Submitted:
23 November 2023
Posted:
24 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Preparing the microporous β-TCP ceramics
2.2. Characterization of the microporous β-TCP ceramics
2.3. Preparation and Characterization of the ADA-gelatin hydrogels
2.3.1. Gel permeation chromatography (GPC)
2.3.2. Rheology
2.4. Loading the microporous β-TCP ceramics
2.5. Release experiments
2.6. Determination of the release kinetics of daptomycin and BMP-2
2.6.1. HPLC
2.6.2. ELISA
2.7. Biocompatibility
2.8. Anti-microbial activity
2.9. Statistics
3. Results
3.1. Characterization of the microporous β-TCP
3.2. Characterization of the ADA-gelatin gel
3.3. Release kinetics of dual release of daptomycin and BMP-2
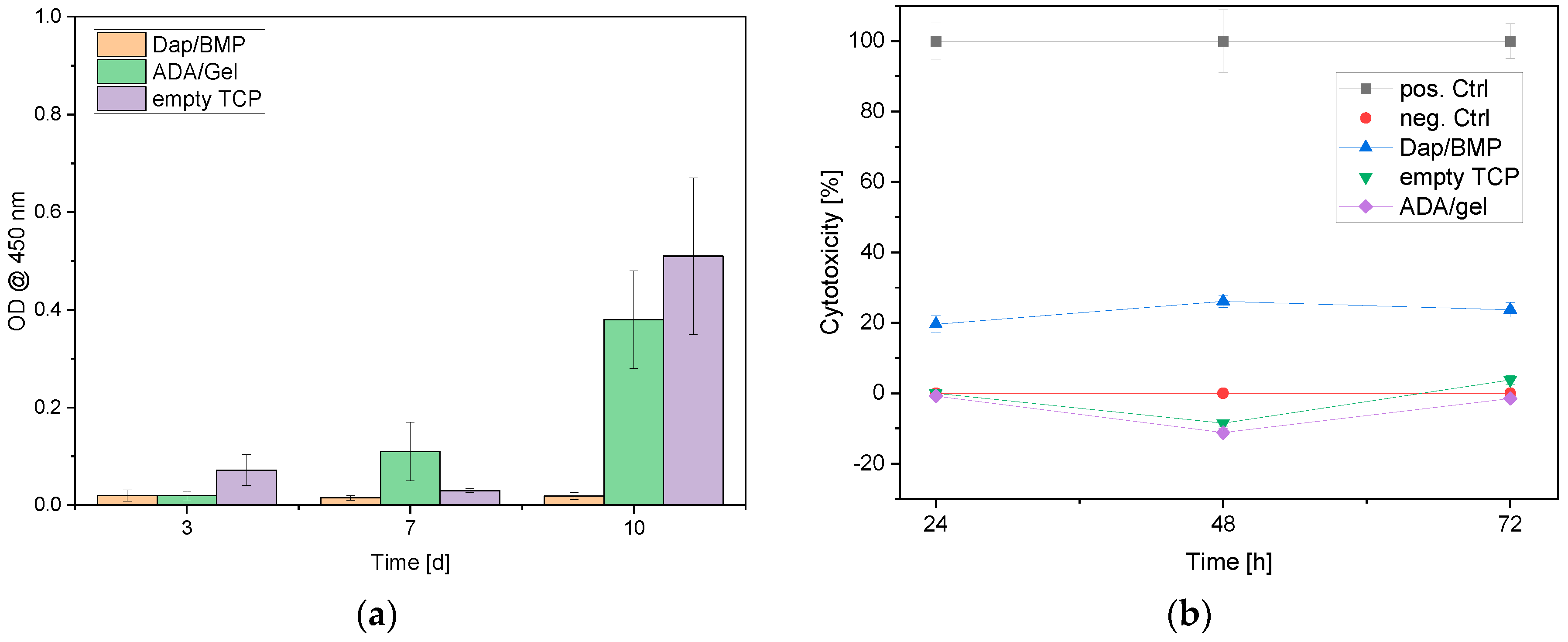
3.4. Biocompatibility
3.5. Antimicrobial activity
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, Y.J.; Sadigh, S.; Mankad, K.; Kapse, N.; Rajeswaran, G.J.Q.I.i.M.; Surgery. The imaging of osteomyelitis. Quantitative Imaging in Medicine and Surgery 2016, 6, 184-198. [CrossRef]
- Oliveira, T.C.; Gomes, M.S.; Gomes, A.C. The crossroads between infection and bone loss. Microorganisms 2020, 8, 1765. [CrossRef]
- Zimmerli, W. Clinical presentation and treatment of orthopaedic implant-associated infection. Journal of Internal Medicine 2014, 276, 111-119. [CrossRef]
- Hatzenbuehler, J.; Pulling, T.J. Diagnosis and management of osteomyelitis. Am. Fam. Physician 2011, 84, 1027-1033.
