1. Introduction
Polycystic ovary syndrome is a complex multisystem disorder arising from exposure to a variety of environmental and lifestyle factors in women with a genetic predisposition (1,2). The core pathophysiological features include chronic low-grade systemic inflammation, insulin resistance, hyperandrogenism, and disruption of the microbiome (3). A range of lifestyle and environmental factors have been implicated in the pathogenesis of PCOS, including diet, exercise, sleep disruption, stress, disturbance to the gastrointestinal microbiome, and endocrine disrupting chemicals (1,4). Recent evidence has identified microparticulate air pollution (MAP), as a factor that can dysregulate internal homeostasis and cause changes that initiate and exacerbate the pathophysiological processes involved in PCOS (5–7).
PCOS is a metabolic and endocrine condition affecting 10-13% of reproductive age women that is thought to be increasing in prevalence globally (8–10). Women with PCOS have an increased risk of subfertility, pregnancy complications (deep venous thrombosis, pre-eclampsia, macrosomia, fetal growth restriction, miscarriage, and preterm labour) psychological disorders (anxiety, depression, and eating disorders) and can progress to a range of other metabolic-related conditions (obesity, gestational diabetes, type 2 diabetes [T2DM], metabolic syndrome, cardiovascular disease, and cancer) (11–13). PCOS therefore makes a significant contribution to the chronic disease epidemic (14). The population attributable risk of PCOS to T2DM alone has been estimated at 19-28% of reproductive age women (15). Environmental factors such as EDC have been associated with PCOS and international professional bodies have recommended all pregnant women be advised of the risks of EDC and that education programs be developed to inform health professionals (16–18). Microparticulate air pollution is emerging as an environmental risk factor with significant health effects.
Microparticulate air pollution (MAP) is a global threat to health and wellbeing throughout the lifespan and has been identified as a possible contributing factor to a number of conditions including PCOS. The Global Burden of Disease Study has listed air pollution as the fourth leading cause of death in females worldwide, and the third cause of disability-adjusted-life-years (19). Previous studies estimate that 4-9 million premature deaths worldwide are attributable to microparticulate air pollution each year (20). The widespread presence of MAP has been identified as a contributing factor to increased risk of chronic disease including respiratory illnesses including asthma and chronic obstructive airway disease (21), T2DM (6), cardiovascular disease (22), dementia (23), autoimmune disease (24), infertility (25), and reduced life expectancy (26).
Recent observational studies have explored the influence of MAP on pulmonary-induced chronic systemic inflammation as a possible contributor to the pathogenesis of PCOS (5). One of the proposed mechanisms of the pathogenesis of PCOS relates to diet-induced increased gastrointestinal permeability which promotes chronic inflammation, insulin resistance (IR), hyperandrogenism and the observed endocrine and metabolic features of PCOS (1,4,27). It has been suggested that these same pathways may be disrupted in women with PCOS exposed to MAP (5). In addition, MAP may have an impact on fetal epigenetic programming and therefore play a role in the transgenerational transmission of PCOS (28).
This study aimed to identify and systematically review evidence from observational studies on the associations between MAP and PCOS and there was no attempt to synthesize the data.
2. Materials and Methods
The literature search was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. This systematic review is based only on published articles, and no patient or ethical approval was required.
We searched PubMed, Cochrane, and Scopus databases for articles published in the English language using the following MeSH search terms: “polycystic ovary syndrome” AND “microparticulate air pollution”; “polycystic ovary syndrome” AND “air pollution”; “PCOS” AND “microparticulate air pollution”; “PCOS” AND “air pollution”; “polycystic ovarian syndrome” AND “microparticulate air pollution”; “polycystic ovarian syndrome” AND “air pollution”.
The selection criteria for the review included original articles (prospective observational studies, retrospective cohort studies and case-control studies). Studies were included if they reported outcomes related to PCOS and MAP exposure. Articles were excluded if they were not in English. Titles and abstracts were examined by two reviewers (NS and JP) and full articles that met the selection criteria were retrieved. A manual search of review articles and cross-references completed the search.
The results are presented as a systematic review of the available evidence examining the relationship between MAP and PCOS. No attempt was made to combine the results in a meta-analysis due to the degree of heterogeneity and the small number of studies identified.
3. Results
3.1. Characteristics of the studies
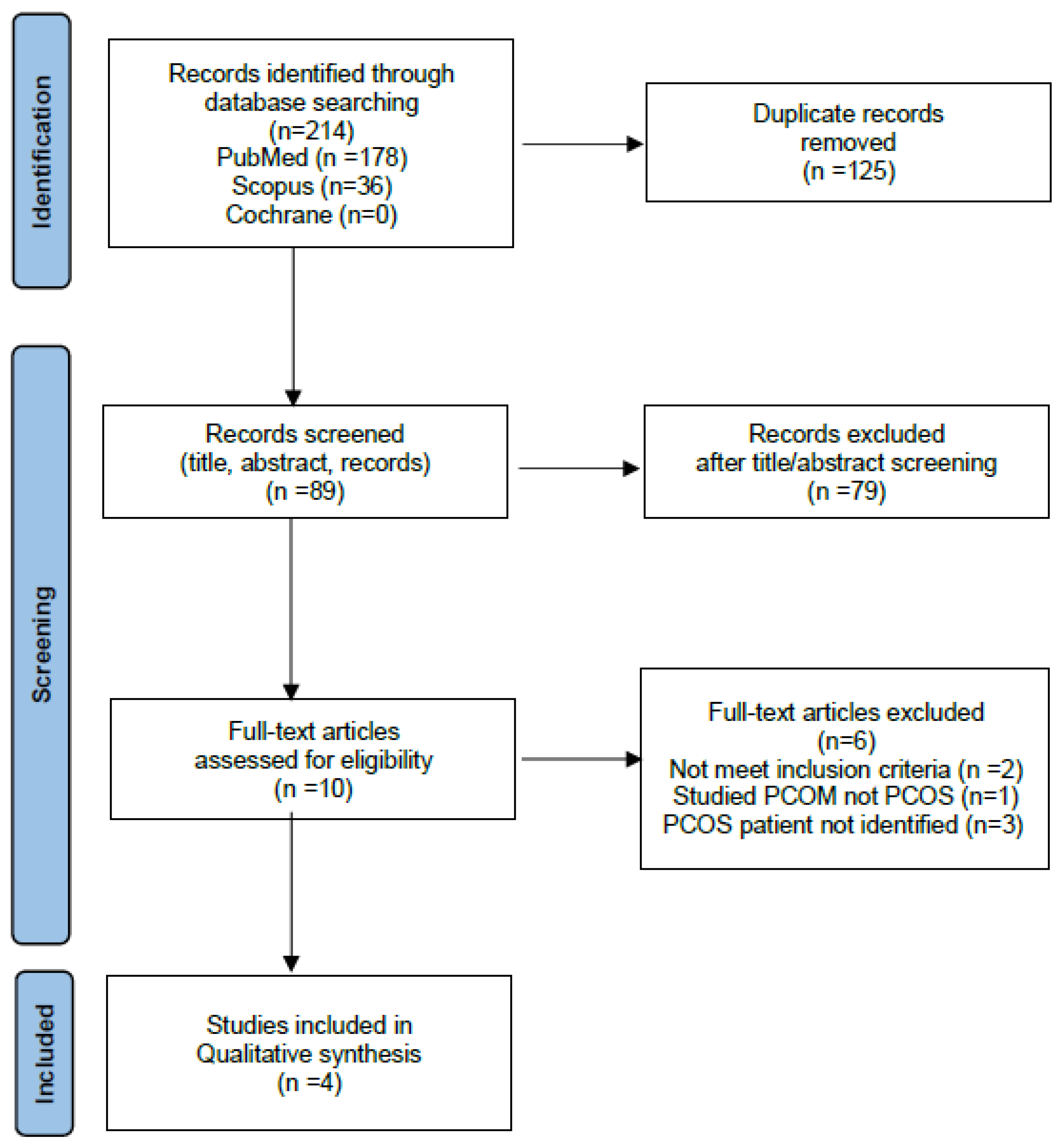
