1. Introduction
Hemophilia A is a rare, congenital disorder with X-linked transmission, characterized by a deficiency in factor VIII (FVIII) caused by a mutation found on the X chromosome. The condition has more clinical significance for males, through spontaneous or post-traumatic bleeding, especially in joints and muscles. Over time, repeated hemarthroses lead to joint destruction, that ultimately results in a negative impact on locomotor function, quality of life and social integration1.
Hemophilia treatment has evolved tremendously in recent decades. From a historic point of view, fresh frozen plasma or cryoprecipitate (with the risk of allergic reactions or transmission of viral diseases) were considered the first, as well as the single, lines of therapy. It was only when researchers successfully purified and concentrated factor VIII compounds, that were able to ultimately expand the perspectives of hemophilia treatment2. The World Federation of Hemophilia (WFH) recommends prophylactic therapy to all people with hemophilia, to prevent joint degradation, avoid life-threatening bleedings and improve the quality of life3. Despite the progress made when using substitution therapy (recombinant products and extended half-life factor concentrates), hemophiliacs will find themselves in a situation where they might still experience clinical or subclinical bleedings 4-7, more importantly, 30% of them may develop anti-FVIII inhibitors8, a complication still feared in the management of the disease. On the other hand, prophylaxis involves regular, frequent (every 2-4 days, or even daily), intravenous injections, a real challenge for the patient/caregiver, as well as for the medical institutions, that tend to overcrowd fast. Consequentially, the compliance to the treatment regimens will decrease, with a negative impact on the therapy’s efficiency and increased risk of developing long-term complications associated with the bleeding patterns9.
People who develop inhibitors (neutralizing antibodies, most predominant IgG) no longer respond to standard factor VIII concentrates and their therapeutic options include an inhibitor eradication protocol or immune tolerance induction (ITI), bypass agents (BPAs): activated prothrombin complex concentrate (aPCC) or recombinant activated factor VII (rFVIIa). Although immune desensibilization induction therapy can eradicate, or at least keep the antibodies under control, it is demanding for patients and their family (with daily administration of high doses of factor VIII for a long time, up to 1.5 years, and frequent monitoring of coagulation tests), with the success dependent on compliance and compliance (optimal success rate of 70-80%10). When talking about the two available bypassing agents, although they have similar hemostatic effectiveness11, they are more difficult to monitor through laboratory tests in comparison with FVIII products, more expensive and the individual response is not predictable. On the other hand, it is known that patients with inhibitors have a higher rate of bleeding episodes (which is even more difficult to treat) and a higher recurrence of acute joint and muscular events, which leads to a faster degradation of the knee joint, with an increased risk of morbidity and disability when compared to patients without inhibitors12-14. Advanced cases of hemophilic arthropathy require major orthopedic interventions, procedures which are often denied due to the associated risks and very high costs.
New therapeutic strategies have been developed to improve the standard in medical care for patients with hemophilia and overcome the barriers represented by incomplete protection, the difficulty of chronic intravenous administration15-17 and low adherence.
Thus, in February 2018, Emicizumab received European Medicines Agency (EMA) authorization to be used prophylactically in patients with hemophilia with inhibitors, and in January 2019, it was approved for patients without inhibitors. In June 2021, it entered the Romanian market for patients with inhibitors and in April 2022, hemophilia patients without inhibitors were also included.
Overview
Emicizumab, a non-factor therapy (NFT), is a humanized, bispecific, IgG4 monoclonal antibody that mimics the action exerted by FVIII by binding to activated factor IX (FIXa) and factor X (FX), leading to the activation of the coagulation cascade and the formation of thrombin18. The half-life of the product is approximately 4-5 weeks19.
Subcutaneous administration and at intervals of 1, 2 or 4 weeks makes it very attractive, especially among patients with low compliance to prophylaxis regimens due to poor venous access and those with frequent bleeding even at optimal doses. Emicizumab is administered at a loading dose of 3 mg per kilogram of body weight (mg/Kg bw), once a week for the first 4 weeks followed by the maintenance dose, once a week - 1.5 mg/Kg bw, every 2 weeks - 3 mg/Kg bw or every once every 4 weeks 6 mg/Kg bw19. All therapeutic regimens have an acceptable safety profile and similar clinical efficacy.
A series of extensive clinical studies (HAVEN 1-5) have put into perspective the safety and efficacy profile of Emicizumab in both pediatric and adult patients, with or without inhibitors. The results showed a clear superiority of Emicizumab treatment compared to prophylaxis with FVIII (68% Annualised Bleed Rate (ABR) reduction) or BPA (79% ABR reduction) and an improvement in the quality of life20,21. It was also found that a higher percentage of patients under treatment with Emicizumab reached zero bleeding (71% of patients with inhibitors) compared to patients under prophylactic treatment (12.5% of those under prophylaxis with BPA). Moreover, the low bleeding rates were maintained and even improved in weeks 24-48 and 48-72, suggesting that long-term prophylaxis with Emicizumab successfully improves joint status22.
The most frequent adverse reactions were at the injection site (mild or moderate), followed by arthralgia, myalgia, headache, diarrhea or fever19,23. A rare and serious complication of Emicizumab is represented by the thrombotic microangiopathy (TMA), which was observed in patients using aPCC concomitant with Emicizumab for bleeding episodes (Oldenburg et al., 2017). The recommendation is that the treatment has to be initiated in a reference center, by doctors with experience in the management of hemophilia A, with or without inhibitors and with the support of a dedicated laboratory. When addressing patients with inhibitors from a therapeutic point of view, aPCC treatment is recommended to be stopped at least 24 hours before the initiation of Emicizumab, and in case of bleeding episodes, rFVIIa should be used as the first-line treatment (aPCC should only be used if it remains the only available option, and the doses should not exceed 50 IU/Kg bw/day due to the risk of thrombosis)26. For patients without inhibitors, prophylaxis with FVIII can undergo in parallel with Emicizumab until the second loading dose, after which, secondary administrations will take place only in case of bleeding20.