- Sia, I.G.; Berbari, E.F. Infection and musculoskeletal conditions: Osteomyelitis. Best practice & research. Clinical rheumatology 2006, 20, 1065-1081. [CrossRef]
- Kapadia, B.H.; Berg, R.A.; Daley, J.A.; Fritz, J.; Bhave, A.; Mont, M.A. Periprosthetic joint infection. Lancet 2016, 387, 386-394. [CrossRef]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369-379. [CrossRef]
- Schmitt, S.K. Osteomyelitis. Infectious disease clinics of North America 2017, 31, 325-338. [CrossRef]
- Fraimow, H.S. Systemic antimicrobial therapy in osteomyelitis. Seminars in Plastic Surgery 2009, 23, 90-99. [CrossRef]
- Gogia, J.S.; Meehan, J.P.; Di Cesare, P.E.; Jamali, A.A. Local antibiotic therapy in osteomyelitis. Semin Plast Surg 2009, 23, 100-107. [CrossRef]
- Blaha, J.D.; Calhoun, J.H.; Nelson, C.L.; Henry, S.L.; Seligson, D.; Esterhai, J.L.J.; Heppenstall, R.B.; Mader, J.; Evans, R.P.; Wilkins, J., et al. Comparison of the clinical efficacy and tolerance of gentamicin pmma beads on surgical wire versus combined and systemic therapy for osteomyelitis. Clinical Orthopaedics and Related Research® 1993, 295,.
- Díez-Peña, E.; Frutos, G.; Frutos, P.; Barrales-Rienda, J.M. Gentamicin sulphate release from a modified commercial acrylic surgical radiopaque bone cement. I. Influence of the gentamicin concentration on the release process mechanism. Chem. Pharm. Bull. (Tokyo) 2002, 50, 1201-1208. [CrossRef]
- Alkhraisat, M.H.; Rueda, C.; Cabrejos-Azama, J.; Lucas-Aparicio, J.; Marino, F.T.; Torres Garcia-Denche, J.; Jerez, L.B.; Gbureck, U.; Cabarcos, E.L. Loading and release of doxycycline hyclate from strontium-substituted calcium phosphate cement. Acta Biomater 2010, 6, 1522-1528.S1742-7061(09)00478-4 . [CrossRef]
- Wisniewska, I.; Slosarczyk, A.; Mysliwiec, L.; Sporniak-Tutak, K. [lincomycin applied to the alveolus on tcp carrier and its effect on wound healing after surgical extraction of a third molar]. Ann Acad Med Stetin 2009, 55, 59-64.