The initial literature review identified 214 records from PubMed, Cochrane, and Scopus databases. 125 duplicates were removed, and 89 reports were assessed by screening titles and abstracts. Ten full text articles were reviewed and 6 were excluded as they did not meet the inclusion criteria. Details of the selection process are shown in the PRISMA flow diagram (
Figure 1). A summary of the study design, exposure investigated, and the main findings are shown in
Table 1. The 4 studies identified in the systematic review are discussed in detail in section 3.0.
3.2. Summary of the studies identified in the systematic review on the relationship between fine particulate air pollution and polycystic ovary syndrome
3.2.1. Taiwan nationwide cohort study (5)
A large nationwide cohort analysis investigated the risk of a diagnosis of new-onset PCOS relative to the level of a range of air pollutants (5). Data were extracted from 2 nation-wide databases: Taiwan Longitudinal Health Insurance Database (LHID), which covers over 95% of Taiwan residents, and Taiwan Air Quality Monitoring Database (TAQMD). Women that already had a diagnosis of PCOS were excluded. A total of 91,803 female participants were enrolled and followed until the first diagnosis of PCOS. They used the International Classification of Diseases to identify women with a diagnosis of PCOS. A total of 2,072 women had a new diagnosis of PCOS during the study period, after a mean follow-up time of 7.76 (+/-3.79) years.
The TAQMDB provides data from 78 air quality monitoring stations distributed across Taiwan. Data on daily concentrations of sulfur dioxide (SO2), nitrogen oxides (NOx), nitrogen monoxide (NO), nitrogen dioxide (NO2), and suspended particulates (PM2.5), were used in the study. The investigators integrated the daily concentrations of air pollutants corresponding to residential address to calculate the annual average exposure to air pollutants. The concentrations of air pollutants were divided into four quartiles. A multivariate Cox proportional hazard regression model was used to adjust for confounding factors such as living area, urbanization level (categorized by population density), monthly income, and occupational class (white-collar, blue-collar, other).
Women in the fourth quartile levels of exposure had a significantly increased risk of developing PCOS when compared with women in the first quartile level of exposure, for all air pollutants studied. The increased risk was 10.31 times to SO2 (95% CI = 8.35-12.7), 3.37 times to NOx (95% CI = 2.86-3.96), 4.18 times to NO (95% CI = 3.57-4.89), 7.46 times to NO2 (95% CI = 6.38-8.71), and 3.56 times to PM2.5 (95% CI = 3.05-4.15). The authors concluded that women exposed to high concentrations of air pollutants, including microparticulate matter, had a high risk of developing PCOS.
3.2.2. Korean population-based cohort study (29)
This retrospective population-based cohort study examined the relationship between the level and duration of air pollution exposure and the risk of developing PCOS (29). Data were extracted from the Korean National Health Information Database (NHID) which provides comprehensive medical services to all Koreans. The investigators used the Korean Informative Classification of Disease to identify women newly diagnosed with PCOS. A total of 237,582 cases of PCOS were analyzed using a spatial prediction model to assess individual-level exposure to air pollutants.
Exposure to air pollutants was determined using nationwide real-time data on outdoor pollutants from 355 monitoring stations provided by the Ministry of Environment Korea. Monthly average concentrations of were used to calculate the risk effect of developing PCOS over 1-year, 2-year, and 3-year exposure to PM10, PM2.5, Ozone (O3), Carbon Monoxide (CO), SO2, and NO2. The residence of participants was matched to the concentration of air pollutants and logistic regression analysis was used to investigate the effect of exposure duration and levels of air pollutants on PCOS risk.
The annual age-adjusted incidence of PCOS progressively increased from 6.7% to 11.97% during the 5-year study period. The risk of PCOS significantly increased following 3-years exposure to PM2.5, O3, and NO2, compared to the 1-year average exposure. The adjusted odds ratio (OR) was 1.32 for 3-year exposure to PM2.5, compared to 1-year (95% CI = 1.27-1.37). However, although the 1-year, 2-year, and 3-year risk of PCOS was increased, the risk of PCOS decreased as the concentration of PM2.5 increased. In contrast to the PM2.5 results, the risk of PCOS increased with increasing concentrations of NO2, SO2, and CO. The authors concluded that the risk of PCOS increased in parallel to the duration of exposure to air pollutants, including PM2.5, but was not increased by exposure to higher concentrations of PM2.5.
The similarities and differences between the Taiwan study and the Korean study demonstrate the challenges presented when assessing the risk of disease occurrence based on population databases and air quality monitoring systems. The reported differences may be due to multiple factors including the diagnosis of PCOS, other compounding variables, accuracy of the database information, exposure duration to air pollutants, measurement method of PM2.5, combination effects of multiple air pollutants in different geographic areas, and assessment models used for data analysis.