Emicizumab monitoring remains a challenge for attending physicians and laboratories. Although in current practice it is not a necessity, monitoring is indicated perioperatively or in cases of spontaneous bleeding, occurring during therapy. One-stage coagulation tests (based on APTT) are not recommended because Emicizumab normalizes APTT at very low concentrations27,31, an effect that is maintained up to 6 months after discontinuation25,26. Also, keeping a track of the inhibitor level, using the Bethesda one-stage method can give false negative results, since Emicizumab is not inactivated by heat or neutralized by anti-FVIII antibodies. The plasmatic level of factor VIII should be evaluated using chromogenic tests that take advantage of bovine reagents, which are not influenced by the presence of Emicizumab28. This is due to the fact that both the FIXa and FX formed during the reaction are of bovine origin and so they only bind to endogenous FVIII from human plasma, thus reflecting the activity of FVIII in the sample and not the hemostatic effect of Emicizumab32. The same kit should be used in the detection of inhibitors31,32. An alternative method is to measure the plasmatic concentration of Emicizumab, that has an effectiveness interval between 30 and 70 mcg/ml.32,33
There is growing interest in specific coagulation tests, such as thromboelastography (TEG) and thrombin generation time (TGT), to monitor the patient's coagulation status, but more data is needed in order to better understand the use cases in this field. The available data, however, showed that TGT displays a linear, dose-dependent increase in generation of thrombin and thus, it can be used to monitor hemostatic response in patients treated with Emicizumab30,32.
A summary of Emicizumab’s advantages and disadvantages is given in
Table 1.
2. Case reports
In the Hematology Department of Fundeni Hospital, 10 adults with Hemophilia A with an average age of 44.7 years have been treated with Emicizumab; 6 patients have presented inhibitors (1 with low plasmatic level) and 4 without. Seven out of these ten patients were previously in a prophylaxis program with factor VIII concentrates or bypassing agents and three patients were under on-demand treatment (very difficult venous access, impossibility of self-administration and/or low educational and financial status) (
Table 2). The inclusion criteria were composed of precarious venous access, presence of bleeding episodes despite prophylaxis therapy, target joints with frequent hemarthroses, an active lifestyle that required increased protection against bleeding, and low compliance to prophylaxis protocols due to difficulties in intravenous administration.
All patients received prophylactic doses of Emicizumab with a loading dose of 3 mg/kg bw once per week for 4 weeks, followed by a maintenance dose of 1.5 mg/kg bw weekly (interval chosen in collaboration with the patients). The first administration was carried out in the hospital by the medical staff, the second was carried out by the patient under medical supervision and the following doses were self-administered at home.
The therapeutic interval had an average of
12.8 months (4-28 months interval). There were no spontaneous bleedings, two patients had minor bleeding of the knee’s joint capsule after physical activity (managed at home with a single dose of 30 mcg/kg bw rFVIIa), one patient required an additional dose of rFVIIa (50 mcg/kg bw) before an invasive dental procedure, one patient with a low plasmatic level of inhibitors required laparoscopic cholecystectomy and two patients with a high plasmatic level of inhibitors underwent major orthopedic surgery (total knee joint arthroplasty and total hip prosthesis revision with bone allograft) more than 6 months after Emicizumab initiation (
Table 3).
The reported adverse reactions included moderate increase in blood pressure (two patients) and moderate arthralgia and myalgia (one patient). One patient stated a mild reaction at the injection site (erythema).
Patient 1
A 22-year-old patient, diagnosed at 1 year and 6 months with severe hemophilia A, with a high plasmatic level of inhibitors since 2009, has been under prophylactic treatment with aPCC since 2015 (3 administrations/week). Due to the unavailability of ITI and the late initiation of the prophylactic treatment, the patient has a poor joint status, with chronic hemophilic arthropathy of his ankles, knees and elbows and presents frequent bleeding (ABR >8). Since 2019, the patient is receiving daily administrations of aPCC 50 IU/kg bw 5 days/week because he became a physically active college student. The joint bleeding continued (ABR ~6.5), especially in the articulation capsule of the knee’s joint, with a negative impact on the patient's quality of life (missed days from classes, long hospitalizations, chronic pain and the need for crutches). Intravenous administration was increasingly difficult due to the lack of venous access and needle phobia. From March 2021, thanks to the patient support program from Roche, the patient was switched on Emicizumab treatment, with weekly subcutaneous administrations. The clinical evolution was clearly favorable: the patient had no more spontaneous joint bleeding (only in the context of intense physical effort) and his joint status improved, with no missed days from classes and no more need for crutches.
Patient 2
A 54-year-old patient with severe hemophilia A, without inhibitors, with associated cardiac pathology (hypertension, aorto-coronary bypass), was receiving chronic dual antiplatelet therapy and prophylactic treatment with pdFVIII 3 days/week since 2017. The patient addresses our service with advanced chronic arthropathy, with frequent bleeding in both knees, ankles and elbows and locomotory deficiency(walking with crutches). Due to difficulty of assuring and maintaining a viable venous access, we decided to insert a port-cath-type central venous catheter. Unfortunately, the patient experienced repeated infectious complications that required prolonged hospitalization for antibiotic therapy and the catheter extraction as a last measure. In total, the patient was fitted with 4 port-type catheters and one long-life Hickman catheter. The last infectious episode was so severe, with sepsis, hypotension, and kidney damage, that the decision was finally to initiate subcutaneous treatment with Emicizumab in May 2022. The treatment was well tolerated (occasional increase in blood pressure, myalgias and arthralgias). The costs of Emicizumab therapy are higher compared with prophylaxis using pdFVIII, but the costs are ultimately elevated by the association with prolonged days of hospitalization for the treatment of infections, bleeding episodes and surgical procedures to implant/extract central venous catheters. Adjacent hemorrhagic risks must also be taken into account. The patient's quality of life greatly improved and he did not experience any bleeding.