- Silverman, L.D.; Lukashova, L.; Herman, O.T.; Lane, J.M.; Boskey, A.L. Release of gentamicin from a tricalcium phosphate bone implant. J Orthop Res 2007, 25, 23-29. [CrossRef]
- DiCicco, M.; Goldfinger, A.; Guirand, F.; Abdullah, A.; Jansen, S.A. In vitro tobramycin elution analysis from a novel beta-tricalcium phosphate-silicate-xerogel biodegradable drug-delivery system. J Biomed Mater Res B Appl Biomater 2004, 70, 1-20. [CrossRef]
- El-Ghannam, A.; Ahmed, K.; Omran, M. Nanoporous delivery system to treat osteomyelitis and regenerate bone: Gentamicin release kinetics and bactericidal effect. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2005, 73B, 277-284. [CrossRef]
- El-Ghannam, A.; Jahed, K.; Govindaswami, M. Resorbable bioactive ceramic for treatment of bone infection. J Biomed Mater Res A 2010, 94, 308-316. [CrossRef]
- Baradari, H.; Damia, C.; Dutreih-Colas, M.; Laborde, E.; Pécout, N.; Champion, E.; Chulia, D.; Viana, M. Calcium phosphate porous pellets as drug delivery systems: Effect of drug carrier composition on drug loading and in vitro release. Journal of the European Ceramic Society 2012, 32, 2679-2690. [CrossRef]
- Laurent, F.; Bignon, A.; Goldnadel, J.; Chevalier, J.; Fantozzi, G.; Viguier, E.; Roger, T.; Boivin, G.; Hartmann, D. A new concept of gentamicin loaded hap/tcp bone substitute for prophylactic action: In vitro release validation. J Mater Sci Mater Med 2008, 19, 947-951. [CrossRef]
- Song, J.; Xu, J.; Filion, T.; Saiz, E.; Tomsia, A.P.; Lian, J.B.; Stein, G.S.; Ayers, D.C.; Bertozzi, C.R. Elastomeric high-mineral content hydrogel-hydroxyapatite composites for orthopedic applications. J. Biomed. Mater. Res. A 2009, 89, 1098-1107. [CrossRef]
- Sago, T.; Mori, Y.; Takagi, H.; Iwata, H.; Murase, K.; Kawamura, Y.; Hirose, H. Local treatment of dacron patch graft contaminated with staphylococcus aureus with antibiotic-releasing porous apatite ceramic: An experimental study in the rabbit. J Vasc Surg 2003, 37, 169-174. [CrossRef]
- Zhang, Y.; Zhang, M. Calcium phosphate/chitosan composite scaffolds for controlled in vitro antibiotic drug release. J Biomed Mater Res 2002, 62, 378-386. [CrossRef]
- Itokazu, M.; Yang, W.; Aoki, T.; Ohara, A.; Kato, N. Synthesis of antibiotic-loaded interporous hydroxyapatite blocks by vacuum method and in vitro drug release testing. Biomaterials 1998, 19, 817-. [CrossRef]
- Kundu, B.; Soundrapandian, C.; Nandi, S.K.; Mukherjee, P.; Dandapat, N.; Roy, S.; Datta, B.K.; Mandal, T.K.; Basu, D.; Bhattacharya, R.N. Development of new localized drug delivery system based on ceftriaxone-sulbactam composite drug impregnated porous hydroxyapatite: A systematic approach for in vitro and in vivo animal trial. Pharm Res 2010, 27, 1659-1676. [CrossRef]
- Fang, T.; Wen, J.; Zhou, J.; Shao, Z.; Dong, J. Poly (ε-caprolactone) coating delays vancomycin delivery from porous chitosan/β-tricalcium phosphate composites. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2012, 100B, 1803-1811. [CrossRef]
- Kumar Giri, T.; Thakur, D.; Alexander, A.; Ajazuddin; Badwaik, H.; Krishna Tripathi, D. Alginate based hydrogel as a potential biopolymeric carrier for drug delivery and cell delivery systems: Present status and applications. Curr. Drug Del. 2012, 9, 539-555. [CrossRef]