In conclusion, the combined results of these two large population-based studies raise concerns about the duration and concentrations of multiple air pollutants, including fine particulate matter, on the risk of developing PCOS. This is a significant concern when considered in the context of the extensive body of research on the effects of air pollution on the pathogenesis of other chronic diseases. These data support the need for further studies, and precautionary action, to mitigate future risk from increasing air pollution in women at risk of developing PCOS.
3.2.3. Exposure to second-hand smoke and PCOS (30)
This study investigated the influence of second-hand smoke (SHS) exposure from husband’s smoke on metabolic, endocrine, fertility and obstetric outcomes in women with PCOS (30). This was a prospective observational study of 500 women that compared 271 women exposed to SHS with 229 women not exposed. The data was a secondary analysis of the Polycystic Ovary Syndrome Acupuncture and Clomiphene randomized controlled trial conducted at 27 hospitals in mainland China.
The study participants were Chinese women with PCOS who were undergoing ovulation induction for anovulatory infertility. PCOS was defined by the Rotterdam criteria and SHS exposure was defined as living with a partner who was a chronic smoker on a daily basis for 6 months. The exposure group was divided into high (> 10 cigarettes/day) and low (< 10 cigarettes/day) exposure groups for analysis. The study cohort consisted of 229 women with PCOS exposed to SHS, of whom 59 were classified as high-exposed and 170 were low-exposed. All participants had baseline anthropomorphic, biochemical, and endocrine assessments.
Women in the SHS exposed group had significantly higher total serum testosterone and free androgen index, and lower sex hormone binding globulin (SHBG), compared to non-exposed women. Further subgroup analysis demonstrated a dose-response effect with women in the high-exposed SHS group having significantly higher total testosterone (1.8 vs 1.5 nmol/L, p<0.001), free androgen index (7.2 vs 4.0, p<0.001), and lower SHBG (vs 35.6 vs 25 nmol/L, p<0.001), compared to women in the low-exposed group. The rate of metabolic syndrome was higher in both high and low-exposed groups, compared to the non-exposed. Exposure to SHS was also associated with lower conception rate in women with PCOS. There were no significant differences in gestational age, birth weight and other obstetric outcomes between the groups.
Cigarette smoke is an aerosol of over 5000 chemicals that consists of noxious gases and volatile chemicals including microparticulate matter and nicotine (32). The particulate matter component consists of thousands of chemicals, including alkaloids, polyaromatic hydrocarbons, tobacco-specific nitrosamines, polonium-210, nickel, cadmium, arsenic, and lead (33). Nicotine has been shown to inhibit aromatase, which is the enzyme responsible for converting testosterone to estrogens (34,35). This may be one mechanism contributing to the finding of increased androgens in the current study. These findings warrant further investigation since altered aromatase activity is known to contribute to hyperandrogenism in women with PCOS (3,36).
The findings of Li et al therefore support previous research on the risk of SHS exposure to a large section of the community. It has been estimated that worldwide 65% of non-smoking adults and 40% of children are exposed to SHS (37). According to a World Health Organization report, SHS exposure causes 1.2 million deaths per year, in non-smokers (38). The percentage of women exposed to SHS has been estimated to be 70% in China (39) and almost 50% in the United States (40). Households of smokers have been found to have higher particulate matter (PM) concentrations than non-smoking houses (41). Exposure to SHS has also been associated with increased risks of implantation failure and live birth rates in infertile women (42).
In summary, the study of Li et al found that biochemical hyperandrogenism and metabolic syndrome were more common, and conception rates were lower, in women with PCOS and anovulatory infertility exposed to SHS. These data are consistent with a large body of epidemiological research showing a range of adverse effects of SHS in women. The authors recommended that smoking partners of infertile women with PCOS should be advised to quit smoking.
3.2.4. Air pollution exposure and pregnancy outcomes (31)
Zhu et al (31) performed a retrospective cohort study of 1,652 women with PCOS compared to a control group of 12,543 women with tubal factor or male factor infertility receiving in-vitro fertilization treatment. The investigators evaluated the average daily concentrations of exposure to PM2.5, PM10, SO2, NO2, CO, and O3, during 6 different exposure periods of the IVF cycle. Daily concentration data were obtained from the National Urban Air Quality Real-time Publishing Platform, in the nearest monitoring station to each patient.
The study examined differences in ultrasound determined clinical pregnancy rates at 35 days after embryo transfer, and live birth rate, defined as at least one infant born alive after 24 weeks of gestation that survived more than 28 days. The results demonstrated that exposure to air pollutants were not associated with reduced clinical pregnancy rates or live birth rates in women with PCOS. In contrast, control women with tubal factor or male factor infertility were found to have lower clinical pregnancy and live birth rates.
However, there were significant differences between the study and control populations. The PCOS women were younger, had more primary infertility, higher body mass index (BMI), less embryo’s transferred, and less fresh embryo transfers. The investigators repeated the sensitivity analysis after adjusting for age, BMI, and first embryo transfer cycle, and no significant difference was found compared with the original analysis. The authors point out a number of limitations with their investigation including the retrospective nature of the study, lack of personal ambient air pollution exposure data, and a range of other potential confounders. Nevertheless, it is concerning that women without PCOS were found to have negative pregnancy outcomes related to air pollutant exposure.
Previous studies have shown that ambient particulate matter can adversely affect sperm quality (43) and other pregnancy outcomes in the general population (31,44). Epidemiological data have linked air pollution exposure to miscarriage, preterm birth (45,46), low birthweight (45), and preeclampsia (47). Proximity to the source of air pollution is also a factor for adverse pregnancy outcomes. However, there are no studies that have found an association between adverse pregnancy outcomes and MAP in women with PCOS. The findings of Zhu et al are consistent with reported adverse effects of air pollution in pregnancy and highlight the need for precautionary action in women with PCOS.
4. Discussion
An increasing body of epidemiological research has identified a range of components in air pollution, including microparticulate matter, as risk factors for the development of chronic disease. Polycystic ovary syndrome is a lifelong condition that can lead to a variety of chronic metabolic diseases that have been associated with MAP exposure. This systematic review has identified preliminary evidence that MAP may be a risk factor in PCOS. Both the concentration and duration of exposure to PM2.5 may be important. In addition, reproductive outcomes during infertility treatment may be impacted by exposure to PM2.5 in air pollution, from second-hand cigarette smoke, and ambient air pollution.
