Submitted:
18 September 2023
Posted:
21 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Historical Perspective on Microfluidic Systems
3. Microfluidic Devices
3.1. Materials
3.1.1. Silicon
3.1.2. Glass
3.1.3. Polymers
3.1.4. Hydrogels
3.1.5. Metals
3.1.6. Paper
3.1.7. Epoxy
3.1.8. Ceramics
3.2. Microfabrication Methods
3.3. System Components
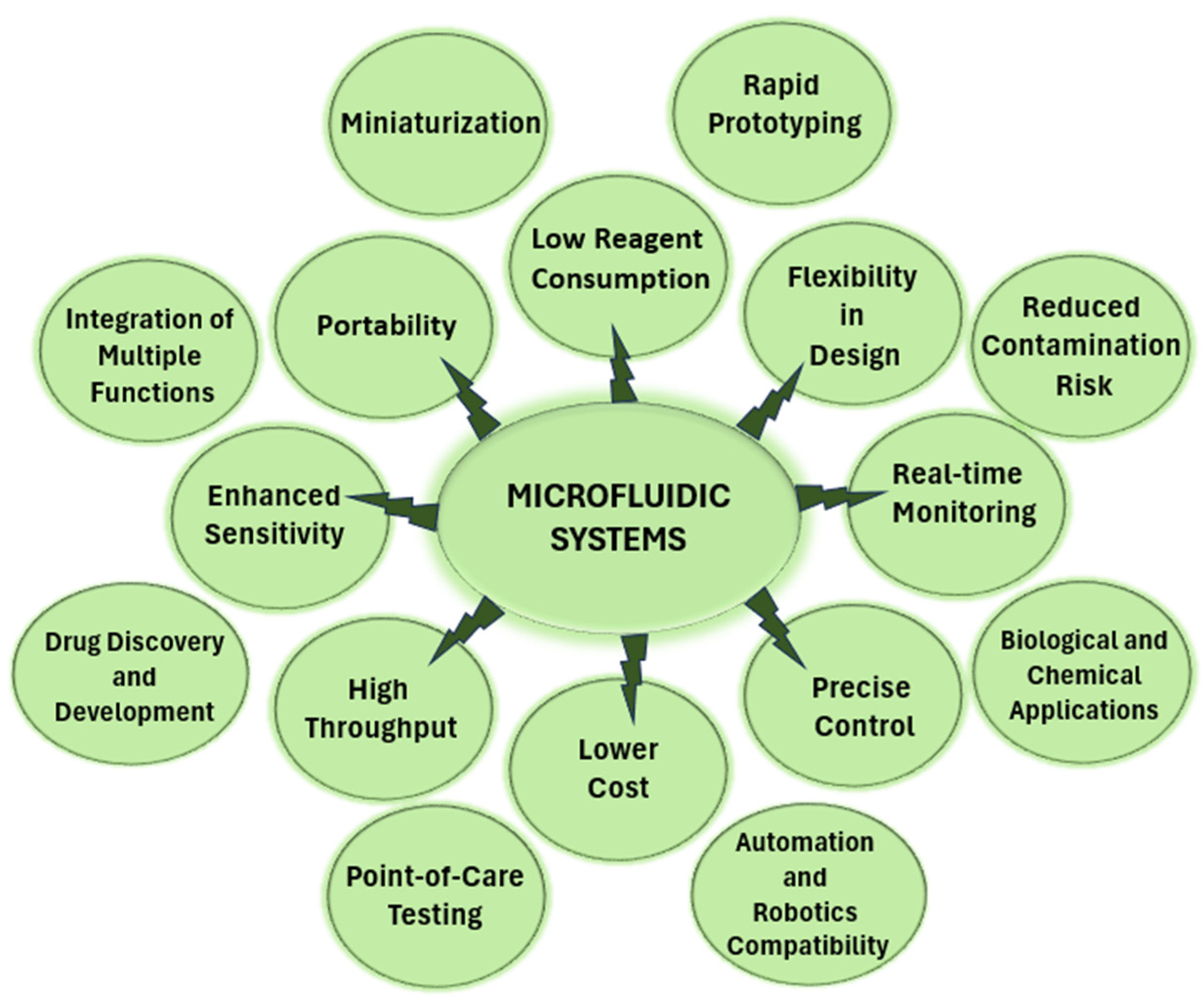
4. Advantages and Disadvantages
5. Polymeric Nanoparticles
6. Lipid Nanoparticles
7. Other Nanoparticle Types
8. Conclusions and future prospects
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Emerich, D.F.; Thanos, C.G. Nanotechnology and medicine. Expert opinion on biological therapy 2003, 3, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: current status and future prospects. The FASEB journal 2005, 19, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, P. Nanoparticles in drug delivery: past, present and future. Advanced drug delivery reviews 2013, 65, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Bawa, R. Nanopharmaceuticals: nanopharmaceuticals. European Journal of Nanomedicine 2010, 3, 34–40. [Google Scholar] [CrossRef]
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic devices: a tool for nanoparticle synthesis and performance evaluation. ACS nano 2023. [Google Scholar] [CrossRef]
- Oroojalian, F.; Charbgoo, F.; Hashemi, M.; Amani, A.; Yazdian-Robati, R.; Mokhtarzadeh, A.; Ramezani, M.; Hamblin, M.R. Recent advances in nanotechnology-based drug delivery systems for the kidney. Journal of controlled release 2020, 321, 442–462. [Google Scholar] [CrossRef]
- Pondman, K.; Le Gac, S.; Kishore, U. Nanoparticle-induced immune response: Health risk versus treatment opportunity? Immunobiology 2022, 152317. [Google Scholar] [CrossRef]
- Illath, K.; Kar, S.; Gupta, P.; Shinde, A.; Wankhar, S.; Tseng, F.-G.; Lim, K.-T.; Nagai, M.; Santra, T.S. Microfluidic nanomaterials: From synthesis to biomedical applications. Biomaterials 2022, 280, 121247. [Google Scholar] [CrossRef]
- Marre, S.; Jensen, K.F. Synthesis of micro and nanostructures in microfluidic systems. Chemical Society Reviews 2010, 39, 1183–1202. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, J.; Chen, C.; Wang, J.; Liu, G.; Nandakumar, K.; Li, Y.; Wang, L. Microfluidic Applications in Drug Development: Fabrication of Drug Carriers and Drug Toxicity Screening. Micromachines 2022, 13, 200. [Google Scholar] [CrossRef] [PubMed]
- Jurin, J. II. An account of some experiments shown before the Royal Society; with an enquiry into the cause of the ascent and suspension of water in capillary tubes. Philosophical Transactions of the Royal Society of London 1717, 30, 739–747. [Google Scholar]
- Castillo-León, J. Microfluidics and lab-on-a-chip devices: history and challenges. In Lab-on-a-Chip Devices and Micro-Total Analysis Systems: A Practical Guide; Springer: 2014; pp. 1-15.
- Sutera, S.P.; Skalak, R. The history of Poiseuille's law. Annual review of fluid mechanics 1993, 25, 1–20. [Google Scholar] [CrossRef]
- Hagen, G. Ueber die Bewegung des Wassers in engen cylindrischen Röhren. Annalen der Physik 1839, 122, 423–442. [Google Scholar] [CrossRef]
- Poiseuille, J.L. Recherches expérimentales sur le mouvement des liquides dans les tubes de très-petits diamètres; Imprimerie Royale: 1844.
- Poiseuille, J. Ecoulement des Liquides: Societe Philomatique de Paris. Extraits des Proces-Verbaux des Seances Pendant I’Annee 1838, 3, 77–81. [Google Scholar]
- Stokes, G.G. On the theories of the internal friction of fluids in motion, and of the equilibrium and motion of elastic solids. 2007.
- Rayleigh, L. On the capillary phenomena of jets. Proc. R. Soc. London 1879, 29, 71–97. [Google Scholar]
- Walker, R. Evolution Flow: The Historical Background of Flow Cytometry. Flow Cytometry, Jan 2013, 28. [Google Scholar]
- Scardamaglia, A. A legal history of lithography. Griffith Law Review 2017, 26, 1–27. [Google Scholar] [CrossRef]
- Tiginyanu, I.; Ursaki, V.; Popa, V. Nanoimprint lithography (NIL) and related techniques for electronics applications. In Nanocoatings and Ultra-Thin Films; Elsevier: 2011; pp. 280-329.
- Rogers, J.A.; Nuzzo, R.G. Recent progress in soft lithography. Materials today 2005, 8, 50–56. [Google Scholar] [CrossRef]
- Kumar, A.; Whitesides, G.M. Features of gold having micrometer to centimeter dimensions can be formed through a combination of stamping with an elastomeric stamp and an alkanethiol ‘‘ink’’followed by chemical etching. Applied physics letters 1993, 63, 2002–2004. [Google Scholar] [CrossRef]
- Kipping, F.S.; Lloyd, L.L. XLVII.—Organic derivatives of silicon. Triphenylsilicol and alkyloxysilicon chlorides. Journal of the Chemical Society, Transactions 1901, 79, 449–459. [Google Scholar] [CrossRef]
- Colas, A. Silicone biomaterials: history and chemistry & medical applications of silicones. 2005.
- Gerami, A.; Alzahid, Y.; Mostaghimi, P.; Kashaninejad, N.; Kazemifar, F.; Amirian, T.; Mosavat, N.; Ebrahimi Warkiani, M.; Armstrong, R.T. Microfluidics for porous systems: fabrication, microscopy and applications. Transport in Porous Media 2019, 130, 277–304. [Google Scholar] [CrossRef]
- Lei, K.F. Introduction: the origin, current status, and future of microfluidics. Microfluidics: fundamental, devices and applications: fundamentals and applications, 2018, 1-18.
- Convery, N.; Gadegaard, N. 30 years of microfluidics. Micro and Nano Engineering 2019, 2, 76–91. [Google Scholar] [CrossRef]
- Andrus, J.; Bond, W. Photoengraving in transistor fabrication. Transistor technology 1958, 3, 151–162. [Google Scholar]
- Panigrahi, P.K. Transport phenomena in microfluidic systems; John Wiley & Sons: 2016.