Patients 3 and 4
The patients, aged 36 and 64 years, with severe hemophilia A with a high plasmatic level of inhibitors, have been receiving prophylactic treatment with rFVIIa/aPCC since 2017; they had chronic hemophilic arthropathy and a history of major orthopedic interventions (total knee and hip arthroplasties) performed under treatment with rFVIIa or aPCC, with difficult evaluation of the coagulation status, requiring additional doses of factor VIII and even blood transfusions in case of the first patient. Both patients started the treatment with Emicizumab in November 2022 and after more than 6 months, both patients required other major orthopedic interventions (total left knee replacement and maintenance for the hip replacement). Perioperatively, in both cases, rFVIIa was used accordingly to the Giangrande protocol34. The preoperative bolus of rFVIIa was 120 mcg/kg bw, followed by 90 mcg/kg bw every 2 hours during first 2-3 days, with a progressive increase in administration interval between doses. Emicizumab administration continued in parallel. No bleeding complications were registered and the total number of days spent in hospital (7 days), as well as the factor consumption were lower compared to previous interventions. No adverse events, especially thrombotic ones, were reported. Postoperative monitoring was performed using chromogenic methods with bovine reagents, and dosing was adjusted based on the serum level of Emicizumab and specific coagulation assays (thromboelastography and thrombin generation time).
Laboratory evaluation
Considering that Emicizumab is an innovative drug, with a different mechanism of action compared to the usual factor concentrates and was only recently introduced into the treatment of romanian hemophilic patients, the hemostasis laboratories played an essential role in the implementation and utilization of this molecule as well as in monitoring of the patients’ response. Extensive research of the literature was necessary in order to better understand the decision-making processes, to purchase special reagents, train and adapt the laboratory staff for working protocols and calibrate the devices.
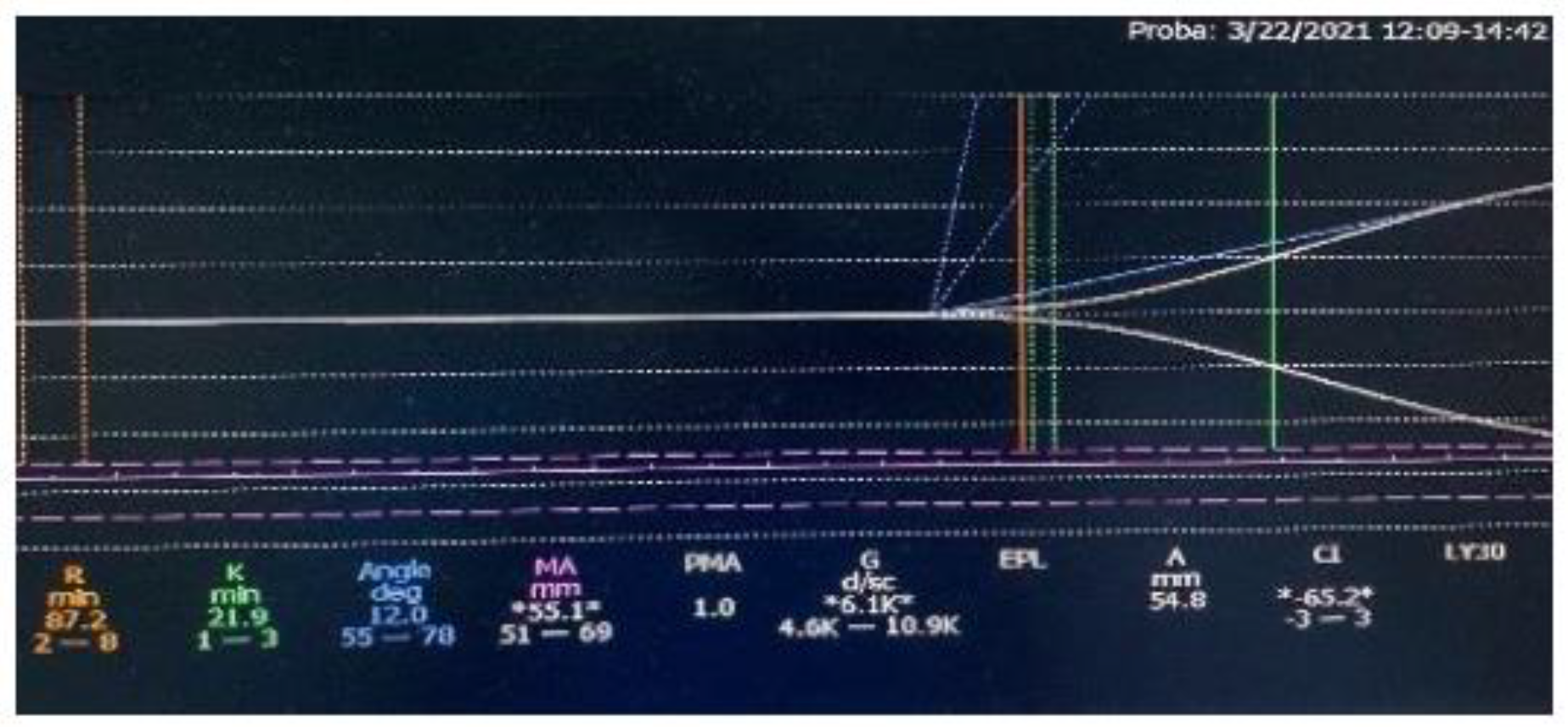
