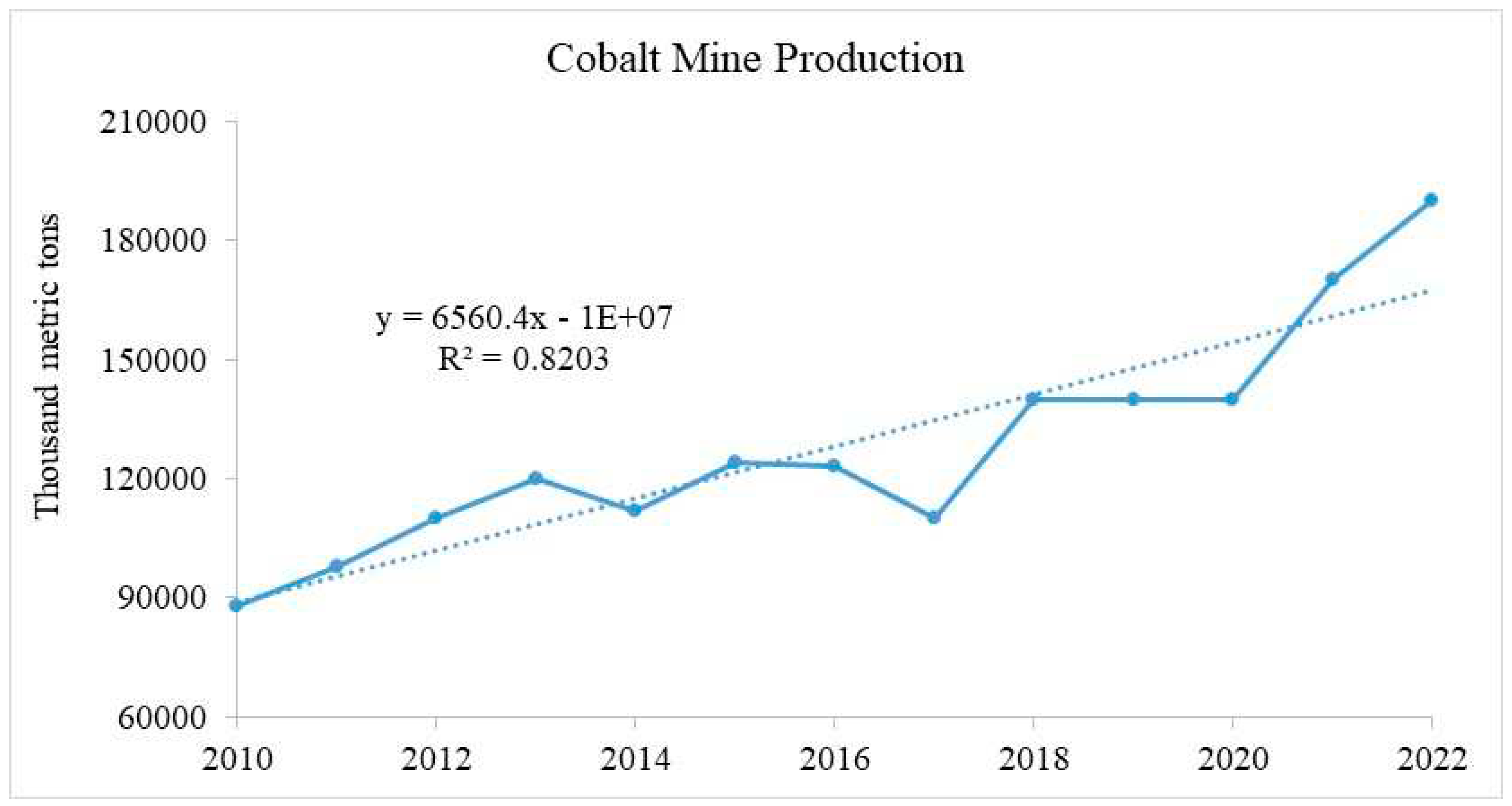
These 1026 observations found 567 increases, 381 decreases, and 1 with no impact on various plant variables/parameters (
Table 2). The soil-plant system's CO behavior is not straightforward, according to meta-analysis. Depending on a number of variables, including the physicochemical characteristics of the soil, the amount of Co that has been applied, and the kind of plant species, it can both decrease and enhance certain variables. The entire trend in the soil-plant system was better understood because of this data analysis.