- Bassous, E.; Taub, H.; Kuhn, L. Ink jet printing nozzle arrays etched in silicon. Applied physics letters 1977, 31, 135–137. [Google Scholar] [CrossRef]
- Terry, S.C.; Jerman, J.H.; Angell, J.B. A gas chromatographic air analyzer fabricated on a silicon wafer. IEEE transactions on electron devices 1979, 26, 1880–1886. [Google Scholar] [CrossRef]
- Whitesides, G. Microfluidics in late adolescence. arXiv preprint arXiv:1802.05595, 2018.
- Grimmer, A.; Wille, R.; Grimmer, A.; Wille, R. Designing Meanders. Designing Droplet Microfluidic Networks: A Toolbox for Designers, 2020, 63-76.
- Kumar, C.S. Microfluidic devices in nanotechnology: Fundamental concepts; John Wiley & Sons: 2010.
- Golay, M.J. A performance index for gas chromatographic columns. Nature 1957, 180, 435–436. [Google Scholar] [CrossRef]
- Van Deemter, J.; Zuiderweg, F.; Klinkenberg, A.v. Longitudinal diffusion and resistance to mass transfer as causes of nonideality in chromatography. Chemical Engineering Science 1956, 5, 271–289. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Karimi, M. Biomedical applications of microfluidic devices; Academic Press: 2020.
- Harrison, D.J.; Fluri, K.; Seiler, K.; Fan, Z.; Effenhauser, C.S.; Manz, A. Micromachining a miniaturized capillary electrophoresis-based chemical analysis system on a chip. Science 1993, 261, 895–897. [Google Scholar] [CrossRef]
- Li, X.J.; Zhou, Y. Microfluidic devices for biomedical applications; Woodhead Publishing: 2021.
- Aryasomayajula, A.; Bayat, P.; Rezai, P.; Selvaganapathy, P.R. Microfluidic devices and their applications. Springer handbook of nanotechnology 2017, 487–536. [Google Scholar]
- Yeo, L.Y.; Chang, H.C.; Chan, P.P.; Friend, J.R. Microfluidic devices for bioapplications. small 2011, 7, 12–48. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Goel, S. Microfluidic devices for synthesizing nanomaterials—A review. Nano Express 2020, 1, 032004. [Google Scholar] [CrossRef]
- Mumtaz, Z.; Rashid, Z.; Ali, A.; Arif, A.; Ameen, F.; AlTami, M.S.; Yousaf, M.Z. Prospects of Microfluidic Technology in Nucleic Acid Detection Approaches. Biosensors 2023, 13, 584. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.S. Microfluidic devices in nanotechnology: applications; John Wiley & Sons: 2010.
- Sommonte, F.; Denora, N.; Lamprou, D.A. Combining 3D printing and microfluidic techniques: A powerful synergy for nanomedicine. Pharmaceuticals 2023, 16, 69. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Fabrication and applications of microfluidic devices: A review. International Journal of Molecular Sciences 2021, 22, 2011. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Yukawa, H.; Baba, Y. Micro-/nano-fluidic devices and in vivo fluorescence imaging based on quantum dots for cytologic diagnosis. Lab on a Chip 2022, 22, 2223–2236. [Google Scholar] [CrossRef] [PubMed]
- Popa, M.L.; Preda, M.D.; Neacșu, I.A.; Grumezescu, A.M.; Ginghină, O. Traditional vs. Microfluidic Synthesis of ZnO Nanoparticles. International Journal of Molecular Sciences 2023, 24, 1875. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Jeon, L.; Kwon, J.; Lee, H. High-Precision Synthesis of RNA-Loaded Lipid Nanoparticles for Biomedical Applications. Advanced Healthcare Materials 2023, 2203033. [Google Scholar] [CrossRef]
- Gale, B.K.; Jafek, A.R.; Lambert, C.J.; Goenner, B.L.; Moghimifam, H.; Nze, U.C.; Kamarapu, S.K. A review of current methods in microfluidic device fabrication and future commercialization prospects. Inventions 2018, 3, 60. [Google Scholar] [CrossRef]
- Hwang, J.; Cho, Y.H.; Park, M.S.; Kim, B.H. Microchannel fabrication on glass materials for microfluidic devices. International Journal of Precision Engineering and Manufacturing 2019, 20, 479–495. [Google Scholar] [CrossRef]
- Scott, S.M.; Ali, Z. Fabrication methods for microfluidic devices: An overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Razavi Bazaz, S.; Rouhi, O.; Raoufi, M.A.; Ejeian, F.; Asadnia, M.; Jin, D.; Ebrahimi Warkiani, M. 3D printing of inertial microfluidic devices. Scientific reports 2020, 10, 5929. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.; Soliman, E.A.; El-Bab, A.M.F.; Matsushita, Y.; Abdel-Mawgood, A.L. Fabrication and characterization of microfluidic devices based on boron-modified epoxy resin using CO2 laser ablation for bio-analytical applications. Scientific Reports 2023, 13, 12623. [Google Scholar] [CrossRef]
- Jayarama, A.; Kannarpady, G.K.; Kale, S.; Prabhu, S.; Pinto, R. Chemical etching of glasses in hydrofluoric Acid: A brief review. Materials Today: Proceedings 2022, 55, 46–51. [Google Scholar]
- Han, X.; Zhang, Y.; Tian, J.; Wu, T.; Li, Z.; Xing, F.; Fu, S. Polymer-based microfluidic devices: A comprehensive review on preparation and applications. Polymer Engineering & Science 2022, 62, 3–24. [Google Scholar]
- Gal-Or, E.; Gershoni, Y.; Scotti, G.; Nilsson, S.M.; Saarinen, J.; Jokinen, V.; Strachan, C.J.; af Gennäs, G.B.; Yli-Kauhaluoma, J.; Kotiaho, T. Chemical analysis using 3D printed glass microfluidics. Analytical Methods 2019, 11, 1802–1810. [Google Scholar] [CrossRef]
- Nie, J.; Fu, J.; He, Y. Hydrogels: the next generation body materials for microfluidic chips? Small 2020, 16, 2003797. [Google Scholar] [CrossRef]
- Alting, L.; Kimura, F.; Hansen, H.N.; Bissacco, G. Micro engineering. CIRP annals 2003, 52, 635–657. [Google Scholar] [CrossRef]
- Brousseau, E.; Dimov, S.S.; Pham, D.T. Some recent advances in multi-material micro-and nano-manufacturing. The International Journal of Advanced Manufacturing Technology 2010, 47, 161–180. [Google Scholar] [CrossRef]
- Madou, M.J. Fundamentals of microfabrication: the science of miniaturization; CRC press: 2002.
- Uriarte, L.; Herrero, A.; Ivanov, A.; Oosterling, H.; Staemmler, L.; Tang, P.T.; Allen, D. Comparison between microfabrication technologies for metal tooling. Proceedings of the Institution of Mechanical Engineers, Part C: Journal of Mechanical Engineering Science 2006, 220, 1665–1676. [Google Scholar] [CrossRef]
- Friend, J.; Yeo, L. Fabrication of microfluidic devices using polydimethylsiloxane. Biomicrofluidics 2010, 4. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Khan, S.; Didar, T.F. Conventional and emerging strategies for the fabrication and functionalization of PDMS-based microfluidic devices. Lab on a Chip 2021, 21, 3053–3075. [Google Scholar] [CrossRef] [PubMed]
- Deb, R.; Sarma, B.; Dalal, A. Magnetowetting dynamics of sessile ferrofluid droplets: a review. Soft Matter 2022, 18, 2287–2324. [Google Scholar] [CrossRef] [PubMed]
- Bragheri, F.; Vazquez, R.M.; Osellame, R. Microfluidics. In Three-dimensional microfabrication using two-photon polymerization; Elsevier: 2020; pp. 493-526.
- Battat, S.; Weitz, D.A.; Whitesides, G.M. An outlook on microfluidics: the promise and the challenge. Lab on a Chip 2022, 22, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.M.; Gitlin, I.; Stroock, A.D.; Whitesides, G.M. Components for integrated poly (dimethylsiloxane) microfluidic systems. Electrophoresis 2002, 23, 3461–3473. [Google Scholar] [CrossRef]
- Huang, C.; Tsou, C. The implementation of a thermal bubble actuated microfluidic chip with microvalve, micropump and micromixer. Sensors and actuators A: Physical 2014, 210, 147–156. [Google Scholar] [CrossRef]
- Beebe, D.J.; Mensing, G.A.; Walker, G.M. Physics and applications of microfluidics in biology. Annual review of biomedical engineering 2002, 4, 261–286. [Google Scholar] [CrossRef]
- Kandlikar, S.; Garimella, S.; Li, D.; Colin, S.; King, M.R. Heat transfer and fluid flow in minichannels and microchannels; elsevier: 2005.
- Kamat, V.; Dey, P.; Bodas, D.; Kaushik, A.; Boymelgreen, A.; Bhansali, S. Active microfluidic reactor-assisted controlled synthesis of nanoparticles and related potential biomedical applications. Journal of Materials Chemistry B 2023. [Google Scholar] [CrossRef]
- Oh, K.W.; Ahn, C.H. A review of microvalves. Journal of micromechanics and microengineering 2006, 16, R13. [Google Scholar] [CrossRef]
- Verma, A.; Bhattacharyya, S. Microfluidics-the state-of-the-art technology for pharmaceutical application. Advanced Pharmaceutical Bulletin 2022, 12, 700. [Google Scholar] [CrossRef]
- Ma, X.; Guo, G.; Wu, X.; Wu, Q.; Liu, F.; Zhang, H.; Shi, N.; Guan, Y. Advances in Integration, Wearable Applications, and Artificial Intelligence of Biomedical Microfluidics Systems. Micromachines 2023, 14, 972. [Google Scholar] [CrossRef] [PubMed]
- Fredrick, S.J.; Gross, E.M. Use of microelectrodes for electrochemiluminescent detection in microfluidic devices. 2009.