Air pollution is an increasing global problem that has been associated with a wide variety of human diseases, including PCOS (5,29–31,48–50). Air polution consists of a range of components including gases (SO2, NOx, NO, NO2) and suspended particulates of different sizes. Particulate matter is classified by the size of the particles into PM10 (diameter < 10 micrometers), PM2.5 (diameter < 2.5 micrometers), and ultrafine particles (diameter < 0.1 micrometers), that are suspended in the atmosphere (48,51). PM2.5 consist of natural (pollens, mineral dust) and anthropomorphic constituents (copper, nickel, carbonaceous materials, persistant organic pollutants, polycyclic aromatic hydrocarbons, gases) (24,52). Second hand smoke is a form of air pollution that contains a large number of toxic compounds, including PM2.5 (30,32). According to the World Health Organization (WHO) guidelines, the maximum mean annual concentrations of PM2.5 should not exceed 5 mg/m3 (49). Microparticulate air pollution is believed to account for a large proportion of the public health impact of air pollution, including reproductive diseases (50,52).
MAP has been associated with menstrual irregularity (53), infertility (25,30), adverse pregnancy outcomes (31), increased incidence of PCOS (5,29), and has also been linked to transgenerational transmission and infant weight gain (54,55). Human studies have shown that metabolites of tobacco (cadmium, cotine) can be found in ovarian follicular fluid and may potentially affect the developing egg (42,53). In-vitro studies have demonstrated that diesel exhaust particles have both estrogenic and androgenic activity, raising the possiblity of adverse hormonal effects in women’s health (56). In summary, all of above health-related effects can occur in women with PCOS and raise significant concerns regarding the effects of MAP in PCOS. Microparticulate air pollution has also been asociated with chronic systemic inflammation (6) and IR (57,58), both of which are known to be core pathophysiological components of PCOS (1,3).
Large systematic reviews confirm an important role for chronic systemic inflammation in the pathogenesis and pathophysiology of PCOS (59,60). Acute inflammation is an evolutionary-conserved physiological survival mechanism that occurs in response to exposure to non-infectious (trauma, reactive oxygen species, uric acid, cytokines, exosomes) and infectious agents (61). Optimal health is achieved when a balance between pro- and anti-inflammatory processes restores homeostasis. Chronic inflammation can be caused by poor-quality diet, disturbance of the microbiome (dysbiosis), endocrine disrupting chemicals, oxidative stress, advanced glycation end-products, neuroendocrine imbalance due to stress, and hyperandrogenism (62). Chronic low-grade systemic inflammation can occur when there is a persistent stimulus that results in failure of homeostatic mechanisms, and is a cornerstone of PCOS pathophysiology (3). MAP can act as a persistent chronic inflammatory stimulus, and has increasingly been recognized as a risk factor in the pathogenesis of PCOS (5,29,30).
Particulate matter can deeply penetrate the respiratory passages and lungs, to lodge in the alveoli (63). The finer particles can translocate across the alveolar epithelial cells where they are absorbed by submucosal immune cells (51). Immune activation can cause oxidative stress, DNA damage, respiratory and systemic inflammation, and changes in the biochemistry and physiology of cells (64). Microparticulate air pollution has also been associated with chronic inflammation, endothelial injury, respiatory disease, and cardiovascular disease (63,65,66). Episodic PM2.5 exposure has been associated with endothelial cell apoptosis, an antiangigenic plasma profile, and elevated levels of monocytes and T-lymphocytes (65). MAP was also associated with increased levels of microparticles in endothelial cells and an increase in systemic antiangiogenic cytokines in healthy adults (65).
Rodent studies have also demonstrated adverse effects of maternal PM2.5 exposure on fetal and placental development (67). Bove et al demonstrated the accumulation of black carbon particles on the fetal side of the placenta (68). The average particle count was increased in high exposed mothers compared to women with lower MAP exposure levels. These data suggest that atmospheric particulate matter can be transported across the placenta and may represent a mechanism that could explain the detrimental health effects of in-utero exposure to MAP, including adverse developmental programming. In summary, these reports raise the possibility that PM2.5 may be a factor in placental dysfunction and adverse pregnancy outcomes in women with PCOS.
Insulin resistance can be defined as decreased tissue sensitivity and cellular response to insulin, and is known to be present in the majority of women with PCOS (69,70). The gold standard for assessing IR is the hyperinsulinemic-euglycemic clamp test (71). A systematic review of hyperinsulinemic-euglycemic clamp studies reported that women with PCOS have a 27% reduction in insulin sensitivity (72). Insulin resistance can be caused by multiple factors including a high-glycaemic diet, metabolic intermediates such as diacylglycerol, hyperinsulinaemia, insulin receptor antagonists, hormones, oxidative stress, advanced-glycation end-products, and inflammatory cytokines (73,74).
Physiological IR has an evolutionary adaptive role that is benficial to women with PCOS in response to environmental challenges (infection, starvation) and internal states (pregnancy, puberty, physical and psychological stress) (75–77). The implementation of IR results in mobilization of fatty acids from adipose tissue, increased hepatic gluconeogenesis, release of glucose from the liver, and redistribution of energy to the brain and immune system (73). Chronic activation of this response can be pathological and lead to type 2 diabetes, metabolic syndrome, metabolic-associated liver disease, and cardiovascular disease, all of which are associated with PCOS (13,15,78). MAP may contribute to the development of IR via a number of biological mechanisms (6), and may be involved in the pathogenesis of PCOS (5).
Epidemiological evidence suggests that air pollution could be a factor in the development of IR (57,58). Air pollutants (NO and PM) have been positively associated with IR in an elderly population (via serum insulin level and homeostasis model assessment index) (79), and in children (with the Pollution Standard Index) (80). Human epidemiological studies have shown an association between air pollution and IR in gestational diabetes (57), type 2 diabetes (81), and obesity (82,83). Niemann et al have published a comprehensive review of the biological mechanisms that can cause IR (84). The authors have outlined the central role of reactive oxygen species (ROS) in the inhibition of insulin signaling and mitochondrial dysfunction, that can lead to IR. Production of reactive oxygen species can be due to a number of nutritional and environmental causes, including MAP.
Insulin resistance is a central component in the pathophysiology of PCOS, and has been associated with MAP in both animal and human studies (57,85). Current evidence suggests that MAP-induced IR is a contributor to metabolic dysfunction in PCOS and related metabolic diseases. Further studies on the role of MAP in the development of IR, and the efficacy of mitigation strategies, should be a research priority.
