Submitted:
21 September 2023
Posted:
22 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Marine Oils: Polyunsaturated Fatty Acids and Polar Lipids
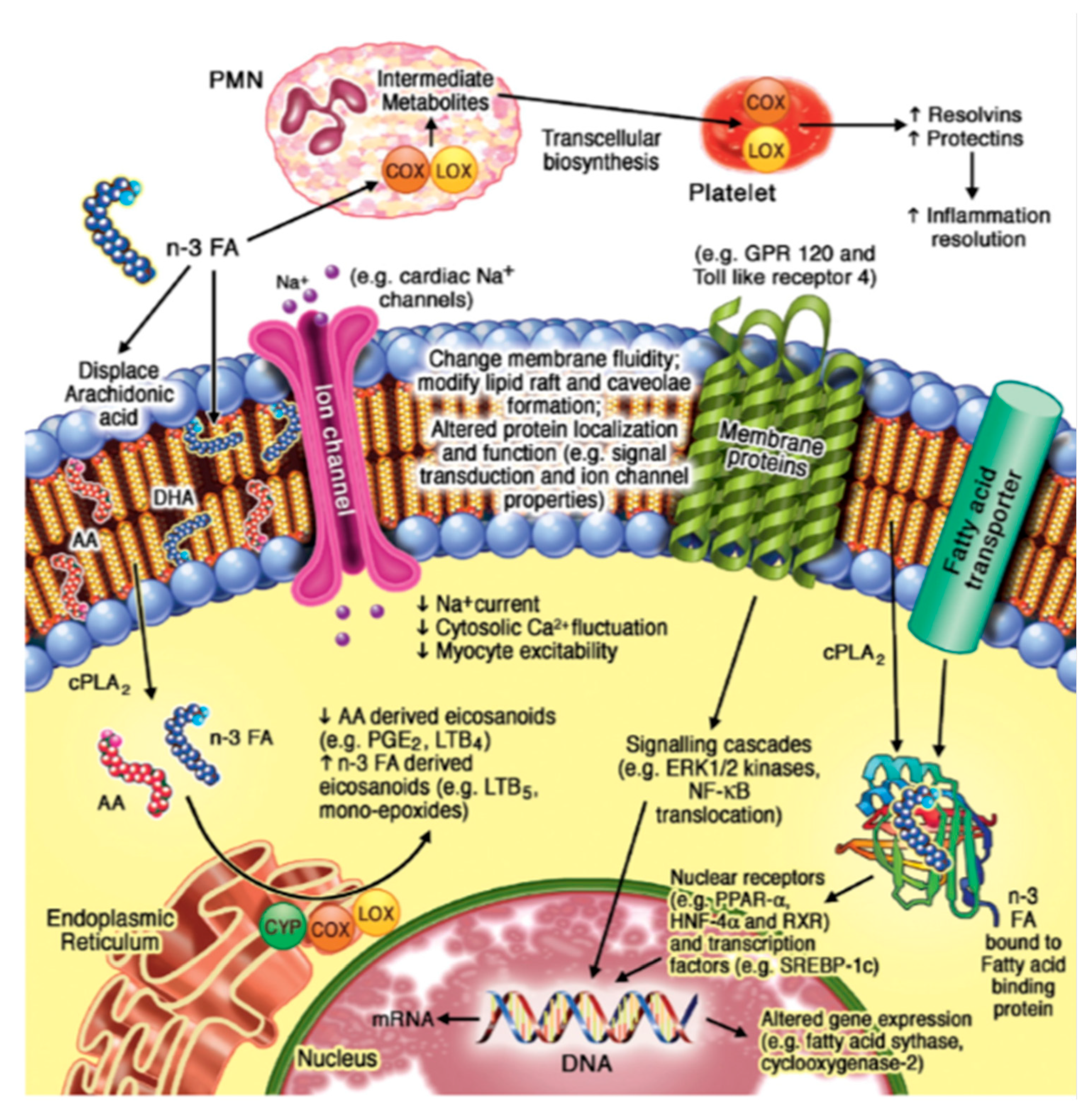
2.1. n-3 PUFA Structure and Function
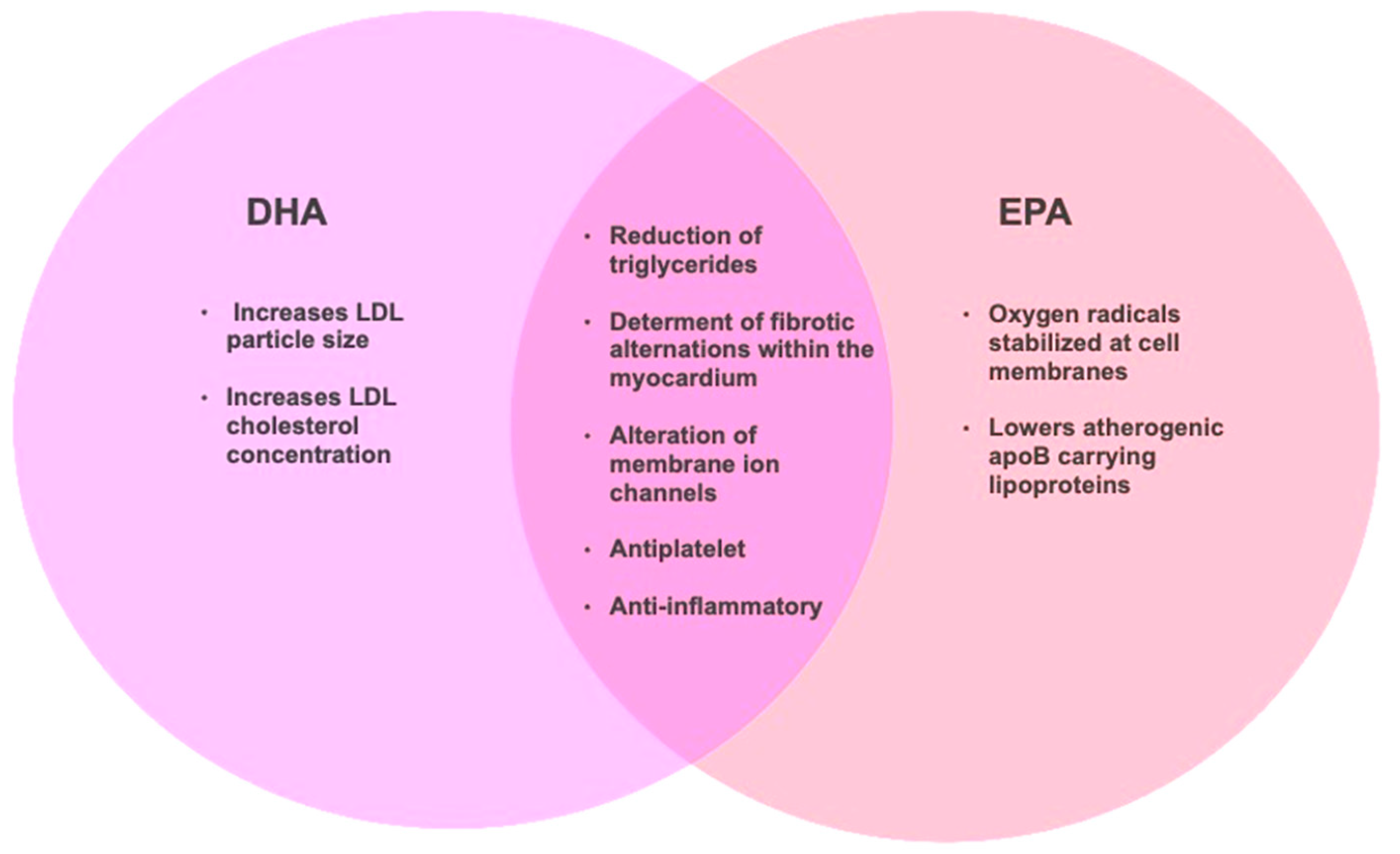
2.2. n-3 PUFA, Eicosapentaenoic Acid (EPA), and Docosahexaenoic Acid (DHA) and Cardiovascualr Health Effects
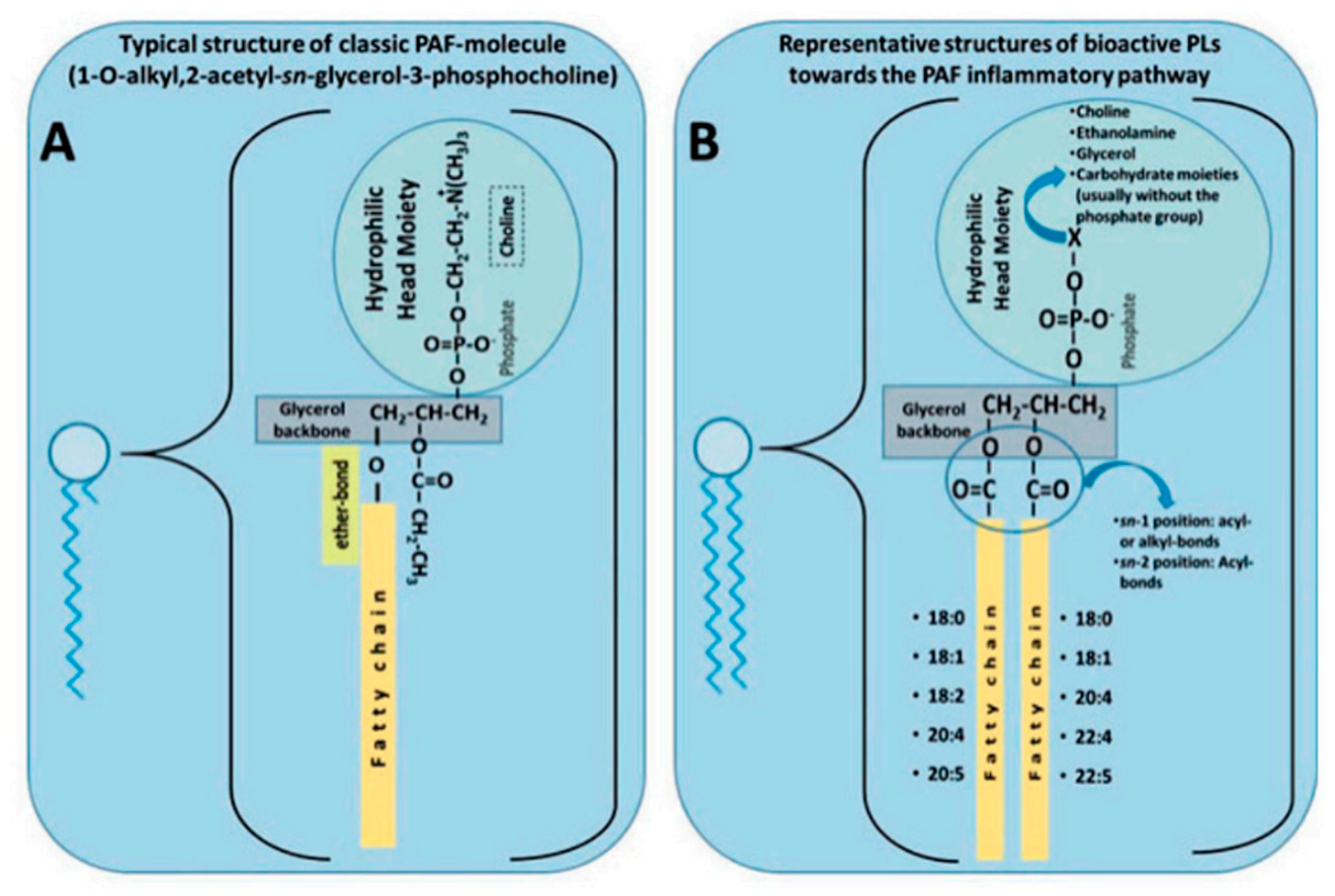
2.3. Polar Lipids Structure and Function
2.4. Polar Lipids and Cardiovascular Health Effects
2.5. Implications of Structural Differences Between n-3 PUFA and Polar Lipids
3. Marine Oils and Cardiovascular Health
3.1. Cardioprotective Marine Oil Supplements Containing n-3 PUFA and Polar lipids
3.2.1. The REDUCE-IT Trial in context
3.2.2. The STRENGTH Trial in context
3.3. What Can We Learn from the STRENGTH and REDUCE-IT Trials
3.4. Marine Oil Polar Lipids and Human Health
5. Conclusions and Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E., et al. Heart Disease and Stroke Statistics—2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93-e621. [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 Fatty Acids and Cardiovascular Disease: Effects on Risk Factors, Molecular Pathways, and Clinical Events. Journal of the American College of Cardiology 2011, 58, 2047-2067. [CrossRef]
- Ravera, A.; Carubelli, V.; Sciatti, E.; Bonadei, I.; Gorga, E.; Cani, D.; Vizzardi, E.; Metra, M.; Lombardi, C. Nutrition and Cardiovascular Disease: Finding the Perfect Recipe for Cardiovascular Health. Nutrients 2016, 8, 363.
