Mégevand, P. Games-Howell post-hoc test for one-way ANOVA. Available online: https://github.com/pierremegevand/games_howell (accessed on 17 September 2023).
- Komar, D.; Smuc, N.R.; Belak, Z.L.; Matešić, S.; Lojen, S.; Kniewald, G.; Vrhovnik, P.; Dolenec, T.; Dolenec, M. Geochemical characteristics and distribution of rare earth elements in makirina bay sediments (n. Dalmatia, Republic of Croatia). Geologica Macedonica 2014, 28, 127–137. [Google Scholar]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell: Oxford, 1985. [Google Scholar]
- Cotton, S.A. Scandium, yttrium & the lanthanides: Inorganic & coordination chemistry. In Encyclopedia of Inorganic Chemistry; Wiley: New York, 2006; the lanthanides: Inorganic &. [Google Scholar] [CrossRef]
- Mao, L.; Mo, D.; Yang, J.; Guo, Y.; Lv, H. Rare earth elements geochemistry in surface floodplain sediments from the Xiangjiang River, middle reach of Changjiang River, China. Quaternary International 2014, 336, 80–88. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C.L. Riverine composition and estuarine geochemistry of particulate metals in China – weathering features, anthropogenic impact and chemical fluxes. Estuarine, Coastal and Shelf Science 2002, 54, 1051–1070. [Google Scholar] [CrossRef]
- Dean, J.R. Bioavailability, Bioaccessibility and Mobility of Environmental Contaminants; Wiley: London, 2007. [Google Scholar]
- Zhang, Y.; Gao, X. Rare earth elements in surface sediments of a marine coast under heavy anthropogenic influence: The Bohai Bay, China. Est. Coast. Shelf Sci. 2015, 164, 86–93. [Google Scholar] [CrossRef]
- Xie, H.G. Advance of application of rare earth elements in agriculture in China. Chin. Sci. Bull. 1991, 36, 561–564. [Google Scholar]
- Akagi, T.; Edanami, K. Sources of rare earth elements in shells and soft-tissues of bivalves from Tokyo bay. Mar. Chem. 2017, 194, 55–62. [Google Scholar] [CrossRef]
- Li, J.X.; Zheng, L.; Sun, C.J.; Jiang, F.H.; Yin, X.F.; Chen, J.H.; Han, B.; Wang, X. Study on ecological and chemical properties of rare earth elements in tropical marine organisms. Chin. J. Anal. Chem. 2016, 44, 1539–1546. [Google Scholar] [CrossRef]
- Akagi, T. Rare earth element (REE)-silicic acid complexes in seawater to explain the incorporation of REEs in opal and the “leftover” REEs in surface water: New interpretation of dissolved REE distribution profiles. Geochim. Cosmochim. Acta 2013, 113, 174–192. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Staszak, K.; Wozniak-Budych, M.J.; Jurga, S. Lanthanides and tissue engineering strategies for bone regeneration. Coord. Chem. Rev. 2019, 388, 248–267. [Google Scholar] [CrossRef]
- Fu, F.; Akagi, T.; Yabuki, S.; Iwaki, M.; Ogura, N. Distribution of rare earth elements in seaweed: Implication of two different sources of rare earth elements and silicon in seaweed. J. Phycology 2000, 36, 62–70. [Google Scholar] [CrossRef]
- Jacinto, J.; Henriques, B.; Duarte, A.C.; Vale, C.; Pereira, E. Removal and recovery of critical rare elements from contaminated waters by living Gracilaria gracilis. J. Hazard. Mater. 2018, 344, 531–538. [Google Scholar] [CrossRef]
- Kovařiková, M.; Tomášková, I.; Soudek, P. Rare earth elements in plants. Biol. Plant. 2019, 63, 20–32. [Google Scholar] [CrossRef]
- Tyler, G.; Olsson, T. Rare earth elements in forest-floor herbs as related to soil conditions and mineral nutrition. Biol. Trace Element Res. 2005, 106, 177–191. [Google Scholar] [CrossRef]
- Gumienna-Kontecka, E.; Rowińska-Zyrek, M.; Luczkowski, M. The role of trace elements in living organisms. In Recent Advances in Trace Elements; Chojnacka, K., Saeid, A., Eds.; Wiley-Blackwell, 2018; pp. 177–206. [Google Scholar] [CrossRef]
- Blinova, I.; Lukjanova, A.; Muna, M.; Vija, H.; Kahru, A. Evaluation of the potential hazard of lanthanides to freshwater microcrustaceans. Sci. Total Environ. 2018, 642, 1100–1107. [Google Scholar] [CrossRef]
- Jalali, J.; Lebeau, T. The role of microorganisms in mobilization and phytoextraction of rare earth elements: A review. Front. Environ. Sci. 2021, 9. [Google Scholar] [CrossRef]