3.1. Molecular understanding of Co uptake by plants
Sessile plants can absorb elements either actively or passively from soils. Plants typically have Co concentrations of 0.1 to 10 mg/kg dry weight [
14]. Plants absorb cobalt from soil in a variety of ways, mostly as di- and trivalent cations. Co uptake by plant roots has an active transport mechanism in higher plants. The enrichment of Co in the soil raises the concentration of cobalt in plants, according to multiple prior studies. This represents the functions of Co concentration in solution and plant absorption of its mobile component. Plant absorption of Co is influenced by its complexation with organic and inorganic ligands and chemical speciation. According to Bhattacharyya
, et al. [
27], co-occurring in OM, carbonate, and residual fractions of cow dung manure (CDM) and municipal solid waste compost (MSWC) does not correlate with its levels in grains or rice straw. However, rice is easily able to absorb co-occurring in Mn and Fe oxides of CDM and MSWC. Similar to this, there is a favorable correlation between the concentration of AAAc-EDTA soluble Co compounds and agricultural plants [
28].
Cobalt uptake by plants greatly varies with the type of plant species. For example, Co was hardly detected in radish whereas a considerable amount was noticed in the shoot of the green beans. Leafy vegetables like Spinacia oleracea, Lactuca sativa, and Brassica oleracea have relatively high cobalt concentrations (>0.6 ppm), whereas fodder crops have a much wider range (0.6 to 3.5 ppm).
After being absorbed by plant roots, cobalt is either transferred to plant shoots or sequestered by the vacuole of root cells. Although poor mobility of Co inside plants limits its transfer toward shoots, roots absorb cobalt through an active transport process, and its distribution in plants may include different chemical molecules [
29,
30,
31].
Mobility of Co within the plant leaf tissue is low and mostly it accumulates in plant roots, but free Co ions can be transported to aerial parts. Page and Feller [
32] studied
57Co transportation in wheat plants and found that 80% of the
57Co was retained in the roots after four days of culture 50% of Co remained in the roots after 50 days and the remaining was translocated towards shoot. The data analysis of the current study revealed an overall 49% accumulation in plant roots and 51% in aerial parts based on 33 observations of different studies (
Table 3,
Table S1). However, the % accumulation ranges 14-94% in roots and 6-86% in aerial parts. This proposes that there exists great variation in Co partitioning between root and shoot tissues. Moreover, data analysis revealed that plants have an effective mechanism to uptake and transfer from root to shoot.
This relative distribution of cobalt in plant roots and aerial tissues greatly varies with the type of plant species. For example, in wheat crops, Co was dispersed in the leaves exactly in the same way as Ca and K through a parallel veins system [
29]. Whereas in tomato leaves Co was not dispersed in a similar way as in wheat crop. The authors proposed that the variation in plant sequestration and defense system for Co can be a possible reason for varied distribution among different plant species. It has also been found that Co ions in higher plants bind with the roots and translocate within the body through passive transport. Cobalt ions move through the plasma membrane enter the cell and might be translocated within the whole plant with the help of IRT1 transporters [
33]. IRT-1 can also transport Co inside cortical cells and vacuoles of the root epidermal thereby sequestrating Co in most outer layers of the roots.
There haven't been any reports of particular Co transporters to yet. Mobile Co can be translocated toward the shoot by FPN1 after being loaded into the xylem [
34]. Cobalt is chelated with ligands before being transferred to shoots. Since phytochelatins have a low affinity for it, Co-S bonds are not expected to be the ligands. According to the Collins and Kinsela [
35], in wheat or tomato plants, Co was complexed with carboxylic acids and moved from the roots to the shoots. Other ligands include non-proteinogenic amino acids like nicotinamide and histidine as well as citrate or malate.
Distribution, transportation, and uptake of Co are mainly dependent on species and governed by several mechanisms (
Figure 2). Intracellular affinity, binding sites, and transport proteins mediate the Co ion uptake through the plasma membrane. Co transport within cell membranes is facilitated by a number of distinct transmembrane proteins. The 1B-ATPase (HMA) family has been described among the different known heavy metal transporters, and this family is involved in the transportation of both divalent (Co
2+/Cd
2+/Zn
2+/Pb
2+) and monovalent (Ag+/Cu+) heavy metals. [
36]. Similarly, HMA2 and HMA3 belong to the P
1B2 sub-group and transport the divalent metals such as Co
2+ and Cd
2+ based upon the phylogenetic analysis, HMA5 fits in the P
1B1 subclass specific for monovalent ions (Cu
+) [
37]. HMA2 is limited in the pericycle and contributes to the movement of metals from root to shoot by loading divalent heavy metals, such as Co, into the xylem [
38], HMA3 and HMA5, however, trap monovalent Co into vacuoles, which helps with metal tolerance [
39].
The AtHMA3 which belongs to the P-type ATPase family is located in the plant vacuolar membrane and is involved in heavy metal transportation to the vacuole [
39] hence enhancing plant tolerance. Previous studies have revealed CPX involvement in Co ion coordination during its transport [
40]. Also unexplored is the metal specificity of these ATPase proteins. Studies on Synechocystis sp. have shown that the Co
2+ tolerance was decreased and intracellular Co
2+ levels were elevated in the presence of the ATPase-encoding gene deletion.