- Tsai, H.-F.; Podder, S.; Chen, P.-Y. Microsystem Advances through Integration with Artificial Intelligence. Micromachines 2023, 14, 826. [Google Scholar] [CrossRef]
- Surappa, S.; Multani, P.; Parlatan, U.; Sinawang, P.D.; Kaifi, J.; Akin, D.; Demirci, U. Integrated “lab-on-a-chip” microfluidic systems for isolation, enrichment, and analysis of cancer biomarkers. Lab on a Chip 2023. [Google Scholar] [CrossRef]
- Gong, L.; Cretella, A.; Lin, Y. Microfluidic systems for particle capture and release: A review. Biosensors and Bioelectronics 2023, 115426. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.J.; Yobas, L.; Lee, G.Y.H.; Ong, C.N.; Lim, C.T. Microdevice for the isolation and enumeration of cancer cells from blood. Biomedical microdevices 2009, 11, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Gao, Y.; Wang, H. Recent advances in drug delivery system fabricated by microfluidics for disease therapy. Bioengineering 2022, 9, 625. [Google Scholar] [CrossRef] [PubMed]
- Forigua, A.; Kirsch, R.L.; Willerth, S.M.; Elvira, K.S. Recent advances in the design of microfluidic technologies for the manufacture of drug releasing particles. Journal of Controlled Release 2021, 333, 258–268. [Google Scholar] [CrossRef]
- Wu, J.; Fang, H.; Zhang, J.; Yan, S. Modular microfluidics for life sciences. Journal of Nanobiotechnology 2023, 21, 1–30. [Google Scholar] [CrossRef]
- Zimmermann, R.; Leal, B.B.J.; Braghirolli, D.I.; Pranke, P. Production of nanostructured systems: main and innovative techniques. Drug Discovery Today 2022, 103454. [Google Scholar] [CrossRef]
- Agha, A.; Waheed, W.; Stiharu, I.; Nerguizian, V.; Destgeer, G.; Abu-Nada, E.; Alazzam, A. A review on microfluidic-assisted nanoparticle synthesis, and their applications using multiscale simulation methods. Discover Nano 2023, 18, 18. [Google Scholar] [CrossRef]
- Maged, A.; Abdelbaset, R.; Mahmoud, A.A.; Elkasabgy, N.A. Merits and advances of microfluidics in the pharmaceutical field: design technologies and future prospects. Drug Delivery 2022, 29, 1549–1570. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Hui, Y.; Ranaweera, S.; Zhao, C.X. Microfluidic nanoparticles for drug delivery. Small 2022, 18, 2106580. [Google Scholar] [CrossRef]
- Fabozzi, A.; Della Sala, F.; di Gennaro, M.; Barretta, M.; Longobardo, G.; Solimando, N.; Pagliuca, M.; Borzacchiello, A. Design of functional nanoparticles by microfluidic platforms as advanced drug delivery systems for cancer therapy. Lab on a Chip 2023, 23, 1389–1409. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Gabold, B.; Chen, S.; Wang, X.; Xu, Z.; Hartschuh, A.; Chiesa, E.; Genta, I.; Ried, C.L.; Merdan, T. Microfluidic mixing as platform technology for production of chitosan nanoparticles loaded with different macromolecules. European Journal of Pharmaceutics and Biopharmaceutics 2023, 188, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Gimondi, S.; Guimarães, C.F.; Vieira, S.F.; Gonçalves, V.M.; Tiritan, M.E.; Reis, R.L.; Ferreira, H.; Neves, N.M. Microfluidic mixing system for precise PLGA-PEG nanoparticles size control. Nanomedicine: Nanotechnology, Biology and Medicine 2022, 40, 102482. [Google Scholar] [CrossRef]
- Saygili, E.; Kaya, E.; Ilhan-Ayisigi, E.; Saglam-Metiner, P.; Alarcin, E.; Kazan, A.; Girgic, E.; Kim, Y.-W.; Gunes, K.; Eren-Ozcan, G.G. An alginate-poly (acrylamide) hydrogel with TGF-β3 loaded nanoparticles for cartilage repair: Biodegradability, biocompatibility and protein adsorption. International Journal of Biological Macromolecules 2021, 172, 381–393. [Google Scholar] [CrossRef]
- Brzeziński, M.; Socka, M.; Makowski, T.; Kost, B.; Cieślak, M.; Królewska-Golińska, K. Microfluidic-assisted nanoprecipitation of biodegradable nanoparticles composed of PTMC/PCL (co) polymers, tannic acid and doxorubicin for cancer treatment. Colloids and Surfaces B: Biointerfaces 2021, 201, 111598. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, Z.; Mohammadnejad, J.; Bazaz, S.R.; Mehrizi, A.A.; Saidijam, M.; Dinarvand, R.; Warkiani, M.E.; Soleimani, M. Promoted chondrogenesis of hMCSs with controlled release of TGF-β3 via microfluidics synthesized alginate nanogels. Carbohydrate Polymers 2020, 229, 115551. [Google Scholar] [CrossRef]
- Kly, S.; Huang, Y.; Moffitt, M.G. Enhancement of Cellular Uptake by Increasing the Number of Encapsulated Gold Nanoparticles in Polymeric Micelles. Journal of Colloid and Interface Science 2023. [Google Scholar] [CrossRef]
- Hu, R.; Li, X.; Xu, J.; Cheng, Y.; Zhang, M.; Shi, G. Continuous Preparation of Semiconducting Polymer Nanoparticles with Varied Sizes for Online Fluorescence Sensing via a Laser-Tailored 3D Microfluidic Chip. Analytical Chemistry 2023, 95, 10422–10429. [Google Scholar] [CrossRef]
- Martin, A.; Lalanne, P.; Weber-Vax, A.; Mutschler, A.; Lecommandoux, S. Controlling Polymersome Size through Microfluidic-Assisted Self-Assembly: Enabling'Ready to Use'formulations for biological applications. International Journal of Pharmaceutics 2023, 123157. [Google Scholar] [CrossRef] [PubMed]
- Gimondi, S.; de Castro, J.V.; Reis, R.L.; Ferreira, H.; Neves, N.M. On the size-dependent internalization of sub-hundred polymeric nanoparticles. Colloids and Surfaces B: Biointerfaces 2023, 225, 113245. [Google Scholar] [CrossRef] [PubMed]
- Behnke, M.; Klemm, P.; Dahlke, P.; Shkodra, B.; Beringer-Siemers, B.; Czaplewska, J.A.; Stumpf, S.; Jordan, P.M.; Schubert, S.; Hoeppener, S. Ethoxy acetalated dextran nanoparticles for drug delivery: A comparative study of formulation methods. International Journal of Pharmaceutics: X 2023, 5, 100173. [Google Scholar] [CrossRef]
- Tenorio-Barajas, A.Y.; Olvera, M.d.l.L.; Romero-Paredes, G.; Altuzar, V.; Garrido-Guerrero, E.; Mendoza-Barrera, C. Chitosan, Chitosan/IgG-Loaded, and N-Trimethyl Chitosan Chloride Nanoparticles as Potential Adjuvant and Carrier-Delivery Systems. Molecules 2023, 28, 4107. [Google Scholar] [CrossRef]
- Bao, Y.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. Preparation of size-tunable sub-200 nm PLGA-based nanoparticles with a wide size range using a microfluidic platform. Plos one 2022, 17, e0271050. [Google Scholar] [CrossRef] [PubMed]
- Roffo, F.; Ponsiglione, A.M.; Netti, P.A.; Torino, E. coupled Hydrodynamic Flow Focusing (cHFF) to Engineer Lipid–Polymer Nanoparticles (LiPoNs) for Multimodal Imaging and Theranostic Applications. Biomedicines 2022, 10, 438. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Wang, J.; Qi, J.; Li, J.; Ma, J.; Chen, L. A rotary multi-positioned cloth/paper hybrid microfluidic device for simultaneous fluorescence sensing of mercury and lead ions by using ion imprinted technologies. Journal of Hazardous Materials 2022, 428, 128165. [Google Scholar] [CrossRef]
- Herranz-Blanco, B.; Ginestar, E.; Zhang, H.; Hirvonen, J.; Santos, H.A. Microfluidics platform for glass capillaries and its application in droplet and nanoparticle fabrication. International journal of pharmaceutics 2017, 516, 100–105. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, T.; Li, Q.; Wang, F.; Li, J. A microfluidic approach for rapid and continuous synthesis of glycoprotein-imprinted nanospheres. Talanta 2022, 239, 123084. [Google Scholar] [CrossRef]