- Seidenstuecker, M.; Kissling, S.; Ruehe, J.; Suedkamp, N.; Mayr, H.; Bernstein, A. Novel method for loading microporous ceramics bone grafts by using a directional flow. J Funct Biomater 2015, 6, 1085. [CrossRef]
- Kissling, S.; Seidenstuecker, M.; Pilz, I.H.; Suedkamp, N.P.; Mayr, H.O.; Bernstein, A. Sustained release of rhbmp-2 from microporous tricalciumphosphate using hydrogels as a carrier. BMC Biotechnol. 2016, 16, 44. [CrossRef]
- Seidenstuecker, M.; Ruehe, J.; Suedkamp, N.P.; Serr, A.; Wittmer, A.; Bohner, M.; Bernstein, A.; Mayr, H.O. Composite material consisting of microporous β-tcp ceramic and alginate for delayed release of antibiotics. Acta Biomater. 2017, 433–446. [CrossRef]
- Schrade, S.; Ritschl, L.; Süss, R.; Schilling, P.; Seidenstuecker, M. Gelatin nanoparticles for targeted dual drug release out of alginate-di-aldehyde-gelatin gels. Gels 2022, 8, 365. [CrossRef]
- Seidenstuecker, M.; Schmeichel, T.; Ritschl, L.; Vinke, J.; Schilling, P.; Schmal, H.; Bernstein, A. Mechanical properties of the composite material consisting of β-tcp and alginate-di-aldehyde-gelatin hydrogel and its degradation behavior. Materials 2021, 14, 1303. [CrossRef]
- Balakrishnan, B.; Jayakrishnan, A.; Kumar, S.S.P.; Nandkumar, A.M. Anti-bacterial properties of an in situ forming hydrogel based on oxidized alginate and gelatin loaded with gentamycin. . Trends Biomater Artificial Organs 2012, 26, 139-145.
- Mayr, H.O.; Klehm, J.; Schwan, S.; Hube, R.; Sudkamp, N.P.; Niemeyer, P.; Salzmann, G.; von Eisenhardt-Rothe, R.; Heilmann, A.; Bohner, M., et al. Microporous calcium phosphate ceramics as tissue engineering scaffolds for the repair of osteochondral defects: Biomechanical results. Acta Biomater. 2013, 9, 4845-4855. [CrossRef]
- Stahli, C.; Bohner, M.; Bashoor-Zadeh, M.; Doebelin, N.; Baroud, G. Aqueous impregnation of porous beta-tricalcium phosphate scaffolds. Acta Biomater. 2010, 6, 2760-2772. [CrossRef]
- Bohner, M.; van Lenthe, G.H.; Grünenfelder, S.; Hirsiger, W.; Evison, R.; Müller, R. Synthesis and characterization of porous -tricalcium phosphate blocks. Biomaterials 2005, 26, 6099-6105. [CrossRef]
- Mayr, H.O.; Hube, R.; Bernstein, A.; Seibt, A.B.; Hein, W.; von Eisenhart-Rothe, R. Beta-tricalcium phosphate plugs for press-fit fixation in acl reconstruction--a mechanical analysis in bovine bone. Knee 2007, 14, 239-244. [CrossRef]
- Ritschl, L.; Schilling, P.; Wittmer, A.; Bohner, M.; Bernstein, A.; Schmal, H.; Seidenstuecker, M. Composite material consisting of microporous beta-tcp ceramic and alginate-dialdehyde-gelatin for controlled dual release of clindamycin and bone morphogenetic protein 2. J. Mater. Sci. Mater. Med. 2023, 34, 39. [CrossRef]
- Voigt, D. Development of a low - temperature sterilization process for thermally unstable medical implants using collagen as example https://www.filkfreiberg.de/forschung-entwicklung/projekte-und-publikationen/projektbibliothek/entwicklung-eines-niedertemperatur-sterilisationsverfahrens-fuer-thermisch-instabile-medizinische-implantate-am-beispiel-von-kollagen (2023-10-23),.