3.2. Beneficial effects of Co on plants
Uncertainty surrounds the direct involvement of cobalt in plant metabolism. Insufficient evidence exists for both Co's direct impact on plants and its bioactive form [
1]. Moreover, the mechanisms of Co-mediated beneficial effects on plants are not well-elucidated. Some studies have reported a positive effect of Co on plants at low applied levels [
11]. Several enzyme and coenzyme processes require cobalt. It is now widely accepted that it assists legumes fix nitrogen fast [
3]. Other advantages include the development of the stem, the lengthening of the coleoptile, the creation of buds, the promotion of plant growth, and the yield when exogenously carried out [
3,
11,
33]. According to several research, Co is essential for plants to produce ethylene. The free-living Azotobacter bacteria, which belong to the genus Rhizobium, use cobalt as one of their coenzymes to bind nitrogen from the air [
41] because redox reactions and the production of nucleoproteins both depend on the production of vitamin B12. Through its interactions with Fe, Ni, and Zn, cobalt plays a significant role in preserving plant cellular homeostasis.
Cobalt is a useful metal for leguminous plants in terms of metabolism, growth, and the formation of root nodules [
33]. Similarly, it improves the development of salinized plants by increasing leaf water potential in comparison to untreated plants. Plant height, branch number, fruit number, and anthocyanin and flavonoid content of Hibiscus sabdariffa rose considerably following application of Co at 20-40 mg/kg [
42]. When increasing levels of Co (50, 100, 150, 200, and 250 mg/kg) were given to maize plants, the root length, shoot height, and number of cobs and seeds per plant increased, but these parameters decreased as the concentration reached 100 mg Co/kg and above [
43]. Cobalt at 10 mg/kg significantly increased the growth, bulb yields, bulb length, and bulb quality of two onion cultivars, including nutritional and essential oil levels. Bulb diameter and weight were considerably larger in the experimental treatment than in the control treatment [
44], However, Co doses greater than 10 mg/kg greatly decreased the pro-inflammatory effects.
Explanations for enhanced plant growth vary, however the following can be summarized: (1) enhanced abiotic stress tolerance, (2) activation of antioxidative enzymes, (3) active metal substitution, and (4) hormesis. Furthermore, Co has been shown to alleviate the effects of drought, salt, and heavy metal stressors, ensuring that plant development is not inhibited by the activation of various enzymes and coenzymes in the presence of Co [
11,
15].
The data analysis of the literature showed an overall 11% and 2% increase, respectively in plant pigment contents (based on 151 observations) and growth parameters (214 observations) (
Table 4,
Tables S2–S4). This reveals that application of Co to plants can enhance plant pigment contents and the growth parameters.
3.3. Cobalt deficiency in plants
Plants respond to Co concentrations in soil in the same way as they do to other critical micronutrients: at low concentrations, it supports plant development, while at greater concentrations, it causes phytotoxicity [
3]. However, it differs from other helpful elements in that plants demonstrate Co shortcoming when soil Co availability is severely limited. Leaf chlorosis and necrosis, growth retardation, and reduced seed germination and crop yield are all symptoms of cobalt deficiency, which is comparable to N deficiency in plants [
45,
46] (
Table 5). Cobalt-deficient legumes have smaller plant size, smaller and pale-yellow leaves, and smaller pods when compared to non-plants [
3]. Furthermore, Co-deficient plants may exhibit nitrogen deficit symptoms. Root development is also hampered by a general decrease in root volume and length. Cobalt shortage reduces methionine synthesis, reducing protein synthesis and contributing to smaller-sized bacteroid. Co-deficient foods include sweet lupin, ground almonds, navy beans, and soy.