- Hibi, Y.; Asai, K.; Arafuka, H.; Hamajima, M.; Iwama, T.; Kawai, K. Molecular structure of La3+-induced methanol dehydrogenase-like protein in Methylobacterium radiotolerans. J. Biosci. Bioeng. 2011, 111, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.H. Interesting and useful biochemical properties of lanthanides. Trends Biochem. Sci. 1983, 8, 445–449. [Google Scholar] [CrossRef]
- Lai, Y.; Wang, Q.; Yang, L.; Huang, B. Subcellular distribution of rare earth elements and characterization of their binding species in a newly discovered hyperaccumulator Pronephrium simplex. Talanta 2006, 70, 26–31. [Google Scholar] [CrossRef]
- Gao, Y.; Zeng, F.; Yi, A.; Ping, S.; Jing, L. Research of the entry of rare earth elements Eu3+ and La3+ into plant cell. Biol. Trace Element Res. 2003, 91, 253–265. [Google Scholar] [CrossRef]
- Grousset, F.E.; Quetel, C.R.; Thomas, B.; Donard, O.F.X.; Lambert, C.E.; Guillard, F.; Monaco, A. Anthropogenic vs. lithogenic origins of trace elements (As, Cd, Pb, Rb, Sb, Sc, Sn, Zn) in water column particles: northwestern Mediterranean Sea. Mar. Chem. 1995, 48, 291–310. [Google Scholar] [CrossRef]
- McLennan, S.M. Rare earth elements in sedimentary rocks: influence of provenance and sedimentary processes. In Geochemistry and Mineralogy of Rare Earth Elements; Lipin B.R., McKay G.A., Eds.; Mineral Soc. Am., Washington, 1989; pp. 169–200. [CrossRef]
- Hannigan, R.; Dorval, E.; Jones, C. The rare earth element chemistry of estuarine surface sediments in the Chesapeake Bay. Chem. Geol. 2010, 272, 20–30. [Google Scholar] [CrossRef]
- Ryabushko, V.I. , Gureeva, E.V.; Kapranov, S.V.; Bobko, N.I.; Prazukin, A.V.; Nekhoroshev, M.V. Rare earth elements in brown algae of the genus Cystoseira (Phaeophyceae) (Black Sea). Eur. J. Phycol. 2022, 57, 433–445. [Google Scholar] [CrossRef]
- Caro, P.; Lemaître-Blaise, M.; Trombe, F. Identification et solubilités des phases solides à l’équilibre sous une atmosphère de gaz carbonique dans les systèmes temaires oxydes de terres rares-gaz carbonique-eau. Comptes rendus hebdomadaires des séances l’Académie des sciences. Série C 1968, 267, 1594–1597. [Google Scholar]
- Liu, X.; Byrne, R.H. Rare earth and yttrium phosphate solubilities in aqueous solution. Geochim. Cosmochim. Acta 1997, 61, 1625–1633. [Google Scholar] [CrossRef]
- Tse, P.-K. China’s rare-earth industry 2011, U.S. Geological Survey Open-File Report 2011–1042. Available online: https://pubs.usgs.gov/of/2011/1042 (accessed on 17 September 2023).
- Goecke, F.; Zachleder, V.; Vítová, M. Rare earth elements and algae: physiological effects, biorefinery and recycling. Algal Biorefineries 2015, 2, 339–363. [Google Scholar] [CrossRef]
- Behera, S.; Singh, R.; Arora, R.; Kumar, S.N.; Shukla, M.; Kumar, S. Scope of algae as third generation biofuels. Front. Bioeng. Biotechnol. 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Sadogurskiy, S.Y.; Belich, T.V.; Sadogurskaya, S.A. The Red Data Book of Crimea and macrophytes stormwrack: how to deal with it? Plant Biology and Horticulture: theory, innovation 2022, 3, 43–49. (In Russian) [Google Scholar] [CrossRef]
- Milchakova, N.A. On the status of seagrass communities in the Black Sea. Aquat. Bot. 1999, 65, 21–32. [Google Scholar] [CrossRef]

| Z. noltei (leaves) | Z. noltei (rhizomes) | Sediments | TFr | TFl | TF’ | |
|---|---|---|---|---|---|---|
| Sc | 373±22a | 560±15a | 1382±77b | 0.405 | 0.270 | 0.666 |
| Y | 153±9a | 1562±18b | 12221±646c | 0.128 | 0.012 | 0.098 |
| La | 243±26a | 761±112a | 17074±614b | 0.045 | 0.014 | 0.320 |
| Ce | 221±9a | 648±25b | 6232±89c | 0.104 | 0.035 | 0.341 |
| Pr | 87±3a | 174±11b | 1162±18c | 0.150 | 0.075 | 0.499 |
| Nd | 38±3a | 267±49b | 2894±37c | 0.092 | 0.013 | 0.145 |
| Sm | 8.2±0.4a | 65±6b | 635±19c | 0.102 | 0.013 | 0.126 |
| Eu | 2.6±0.4a | 21.7±1.3b | 175±6c | 0.124 | 0.015 | 0.122 |
| Gd | 10.4±0.3a | 75±6b | 671±16c | 0.112 | 0.015 | 0.138 |
| Tb | 6±1a | 22±3b | 184±8c | 0.121 | 0.033 | 0.270 |
| Dy | 11±1a | 72±7b | 551±27c | 0.131 | 0.019 | 0.146 |
| Ho | 2.4±0.3a | 19±1b | 131±4c | 0.144 | 0.018 | 0.125 |
| Er | 7.3±0.6a | 53±4b | 386±13c | 0.136 | 0.019 | 0.139 |
| Tm | 1.5±0.1a | 9±2b | 49±2c | 0.190 | 0.031 | 0.164 |
| Yb | 5.1±0.7a | 35±2b | 241±13c | 0.145 | 0.021 | 0.146 |
| Lu | 1.7±0.2a | 9.3±3.4b | 45±2c | 0.206 | 0.038 | 0.183 |
| ΣREE | 1171.2 | 4353 | 44033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