- EUCAST. Breakpoint tables for interpretation of mics and zone diameters. The European Committee on Antimicrobial Susceptibility Testing - EUCAST: Växjö, 2023;
- Diederen, B.M.; van Duijn, I.; Willemse, P.; Kluytmans, J.A. In vitro activity of daptomycin against methicillin-resistant staphylococcus aureus, including heterogeneously glycopeptide-resistant strains. Antimicrob. Agents Chemother. 2006, 50, 3189-3191. [CrossRef]
- Kuehling, T.; Schilling, P.; Bernstein, A.; Mayr, H.O.; Serr, A.; Wittmer, A.; Bohner, M.; Seidenstuecker, M. A human bone infection organ model for biomaterial research. Acta Biomater. 2022, 230-241. [CrossRef]
- Bernstein, A.; Niemeyer, P.; Salzmann, G.; Südkamp, N.P.; Hube, R.; Klehm, J.; Menzel, M.; von Eisenhart-Rothe, R.; Bohner, M.; Görz, L., et al. Microporous calcium phosphate ceramics as tissue engineering scaffolds for the repair of osteochondral defects: Histological results. Acta Biomater. 2013, 9, 7490-7505. [CrossRef]
- Sarker, B.; Rompf, J.; Silva, R.; Lang, N.; Detsch, R.; Kaschta, J.; Fabry, B.; Boccaccini, A.R. Alginate-based hydrogels with improved adhesive properties for cell encapsulation. International Journal of Biological Macromolecules 2015, 78, 72-78. [CrossRef]
- Sarker, B.; Papageorgiou, D.G.; Silva, R.; Zehnder, T.; Gul-E-Noor, F.; Bertmer, M.; Kaschta, J.; Chrissafis, K.; Detsch, R.; Boccaccini, A.R. Fabrication of alginate-gelatin crosslinked hydrogel microcapsules and evaluation of the microstructure and physico-chemical properties. Journal of Materials Chemistry B 2014, 2, 1470-1482. [CrossRef]
- Blankenburg, J.; Vinke, J.; Riedel, B.; Zankovic, S.; Schmal, H.; Seidenstuecker, M. Alternative geometries for 3d bioprinting of calcium phosphate cement as bone substitute. Biomedicines 2022, 10, 3242. [CrossRef]
- Hall, E.W.; Rouse, M.S.; Jacofsky, D.J.; Osmon, D.R.; Hanssen, A.D.; Steckelberg, J.M.; Patel, R. Release of daptomycin from polymethylmethacrylate beads in a continuous flow chamber. Diagn. Microbiol. Infect. Dis. 2004, 50, 261-265. [CrossRef]