3.4. Toxic effects of excess cobalt on the plants
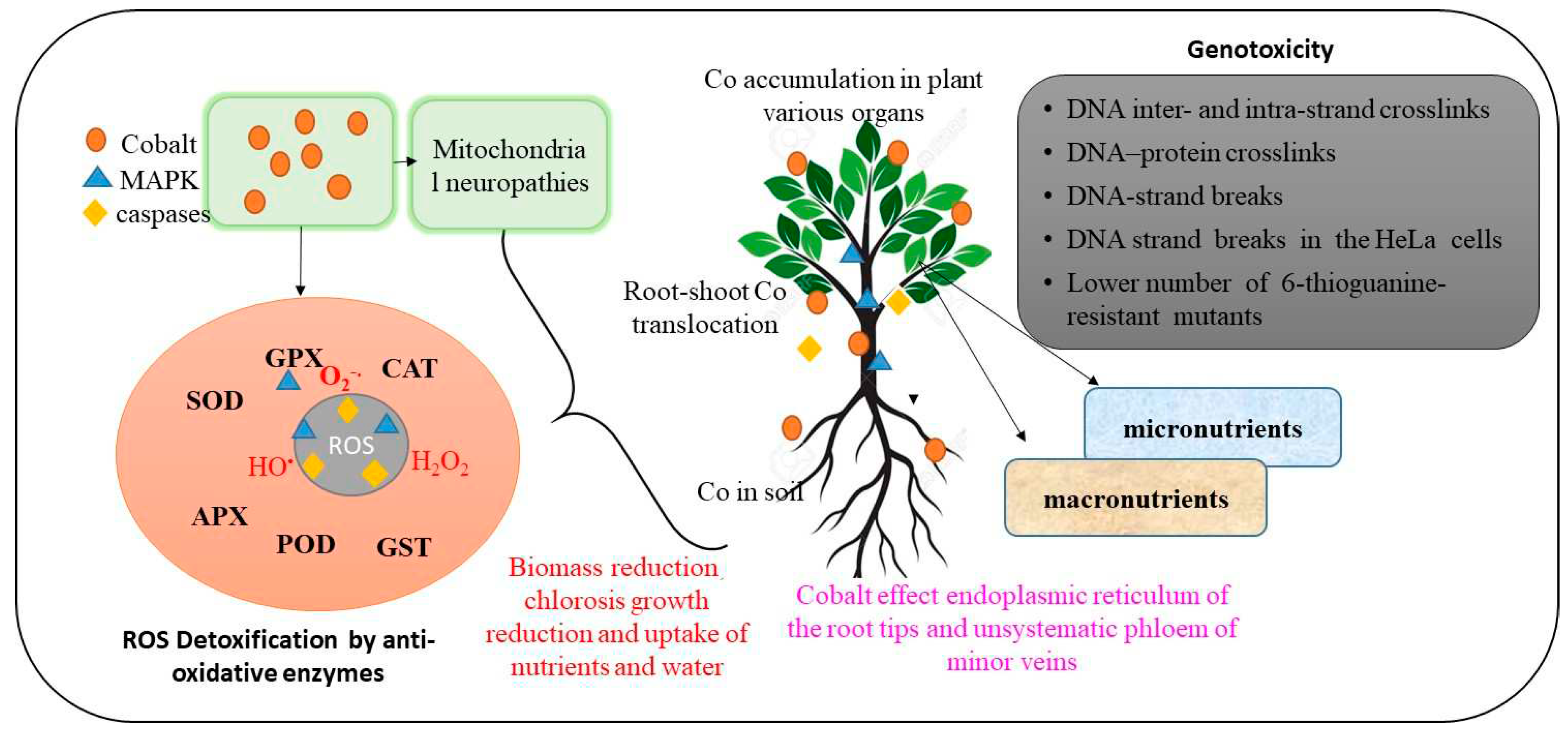
Heavy metals including Co have been recognized for their toxic effects on plant biochemical attributes. Cobalt is not considered an essential metal for plants but rather designated as a beneficial element [
47]. Plant toxicity to cobalt is rare in natural soils, but occurs when plants grow in Co-contaminated soils. Higher concentrations of cobalt in plant tissues can cause impairment to cell membranes, which results in biomass reduction, chlorosis, reduction in growth, and uptake of nutrients and water, and hence induce cellular toxicity [
6,
48,
49,
50,
51]. Excess Co exposure causes chlorosis (pale-white coloring) on young leaves of leguminous plants and interveinal chlorosis (scattered chlorosis) on young leaves of tomatoes [
3]. However, the main plant reaction to greater Co concentrations is interveinal chlorosis, which has been connected to Fe chlorosis. Phytotoxicity study of Co concentration in
Hordeum Vulgare L.,
Lycopersicon esculentum L., and
Brassica napus L. has revealed the adverse effect on plant shoot and biomass growth [
51]. According to Sree
, et al. [
52], Co may reduce the activity of enzymes involved in the production of chlorophyll intermediates such 5-aminolevulinic acid and protoporphyrin, resulting in a decrease in net photosynthetic activities.
Plants with higher Co concentrations had lower Fe concentrations, protein, chlorophyll, and CAT activity in their leaves. Jayakumar et al. [
5] showed the increased chlorophyll concentration at lower Co levels. Higher Co levels limit the Fe incorporation in protoporphyrin which causes a decrease in chlorophyll content. This was reinforced by the fact that high concentrations of other heavy metals, including Co [
53], caused chlorosis in plants, which was comparable to the chlorosis caused by Fe shortage. Excess Co can limit transpiration and water potential rate substantially. Cauliflower leaves exhibited diffusive resistance and greater water content when exposed to high Co concentrations [
6].