- Abad, M.; Mendoza, G.; Usón, L.; Arruebo, M.; Piñol, M.; Sebastián, V.; Oriol, L. Microfluidic Synthesis of Block Copolymer Micelles: Application as Drug Nanocarriers and as Photothermal Transductors When Loading Pd Nanosheets. Macromolecular Bioscience 2022, 22, 2100528. [Google Scholar] [CrossRef]
- Gimondi, S.; Reis, R.L.; Ferreira, H.; Neves, N.M. Microfluidic-driven mixing of high molecular weight polymeric complexes for precise nanoparticle downsizing. Nanomedicine: Nanotechnology, Biology and Medicine 2022, 43, 102560. [Google Scholar] [CrossRef] [PubMed]
- Tiboni, M.; Tiboni, M.; Pierro, A.; Del Papa, M.; Sparaventi, S.; Cespi, M.; Casettari, L. Microfluidics for nanomedicines manufacturing: An affordable and low-cost 3D printing approach. International Journal of Pharmaceutics 2021, 599, 120464. [Google Scholar] [CrossRef]
- Cesur, S.; Cam, M.E.; Sayın, F.S.; Su, S.; Harker, A.; Edirisinghe, M.; Gunduz, O. Metformin-loaded polymer-based microbubbles/nanoparticles generated for the treatment of type 2 diabetes mellitus. Langmuir 2021, 38, 5040–5051. [Google Scholar] [CrossRef] [PubMed]
- Lari, A.S.; Zahedi, P.; Ghourchian, H.; Khatibi, A. Microfluidic-based synthesized carboxymethyl chitosan nanoparticles containing metformin for diabetes therapy: In vitro and in vivo assessments. Carbohydrate Polymers 2021, 261, 117889. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, E.; Greco, A.; Dorati, R.; Conti, B.; Bruni, G.; Lamprou, D.; Genta, I. Microfluidic-assisted synthesis of multifunctional iodinated contrast agent polymeric nanoplatforms. International Journal of Pharmaceutics 2021, 599, 120447. [Google Scholar] [CrossRef]
- Baby, T.; Liu, Y.; Yang, G.; Chen, D.; Zhao, C.-X. Microfluidic synthesis of curcumin loaded polymer nanoparticles with tunable drug loading and pH-triggered release. Journal of Colloid and Interface Science 2021, 594, 474–484. [Google Scholar] [CrossRef]
- Solomun, J.I.; Totten, J.D.; Wongpinyochit, T.; Florence, A.J.; Seib, F.P. Manual versus microfluidic-assisted nanoparticle manufacture: impact of silk fibroin stock on nanoparticle characteristics. ACS Biomaterials Science & Engineering 2020, 6, 2796–2804. [Google Scholar]
- Wan, F.; Bohr, S.S.-R.; Kłodzińska, S.N.; Jumaa, H.; Huang, Z.; Nylander, T.; Thygesen, M.B.; Sørensen, K.K.; Jensen, K.J.; Sternberg, C. Ultrasmall TPGS–PLGA hybrid nanoparticles for site-specific delivery of antibiotics into Pseudomonas aeruginosa biofilms in lungs. ACS applied materials & interfaces 2019, 12, 380–389. [Google Scholar]
- Huang, Y.; Moini Jazani, A.; Howell, E.P.; Oh, J.K.; Moffitt, M.G. Controlled microfluidic synthesis of biological stimuli-responsive polymer nanoparticles. ACS applied materials & interfaces 2019, 12, 177–190. [Google Scholar]
- Kim, S.; Choi, B.; Kim, Y.; Shim, G. Immune-Modulating Lipid Nanomaterials for the Delivery of Biopharmaceuticals. Pharmaceutics 2023, 15, 1760. [Google Scholar] [CrossRef]
- Vogelaar, A.; Marcotte, S.; Cheng, J.; Oluoch, B.; Zaro, J. Use of Microfluidics to Prepare Lipid-Based Nanocarriers. Pharmaceutics 2023, 15, 1053. [Google Scholar] [CrossRef]
- Lin, Z.; Zou, Z.; Pu, Z.; Wu, M.; Zhang, Y. Application of microfluidic technologies on COVID-19 diagnosis and drug discovery. Acta Pharmaceutica Sinica B 2023. [Google Scholar] [CrossRef]
- Prakash, G.; Shokr, A.; Willemen, N.; Bashir, S.M.; Shin, S.R.; Hassan, S. Microfluidic fabrication of lipid nanoparticles for the delivery of nucleic acids. Advanced drug delivery reviews 2022, 184, 114197. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.G.; Ceccato, B.T.; Michelon, M.; Han, S.W.; de la Torre, L.G. Advanced microfluidic technologies for lipid nano-microsystems from synthesis to biological application. Pharmaceutics 2022, 14, 141. [Google Scholar] [CrossRef]
- van Der Meel, R.; Chen, S.; Zaifman, J.; Kulkarni, J.A.; Zhang, X.R.S.; Tam, Y.K.; Bally, M.B.; Schiffelers, R.M.; Ciufolini, M.A.; Cullis, P.R. Modular lipid nanoparticle platform technology for siRNA and lipophilic prodrug delivery. Small 2021, 17, 2103025. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, W.; Li, Q.; He, C.; Liu, Y.; Liu, J.; Wang, R.; Wu, J.; Xiang, D.; Chen, C. Coaxial electrostatic spray-based preparation of localization missile liposomes on a microfluidic chip for targeted treatment of triple-negative breast cancer. International Journal of Pharmaceutics 2023, 643, 123220. [Google Scholar] [CrossRef] [PubMed]
- Fathordoobady, F.; Sannikova, N.; Guo, Y.; Singh, A.; Kitts, D.D.; Pratap-Singh, A. Comparing microfluidics and ultrasonication as formulation methods for developing hempseed oil nanoemulsions for oral delivery applications. Scientific Reports 2021, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lee, R.J.; Huang, X.; Li, Y.; Lv, B.; Wang, T.; Qi, Y.; Hao, F.; Lu, J.; Meng, Q. Single-step microfluidic synthesis of transferrin-conjugated lipid nanoparticles for siRNA delivery. Nanomedicine: Nanotechnology, Biology and Medicine 2017, 13, 371–381. [Google Scholar] [CrossRef]
- Ran, R.; Wang, H.; Liu, Y.; Hui, Y.; Sun, Q.; Seth, A.; Wibowo, D.; Chen, D.; Zhao, C.-X. Microfluidic self-assembly of a combinatorial library of single-and dual-ligand liposomes for in vitro and in vivo tumor targeting. European Journal of Pharmaceutics and Biopharmaceutics 2018, 130, 1–10. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Han, X.; Mukalel, A.J.; El-Mayta, R.; Thatte, A.S.; Wu, J.; Padilla, M.S.; Alameh, M.-G.; Srikumar, N.; Lee, D. Throughput-scalable manufacturing of SARS-CoV-2 mRNA lipid nanoparticle vaccines. Proceedings of the National Academy of Sciences 2023, 120, e2303567120. [Google Scholar] [CrossRef]
- O’Brien Laramy, M.N.; Costa, A.P.; Cebrero, Y.M.; Joseph, J.; Sarode, A.; Zang, N.; Kim, L.J.; Hofmann, K.; Wang, S.; Goyon, A. Process Robustness in Lipid Nanoparticle Production: A Comparison of Microfluidic and Turbulent Jet Mixing. Molecular Pharmaceutics 2023, 20, 4285–4296. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, V.; Boso, D.P.; Moore, T.L.; Schrefler, B.A.; Decuzzi, P. Machine Learning Instructed Microfluidic Synthesis of Curcumin-loaded Liposomes. 2023.
- Chen, Y.; Chen, W.; Ren, Y.; Li, S.; Liu, M.; Xing, J.; Han, Y.; Chen, Y.; Tao, R.; Guo, L. Lipid nanoparticle-encapsulated VEGFa siRNA facilitates cartilage formation by suppressing angiogenesis. International Journal of Biological Macromolecules 2022, 221, 1313–1324. [Google Scholar] [CrossRef]
- Cui, L.; Renzi, S.; Quagliarini, E.; Digiacomo, L.; Amenitsch, H.; Masuelli, L.; Bei, R.; Ferri, G.; Cardarelli, F.; Wang, J. Efficient delivery of DNA using lipid nanoparticles. Pharmaceutics 2022, 14, 1698. [Google Scholar] [CrossRef] [PubMed]