In the case of the first patient, to whom we initiated Emicizumab therapy through the patient support program, we could not use the specific chromogenic tests or monitor the Emicizumab serum level from the beginning, although these measurements were performed later. We used thrombelastography (TEG) and the thrombin generation time (TGT) to evaluate the coagulation response during the first months of treatment. Before the therapy introduction, APTT was 94.3s, FVIII was <1% and TEG indicated poor global coagulation (
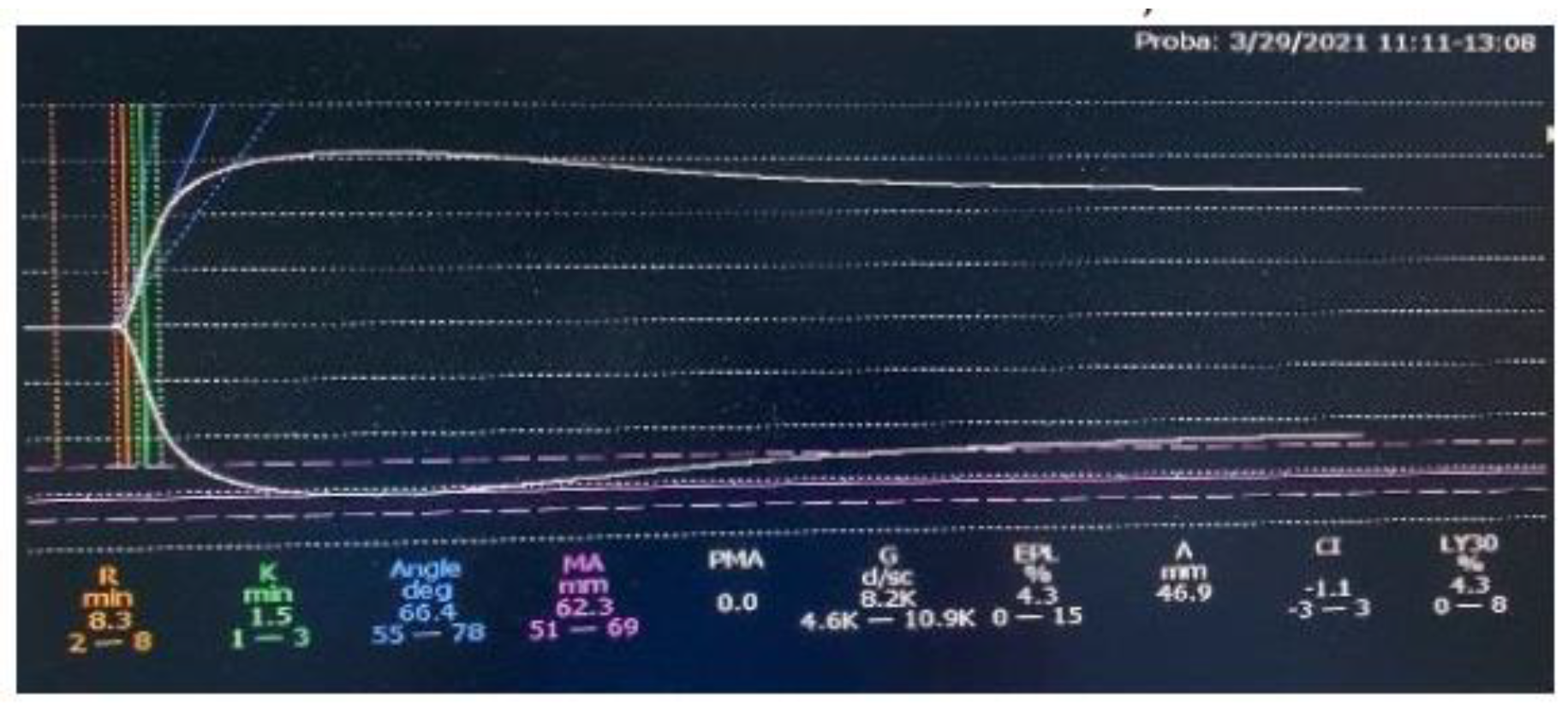
Figure 1). After 2 months of treatment, the TEG result was within normal limits (
Figure 2). The typical, one-stage assays showed that APTT and FVIII activity normalized since the beginning of Emicizumab administration, as found in the literature
28.
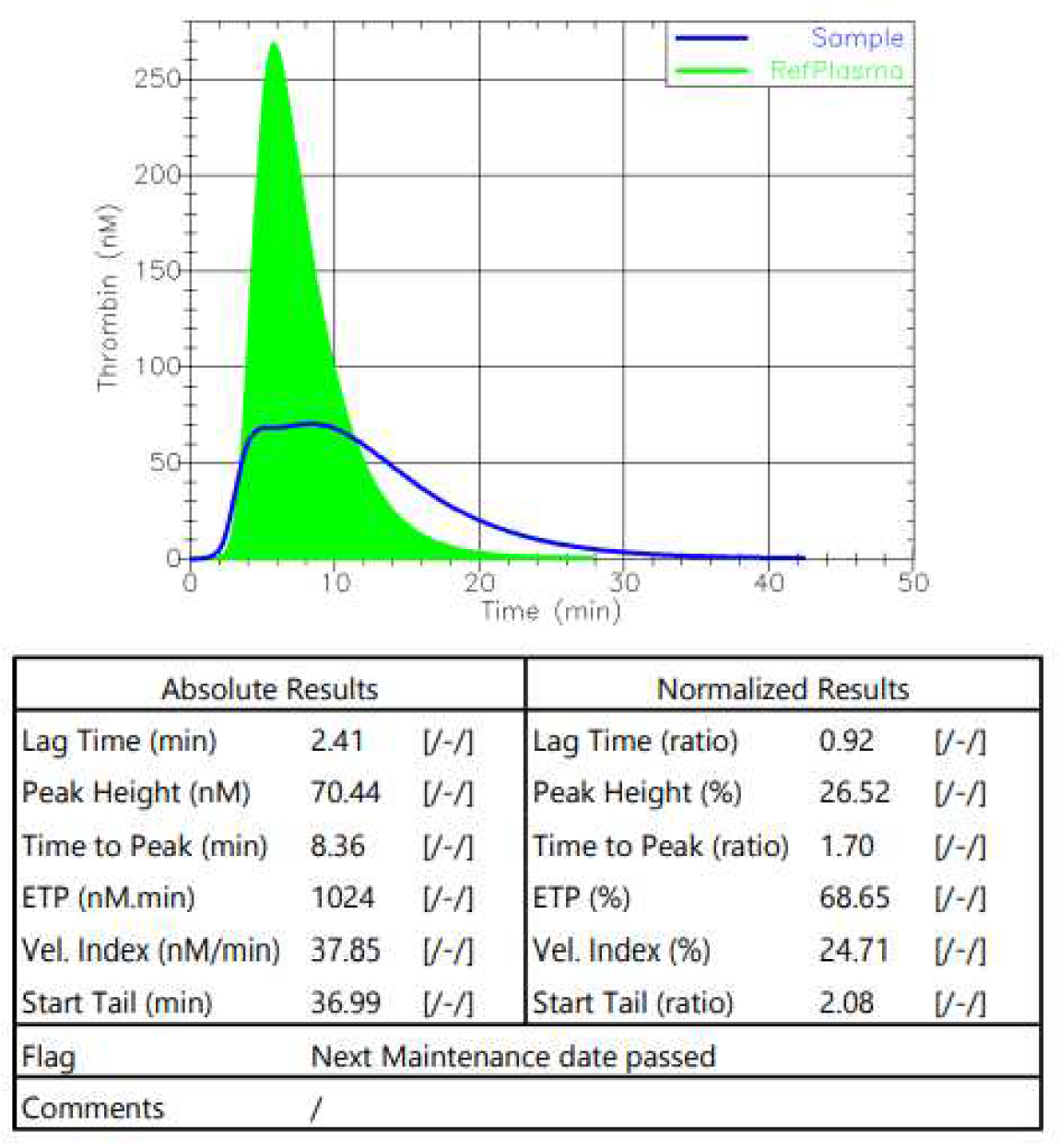
The thrombin generation time (TGT) was measured before the initiation of the therapy (
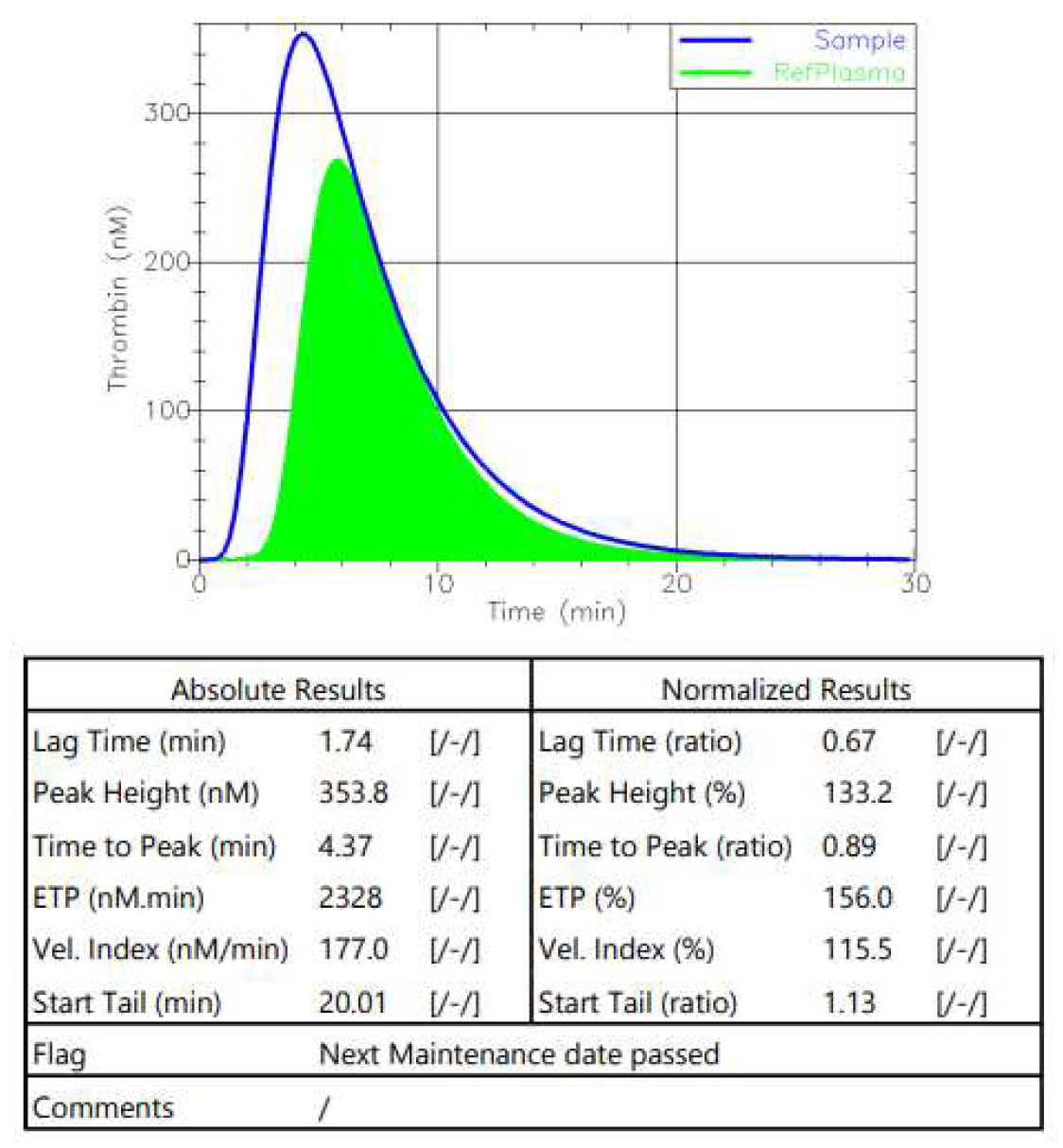
Figure 3) and after 2 months of Emicizumab (
Figure 4). The normalization of the tested parameters was observed.