Higher concentration of Co also affects root growth by hindering the cell division and retarding the nutrient and water uptake. Reduced germination was also observed in wheat seeds due to the higher Co exposure [
54]. Similar findings of reduction in germination due to Co exposure had been stated in several crops such as
Vigna mungo [
55], and ragi [
56]. All of these elements, when combined, can cause phytotoxicity and drastically limit plant development. However, phytotoxicity varies according to plant species and Co concentration in plant organs.
The data analysis revealed both increase and decrease in plant pigment and growth parameters under Co exposure. Out of 151 observations, 92 showed a decrease while 54 showed an increase in plant pigments (
Table 4). In the case of plant growth parameters, 106 observations demonstrated a decrease and 103 represented an increase. The intensity of increase and decrease was, respectively 94% and 1587% for pigments and 169% and 214% for plant growth factors. Hence, Co exposure can mediate both positive and negative effects on plant growth. Therefore, it is highly necessary to monitor Co soil levels, possible soil-plant transfer index, accumulation in different plant tissues, and toxicity.
3.6. Cobalt effects on plant macronutrients
Excessive soil Co level might impact plant absorption of other necessary elements, in addition to decreasing plant growth and development [
15,
61]. Soil pollution with Co-produced a reduction in the quantity of K, P, Na, Mg, and mainly Ca in the aboveground sections of oats in a research by Wyszkowski
, et al. [
62], Soil pollution with Co reduced the level of K, P, Na, Mg, and, in particular, Ca in the aboveground sections of oats. Low Co dosages (10-20 mg Co/kg soil) showed a minor influence on macronutrient composition in spring barley in another experiment [
63]. High dosages (320 mg Co/kg soil) enhanced the concentration of all macronutrients, particularly Ca, Na, and N, in the plant's aboveground portions [
64] discovered a significant rise in P content in plants when exposed to Co.. Chaudhari
, et al. [
65] discovered that increasing Co levels beyond 10.0 mg kg
-1 resulted in a decrease in the promoter impact on macronutrients (N, P, and K), except when Co was applied in the oxide form, which marginally increased the N, P, and K content. These findings are consistent with those of Gad and Kandil [
66], who discovered that Co content in wheat plants had a favorable influence on the status of all minerals. Basu
, et al. [
67] discovered that co-treatment increased nutrient absorption by groundnut seeds when compared to the control.
When compared to the control, 12 mg kg
-1 of Co had a substantial primitive influence on the plant macronutrient (N, P, and K) content of soybean with all sources of N
2 fertilizers [
57]. Increasing Co content in plant medium by more than 12 mg kg
-1, on the other hand, resulted in a considerable drop in these nutritive element quantities. This drop appeared to be connected to the Co concentration. Co also has an adverse effect on the movement of P, S, Cu, Mn, and Zn from roots to shoots [
6].
We calculated the effect of Co on the uptake of other elements. Data analysis of 148 observations revealed an overall 46 increase in element contents in the presence of Co (
Table 6,
Table S5-S6). The effect varied from -48% to 2316% with 43 and 105 observations showing, respectively decrease and increase in element contents. Hence, Co co-presence can affect the uptake of different elements by plants, both positively and negatively. Generally, the effect greatly varied for different elements, plants, and soil types.
Moreover, the presence of other elements also mediates an overall increase in Co plant uptake by 53% (range -32% to 612%) (
Table 6). Out of 119 observations, 18 showed a decrease while 101 showed an increase in Co phytouptake.
3.7. Cobalt-induced oxidative stress and lipid peroxidation
Excess cobalt is known to function as a catalyst in the formation of hazardous reactive oxygen species such as superoxide (O
2), hydroxyl radical (OH), hydrogen peroxide (H
2O
2), and alkoxy radical (RO) [
8]. The generation of increased levels of ROS in plants is thought to be the most damaging and early impact of heavy metal poisoning. The Fenton reaction produces a hydroxyl radical (OH), which promotes lipid peroxidation and degrades the integrity and fluidity of cell membranes [
68]. ROS induce oxidative damage to biological macromolecules such as nucleic acids, lipids, proteins, and so on [
69,
70]. Excess Co also causes proline buildup and has the potential to disrupt antioxidative defense mechanisms [
71]. According to Catalani
, et al. [
72], Co ions are capable of inducing the formation of ROS in vitro and in vivo. Cobalt catalyzes the formation of OH from H
2O
2 in a Fenton-type reaction. Cobalt exposure can produce oxidative stress, lower glutathione levels, increase oxidized glutathione levels, promote hexose monophosphate shunt, and cause DNA free-radical damage [
73,
74]. The mechanisms of action include co-interaction with the mitochondria and ROS generation, which may result in mitochondrial neuropathies. It is believed that mitochondrial dysfunction can be impacted by specific mutations in mitochondrial DNA and aggravated by a variety of environmental variables. The data analysis confirmed that Co-mediated enhanced ROS production (average 59% increase with a range of -35% to 352). Out of 35 observations, 11 showed a decrease and 24 exhibited an increase in ROS contents by Co. Hence, it is anticipated that Co presence in plant tissues mediates increased production of ROS. However, it is not fully explored whether this increase always induced negative effects or not. Indeed, ROS plays several beneficial roles inside plants up to their optimal level. However, these radicles become highly toxic when present at supra-optimal levels. Therefore, a Co-induced increase in ROS contents can cause both beneficial and negative effects, depending on the ROS contents and the defense mechanism of the plant.