- Yathindranath, V.; Safa, N.; Sajesh, B.V.; Schwinghamer, K.; Vanan, M.I.; Bux, R.; Sitar, D.S.; Pitz, M.; Siahaan, T.J.; Miller, D.W. Spermidine/Spermine N1-Acetyltransferase 1 (SAT1)—A Potential Gene Target for Selective Sensitization of Glioblastoma Cells Using an Ionizable Lipid Nanoparticle to Deliver SiRNA. Cancers 2022, 14, 5179. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Sato, Y.; Iwakawa, K.; Sasaki, K.; Okabe, N.; Maeki, M.; Tokeshi, M.; Harashima, H. On the size-regulation of RNA-loaded lipid nanoparticles synthesized by microfluidic device. Journal of Controlled Release 2022, 348, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Han, J.P.; Kim, M.; Choi, B.S.; Lee, J.H.; Lee, G.S.; Jeong, M.; Lee, Y.; Kim, E.-A.; Oh, H.-K.; Go, N. In vivo delivery of CRISPR-Cas9 using lipid nanoparticles enables antithrombin gene editing for sustainable hemophilia A and B therapy. Science advances 2022, 8, eabj6901. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Chen, S.; Tam, Y.Y.C. Scalable production of lipid nanoparticles containing Amphotericin B. Langmuir 2021, 37, 7312–7319. [Google Scholar] [CrossRef]
- Terada, T.; Kulkarni, J.A.; Huynh, A.; Chen, S.; van der Meel, R.; Tam, Y.Y.C.; Cullis, P.R. Characterization of lipid nanoparticles containing ionizable cationic lipids using design-of-experiments approach. Langmuir 2021, 37, 1120–1128. [Google Scholar] [CrossRef]
- Younis, M.A.; Khalil, I.A.; Elewa, Y.H.; Kon, Y.; Harashima, H. Ultra-small lipid nanoparticles encapsulating sorafenib and midkine-siRNA selectively-eradicate sorafenib-resistant hepatocellular carcinoma in vivo. Journal of Controlled Release 2021, 331, 335–349. [Google Scholar] [CrossRef]
- Forbes, N.; Hussain, M.T.; Briuglia, M.L.; Edwards, D.P.; Ter Horst, J.H.; Szita, N.; Perrie, Y. Rapid and scale-independent microfluidic manufacture of liposomes entrapping protein incorporating in-line purification and at-line size monitoring. International journal of pharmaceutics 2019, 556, 68–81. [Google Scholar] [CrossRef]
- Ayad, C.; Libeau, P.; Lacroix-Gimon, C.; Ladavière, C.; Verrier, B. Lipoparticles: lipid-coated PLA nanoparticles enhanced in vitro mRNA transfection compared to liposomes. Pharmaceutics 2021, 13, 377. [Google Scholar] [CrossRef] [PubMed]
- Quagliarini, E.; Renzi, S.; Digiacomo, L.; Giulimondi, F.; Sartori, B.; Amenitsch, H.; Tassinari, V.; Masuelli, L.; Bei, R.; Cui, L. Microfluidic formulation of DNA-loaded multicomponent lipid nanoparticles for gene delivery. Pharmaceutics 2021, 13, 1292. [Google Scholar] [CrossRef] [PubMed]
- Connerty, P.; Moles, E.; de Bock, C.E.; Jayatilleke, N.; Smith, J.L.; Meshinchi, S.; Mayoh, C.; Kavallaris, M.; Lock, R.B. Development of siRNA-loaded lipid nanoparticles targeting long non-coding RNA LINC01257 as a novel and safe therapeutic approach for t (8; 21) pediatric acute myeloid leukemia. Pharmaceutics 2021, 13, 1681. [Google Scholar] [CrossRef]
- Khadke, S.; Roces, C.B.; Donaghey, R.; Giacobbo, V.; Su, Y.; Perrie, Y. Scalable solvent-free production of liposomes. Journal of Pharmacy and Pharmacology 2020, 72, 1328–1340. [Google Scholar] [CrossRef] [PubMed]
- Gkionis, L.; Campbell, R.A.; Aojula, H.; Harris, L.K.; Tirella, A. Manufacturing drug co-loaded liposomal formulations targeting breast cancer: Influence of preparative method on liposomes characteristics and in vitro toxicity. International journal of pharmaceutics 2020, 590, 119926. [Google Scholar] [CrossRef]
- Billingsley, M.M.; Singh, N.; Ravikumar, P.; Zhang, R.; June, C.H.; Mitchell, M.J. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano letters 2020, 20, 1578–1589. [Google Scholar] [CrossRef]
- Sato, Y.; Okabe, N.; Note, Y.; Hashiba, K.; Maeki, M.; Tokeshi, M.; Harashima, H. Hydrophobic scaffolds of pH-sensitive cationic lipids contribute to miscibility with phospholipids and improve the efficiency of delivering short interfering RNA by small-sized lipid nanoparticles. Acta biomaterialia 2020, 102, 341–350. [Google Scholar] [CrossRef]
- Zukancic, D.; Suys, E.J.; Pilkington, E.H.; Algarni, A.; Al-Wassiti, H.; Truong, N.P. The importance of poly (Ethylene glycol) and lipid structure in targeted gene delivery to lymph nodes by lipid nanoparticles. Pharmaceutics 2020, 12, 1068. [Google Scholar] [CrossRef]
- Hashiba, A.; Toyooka, M.; Sato, Y.; Maeki, M.; Tokeshi, M.; Harashima, H. The use of design of experiments with multiple responses to determine optimal formulations for in vivo hepatic mRNA delivery. Journal of Controlled Release 2020, 327, 467–476. [Google Scholar] [CrossRef]
- Roces, C.B.; Lou, G.; Jain, N.; Abraham, S.; Thomas, A.; Halbert, G.W.; Perrie, Y. Manufacturing considerations for the development of lipid nanoparticles using microfluidics. Pharmaceutics 2020, 12, 1095. [Google Scholar] [CrossRef]
- Hamano, N.; Böttger, R.; Lee, S.E.; Yang, Y.; Kulkarni, J.A.; Ip, S.; Cullis, P.R.; Li, S.-D. Robust microfluidic technology and new lipid composition for fabrication of curcumin-loaded liposomes: effect on the anticancer activity and safety of cisplatin. Molecular Pharmaceutics 2019, 16, 3957–3967. [Google Scholar] [CrossRef]
- Dong, Y.-D.; Tchung, E.; Nowell, C.; Kaga, S.; Leong, N.; Mehta, D.; Kaminskas, L.M.; Boyd, B.J. Microfluidic preparation of drug-loaded PEGylated liposomes, and the impact of liposome size on tumour retention and penetration. Journal of liposome research 2019, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Sung, J.; Chang, Y.; Alfeche, A.; Leal, C. Microfluidics synthesis of gene silencing cubosomes. ACS nano 2018, 12, 9196–9205. [Google Scholar] [CrossRef]
- Kimura, N.; Maeki, M.; Sato, Y.; Note, Y.; Ishida, A.; Tani, H.; Harashima, H.; Tokeshi, M. Development of the iLiNP device: fine tuning the lipid nanoparticle size within 10 nm for drug delivery. ACS omega 2018, 3, 5044–5051. [Google Scholar] [CrossRef] [PubMed]
- Balbino, T.A.; Serafin, J.M.; Radaic, A.; de Jesus, M.B.; de la Torre, L.G. Integrated microfluidic devices for the synthesis of nanoscale liposomes and lipoplexes. Colloids and surfaces B: biointerfaces 2017, 152, 406–413. [Google Scholar] [CrossRef]
- Krzysztoń, R.; Salem, B.; Lee, D.; Schwake, G.; Wagner, E.; Rädler, J. Microfluidic self-assembly of folate-targeted monomolecular siRNA-lipid nanoparticles. Nanoscale 2017, 9, 7442–7453. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Hussain, M.T.; Roces, C.B.; Anderluzzi, G.; Kastner, E.; Salmaso, S.; Kirby, D.J.; Perrie, Y. Microfluidics based manufacture of liposomes simultaneously entrapping hydrophilic and lipophilic drugs. International journal of pharmaceutics 2016, 514, 160–168. [Google Scholar] [CrossRef]
- Sato, Y.; Note, Y.; Maeki, M.; Kaji, N.; Baba, Y.; Tokeshi, M.; Harashima, H. Elucidation of the physicochemical properties and potency of siRNA-loaded small-sized lipid nanoparticles for siRNA delivery. Journal of controlled release 2016, 229, 48–57. [Google Scholar] [CrossRef]
- Kastner, E.; Verma, V.; Lowry, D.; Perrie, Y. Microfluidic-controlled manufacture of liposomes for the solubilisation of a poorly water soluble drug. International journal of pharmaceutics 2015, 485, 122–130. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, B.; Zhu, J.; Huang, X.; Xie, J.; Xu, S.; Yang, X.; Wang, X.; Yung, B.C.; Lee, L.J. A microfluidic method to synthesize transferrin-lipid nanoparticles loaded with siRNA LOR-1284 for therapy of acute myeloid leukemia. Nanoscale 2014, 6, 9742–9751. [Google Scholar] [CrossRef]
- Balbino, T.A.; Azzoni, A.R.; de La Torre, L.G. Microfluidic devices for continuous production of pDNA/cationic liposome complexes for gene delivery and vaccine therapy. Colloids and Surfaces B: Biointerfaces 2013, 111, 203–210. [Google Scholar] [CrossRef]