Both individual and public health strategies have been proposed to minimize exposure to MAP (86). Individual strategies include behavioral approaches (ensure good ventilation in-doors, avoid living and commuting in high traffic areas, consider HEPA air purifiers, avoid second-hand smoke exposure, replace scented cleaning products with natural products) (87), and nutritional practices to increase consumption of a healthy plant-based diet containing multiple antioxidants (vitamins A, B6, C, D, E, and omega-3 polyunsaturated fatty acids) (88). Public health strategies to reduce air pollution in urban areas, and education campaigns to limit sources of pollution, should be a priority (86).
Reproductive-age women should be counselled about the possible risks related to air pollution and advised to limit their exposure to areas known to have high concentrations of pollutants and avoid smoking. Women with PCOS-associated infertility, and their partners, should be counselled about the risk of second-hand smoke exposure to both fertility and pregnancy outcomes. Partners of infertile women with PCOS should be advised and supported to quit smoking.
Future research on the health effects of air pollution should include cohorts of women with PCOS, as they constitute 10-13% of reproductive-age women and are an important link to progressive chronic disease and the future health of their offspring. Research towards decreasing exposure and smoking cessation of pregnant women and their partners should be prioritized. The potential risk of air pollution on the incidence of PCOS and fertility and pregnancy outcomes should be highlighted in discussions aimed to mitigate the risk of anthropomorphic induced air pollution and climate change.
5. Conclusions
Microparticulate air pollution has been found to play a role in many metabolic-associated chronic diseases. Preliminary data from the present systematic review suggests that MAP may be contributing to an increased risk of PCOS, in addition to other established lifestyle, nutritional and environmental risk factors. These data add weight to the increasing global concern about the human health effects of air pollution and climate change. Future research into the effects of MAP should include cohorts of women with PCOS. Mitigation strategies to reduce individual exposure to MAP and exposure to smoking should be considered when counselling women with PCOS. Partners of women with PCOS should be advised to quit smoking. MAP should be included in the risk assessment of women with PCOS.
Author Contributions
N.S. conceptualize, design, data extraction and analysis, writing original draft. V.K. Conceptualize, review, and edit. J.P. conceptualize, design, data extraction and analysis, writing. All authors critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parker, J.; O’brien, C.; Hawrelak, J.; Gersh, F.L. Polycystic Ovary Syndrome: An Evolutionary Adaptation to Lifestyle and the Environment. Int. J. Environ. Res. Public Heal. 2022, 19, 1336. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Abbott, D.H.; Chazenbalk, G.D. An Evolutionary Model for the Ancient Origins of Polycystic Ovary Syndrome. J. Clin. Med. 2023, 12, 6120. [Google Scholar] [CrossRef] [PubMed]
- Parker, J. Pathophysiological Effects of Contemporary Lifestyle on Evolutionary-Conserved Survival Mechanisms in Polycystic Ovary Syndrome. Life 2023, 13, 1056. [Google Scholar] [CrossRef]
- Parker, J.; O'Brien, C.; Hawrelak, J. A narrative review of the role of gastrointestinal dysbiosis in the pathogenesis of polycystic ovary syndrome. Obstet. Gynecol. Sci. 2022, 65, 14–28. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Yang, Y.-C.; Chang, C.Y.-Y.; Lin, C.-C.; Hsu, W.-H.; Ju, S.-W.; Hsu, C.-Y.; Kao, C.-H. Risk of Polycystic Ovary Syndrome in Women Exposed to Fine Air Pollutants and Acidic Gases: A Nationwide Cohort Analysis. Int. J. Environ. Res. Public Heal. 2019, 16, 4816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Mwiberi, S.; Pickford, R.; Breitner, S.; Huth, C.; Koenig, W.; Rathmann, W.; Herder, C.; Roden, M.; Cyrys, J.; et al. Longitudinal associations between ambient air pollution and insulin sensitivity: results from the KORA cohort study. Lancet Planet. Heal. 2021, 5, e39–e49. [Google Scholar] [CrossRef]
- Chuang, K.-J.; Chan, C.-C.; Su, T.-C.; Lee, C.-T.; Tang, C.-S. The Effect of Urban Air Pollution on Inflammation, Oxidative Stress, Coagulation, and Autonomic Dysfunction in Young Adults. Am. J. Respir. Crit. Care Med. 2007, 176, 370–376. [Google Scholar] [CrossRef]
- Pathak, G.; Nichter, M. Polycystic ovary syndrome in globalizing India: An ecosocial perspective on an emerging lifestyle disease. Soc. Sci. Med. 2015, 146, 21–28. [Google Scholar] [CrossRef]
- Parker, J.; O’Brien, C. Evolutionary and genetic antecedents to the pathogenesis of polycystic ovary syndrome (PCOS). J ACNEM. 2021, 40, 12–20. [Google Scholar]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; on behalf of theInternational PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef]
- Palomba, S.; De Wilde, M.A.; Falbo, A.; Koster, M.P.; La Sala, G.B.; Fauser, B.C. Pregnancy complications in women with polycystic ovary syndrome. Hum. Reprod. Updat. 2015, 21, 575–592. [Google Scholar] [CrossRef] [PubMed]
- Brutocao, C.; Zaiem, F.; Alsawas, M.; Morrow, A.S.; Murad, M.H.; Javed, A. Psychiatric disorders in women with polycystic ovary syndrome: a systematic review and meta-analysis. Endocrine 2018, 62, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Zore, T.; Joshi, N.V.; Lizneva, D.; Azziz, R. Polycystic Ovarian Syndrome: Long-Term Health Consequences. Semin. Reprod. Med. 2017, 35, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Parker, J. NEM : A New Paradigm for Understanding the Common Origins of the Chronic Disease Epidemic. ACNEM J. 2018, 37, 6–11. [Google Scholar]
- Rodgers, R.J.; Avery, J.C.; Moore, V.M.; Davies, M.J.; Azziz, R.; Stener-Victorin, E.; Moran, L.J.; A Robertson, S.; Stepto, N.K.; Norman, R.J.; et al. Complex diseases and co-morbidities: polycystic ovary syndrome and type 2 diabetes mellitus. Endocr. Connect. 2019, 8, R71–R75. [Google Scholar] [CrossRef]
- Di Renzo, G.C.; Conry, J.A.; Blake, J.; DeFrancesco, M.S.; DeNicola, N.; Martin, J.N.; McCue, K.A.; Richmond, D.; Shah, A.; Sutton, P.; et al. International Federation of Gynecology and Obstetrics opinion on reproductive health impacts of exposure to toxic environmental chemicals. Int. J. Gynecol. Obstet. 2015, 131, 219–225. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Bellingham M, Sharpe R. Chemical Exposures During Pregnancy: Dealing with Potential, but Unproven, Risks to Child Health. R Coll Obstet Gynaecol [Internet]. 2013;(37). Available from: http://www.rcog.org.uk/files/rcog-corp/5.6.13ChemicalExposures.pdf.