It is well-established that enhanced ROS production is associated with peroxidation of lipids. The data analysis of the current study supported the hypothesis by showing an overall 47% increase (range, -88% to 436%) in LPO after Co exposure (
Table 7,
Table S7-S8). Data analysis showed that Co exposure caused an increase in LOP by 82 observations and a decrease by 72 observations. Hence, Co-mediated overproduction of ROS may cause LPO in plants.
3.8. Cobalt genotoxicity
Cobalt is implicated in the creation of ROS in plants, which can interact with plant DNA directly or indirectly to cause strand breaks. According to Faisal
, et al. [
75], CoO-NPs may have indirectly caused DNA damage in eggplant, as demonstrated by an increase in ROS, mitochondrial membrane damage, and an enhanced esterase level. So far, in vitro studies on Co nanoparticle toxicity has revealed DNA damage [
76], oxidative stress [
77,
78], genotoxic effects [
79] and inflammatory responses [
80]. Cobalt is a genotoxic metal both in vitro and in vivo, and can potentially cause inhibition of DNA repair and oxidative stress [
81,
82,
83,
84]. The genomic instabilities in RAPD (randomly amplified polymorphic DNA) patterns are said to indicate DNA damage caused by genotoxins at Co doses of 5 mM, 10 mM, and 20 mM. SDS-PAGE examination of protein profiles revealed similar alterations [
85]. When organisms are exposed to high levels of Co, it directly causes DNA damage, DNA-protein cross-links, and sister chromatid exchange. Furthermore, Co disrupts the DNA repair machinery (Leonard et al., 1998). Co-exposed seeds of various Fabaceae plants revealed individual polymorphism in a research on
V. faba plants to evaluate the influence of Co-treatment on polymorphism [
86]. However, Co genotoxicity is still a contentious issue.
3.9. Antioxidant enzymes
Plants have an effective tolerance and detoxifying system for dealing with increased levels of ROS caused by biotic and abiotic stress [
87,
88,
89]. Cobalt effect on plant antioxidative enzyme activities is contradictory and both their inhibition and induction have been found in different plant species such as Brassica napus [
7], dark tea [
4], Glycine max [
90],
Vigna radiata L. [
91],
Brassica oleracea [
6,
49,
50,
92] and
Lycopersicon esculentum L. [
50]. In a study, a reduction in the CAT activity which coincided with a significant elevation in the H
2O
2 content in mustard leaves [
93]. The reduced CAT activity under Co stress does not induce the scavenging of H
2O
2 in mustard leaves. The results confirmed prior findings using co-treated tomato [
50], spinach [
49], and cabbage [
94]. Furthermore, the reduction in enzyme activity may be due to enzyme deactivation caused by direct interaction with metal ions or by higher amounts of damaging ROS [
95].
Cobalt, at the right amounts, can activate antioxidative enzymes, minimizing ROS-caused damage. The mitogen-activated protein kinase (MAPK) cascades are crucial pathways in the response of plants to a variety of stressors [
96]. Heavy metals such as Hg, Zn, Cd, and Cu have been found to participate in plant signal transduction through MAPK in Medicago sativa [
97] and
Oryza sativa [
98,
99]. Despite our expertise and data on Co toxicity and detoxification procedures, there is still a scarcity of information on Co-induced signal transduction pathways. The role of MAPKs (activation of the 44 and 46 kDa MAPKs) in signal transduction in response to excess Co through ROS cannot be ruled out. Several prior studies in various plant species have found evidence of MAPK activation by heavy metals and ROS [
97,
100]. As a result, the total relevance of the MAPK signaling pathways in the response to heavy metal stress remains unknown. The data analysis of the current study (155 observations) revealed an overall 122% enhanced antioxidant activities with a range of -99% to 3750% (
Table 8,
Table S9-14). However, 72 observations revealed an increase while 82 observations highlighted a decrease in the activities of antioxidants. Thus, Co generally enhances antioxidant activities, but it may also decrease antioxidant activities. However, the effect depends on the type of enzyme, plant, plant tissue, and the applied Co level.