- Belliveau, N.M.; Huft, J.; Lin, P.J.; Chen, S.; Leung, A.K.; Leaver, T.J.; Wild, A.W.; Lee, J.B.; Taylor, R.J.; Tam, Y.K. Microfluidic synthesis of highly potent limit-size lipid nanoparticles for in vivo delivery of siRNA. Molecular Therapy-Nucleic Acids 2012, 1. [Google Scholar] [CrossRef]
- Aftab, A.; Ahmad, B.; Bashir, S.; Rafique, S.; Bashir, M.; Ghani, T.; Gul, A.; Shah, A.U.; Khan, R.; Sajini, A.A. Comparative study of microscale and macroscale technique for encapsulation of Calotropis gigantea extract in metal-conjugated nanomatrices for invasive ductal carcinoma. Scientific Reports 2023, 13, 13474. [Google Scholar] [CrossRef]
- Alhalili, Z. Metal Oxides Nanoparticles: General Structural Description, Chemical, Physical, and Biological Synthesis Methods, Role in Pesticides and Heavy Metal Removal through Wastewater Treatment. Molecules 2023, 28, 3086. [Google Scholar] [CrossRef]
- Acharya, G.; Mitra, A.K.; Cholkar, K. Nanosystems for diagnostic imaging, biodetectors, and biosensors. In Emerging nanotechnologies for diagnostics, drug delivery and medical devices; Elsevier: 2017; pp. 217-248.
- Wacker, J.B.; Lignos, I.; Parashar, V.K.; Gijs, M.A. Controlled synthesis of fluorescent silica nanoparticles inside microfluidic droplets. Lab on a Chip 2012, 12, 3111–3116. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, T.; Campos, M.G.N.; Smith, S.; Doomra, M.; Thwin, Z.; Santra, S. Quantum dots. In Nanoparticles for Biomedical Applications; Elsevier: 2020; pp. 243-265.
- Shmeis, R.M.A. Nanotechnology in wastewater treatment. In Comprehensive Analytical Chemistry; Elsevier: 2022; Volume 99, pp. 105-134.
- Patel, K.D.; Singh, R.K.; Kim, H.-W. Carbon-based nanomaterials as an emerging platform for theranostics. Materials Horizons 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Khizar, S.; Zine, N.; Errachid, A.; Jaffrezic-Renault, N.; Elaissari, A. Microfluidic-based nanoparticle synthesis and their potential applications. Electrophoresis 2022, 43, 819–838. [Google Scholar] [CrossRef] [PubMed]
- Imanparast, A.; Shaegh, S.A.M.; Attaran, N.; Ameri, A.R.; Sazgarnia, A. Opto-microfluidic assisted synthesis of photo-protoporphyrin (pPP) conjugated to hollow gold-albumin hybrid nanoshells to enhance the efficiency of photodynamic therapy of triple negative breast cancer cells. Photodiagnosis and Photodynamic Therapy 2023, 103632. [Google Scholar] [CrossRef] [PubMed]
- Bîrcă, A.C.; Gherasim, O.; Niculescu, A.-G.; Grumezescu, A.M.; Neacșu, I.A.; Chircov, C.; Vasile, B.Ș.; Oprea, O.C.; Andronescu, E.; Stan, M.S. A Microfluidic Approach for Synthesis of Silver Nanoparticles as a Potential Antimicrobial Agent in Alginate–Hyaluronic Acid-Based Wound Dressings. International Journal of Molecular Sciences 2023, 24, 11466. [Google Scholar] [CrossRef]
- Podlesnaia, E.; Gerald Inangha, P.; Vesenka, J.; Seyring, M.; Hempel, H.J.; Rettenmayr, M.; Csáki, A.; Fritzsche, W. Microfluidic-Generated Seeds for Gold Nanotriangle Synthesis in Three or Two Steps. Small 2023, 2204810. [Google Scholar] [CrossRef]
- Ochoa-Vazquez, G.; Kharisov, B.; Arizmendi-Morquecho, A.; Cario, A.; Aymonier, C.; Marre, S.; López, I. Continuous segmented-flow synthesis of Ag and Au nanoparticles using a low cost microfluidic PTFE tubing reactor. IEEE Transactions on NanoBioscience 2021, 21, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Kafali, M.; Şahinoğlu, O.B.; Tufan, Y.; Orsel, Z.C.; Aygun, E.; Alyuz, B.; Saritas, E.U.; Erdem, E.Y.; Ercan, B. Antibacterial properties and osteoblast interactions of microfluidically synthesized chitosan–SPION composite nanoparticles. Journal of Biomedical Materials Research Part A 2023. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Leem, J.; Yoon, S.Y.; Sung, H.J. Continuous synthesis of zinc oxide nanoparticles in a microfluidic system for photovoltaic application. Nanoscale 2014, 6, 2840–2846. [Google Scholar] [CrossRef]
- Lee, S.-K.; Liu, X.; Cabeza, V.S.; Jensen, K.F. Synthesis, assembly and reaction of a nanocatalyst in microfluidic systems: a general platform. Lab on a Chip 2012, 12, 4080–4084. [Google Scholar] [CrossRef]
- Lee, W.-B.; Weng, C.-H.; Cheng, F.-Y.; Yeh, C.-S.; Lei, H.-Y.; Lee, G.-B. Biomedical microdevices synthesis of iron oxide nanoparticles using a microfluidic system. Biomedical microdevices 2009, 11, 161–171. [Google Scholar] [CrossRef]
- Frenz, L.; El Harrak, A.; Pauly, M.; Bégin-Colin, S.; Griffiths, A.D.; Baret, J.C. Droplet-based microreactors for the synthesis of magnetic iron oxide nanoparticles. Angewandte Chemie International Edition 2008, 47, 6817–6820. [Google Scholar] [CrossRef]
- Li, Q.; Wang, M.; Jin, Y.; Lu, Y.; Xiong, S.; Wang, M.; Xu, J.; Wei, C.; Li, J. Microfluidic synthesis of pH-responsive molecularly imprinted silica nanospheres for fluorescence sensing target glycoprotein. Food Chemistry 2023, 136570. [Google Scholar] [CrossRef]
- Lisina, S.; Inam, W.; Huhtala, M.; Howaili, F.; Zhang, H.; Rosenholm, J.M. Nano Differential Scanning Fluorimetry as a Rapid Stability Assessment Tool in the Nanoformulation of Proteins. Pharmaceutics 2023, 15, 1473. [Google Scholar] [CrossRef]
- Küçüktürkmen, B.; Inam, W.; Howaili, F.; Gouda, M.; Prabhakar, N.; Zhang, H.; Rosenholm, J.M. Microfluidic-assisted fabrication of dual-coated pH-sensitive mesoporous silica nanoparticles for protein delivery. Biosensors 2022, 12, 181. [Google Scholar] [CrossRef]
- He, P.; Greenway, G.; Haswell, S.J. The on-line synthesis of enzyme functionalized silica nanoparticles in a microfluidic reactor using polyethylenimine polymer and R5 peptide. Nanotechnology 2008, 19, 315603. [Google Scholar] [CrossRef]
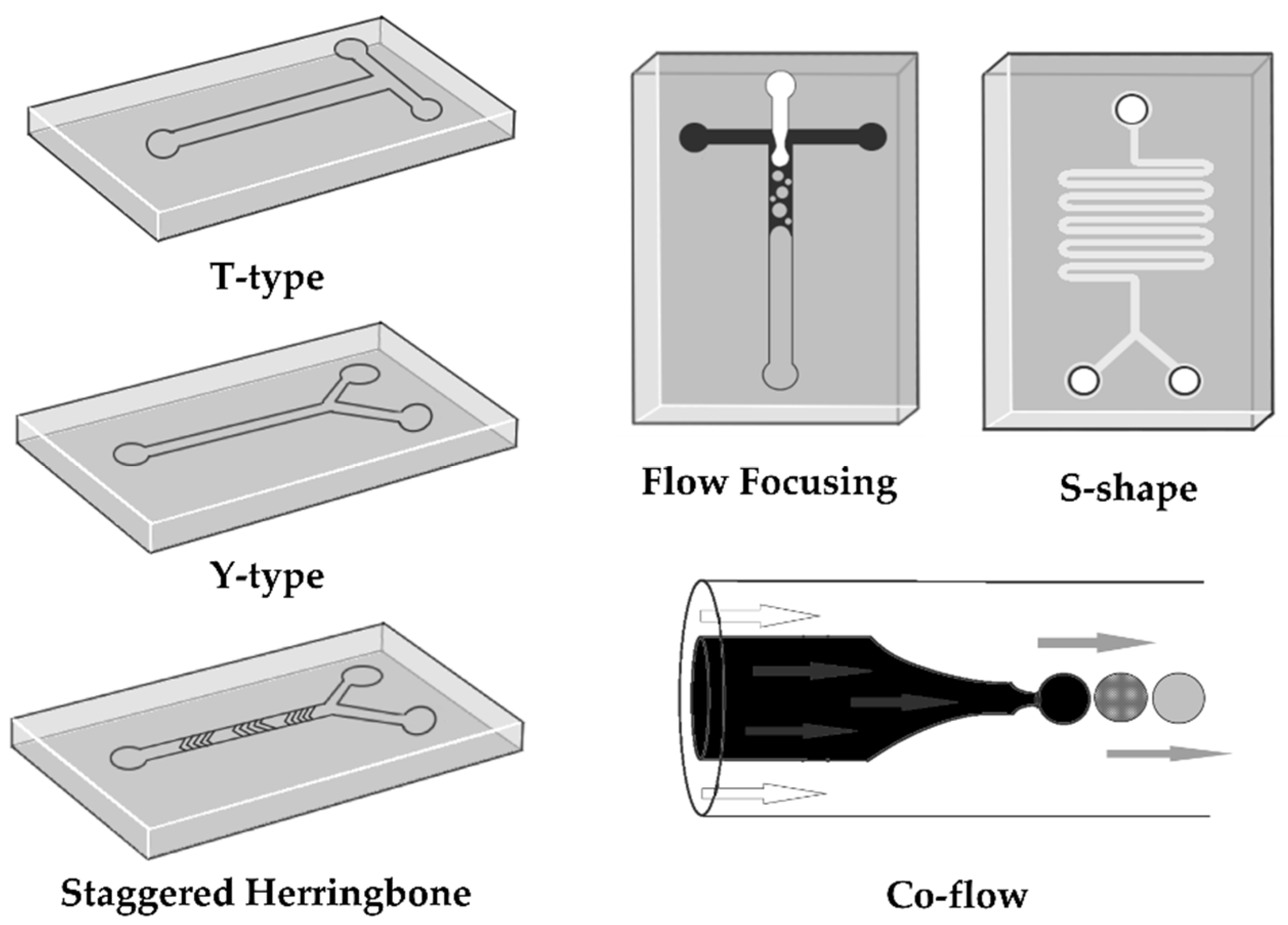
| Polymers | NP Types | Device Properties | Flow Conditions | Size [nm] | PDI | EE [%] | Year | Ref. |
|---|---|---|---|---|---|---|---|---|
| PCL PEG |
Gold nanoparticle (GNP)-loaded micellar polymer nanoparticles (GNP-PNPs) |
PDMS-based microfluidic mixer |
FRR: 1:1:1 TFR: 50, 100, or 200 μL/min |
< 50 | ≤ 0.3 | - | 2023 | [96] |
| Chitosan | Chitosan NPs | Microfluidic mixing chip “Fluidic 186” | bGal CSTPP NPs: FRR: 1:2.3 TFR: 0.5 siRNA CS NPs: FRR:1:2.3 TFR: 0.5 siRNA CSTPP NPs: FRR:1:2.3 TFR: 0.5 mRNA CS NPs: FRR:1:3 TFR: 0.6 mRNA CSTPP NPs: FRR:1:3 TFR: 0.6 (TFR values are mL/min) |
~80 99.48 ± 9.89 77.64±0.43 104.72 ± 8.82 67.51±3.34 |
<0.2 0.15 ± 0.010 0.15 ± 0.001 0.22 ± 0.050 0.19 ± 0.020 |
89.86 ± 4.30 98.14 ± 0.03 99.19 ± 0.042 94.72 ± 8.05 93.91 ± 0.19 |
2023 | [91] |
| F8BT, PFO, MEH-PPV, PSMA | Semiconducting polymer nanoparticles (SPNs) |
Laser-Tailored 3D Microfluidic Chip | FRR:1:1, 2:1, 3:1, 5:1 TFR: 60, 150, 300, 500, and 750 μL/min. |
Ultrasmall SPNs: <3 | - | - | 2023 | [97] |
| PEG-b-PTMC | Polymersome | Two microfluidic chips with different flow regimes (micromixer and Herringbone) | FRR:4:1 TFR: 100 to 1000 µL/min |
76 nm to 224 nm |
<0.2 | - | 2023 | [98] |
| PLGA-PEG | Polymeric NPs | Microfluidic Mixer Chip |
FRR:10:1 TFR: 550 µL/min |
≈ 30, 50, and 70 | PDI < 0.2 | - | 2023 | [99] |
| Ace-DEX | Polymer-based NPs | Three staggered herringbone | FRR: 4:1 TFR: 2000:500 μL/min |
182 | 0.06 | - | 2023 | [100] |