- Silva, N.C.; Silva, S.; Sarmento, B.; Pintado, M. Chitosan nanoparticles for daptomycin delivery in ocular treatment of bacterial endophthalmitis. Drug Deliv. 2015, 22, 885-893. [CrossRef]
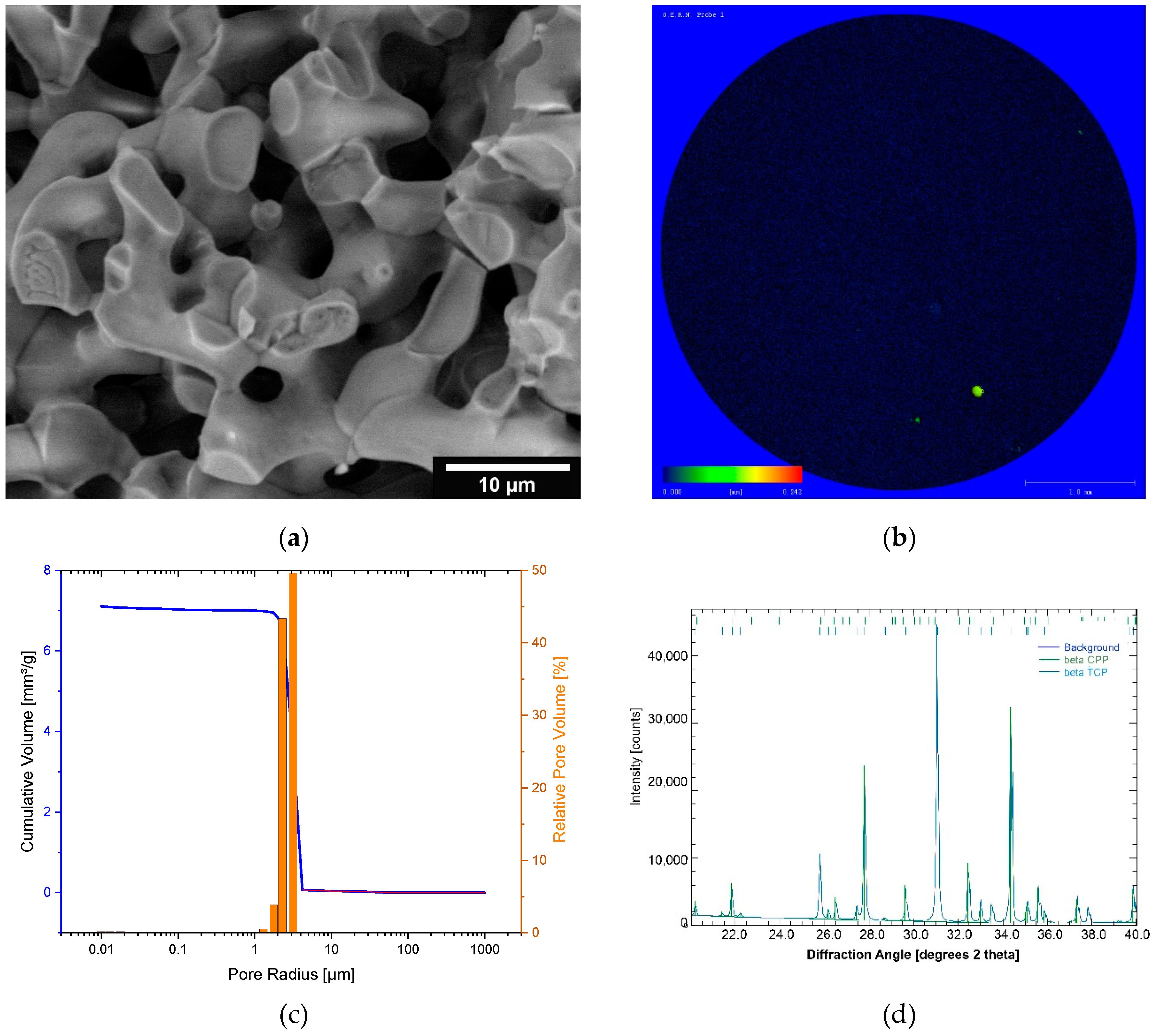
| Sample | Mn [kDa] | Mw [kDa] | Mz [kDa] | PDI (= Mw/Mn) |
|---|---|---|---|---|
| Alginate before plasma sterilization | 198 ± 23 | 729 ± 89 | 1440 ± 99 | 3.68 |
| Alginate after plasma sterilization | 201 ± 18 | 767 ± 57 | 1530 ± 187 | 3.81 |
| ADA unsterile | 52.4 ± 6,8 | 316 ± 26 | 1280 ± 111 | 6.03 |
| ADA out of plasma-sterile alginate | 55.3 ± 5.3 | 298 ± 17 | 1160 ± 53 | 5.38 |
| ADA out of unsterile alginate, plasma sterilized after manufacturing | 6.91 ± 0.87 | 17.8 ± 2.1 | 39.8 ± 2.5 | 2.57 |
| ADA out of plasma-sterile alginate and plasma sterilized again after manufacturing | 13.1 ± 1.4 | 41 ± 9 | 116 ± 12 | 3.13 |
| Gelatin before plasma sterilization | 16.3 ± 2.7 | 114 ± 9 | 254 ± 22 | 6.99 |
| Gelatine after plasma sterilization | 15.9 ± 3.3 | 105 ± 8 | 231 ± 16 | 6.61 |
| Release period [d] | Daptomycin-Release [µg/mL] | BMP-2 Release [ng/mL] |
|---|---|---|
| 1 | 1026.05 ± 84.28 | 59.80 ± 90.0 |
| 2 | 318.01 ± 34.85 | 101.86 ± 92.04 |
| 3 | 72.43 ± 48.64 | 669.00 ± 148.40 |
| 6 | 8.62 ± 2.65 | 208.96 ± 43.20 |
| 9 | 2.76 ± 0.18 | 71.54 ± 47.63 |
| 14 | 1.45 ± 1.30 | 148.31 ± 100.86 |
| 21 | 0.37 ± 0.98 | 1.75 ± 6.14 |
| 28 | 0.18 ± 0.67 | 2.63 ± 9.15 |
| Recovery [%]: | 105.13 ± 9.14 | 0.72 ± 0.16 |
| Sample | Relative cell count [%] | ||
| Alive | Intermediate | Dead | |
| Day3 | |||
| Dap/BMP-2 | 74.85 ± 48.16 | 5.56 ± 2.82 | 19.59 ± 9.08 |
| Control (empty ceramics) | 95.93 ± 14.21 | 0 ± 0 | 4.07 ± 1.20 |
| Control 2 (ADA/Gel no drugs) | 96.48 ± 31,22 | 0 ± 0 | 3.52 ± 1.93 |
| Day 7 | |||
| Dap/BMP-2 | 65.83 ± 27.14 | 0 ± 0 | 34.17 ± 23.41 |