To check the effect of Co exposure on different types of antioxidant enzymes, a meta-analysis was also performed for each antioxidant. The overall effect was 456%, 64%, 49%, 49%, and -7%, respectively for APX, CAT, GR, POD, and SOD (
Table 9,
Table S9-S13). This revealed a great variation regarding the effect of Co exposure on the activities of different enzymes. Different enzymes are involved in different reactions to mediate the conversion of different types of ROS. Hence, exposure to CO can differently affect the activities of enzymes.
3.10. Non-enzymatic antioxidants
Co exposure alters the quantity of non-enzymatic antioxidants in addition to antioxidants. Non-enzymatic antioxidants, such as phytochelatins and glutathione, have been shown to scavenge ROS in the presence of heavy metals [
101,
102]. The role of cysteine as a modifying element in Co stress is particularly intriguing. The activation of the GSH production pathway in response to metal stress is essential for the synthesis of Cys-residual rich peptides, phytochelatins (PC), which naturally bind to and detoxify the detrimental toxic metals. One of the heavy metals that does not activate PC-synthase is cobalt [
103], and the activation caused by Co is quite little [
104]. Citrate and cysteine concentrations were observed to be greater in the non-accumulators
Silene cucubalus and
Rauvolfia serpentina, as well as the hyperaccumulator
Crotolaria cobalticola, following Co exposure. Free cysteine was found to be involved in Co complexation in plant cells using size exclusion chromatography [
105]. A recent research found that Co treatments significantly increased PC, GSH, and GSSG contents in both roots and shoots of the two barley genotypes when compared to controls [
106].
Data analysis observed a Co-mediated increase in the activities of non-enzymatic antioxidants (average 100%, range -33% to 1900%) (
Table 8,
Table S9-S14). Overall, 58 observations showed an increase while 7 observations showed a decrease in the activities of non-enzymatic antioxidants. Hence, like enzymatic antioxidants, the activities of non-enzymatic antioxidants also increased under Co exposure in plants. This increase can ultimately induce some beneficial or tolerance effects inside plants due to the role of different types of antioxidants in various biochemical processes/reactions involved in plant homeostasis/tolerance as well as growth improvement.
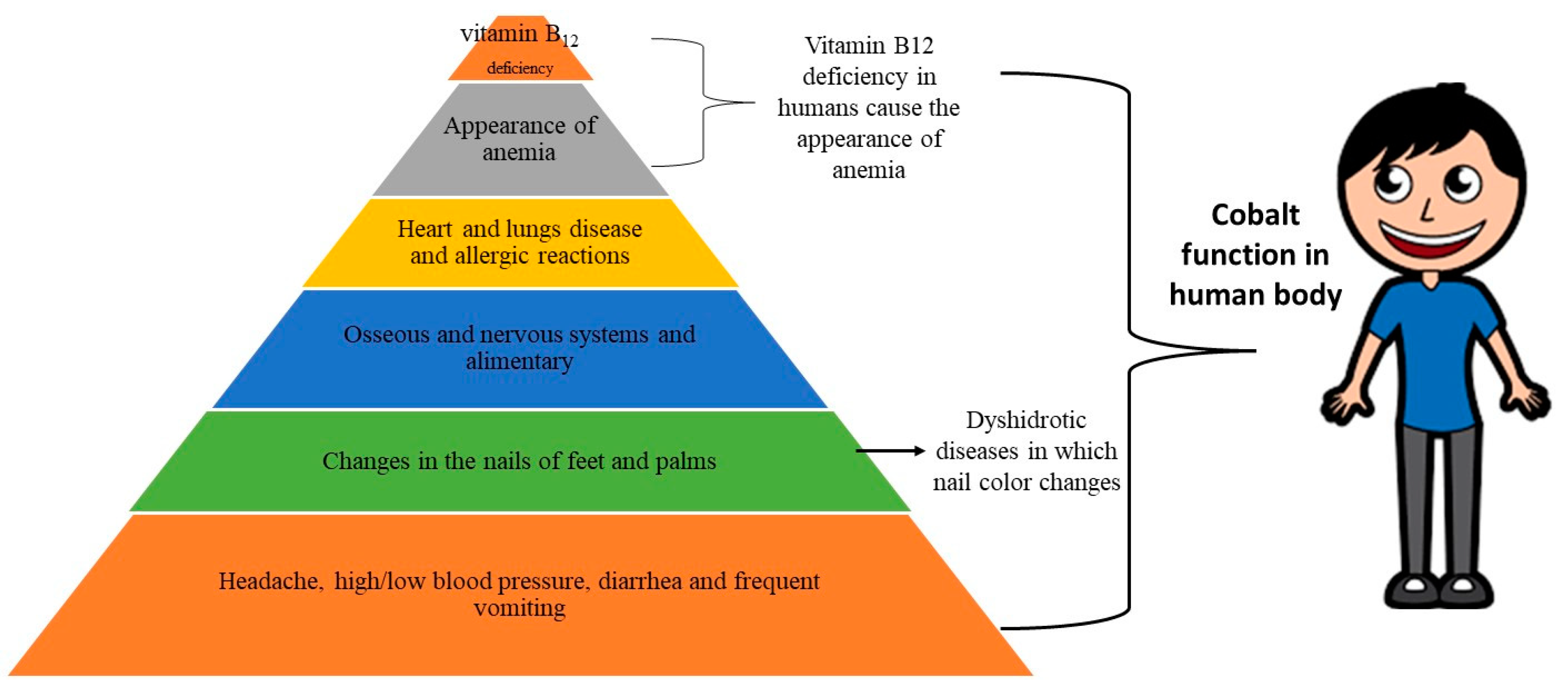
3.11. Cobalt in the human body
Co has a wide range of effects on people. Cobalt is essential for the production of vitamin B
12 in humans [
107]. An adult person typically carries 1 mg of Co, with vitamin B12 accounting for 85% of it. According to Leyssens
, et al. [
108], 0.1 mol concentration of Co in vitamin B12 forms provides the human body with the appropriate quantity of Co. Cobalt intake by humans varies between 5 and 50 μg day
−1 and is mainly in the form of inorganic Co. The maximum Co intake up to 1800 µmol does not cause any negative effect. Another manifestation of the deficiency of Co in humans is disturbed functions of the osseous nervous systems and alimentary (