| Chitosan | Chitosan NPs | poly-dimethylsiloxane ψ-shaped microfluidic device | CS flow:270 mbar Oil flow:282 mbar |
~80 nm | - | ~100 | 2023 | [101] |
| PEG-PLGA, PLGA | PLGA-based NPs | PDMS-based microfluidic device |
FRR: ranges from 3:1 to 9:1 TFR: ranges from 50 to 500 μL/min |
40–114 | <0.2 | - | 2022 | [102] |
| PLGA, PEG | Polymeric NPs | Microfluidic device (rapid mixing of three fluid streams) |
FRR: 10:1 TFR: 550 μL/min |
30.5 ± 0.5 50.1 ± 0.3 72.3 ± 0.6 |
0.126 ± 0.02 0.088 ± 0.02 0.114 ± 0.02 |
40 ± 0.6 56 ± 4.0 72 ± 1.3 |
2022 | [92] |
| Chitosan | Lipid–Polymer nanoparticles (LiPoNs) |
coupled Hydrodynamic Flow Focusing (cHFF) | FRR:3:41 TFR: 44 µL/min |
LiPoNs: 77.4 Gd-DTPA LiPoNs: 95.3 IRI-Gd-DTPA LiPoNs: 112.8 |
0.22 0.30 0.28 |
- 78 79 |
2022 | [103] |
| Gellan, chitosan, hydrolyzed chitosan | Nanocomplexes | hydrodynamic flow-focusing microfluidic device (HFF) | FRR: 7:3 or 8:2 TFR: 10 to 20 μL/min |
~200 nm | ~ 0.1 | 2022 | [104] | |
| Glycoprotein | Nanospheres | Y-shape | FRR:- TFR: 2, 5 and 10 mL/h |
473 ± 18 | 0.17 ± 0.04 | - | 2022 | [106] |
| PEG-b-PDAP | Block Copolymer Micelles | Microfluidic device | FRR: 3:2 7:3 4:1 9:1 19:1 TFR: 10 mL/min |
34 ± 14 29 ± 12 25 ± 11 26 ± 10 23 ± 7 |
0.17 0.17 0.19 0.15 0.09 |
3.4 ± 0.4 (wt%) |
2022 | [107] |
| Chitosan (CHIT), hyaluronic acid (HA) | Polymeric NPs | 12 mixing stage of micro-sized channels | FRR:1:5:1 TFR: 350 μL/min |
~100 nm | <0.2 | - | 2022 | [108] |
| PLGA | Polymeric NPs | Zigzag chip (Z chip) combined with a T-junction and split and recombine sub-channels (C chip) |
FRR: 3:1 and 1:1 TFR: 10 and 12 mL/min |
CBD-loaded: ~190 |
~0.12 | - | 2021 | [109] |
| PLGA | Hydrogel (with nanoparticles) |
T-junction microfluidic platform | FRR:60:1 TFR: 50, 100, 200, 400, and 600 μL/min |
PLGA NPs: 81.44 ± 9.2 NPTGF-β3: 126 ± 4.52 |
0.194 0.137 |
77.7 | 2021 | [93] |
| PVA, SA | Polymer-based NPs | T-junction microfluidic device | FRR:- TFR:110 μL/min |
70 ± 5 | - | 78 | 2021 | [110] |
| alginate | nanogel | hydrodynamic flow focusing microfluidic | FRR: 0.01:1 to 0.2:1 TFR:3.03 to 3.6 mL/h |
43 ± 4 to 125 ± 7 | ≤0.2 | 50 to 68 | 2019 | [95] |
| CMCS | Polymeric NPs | PMMA microfluidic device | FRR:20:1 TFR:10,2 mL/h |
77 ± 19 nm | ≤ 0.2 | ~90 % | 2021 | [111] |
| PEGME-b-PDLLA PTMC, PCL, P(CL-co-TMC) |
Biodegradable NPs | Microfluidic flow focusing glass capillary device. |
FRR:10:1 TFR:550 to 1800 μL/min |
Empty NPs:143-194 DOX-loaded: 177-207 DOX and TA-loaded:202-229 |
≤0.1 ≤0.14 ≤0,21 |
- 35.6- 51.4 23.3-46.1 |
2021 | [94] |
| PLGA, PLGA-PEG |
iodinated polymeric nanoparticles | Y-shape staggered herringbone micromixer (SHM) | FRR: 4:1 TFR: 6 and 13 mL/min |
67 ± 2.8 | < 0.2 | IOX:38 NAC:20 |
2021 | [112] |
| Shellac | Polymeric NPs | 3-Dimensional hydrodynamic flow-focusing (3D-HFF) microfluidic tubing device | FRR:250:17 TFR: 290 mL/min |
137 ± 2 | 0.09 | 50%. (DL%) |
2021 | [113] |
| Degummed silk | silk nanoparticles | microfluidic setup | FRR: 5:1 TFR: 1 mL/min |
~110 | ~0.1 | - | 2020 | [114] |
| PLGA,TPGS | Ultrasmall TPGS−PLGA Hybrid NPs | 3D focusing pattern | FRR:5:1:5 TFR: 11mL/min |
< 100 | ≤0.17 | 14 to 24 | 2019 | [115] |
| PEO-ss-PHMssEt block copolymer | Stimuli-Responsive Polymer NPs |
two-phase microfluidic reactor | FRR:1:1:1 TFR: 50, 100, and 200 μL/min |
31 to 291 | - | - | 2019 | [116] |
| Lipids | NP Types | Device Properties | Flow Conditions | Size [nm] | PDI | EE [%] | Year | Ref. |
|---|---|---|---|---|---|---|---|---|
| DLin-MC3-DMA (MC3) | Lipid nanoparticles | Slicon Scalable Lipid Nanoparticle Genetation Platform (SCALAR) | FRR: 3:1 TFR: 0.07 L/h to 17 L/h |
~70 nm | - | >85% | 2023 | [127] |
| DPPC, DPPG, Chol, cRGD-DSPE-PEG2000 | Liposome | Microfluidic chip-based coaxial electrostatic spray method | FRR: 5:1:1 TFR: 14 μL/ min |
PTX/DOX@Lipo: 167.2 ± 1.7 PTX/DOX@cRGD-Lipo: 179.5 ± 0.3 |
0.098 ± 0.054 0.119 ± 0.027 |
90.85±1.87(DOX) 98.47±1.21 (PTX) 90.47±0.31(DOX) 99.15±0.43 (PTX) |
2023 | [123] |
| DLin-MC3-DMA, DSPC, Chol, DMG-PEG2000, | Lipid nanoparticles | Staggered herringbone micromixer (SHM) | FRR: 3:1, 4.5:1, or 6:1 TFR: 12, 16.5, or 21 mL/min. |
varied between 60 to 110 | ≤ 0.5 | 55% of the samples ≥85% 20% of samples <70% |
2023 | [128] |
| DPPC, Chol, and DSPE-PEG2000 |
Liposome | Benchtop microfluidic system | FRR: ranging from 3:1 to 9:1 TFR: between 1 and 16 ml/min |
empty liposomes: 35 to 183 curcumin-loaded: 52 to 183 |
≤ 0.220 | - | 2023 | [129] |
| DLin-MC3-DMA, cholesterol, DSPC, and DMG-PEG2000 | Lipid nanoparticles | microfluidic device | FRR: 1:3 TFR:- |
LNPs: 65.64 ± 5.31 siRNA- LNPs: 71.04 ± 6.18 |
- | 87.6 ± 4.5 % | 2022 | [130] |
| DOTAP, DC-Chol, DOPE, DOPC, | Lipid nanoparticles | Y-shape staggered herringbone micromixer | FRR: 3:1 TFR: 2 mL/min |
LNP10 :120 LNP20 :130 |
0.12 0.27 |
>60 | 2022 | [131] |
| DODAP, DSPC, DSPE-PEG, Chol, DiO |
Lipid nanoparticles | NanoAssemblr Benchtop instrument | FRR: 1:3 TFR: 12 mL/min |
LNP-siSAT1: 82 | 0.16 | 100% | 2022 | [132] |
| CL4H6, Chol, DSPC, DOPC, DOPE, PEG-DMG, DiO | Lipid nanoparticles |
iLiNP | FRR: 3:1 to 9:1 TFR: 0.1 to 0.5 mL/min |
137 ± 39 | 0.11 ± 0.01 | 84.2 ± 4.8 | 2022 | [133] |
| DOPE, Chol, PEG lipid, 246C10 | Lipid nanoparticles | NanoAssemblr Benchtop Instrument | FRR:3:1 TFR: 12 ml/min |
75.3 | 0.082 | 92.2 | 2022 | [134] |
| DSPC, POPC | Lipid nanoparticles |
NanoAssmblr and T-junction mixer |
FRR: 1:3 TFR: 12 mL/min for NanoAssmblr, 24 mL/min for T-junction mixer |
KC2-AmpB: 54.3 TO-AmpB: 25.5 PC-AmpB: 39.2 |
0.109 0.235 0.115 |
80.2 77.2 88.0 |
2021 | [135] |
| HSPC, Chol, PEG-DSPE, DODAP | Lipid nanoparticles |
Staggered herringbone micromixer (SHM) | FRR: 1:1 to 5:1 TFR: 1 to 3 mL/min |
~30 | ~0.15 | 90 | 2021 | [136] |
| SPC, Chol | Liposome | Zigzag chip (Z chip) combined with a T-junction and split and recombine sub-channels (C chip) |
FRR: 1:3 and 1:5 TFR: 10 and 15 mL/min |
CBD-loaded: ~110 | ~0.13 | - | 2021 | [109] |
| DSPC, Chol, DLin-MC3-DMA, PEG-DSG, PEG-DMG | Lipid nanoparticles |
T-junction | FRR: 3:1 TFR: 28 mL/min |
~50 nm | ~0.07 | >90% | 2021 | [122] |
| YSK05, DOPE, DOPC, EPC, DSPE-PEG2K | Ultra-small lipid nanoparticles (usLNPs) |
iLiNP microfluidic device | FRR: 2:1 to 4:1 TFR: 500 μL/min |
Optimized usLNPs: 60.47 ± 6.9 | 0.101 ± 0.011 | SOR: 96.5 ± 4.8 SiRNA: 94.5 ±6.5 |
2021 | [137] |
| PC, DMPC, DPPC, DSPC, PS and hol | Liposome | Herringbone micromixer chip | FRR: 3:1 TFR: 15 mL/min |
Insulin-loaded: ~57 OVA-loaded: ~53 BSA-loaded: ~65 |
0.087 ± 0.062 0.219 ± 0.011 0.144 ± 0.021 |
36.8 ± 2.7 34.2 ± 4.9 25.2 ± 2.8 |
2019 | [138] |
| DSPC, DOTAP | LipoParticles (LP) | NanoAssemblr™ benchtop instrument | FRR:- TFR:- |
245 ± 14 | 0.135 ± 0.007 | - | 2021 | [139] |
| DOTAP, DC-Chol, DOPE, Chol, PEG-lipid | Lipid nanoparticles |
Y-shape staggered herringbone micromixer (SHM) |
FRR: 3:1 TFR: 2 mL/min and 8 mL/min |
<200 | 0.113 ± 0.001 | ~80 | 2021 | [140] |
| Chol, DSPC, DMG-PEG, DOPE-Rho, D-Lin-MC3-DMA, | Lipid nanoparticles |
NanoAssemblr™ Spark | FRR: 3:1 TFR: 12 mL/min |
~65 | ≤ 0.22 | >85% | 2021 | [141] |
| Hempseed oil, lecithin, poloxamer 188 | Nanoemulsion | NanoAssemblr Benchtop instrument | FRR: 4:1 TFR:12 mL/min |
< 62.0 nm | 0.032 ± 0.014 | >99% | 2021 | [124] |