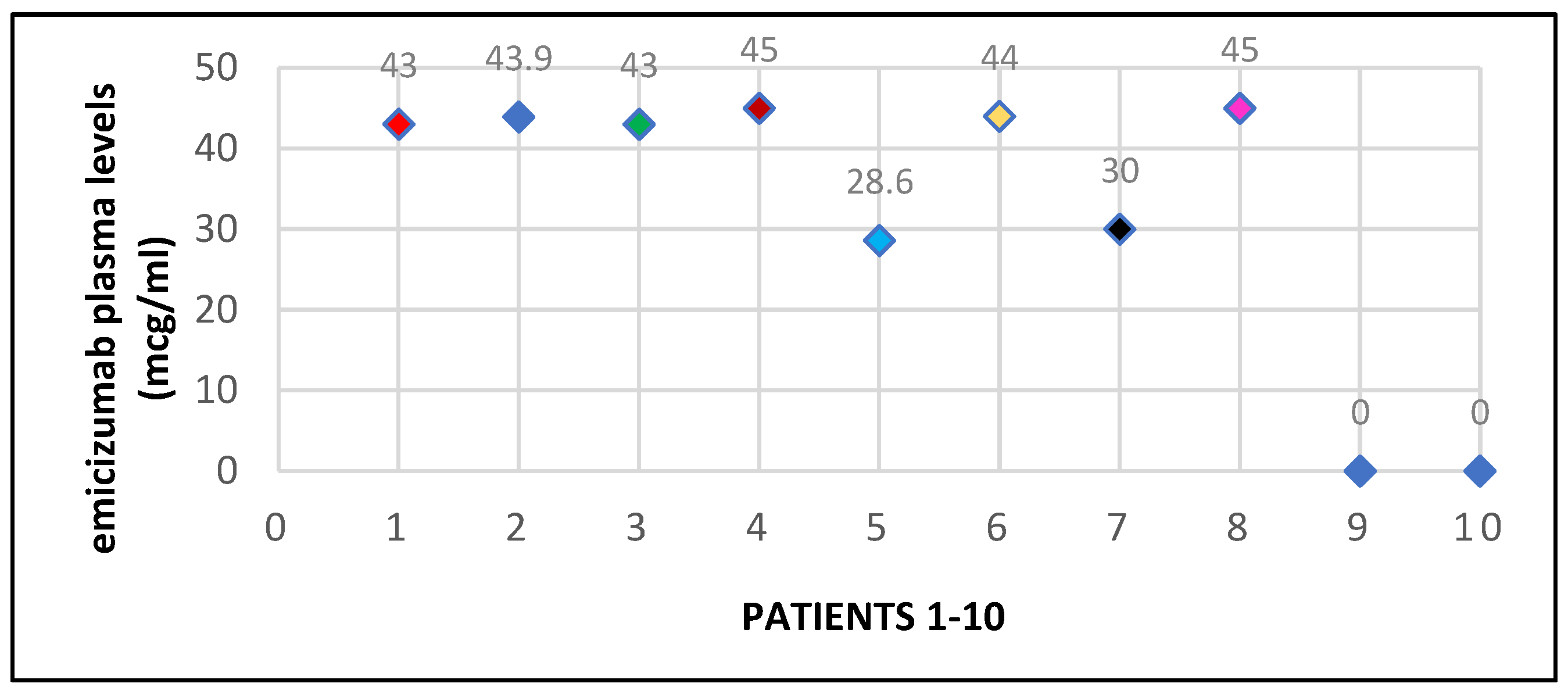
Later, the chromogenic tests with bovine reagents were also used in our clinic, accompanied by the measurement of the residual level of endogenous FVIII, APTT and the inhibitor plasmatic level. The Emicizumab level at 6 months after initiation varied between
28.6 and 45 mcg/ml, with a median of
40,31 mcg/ml (
Chart 1).
3. Discussions and Conclusions
Emicizumab is a novel therapeutic agent with reproduceable good tolerance and efficacy in preventing spontaneous, breakthrough bleeding episodes in both inhibitor and non-inhibitor hemophilia A patients.
Here, we reported our experience with prophylaxis Emicizumab for 10 adult hemophilia A patients, with and without inhibitors. The introduction of Emicizumab therapy was associated with a significant improvement in bleeding rates and in quality of life for our patients. The ease of subcutaneous administration represented a great advantage, especially among those with very difficult venous access or with a lack of self-administration skills. The increased compliance to the treatment had beneficial repercussions on the efficiency of the therapy, with an improvement in the joint status, reduction of chronic pain and improvement of quality of life.
The treatment was well tolerated, without major adverse reactions, without thrombotic events or specific antibodies formation. The impact of Emicizumab was also felt in terms of major surgical interventions, which occurred in optimal conditions in association with factor VIII or BPA and without spontaneous bleeding that would require additional measures.
The major challenge that remained was laboratory monitoring since the specific reagents were not available in our hospital from the beginning of treatment initiation and laboratory staff had to receive special training in performing these assays. Emicizumab level was measured in 8 out of 10 patients and the results were very satisfying, 75% of patients (6/8) having therapeutic levels above 40 mcg/ml. Two out of the 8 patients had lower levels (28.6 and 30 mcg/ml), but they reported no spontaneous bleeding events, suggesting that lower levels may also be effective in preventing bleeding events. Thrombin generation time and thromboelastography are useful tools in evaluating hemostatic responses, especially during major surgeries in patients with inhibitors, but they are not available in all hospitals.
The results of our study are comparable with previously reported data from both clinical trials and real-life experience, with similar decrease in ABR, highlighting the beneficial effects of Emicizumab therapy on hemophilia patients. Even if the number of patients is low, our study provides good clinical practice data for other hemophilia centers in Romania and will support the increasing demand for enrolling patients on Emicizumab and overcome the fear of associated laboratory challenges.
Author Contributions
Melen Brinza: Conceptualisation, methodology, validation, Writing – original draft, project administration; Valentina Uscatescu: resources, Writing –review & editing, validation, supervision; Mihai Hemcinschi: writing- review & editing; Elisabeta Chiriac: resources; Georgiana Gherghe: resources; Daniel Coriu: conceptualization, validation, writing – review & editing, supervision, project administration.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Local Ethics Committee (no. 41066/08.08.2023).
Informed Consent Statement
All patients consented to the use of their medical data.
Acknowledgments
We are grateful to ROCHE for their support in acquiring special reagents for lab monitoring during Emicizumab therapy. Most of all, we would like to thank the patients for taking part in this study.