- Abbafati C, Abbas KM, Abbasi-Kangevari M, Abd-Allah F, Abdelalim A, Abdollahi M, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020, 396, 1223–49. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Schraufnagel, D.E.; Balmes, J.R.; Cowl, C.T.; De Matteis, S.; Jung, S.-H.; Mortimer, K.; Perez-Padilla, R.; Rice, M.B.; Riojas-Rodriguez, H.; Sood, A.; et al. Air Pollution and Noncommunicable Diseases. Chest 2018, 155, 409–416. [Google Scholar] [CrossRef]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Power, M.C.; Adar, S.D.; Yanosky, J.D.; Weuve, J. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: A systematic review of epidemiologic research. NeuroToxicology 2016, 56, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Adami, G.; Pontalti, M.; Cattani, G.; Rossini, M.; Viapiana, O.; Orsolini, G.; Benini, C.; Bertoldo, E.; Fracassi, E.; Gatti, D.; et al. Association between long-term exposure to air pollution and immune-mediated diseases: a population-based cohort study. RMD Open 2022, 8, e002055. [Google Scholar] [CrossRef] [PubMed]
- Carré, J.; Gatimel, N.; Moreau, J.; Parinaud, J.; Léandri, R. Does air pollution play a role in infertility?: a systematic review. Environ. Health 2017, 16, 82. [Google Scholar] [CrossRef]
- Dockery, DW. Pope, CA. Xu, X. Spegler, JD. Ware, JH. Fay, ME. Ferris, BG. Speizer F. An association between air pollution and mortality in six us cities. N Engl J Med. 1993, 29, 1753–9. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K.; Pearce, K. Dysbiosis of Gut Microbiota (DOGMA) – A novel theory for the development of Polycystic Ovarian Syndrome. Med Hypotheses 2012, 79, 104–112. [Google Scholar] [CrossRef]
- Ghazi, T.; Naidoo, P.; Naidoo, R.N.; Chuturgoon, A.A. Prenatal Air Pollution Exposure and Placental DNA Methylation Changes: Implications on Fetal Development and Future Disease Susceptibility. Cells 2021, 10, 3025. [Google Scholar] [CrossRef]
- Kim, J.-H.; Hong, S.-H.; Moon, N.-L.; Kang, D.-R. Effects of Exposure Duration and Exposure Levels of Ambient Air Pollutants on the Risk of Polycystic Ovarian Syndrome: A 2015–2019 Korean Population-Based Cohort Study. Toxics 2022, 10, 542. [Google Scholar] [CrossRef]
- Li, J.; Wu, Q.; Wu, X.-K.; Zhou, Z.-M.; Fu, P.; Chen, X.-H.; Yan, Y.; Wang, X.; Yang, Z.-W.; Li, W.-L.; et al. Effect of exposure to second-hand smoke from husbands on biochemical hyperandrogenism, metabolic syndrome and conception rates in women with polycystic ovary syndrome undergoing ovulation induction. Hum. Reprod. 2018, 33, 617–625. [Google Scholar] [CrossRef]
- Zhu, Q.; Cai, J.; Guo, H.; Zhao, Y.; Lin, J. Air pollution exposure and pregnancy outcomes among women with polycystic ovary syndrome. Front. Public Heal. 2022, 10, 1066899. [Google Scholar] [CrossRef]
- Borgerding, M.; Klus, H. Analysis of complex mixtures – Cigarette smoke. Exp. Toxicol. Pathol. 2005, 57, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee on Secondhand Smoke Exposure and Acute Coronary Events. Secondhand Smoke Exposure and Cardiovascular Effects: Making Sense of the Evidence. National Academies Press (US); 2010.
- Nelson, L.R.; Bulun, S.E. Estrogen production and action. J. Am. Acad. Dermatol. 2001, 45, S116–S124. [Google Scholar] [CrossRef]
- Biegon, A.; Alia-Klein, N.; Fowler, J.S. Potential Contribution of Aromatase Inhibition to the Effects of Nicotine and Related Compounds on the Brain. Front. Pharmacol. 2012, 3, 185. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Nabi, M.; Rasool, S.U.A.; Rashid, F.; Amin, S. Hyperandrogenism in polycystic ovarian syndrome and role of CYP gene variants: a review. Egypt. J. Med Hum. Genet. 2019, 20, 1–10. [Google Scholar] [CrossRef]
- Öberg, M.; Jaakkola, M.S.; Woodward, A.; Peruga, A.; Prüss-Ustün, A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet 2011, 377, 139–146. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ambient (outdoor) air pollution. 2022. p. Accessed 1 September, 2023.
- Yao T, Sung HY, Mao Z, Hu T wei, Max W. Secondhand smoke exposure at home in rural China. Cancer Causes Control. 2012, 23 (Suppl 1), 109–15.