Figure 3) [
109]. Although, there is no suggested dietary threshold of Co for humans, but its deficiency can induce pernicious anemia. It is reported that the total body contents of Co in humans are 1.1 and 1.5 mg [
110].
Its excess concentration can cause hyperglycemia, gastric disturbance, and polycythemia, and lead to reproductive changes. Cobalt and its compound have higher allergic potential and cause allergic dermatitis. The disease symptoms due to higher levels of Co include heart and lung disease and allergic reactions [
111,
112,
113]. Other symptoms of excess Co exposure include headache, high/low blood pressure, diarrhea, and frequent vomiting. High Co concentration also affects the immunological system [
112,
114,
115]. Cobalt can also mediate significant allergenic problems. interstitial lung disease, asthma, and alveolitis [
115].
Intensification of the above diseases is mostly through the plants near industrial sites where human exposure to harmful levels of Co is much greater than at any other site. The frequent use of instruments with high Co admixtures has a considerable impact on the induction of allergic responses, particularly on the hands and skin. Workers and carpenters are particularly vulnerable to harmful metal exposure [
116]. Concerns have been raised concerning the long-term effects of exposure to compounds containing tungsten carbide (80-95%) in matrices containing Ni (0-5%) and Co (5-20%) [
117,
118,
119,
120,
121].
The harmful effects of Co on humans are majorly due to the intake of Co-contaminated water and food. Mainly, pregnant women can accumulate a significant Co quantity in their bodies from food consumption, which ultimately affects the growing fetus. Chan-Hon-Tong
, et al. [
122] studied pregnant women and showed that women who had higher blood Co levels were eating only fish in comparison to women who were eating milk products, fruits, soups, and sweats.
Cobalt is a proven carcinogen in humans; however, it is only weakly genotoxic. Cobalt produces DNA breaks, changes in DNA basis, and sister chromatid exchanges in human aneuploidy and lymphocytes but not chromosomal abnormalities [
123] or a substantial increase in DNA migration when the comet' test was used [
124]. In HeLa cells, cobalt causes DNA strand breaks, a smaller proportion of 6-thioguanine-resistant mutants, and a higher frequency of sister chromatid exchanges than in V79 Chinese hamster cells [
30]. Cobalt compounds help to produce sticky bridges in binucleate cells and anaphase, as well as fragmentation and chromatin bridges. High Co concentration inhibits RNA synthesis and reduces DNA and RNA quantities, most likely through altering the activities of a large number of exo- and endonucleases [
31].
When Co interacts with other mutagens, its genotoxicity may become more dangerous. In the presence of H
2O
2, cobalt causes alterations in the DNA of chromatin isolated from grown human cells [
125]. Cobalt inhibits DNA repair after it has been damaged by genotoxic substances such as X-rays, ultraviolet light, and alkylating compounds [
126,
127] even at lower cytotoxic content [
128].