Disclosures
The authors did not receive funding for this research and are not employed by any pharmaceutical company. The authors deny any conflict of interest interfering with the current study. Two patients received medication for the first 3 months thanks to the patients support program by ROCHE. The manuscript has been read and approved by all the authors and each author believes the manuscript represents honest work.
List of abbreviations:
aPCC—activated prothrombin complex concentrate
ABR—annual bleeding rate
APTT—activated partial thromboplastin time
BPA—bypassing agent
BU—Bethesda units
FVIII—factor VIII
FIXa—activated Factor IX
FX – factor X
ITI—immune tolerance induction
IU—international units
kg BW—kilograms of body weight
mcg – microgram
ml - milliliter
NTFs—non-factor therapies
pdFVIII—plasma-derived factor VIII
rFVIIa—recombinant activated factor VII
TEG—thromboelastography
TGA—time for the generation of thrombin assay
TMA—thrombotic microangiopathy
References
- Srivastava, A.; Brewer, A.K.; Mauser-Bunschoten, E.P.; Key, N.S.; Kitchen, S.; Llinas, A.; Ludlam, C.A.; Mahlangu, J.N.; Mulder, K.; Poon, M.C.; et al. Guidelines for the management of hemophilia. Haemophilia 2013, 19, e1–e47. [Google Scholar] [CrossRef]
- Carcao, M.D. The Diagnosis and Management of Congenital Hemophilia. Semin. Thromb. Hemost. 2012, 38, 727–734. [Google Scholar] [CrossRef]
- Srivastava, A.; Santagostino, E.; Dougall, A.; Kitchen, S.; Sutherland, M.; Pipe, S.W.; Carcao, M.; Mahlangu, J.; Ragni, M.V.; Windyga, J.; et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia 2021, 27 (Suppl. 6), 699. [Google Scholar] [CrossRef] [PubMed]
- Khair, K.; Mazzucconi, M.G.; Parra, R.; Santagostino, E.; Tsakiris, D.A.; Hermans, C.; Oldenburg, J.; Spotts, G.; Steinitz-Trost, K.; Gringeri, A.; et al. Pattern of bleeding in a large prospective cohort of haemophilia A patients: A three-year follow-up of the AHEAD (Advate in HaEmophilia A outcome Database) study. Haemophilia 2018, 24, 85–96. [Google Scholar] [CrossRef]
- Oldenburg, J.; Khair, K.; Mazzucconi, M.G.; et al. Real World prospective data on bleeding frequency in 1,000 patients with hemophilia A – is the goal of zero bleeds achievable? In Proceedings of the 10th Annual Congress of the European Association for Haemophilia and Allied Disorders 2017, Paris, France, February 2017; pp. 1–3. [Google Scholar]
- Manco-Johnson, M.J.; Abshire, T.C.; Shapiro, A.D.; Riske, B.; Hacker, M.R.; Kilcoyne, R.; Ingram, J.D.; Manco-Johnson, M.L.; Funk, S.; Jacobson, L.; et al. Prophylaxis versus Episodic Treatment to Prevent Joint Disease in Boys with Severe Hemophilia. New Engl. J. Med. 2007, 357, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Manco-Johnson, M.J.; Lundin, B.; Funk, S.; Peterfy, C.; Raunig, D.; Werk, M.; Kempton, C.L.; Reding, M.T.; Goranov, S.; Gercheva, L.; et al. Effect of late prophylaxis in hemophilia on joint status: a randomized trial. J. Thromb. Haemost. 2017, 15, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; You, C.W. The prevalence and risk factors of inhibitor development of FVIII in previously treated patients with hemophilia A. BLOOD Res. 2019, 54, 204–209. [Google Scholar] [CrossRef]
- Petrini, P.; A Valentino, L.; Gringeri, A.; Re, W.M.; Ewenstein, B. Individualizing prophylaxis in hemophilia: a review. Expert Rev. Hematol. 2015, 8, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Giangrande, P.L.F.; Hermans, C.; O’mahony, B.; de Kleijn, P.; Bedford, M.; Batorova, A.; Blatný, J.; Jansone, K.; on behalf of the European Haemophilia Consortium (EHC) and the European Association for Haemophilia and Allied Disorders (EAHAD). European principles of inhibitor management in patients with haemophilia. Orphanet J. Rare Dis. 2018, 13, 66. [Google Scholar] [CrossRef]
- Matino, D.; Makris, M.; Dwan, K.; D'Amico, R.; Iorio, A. Recombinant factor VIIa concentrate versus plasma-derived concentrates for treating acute bleeding episodes in people with haemophilia and inhibitors. Cochrane Database Syst. Rev. 2015, 2015, CD004449. [Google Scholar] [CrossRef]
- Gringeri, A.; Mantovani, L.G.; Scalone, L.; Mannucci, P.M.; COCIS Study Group. Cost of care and quality of life for patientswith hemophilia complicated by inhibitors: the COCIS Study Group. Blood 2003, 102, 2358–2363. [Google Scholar] [CrossRef]
- Scalone, L.; Mantovani, L.G.; Mannucci, P.M.; Gringeri, A. THE COCIS STUDY INVESTIGATORS1 Quality of life is associated to the orthopaedic status in haemophilic patients with inhibitors. Haemophilia 2006, 12, 154–162. [Google Scholar] [CrossRef]
- Morfini, M.; Haya, S.; Tagariello, G.; Pollmann, H.; Quintana, M.; Siegmund, B.; Stieltjes, N.; Dolan, G.; Tusell, J. European Study on Orthopaedic Status of haemophilia patients with inhibitors. Haemophilia 2007, 13, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Manco-Johnson, M.J.; Lundin, B.; Funk, S.; Peterfy, C.; Raunig, D.; Werk, M.; Kempton, C.L.; Reding, M.T.; Goranov, S.; Gercheva, L.; et al. Effect of late prophylaxis in hemophilia on joint status: a randomized trial. J. Thromb. Haemost. 2017, 15, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Petrini, P.; A Valentino, L.; Gringeri, A.; Re, W.M.; Ewenstein, B. Individualizing prophylaxis in hemophilia: a review. Expert Rev. Hematol. 2015, 8, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Valentino, L.A.; Kawji, M.; Grygotis, M. Venous access in the management of hemophilia. Blood Rev. 2011, 25, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, T.; Igawa, T.; Sampei, Z.; Muto, A.; Kojima, T.; Soeda, T.; Yoshihashi, K.; Okuyama-Nishida, Y.; Saito, H.; Tsunoda, H.; et al. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat. Med. 2012, 18, 1570–1574. [Google Scholar] [CrossRef]
- HEMLIBRA product characteristics:https://www.ema.europa.eu/en/documents/product-information/hemlibra-epar-product-information_ro.pdf.