- Fischer, F.; Kraemer, A. Secondhand smoke exposure at home among middle and high school students in the United States - does the type of tobacco product matter? BMC Public Heal. 2017, 17, 98. [Google Scholar] [CrossRef]
- Van Deusen, A.; Hyland, A.; Travers, M.J.; Wang, C.; Higbee, C.; King, B.A.; Alford, T.; Cummings, K.M. Secondhand smoke and particulate matter exposure in the home. Nicotine Tob. Res. 2009, 11, 635–641. [Google Scholar] [CrossRef]
- Benedict, M.D.; Missmer, S.A.; Vahratian, A.; Berry, K.F.; Vitonis, A.F.; Cramer, D.W.; Meeker, J.D. Secondhand tobacco smoke exposure is associated with increased risk of failed implantation and reduced IVF success. Hum. Reprod. 2011, 26, 2525–2531. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Q.; Lin, J.; Cai, J. Association of Exposure to Particulate Matter Air Pollution With Semen Quality Among Men in China. JAMA Netw. Open 2022, 5, e2148684–e2148684. [Google Scholar] [CrossRef]
- Decrue, F.; Townsend, R.; Miller, M.R.; E Newby, D.; Reynolds, R.M. Ambient air pollution and maternal cardiovascular health in pregnancy. Hear. 2023. [Google Scholar] [CrossRef]
- Lamichhane, D.K.; Leem, J.-H.; Lee, J.-Y.; Kim, H.-C. A meta-analysis of exposure to particulate matter and adverse birth outcomes. Environ. Heal. Toxicol. 2015, 30, e2015011–e2015011. [Google Scholar] [CrossRef] [PubMed]
- Malley, C.S.; Kuylenstierna, J.C.; Vallack, H.W.; Henze, D.K.; Blencowe, H.; Ashmore, M.R. Preterm birth associated with maternal fine particulate matter exposure: A global, regional and national assessment. Environ. Int. 2017, 101, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Halldorsson, T.I.; Olsen, S.F.; Hjortebjerg, D.; Ketzel, M.; Grandström, C.; Raaschou-Nielsen, O.; Sørensen, M. Impact of Road Traffic Pollution on Pre-eclampsia and Pregnancy-induced Hypertensive Disorders. Epidemiology 2017, 28, 99–106. [Google Scholar] [CrossRef]
- Quarato, M.; De Maria, L.; Gatti, M.F.; Caputi, A.; Mansi, F.; Lorusso, P.; Birtolo, F.; Vimercati, L. Air Pollution and Public Health: A PRISMA-Compliant Systematic Review. Atmosphere 2017, 8, 183. [Google Scholar] [CrossRef]
- Kan, H. World Health Organization air quality guidelines 2021: implication for air pollution control and climate goal in China. Chin. Med J. 2022, 135, 513–515. [Google Scholar] [CrossRef]
- Wu, Y.; Fu, R.; Lei, C.; Deng, Y.; Lou, W.; Wang, L.; Zheng, Y.; Deng, X.; Yang, S.; Wang, M.; et al. Estimates of Type 2 Diabetes Mellitus Burden Attributable to Particulate Matter Pollution and Its 30-Year Change Patterns: A Systematic Analysis of Data From the Global Burden of Disease Study 2019. Front. Endocrinol. 2021, 12. [Google Scholar] [CrossRef]
- Schraufnagel, D.E. The health effects of ultrafine particles. Exp. Mol. Med. 2020, 52, 311–317. [Google Scholar] [CrossRef]
- De Marco, A.; Amoatey, P.; Khaniabadi, Y.O.; Sicard, P.; Hopke, P.K. Mortality and morbidity for cardiopulmonary diseases attributed to PM2.5 exposure in the metropolis of Rome, Italy. Eur. J. Intern. Med. 2018, 57, 49–57. [Google Scholar] [CrossRef]
- Mahalingaiah, S.; E Missmer, S.; Cheng, J.J.; Chavarro, J.; Laden, F.; E Hart, J. Perimenarchal air pollution exposure and menstrual disorders. Hum. Reprod. 2018, 33, 512–519. [Google Scholar] [CrossRef]
- Wang, C.; Plusquin, M.; Ghantous, A.; Herceg, Z.; Alfano, R.; Cox, B.; Nawrot, T.S. DNA methylation of insulin-like growth factor 2 and H19 cluster in cord blood and prenatal air pollution exposure to fine particulate matter. Environ. Heal. 2020, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-J.; Lee, S.-H.; Lee, S.-Y.; Kim, H.-C.; Kim, H.-B.; Park, M.J.; Yoon, J.; Jung, S.; Yang, S.-I.; Lee, E.; et al. Mid-pregnancy PM2.5 exposure affects sex-specific growth trajectories via ARRDC3 methylation. Environ. Res. 2021, 200, 111640. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.M.; Ryu, B.T.; Chung, K.H. Identification of estrogenic and antiestrogenic activities of respirable diesel exhaust particles by bioassay-directed fractionation. Arch. Pharmacal Res. 2008, 31, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Q.; He, S.; Wu, K.; Ren, M.; Dong, H.; Di, J.; Yu, Z.; Huang, C. Ambient air pollution and gestational diabetes mellitus: A review of evidence from biological mechanisms to population epidemiology. Sci. Total. Environ. 2020, 719, 137349. [Google Scholar] [CrossRef]
- Wolf, K.; Popp, A.; Schneider, A.; Breitner, S.; Hampel, R.; Rathmann, W.; Herder, C.; Roden, M.; Koenig, W.; Meisinger, C.; et al. Association Between Long-term Exposure to Air Pollution and Biomarkers Related to Insulin Resistance, Subclinical Inflammation, and Adipokines. Diabetes 2016, 65, 3314–3326. [Google Scholar] [CrossRef]
- Aboeldalyl, S.; James, C.; Seyam, E.; Ibrahim, E.M.; Shawki, H.E.-D.; Amer, S. The Role of Chronic Inflammation in Polycystic Ovarian Syndrome—A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2021, 22, 2734. [Google Scholar] [CrossRef]
- Szukiewicz, D.; Trojanowski, S.; Kociszewska, A.; Szewczyk, G. Modulation of the Inflammatory Response in Polycystic Ovary Syndrome (PCOS)—Searching for Epigenetic Factors. Int. J. Mol. Sci. 2022, 23, 14663. [Google Scholar] [CrossRef]
- Khan RN, Hay DP. A clear and present danger: Inflammasomes DAMPing down disorders of pregnancy. Hum Reprod Update. 2015, 21, 388–405. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, F.; Liu, F.; Zhao, J.; Huang, X.; Luo, D.; Guo, J. Metaflammation in glucolipid metabolic disorders: Pathogenesis and treatment. Biomed. Pharmacother. 2023, 161, 114545. [Google Scholar] [CrossRef]
- Dominski, F.H.; Lorenzetti Branco, J.H.; Buonanno, G.; Stabile, L.; Gameiro da Silva, M.; Andrade, A. Effects of air pollution on health: A mapping review of systematic reviews and meta-analyses. Environ. Res. 2021, 201, 111487. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Pope CA, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, O’Toole T. Exposure to Fine Particulate Air Pollution Is Associated with Endothelial Injury and Systemic Inflammation. Circ Res. 2016, 119, 1204–14. [Google Scholar] [CrossRef] [PubMed]