| Control (empty ceramics) | 93.53 ± 11.33 | 0 ± 0 | 6.47 ± 1.48 |
| Control 2 (ADA/Gel no drugs) | 82.95 ± 31.48 | 0 ± 0 | 17.05 ± 4.80 |
| Day 10 | |||
| Dap/BMP-2 | 77.41 ± 63.13 | 0 ± 0 | 22.59 ± 22.97 |
| Control (empty ceramics) | 89.07 ± 53.61 | 0 ± 0 | 10.93 ± 3.84 |
| Control 2 (ADA/Gel no drugs) | 82.79 ± 27.79 | 0 ± 0 | 17.21 ± 10.67 |
| Sample | Dilution levels of the original concentration [mg/l] | ||||||||||||
| 16 | 8 | 4 | 2 | 1 | 0.5 | 0.25 | 0.125 | 0.063 | 0.031 | GC | EW | MIC | |
| Control | - | - | - | - | - | - | +++ | +++ | +++ | +++ | +++ | - | 0.5 |
| 1291 | 8 | 4 | 2 | 1 | 0.5 | 0.25 | 0.125 | 0.063 | 0.031 | GC | EW | MIC | |
| D1P1 | - | - | - | - | - | - | +++ | +++ | +++ | +++ | +++ | - | 0.5 |
| 308 | 8 | 4 | 2 | 1 | 0.5 | 0.25 | 0.125 | 0.063 | 0.031 | GC | EW | MIC | |
| D2P1 | - | - | - | - | - | - | +++ | +++ | +++ | +++ | +++ | - | 0.5 |
| 110.5 | 8 | 4 | 2 | 1 | 0.5 | 0.25 | 0.125 | 0.063 | 0.031 | GC | EW | MIC | |
| DT3P1 | - | - | - | - | - | +++ | +++ | +++ | +++ | +++ | +++ | - | 1 |
| 18.59 | 8 | 4 | 2 | 1 | 0.5 | 0.25 | 0.125 | 0.063 | 0.031 | GC | EW | MIC | |
| D6P1 | - | - | - | - | - | - | +++ | +++ | +++ | +++ | +++ | - | 0.5 |
| - | 0.95 | 0.475 | 0.238 | 0.119 | 0.059 | 0.03 | 0.015 | 0.007 | 0.004 | GC | EW | MIC | |
| D9P1 | / | - | - | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | - | 0.475 |
| - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | GC | EW | MIC | |
| D14P1 | / | ++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | - | > 0 |
| - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | GC | EW | MIC | |
| D21P1 | / | ++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | - | > 0 |
| - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | GC | EW | MIC | |
| D28P1 | / | ++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | - | > 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