| PC, HSPC, DSPC, DSPG, DSPE-PEG 2000, Chol | Liposome | Microfluidizer | FRR: 95:5 - 5:95 -95:5 (cycles) TFR: 1 mL/min |
amphotericin B-loaded: 106 ± 2 Doxorubicin-loaded: 100–110 |
0.2 ± 0.1 <0.2 |
98 ± 4 97–98 (drug loading %) |
2020 | [142] |
| DSPC, Chol, and DSPE-PEG2000 |
Liposome | 5-Input Chip | FRR: 1:16 TFR: 500 µL/min |
Drug-free: 152 ± 1 DOX-passive loading: 176 ± 5 DOX-active loading: 146 ± 4 DOX- UMB co-loaded: 227 ± 1 |
0.20 ± 0.01 0.15 ± 0.03 0.13 ± 0.01 0.20 ± 0.01 |
- 81.0 ± 0.6 81.0 ± 6.9 74.0 ± 5.8, 47.0 ± 1.7 (EE %) |
2020 | [143] |
| DOPE, Chol, C14−4 | ionizable lipid nanoparticles |
Microfluidic device | FRR: 3:1 TFR: - |
65.19 ± 0.83 | 0.189 ± 0.014 | 86.3% | 2020 | [144] |
| CL15A6, CL15H6, Chol or ESM, PEG-DMG | Lipid nanoparticles |
Staggered herringbone micromixer (SHM) | FRR:3:1 TFR: 1.5 mL/min |
~22 | - | >90% | 2020 | [145] |
| DLin-MC3-DMA, DSPC, Chol, PEG-DSPE | Lipid nanoparticles |
Staggered herringbone mixer (SHM) | FRR:- TFR: 8 mL/min |
PEG-DSPE used: ~130 Tween 80 used: ~180 Tween 20 used: ~150 |
<0.2 | >50% | 2020 | [146] |
| CL4H6, CL15H6, DLin-MC3-DMA (MC3), Chol, ESM, DSPC, DOPE, PEG-DMG, DiD | Lipid nanoparticles |
iLiNP device | FRR:3:1 TFR: 0.5 mL/min |
63.9 | <0.15 | 95.5 | 2020 | [147] |
| DSPC, DSPE-PEG2000, DOTAP, DDAB, DMG-PEG2000, Chol, HSPC, MC3 | Lipid nanoparticles |
Staggered herringbone (SHM) and toroidal mixer (TrM) | FRR: 1:1 to 5:1 TFR: 5 to 20 mL/min |
DOTAP-LNPs: 60 to 45 MC3-iLNPs: 68 to 50 DDAB-LNPs: 88 to 75 |
≤0.2 | ≥ 95% | 2020 | [148] |
| DMPC, DPPC, and DSPC |
Liposome | Staggered Herringbone Micromixer (SHM) | FRR: 1:5 TFR: 17 mL/min |
Curcumin-loaded: 124.7±4.2 | 0.100 ± 0.02 | 17.1 ± 2.1 | 2019 | [149] |
| HSPC, Chol, and DSPE-PEG2000 |
Liposome | microfluidicmixer | FRR: 9:1 TFR: 10 mL/min |
Doxorubicin-loaded: 50.6 ± 2.6 |
0.37 ± 0.01 | 80.45 ± 0.194 | 2019 | [150] |
| DOTAP, GMO, GMO-PEG | Cubosomes and cuboplexes | Staggered herringbone mixer (SHM) | FRR: 6:1 TFR: 0.05 and 4 mL/min |
77 | 0.06 | >90 | 2018 | [151] |
| YSK05, Chol, PEG-DMG | Lipid nanoparticles |
Baffle mixer (iLiNP device) |
FRR: 3:1 to 9:1 TFR: 50 to 500 μL/min |
60 to 80 | <0.1 | >90 | 2018 | [152] |
| DMPC, DEPE-PEG2000, Chol, DSPE-PEG2000-FA, DSPE-PEG2000-TAT | Liposomes | Hydrodynamic flow focusing (HFF) | FRR: 8:1 TFR: 28.8 µL/min |
PEG-Lip: 67.9 ± 2.0 FA-Lip: 72.8 ± 1.8 TAT-Lip: 66.0 ± 0.7 FA-TAT-Lip: 73.2 ± 1.6 |
<0.3 | 60 to 80 | 2018 | [126] |
| DODMA, DOTMA, PC, Chol and mPEG-Chol |
Tf-LNPs | Staggered herringbone micromixer (SHM) combined with a Y-junction |
FRR: 3:1:1 TFR: - |
LNPs: 94 ± 0.9 Tf-LNPs:132.6 ± 1.6 |
0.072 0.129 |
- | 2017 | [125] |
| EPC, DOPE, DOTAP | Liposomes and Lipoplexes | hydrodynamic flow focusing microfluidic (HFF) |
FRR: 10:1 to 16:1 TFR: 100 μL/min and 150 μL/min |
Lipoplexes: ~195-205 Liposomes: ~150-170 |
<2.5 <1.5 |
- | 2017 | [153] |
| DOPE, DOTAP, DOPC and DSPE-PEG(2000) | Lipid nanoparticles |
Hydrodynamic-focusing microfluidic chip (HFF) | FRR: 9:1 TFR: 1 mL/hour |
~40 | - | ~60 | 2017 | [154] |
| PC, DMPC, DPPC, and DSPC |
Liposome | Staggered Herringbone Micromixer (SHM) | FRR: 3:1 TFR: 15 mL/min |
Metformin-loaded: 53±2 Glipizide-loaded: 55±5 Metformin + Glipizide: 64±6 |
0.11 ± 0.01 0.18 ± 0.01 0.15 ± 0.02 |
20.7 ± 2.6 42.8 ± 0.4 24.1 ± 2.0, 40.6 ± 1.9 (Drug loading%) |
2016 | [155] |
| YSK05, Chol, PEG-DMG | Lipid nanoparticles |
Staggered Herringbone Micromixer (SHM) | FRR: 3:1 TFR: 1.5 mL/min |
~30-70 | - | ~100 | 2016 | [156] |
| PC and CHOL | Liposome | Chaotic advection micromixer (Staggered herringbone micromixer (SHM)) | FRR: 1:3 TFR: 2 mL/min |
Propofol-loaded: ~50 | 0.17 ± 0.01 | 41 ± 4 (mol%) | 2015 | [157] |
| DOTMA, DODMA, mPEG-DSPE, DC-Chol, PC | Lipid nanoparticles |
Microfluidic hydrodynamic focusing (MHF) | FRR: 5:1 TFR: between 0.025−2.000 |
DC-Chol based: ~150 DOTMA based: ~110 DODMA based: ~80 |
- | - - 91.5 ± 4.5 |
2014 | [158] |
| DOTAP, EPC and DOPE | Liposome | Simple straight hydrodynamic flow focusing (SMD) and patterned microfluidic device (PMD) |
FRR: 5:1 TFR:140 mm/s |
SMD: 119.8 ± 1.6 PMD: 118.8 ± 7.9 |
0.22 ± 0.02 0.23 ± 0.03 |
- | 2013 | [159] |
| DLinKC2-DMA, DSPC, Chol, PEG-c-DMA | Lipid nanoparticles |
Staggered herringbone micromixer (SHM) |
FRR: 3:1 TFR: 0.02 to 4 ml/min. |
Between 28 - 54 | <0.1 | ~100 | 2012 | [160] |
| NP Types | Device Properties | Flow Conditions | Size [nm] | PDI | Year | Ref. |
|---|---|---|---|---|---|---|
| Metal-based NPs | ||||||
| hollow gold-albumin hybrid nanoshells |
Opto-microfluidic | FRR:1:1 TFR: 200 μl/min |
HGN-BSA-CTAB-PPP:120 | 0.357 | 2023 | [169] |
| Metal-conjugated nanomatrices | Droplet-based microfluidic system | FRR: 1:1.5, and 1:3 TFR: |
silver NPs: 92 ± 19 | - | 2023 | [161] |
| Silver NPs | microfluidic platform with a cross-shape design | FRR:3:2 TFR: 25 RPM |
42.249 | - | 2023 | [170] |
| Gold Nanotriangle | microfluidic setup | FRR:1:1 TFR: 80 µL/s |
Triangles edge lenght ca.: 40-150 | ≤1.1 | 2023 | [171] |
| Ag and Au spherical-shaped nanoparticles | Microfluidic PTFE Tubing Reactor | FRR:1:1 TFR:2.6 to 44 mL/h |
Ag NPs: 4.2 ± 1.4 Au NPs: 15 ± 0.6 |
- | 2021 | [172] |
| Metal oxide NPs | ||||||
| chitosan - superparamagnetic iron oxide composite nanoparticles (Ch - SPIONs) |
multi-step PDMS microfluidic reactor | FRR:3.1:6.2:2.2 TFR:11.5 μL/min |
8.8 ± 1.2 | - | 2023 | [173] |
| zinc oxide nanoparticles (ZnO NPs) |
a silicon microfluidic prepared by deep reactive ion etching (DRIE) techniques | various mixing frequencies: (1, 3, and 5 Hz) | 3–5 (crystalline structure) |
- | 2014 | [174] |
| platinum nanoparticles immobilized uniformly onto iron oxide/silica core–shell nanospheres | silicon microfluidic drop generator | FRR:2:5 TFR:140 μL/min |
85 | - | 2012 | [175] |
| iron oxide NPs | double-loop rotary micromixer | FRR:1:10:4 TFR:30 μL/min FRR:5:23:5 TFR:33 Pt-NPs FRR: 8:25:2 TFR:35 μL/min |
5.24±0.85 6.69±1.15 4.83±1.20 |
- | 2009 | [176] |
| Magnetic Iron Oxide Nanoparticles |
Droplet-based microreactors | FRR:3:40:5, 3:25:5, 1:8:2, 4:16:5, 1:8:5 TFR: 480, 660, 1100, 1250, 1400 µL/h |
4 nm | - | 2008 | [177] |
| SILICA NPs | ||||||
| pH-responsive molecularly imprinted silica nanospheres | PTFE capillary columns as microfluidic reactor | FRR:1:1:1 TFR: 15 mL/h |
FFMIPs: 310-359 RFMIPs: 275-732 |
0.116±0.092 to 0.226±0.067 |
2023 | [178] |
| 3D dendritic mesoporous silica nanoparticle | three-inlet coflow Glass Capillary microfluidics device |
FRR: 2:0.1:20 TFR: 22.1mL/h. |
MSN-PEI–BSA–NaPSS: 274.8 ± 8.43 MSN-PEI–BSA–NaPSS–SpAcDEX: 296.9 ± 8.98 |
0.134 ± 0.06 0.208 ± 0.05 |
2023 | [179] |
| Dual-Coated pH-Sensitive Mesoporous Silica Nanoparticles |
Continuous flow in microfluidic channels | FRR::2:20, 2:40,2:60 and 2:80 TFR: 22, 42, 62 and 82 mL/h |
96.16 ± 4.09 to 792.5 ± 152.0 |
0.151 ± 0.054 to 0.827 ± 0.300 |
2022 | [180] |
| enzyme functionalized silica nanoparticles |
continuous flow microfluidic (CFM) reactor system with a Y-shape connector |
FRR:1:1 TFR: 180 μl/min |
362 - 517 nm | - | 2008 | [181] |
| fluorescent silica nanoparticles |
Microfluidic droplets | FRR:50:3:3, 50:3:6 TFR: 0.506 and 0.509 μl/s |
50–350 | - | 2012 | [164] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