- Siponen, T.; Yli-Tuomi, T.; Aurela, M.; Dufva, H.; Hillamo, R.; Hirvonen, M.-R.; Huttunen, K.; Pekkanen, J.; Pennanen, A.; Salonen, I.; et al. Source-specific fine particulate air pollution and systemic inflammation in ischaemic heart disease patients. Occup. Environ. Med. 2014, 72, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Peng, J.; Zhang, W.; Wu, Y.; Hu, R.; Chen, R.; Gu, W.; Zhang, L.; Qin, L.; Zhong, M.; et al. Ambient fine particulate matter exposure disrupts placental autophagy and fetal development in gestational mice. Ecotoxicol. Environ. Saf. 2022, 239, 113680. [Google Scholar] [CrossRef]
- Bové, H.; Bongaerts, E.; Slenders, E.; Bijnens, E.M.; Saenen, N.D.; Gyselaers, W.; Van Eyken, P.; Plusquin, M.; Roeffaers, M.B.J.; Ameloot, M.; et al. Ambient black carbon particles reach the fetal side of human placenta. Nat. Commun. 2019, 10, 3866. [Google Scholar] [CrossRef] [PubMed]
- Stener-Victorin, E.; Padmanabhan, V.; A Walters, K.; E Campbell, R.; Benrick, A.; Giacobini, P.; A Dumesic, D.; Abbott, D.H. Animal Models to Understand the Etiology and Pathophysiology of Polycystic Ovary Syndrome. Endocr. Rev. 2020, 41. [Google Scholar] [CrossRef]
- Stepto, N.K.; Cassar, S.; Joham, A.E.; Hutchison, S.K.; Harrison, C.L.; Goldstein, R.F.; Teede, H.J. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum. Reprod. 2013, 28, 777–784. [Google Scholar] [CrossRef]
- Tam, C.S.; Xie, W.; Johnson, W.D.; Cefalu, W.T.; Redman, L.M.; Ravussin, E. Defining Insulin Resistance From Hyperinsulinemic-Euglycemic Clamps. Diabetes Care 2012, 35, 1605–1610. [Google Scholar] [CrossRef]
- Cassar, S.; Misso, M.L.; Hopkins, W.G.; Shaw, C.S.; Teede, H.J.; Stepto, N.K. Insulin resistance in polycystic ovary syndrome: a systematic review and meta-analysis of euglycaemic–hyperinsulinaemic clamp studies. Hum. Reprod. 2016, 31, 2619–2631. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Gorjão, R.; Takahashi, H.K.; Pan, J.A.; Hirabara, S.M. Molecular Mechanisms Involved in Inflammation and Insulin Resistance in Chronic Diseases and Possible Interventions. J. Biomed. Biotechnol. 2012, 2012, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Zhou MS, Wang A, Yu H. Link between insulin resistance and hypertension: What is the evidence from evolutionary biology? Diabetol Metab Syndr. 2014, 6, 1–8. [Google Scholar]
- Wang, P.; Mariman, E.C. Insulin resistance in an energy-centered perspective. Physiol. Behav. 2008, 94, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Tsatsoulis, A.; Mantzaris, M.D.; Bellou, S.; Andrikoula, M. Insulin resistance: An adaptive mechanism becomes maladaptive in the current environment — An evolutionary perspective. Metabolism 2013, 62, 622–633. [Google Scholar] [CrossRef]
- Wu, J.; Yao, X.-Y.; Shi, R.-X.; Liu, S.-F.; Wang, X.-Y. A potential link between polycystic ovary syndrome and non-alcoholic fatty liver disease: an update meta-analysis. Reprod. Heal. 2018, 15, 1–9. [Google Scholar] [CrossRef]
- Kim JH, Hong Y. GSTM1, GSTT1, and GSTP1 Polymorphisms and Associations between Air Pollutants and Markers of Insulin Resistance in Elderly Koreans. Environ Health Perspect. 2012, 120, 1378–84. [Google Scholar] [CrossRef]
- Kelishadi, R.; Mirghaffari, N.; Poursafa, P.; Gidding, S.S. Lifestyle and environmental factors associated with inflammation, oxidative stress and insulin resistance in children. Atherosclerosis 2009, 203, 311–319. [Google Scholar] [CrossRef]
- Qin, G.-Q.; Chen, L.; Zheng, J.; Wu, X.-M.; Li, Y.; Yang, K.; Liu, T.-F.; Fang, Z.-Z.; Zhang, Q. Effect of passive smoking exposure on risk of type 2 diabetes: a systematic review and meta-analysis of prospective cohort studies. Front. Endocrinol. 2023, 14, 1195354. [Google Scholar] [CrossRef]
- Liu, X.; Tu, R.; Qiao, D.; Niu, M.; Li, R.; Mao, Z.; Huo, W.; Chen, G.; Xiang, H.; Guo, Y.; et al. Association between long-term exposure to ambient air pollution and obesity in a Chinese rural population: The Henan Rural Cohort Study. Environ. Pollut. 2020, 260, 114077. [Google Scholar] [CrossRef]
- Huang, C.; Li, C.; Zhao, F.; Zhu, J.; Wang, S.; Sun, G. The Association between Childhood Exposure to Ambient Air Pollution and Obesity: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Heal. 2022, 19, 4491. [Google Scholar] [CrossRef]
- Niemann, B.; Rohrbach, S.; Miller, M.R.; Newby, D.E.; Fuster, V.; Kovacic, J.C. Oxidative Stress and Cardiovascular Risk: Obesity, Diabetes, Smoking, and Pollution: Part 3 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 230–251. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, C.; Xu, Z.; Tzan, K.; Zhong, M.; Wang, A.; Lippmann, M.; Chen, L.-C.; Rajagopalan, S.; Sun, Q. Long-term Exposure to Ambient Fine Particulate Pollution Induces Insulin Resistance and Mitochondrial Alteration in Adipose Tissue. Toxicol. Sci. 2011, 124, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, D.I.; Ustáriz, J.; Marín, R.; Carrasco-Wong, I.; Farías, M.; Giordano, A.; Gallardo, F.S.; Illanes, S.E.; Gutiérrez, J. Cellular mechanisms linking to outdoor and indoor air pollution damage during pregnancy. Front. Endocrinol. 2023, 14, 1084986. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.-Y.; Wu, M.; Han, R.-Q.; Zhang, X.-F.; Wang, X.-S.; Liu, A.-M.; Zhou, J.-Y.; Lu, Q.-Y.; Kim, C.H.; Mu, L.; et al. Household Ventilation May Reduce Effects of Indoor Air Pollutants for Prevention of Lung Cancer: A Case-Control Study in a Chinese Population. PLOS ONE 2014, 9, e102685. [Google Scholar] [CrossRef] [PubMed]
- Péter, S.; Holguin, F.; Wood, L.G.; Clougherty, J.E.; Raederstorff, D.; Antal, M.; Weber, P.; Eggersdorfer, M. Nutritional Solutions to Reduce Risks of Negative Health Impacts of Air Pollution. Nutrients 2015, 7, 10398–10416. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).