- Mahlangu, J.; Oldenburg, J.; Paz-Priel, I.; Negrier, C.; Niggli, M.; Mancuso, M.E.; Schmitt, C.; Jiménez-Yuste, V.; Kempton, C.; Dhalluin, C.; et al. Emicizumab Prophylaxis in Patients Who Have Hemophilia A without Inhibitors. New Engl. J. Med. 2018, 379, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, J.; Mahlangu, J.N.; Kim, B.; Schmitt, C.; Callaghan, M.U.; Young, G.; Santagostino, E.; Kruse-Jarres, R.; Negrier, C.; Kessler, C.; et al. Emicizumab Prophylaxis in Hemophilia A with Inhibitors. New Engl. J. Med. 2017, 377 (Supplementary Appendix), 809–818. [Google Scholar] [CrossRef]
- Callaghan, M.U.; Negrier, C.; Paz-Priel, I.; Chang, T.; Chebon, S.; Lehle, M.; Mahlangu, J.; Young, G.; Kruse-Jarres, R.; Mancuso, M.E.; et al. Long-term outcomes with emicizumab prophylaxis for hemophilia A with or without FVIII inhibitors from the HAVEN 1-4 studies. Blood 2021, 137, 2231–2242. [Google Scholar] [CrossRef]
- Gelbenegger, G.; Schoergenhofer, C.; Knoebl, P.; Jilma, B. Bridging the Missing Link with Emicizumab: A Bispecific Antibody for Treatment of Hemophilia A. Thromb Haemost. 2020, 120, 1357–1370. [Google Scholar] [CrossRef]
- Paz-Priel, I.; Chang, T.; Asikanius, E.; Chebon, S.; Emrich, T.; Fernandez, E.; Kuebler, P.; Schmitt, C. Immunogenicity of Emicizumab in People with Hemophilia A (PwHA): Results from the HAVEN 1-4 Studies. Blood 2018, 132 (Suppl. S1). [Google Scholar] [CrossRef]
- Food and Drug Administration. HEMLIBRA® (emicizumab-kxwh) injection for subcutaneous use, prescribing information. Initial U.S. approval: 2017. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761083s000lbl.pdf. Accessed January 9, 2019. 9 January 2019.
- Sampei, Z.; Igawa, T.; Soeda, T.; et al. Identification and multidimensional optimization of an asymmetric bispecific IgG antibody mimicking the function of factor VIII cofactor activity. PLoS One 2013, 8, e57479. [Google Scholar] [CrossRef] [PubMed]
- Lenting, P.J.; Denis, C.V.; Christophe, O.D. Emicizumab, a bispecific antibody recognizing coagulation factors IX and X: how does it actually compare to factor VIII? Blood 2017, 130, 2463–2468. [Google Scholar] [CrossRef] [PubMed]
- Castaman, G.; Santoro, C.; Coppola, A.; E Mancuso, M.; Santoro, R.C.; Bernardini, S.; Pugliese, F.R.; Lubrano, R.; Golato, M.; Tripodi, A.; et al. Emergency management in patients with haemophilia A and inhibitors on prophylaxis with emicizumab: AICE practical guidance in collaboration with SIBioC, SIMEU, SIMEUP, SIPMeL and SISET. Blood Transfus. 2020, 18, 143–151. [Google Scholar] [CrossRef]
- Jonsson, F.; Mercier, F.; et al. Exposure-response modeling of emicizumab for the prophylaxis of bleed counts in hemophilia A patients. In Proceedings of the 27th Meeting of the Population Approach Group in Europe (PAGE), Montreux, Switzerland; 2018. [Google Scholar]
- Müller, J.; Pekrul, I.; Pötzsch, B.; Berning, B.; Oldenburg, J.; Spannagl, M. Laboratory Monitoring in Emicizumab-Treated Persons with Hemophilia A. Thromb Haemost. 2019, 119, 1384–1393. [Google Scholar] [CrossRef]
- Adamkewicz, J.I.; Chen, D.C.; Paz-Priel, I. Effects and Interferences of Emicizumab, a Humanised Bispecific Antibody Mimicking Activated Factor VIII Cofactor Function, on Coagulation Assays. Thromb Haemost. 2019, 119, 1084–1093. [Google Scholar] [CrossRef]
- Nardi, M.A. Emicizumab and the clinical laboratory. American Society for Clinical Laboratory Science 2020, ascls.119. [Google Scholar] [CrossRef]
- Jonsson, F.; Schmitt, C.; Petry, C.; Mercier, F.; Frey, N.; Retout, S. Exposure–Bleeding Count Modeling of Emicizumab for the Prophylaxis of Bleeding in Persons with Hemophilia A with/Without Inhibitors Against Factor VIII. Clin. Pharmacokinet. 2021, 60, 931–941. [Google Scholar] [CrossRef]
- Giangrande, P.L.F.; Wilde, J.T.; Madan, B.; Ludlam, C.A.; Tuddenham, E.G.D.; Goddard, N.J.; Dolan, G.; Ingerslev, J. Consensus protocol for the use of recombinant activated factor VII [eptacog alfa (activated); NovoSeven®] in elective orthopaedic surgery in haemophilic patients with inhibitors. Haemophilia 2009, 15, 501–508. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).