- Yu, E.; Malik, V.S.; Hu, F.B. Cardiovascular disease prevention by diet modification: JACC Health Promotion Series. Journal of the American College of Cardiology 2018, 72, 914-926. [CrossRef]
- Bhupathiraju, S.N.; Tucker, K.L. Coronary heart disease prevention: Nutrients, foods, and dietary patterns. Clinica Chimica Acta 2011, 412, 1493-1514. [CrossRef]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab Syndr Relat Disord 2015, 13, 423-444. [CrossRef]
- Stokes, K.Y.; Granger, D.N. Platelets: a critical link between inflammation and microvascular dysfunction. The Journal of Physiology 2012, 590, 1023-1034. [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Platelet activation and prothrombotic mediators at the nexus of inflammation and atherosclerosis: Potential role of antiplatelet agents. Blood Rev. 2020, 100694. [CrossRef]
- Aggarwal, A.; Jennings, C.L.; Manning, E.; Cameron, S.J. Platelets at the Vessel Wall in Non-Thrombotic Disease. Circulation Research 2023, 132, 775-790. [CrossRef]
- Adili, R.; Hawley, M.; Holinstat, M. Regulation of platelet function and thrombosis by omega-3 and omega-6 polyunsaturated fatty acids. Prostaglandins & Other Lipid Mediators 2018, 139, 10-18. [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: potential to reduce the incidence of chronic diseases. Marine drugs 2011, 9, 1056-1100. [CrossRef]
- Damaiyanti, D.W.; Tsai, Z.-Y.; Masbuchin, A.N.; Huang, C.-Y.; Liu, P.-Y. Interplay between fish oil, obesity and cardiometabolic diabetes. Journal of the Formosan Medical Association 2023, 122, 528-539. [CrossRef]
- Sunkara, A.; Raizner, A. Supplemental Vitamins and Minerals for Cardiovascular Disease Prevention and Treatment. Methodist Debakey Cardiovasc J 2019, 15, 179-184. [CrossRef]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662.
- Vidal, N.P.; Dermiki, M.; Lordan, R. Chapter 11 - Fish-derived functional foods and cardiovascular health: An overview of current developments and advancements. In Functional Foods and Their Implications for Health Promotion, Zabetakis, I., Tsoupras, A., Lordan, R., Ramji, D., Eds. Academic Press: 2023. 303-316. [CrossRef]
- Burri, L.; Hoem, N.; Banni, S.; Berge, K. Marine omega-3 phospholipids: Metabolism and biological activities. International Journal of Molecular Sciences 2012, 13, 15401. [CrossRef]
- Cook, C.M.; Hallaråker, H.; Sæbø, P.C.; Innis, S.M.; Kelley, K.M.; Sanoshy, K.D.; Berger, A.; Maki, K.C. Bioavailability of long chain omega-3 polyunsaturated fatty acids from phospholipid-rich herring roe oil in men and women with mildly elevated triacylglycerols. Prostaglandins, Leukotrienes and Essential Fatty Acids 2016, 111, 17-24. [CrossRef]
- Lordan, R.; Redfern, S.; Tsoupras, A.; Zabetakis, I. Inflammation and cardiovascular disease: are marine phospholipids the answer? Food Funct. 2020, 11, 2861 - 2885. [CrossRef]
- Chopra, A.S.; Lordan, R.; Horbańczuk, O.K.; Atanasov, A.G.; Chopra, I.; Horbańczuk, J.O.; Jóźwik, A.; Huang, L.; Pirgozliev, V.; Banach, M., et al. The current use and evolving landscape of nutraceuticals. Pharma. Res. 2022, 175, 106001. [CrossRef]
- Lordan, R. Dietary supplements and nutraceuticals market growth during the coronavirus pandemic - Implications for consumers and regulatory oversight. PharmaNutrition 2021, 18, 100282. [CrossRef]
- Grand View Research. Nutraceuticals Market Analysis by Product (Dietary Supplements, Functional Food, Functional Beverage), By Region (North America, Asia Pacific, Europe, CSA, MEA), And Segment Forecasts, 2020 - 2027. . Availabe online: https://www.grandviewresearch.com/industry-analysis/nutraceuticals-market (accessed on 18 Aug).
- Liao, J.; Xiong, Q.; Yin, Y.; Ling, Z.; Chen, S. The Effects of Fish Oil on Cardiovascular Diseases: Systematical Evaluation and Recent Advance. Frontiers in Cardiovascular Medicine 2022, 8. [CrossRef]
- Clarke, T.C.; Black, L.I.; Stussman, B.J.; Barnes, P.M.; Nahin, R.L. Trends in the use of complementary health approaches among adults: United States, 2002-2012. National Health Statistics Reports 2015, 1-16.
- Black, L.I.; Clarke, T.C.; Barnes, P.M.; Stussman, B.J.; Nahin, R.L. Use of complementary health approaches among children aged 4-17 years in the United States: National Health Interview Survey, 2007-2012. Natl Health Stat Report 2015, 1-19.
- Grand view Research. Global Fish Oil Market is expected to value around US$ 3.62 Billion by 2030. Availabe online: https://www.globenewswire.com/news-release/2023/06/28/2696298/0/en/Global-Fish-Oil-Market-is-expected-to-value-around-US-3-62-Billion-by-2030.html (accessed on 18 Aug).
- Pike, I.H.; Jackson, A. Fish oil: production and use now and in the future. Lipid Technology 2010, 22, 59-61. [CrossRef]
- Research, G.V. Omega 3 supplements market size & share report 2020-2027 Availabe online: https://www.grandviewresearch.com/industry-analysis/omega-3-supplement-market (accessed on 15 Aug).
- Nasopoulou, C.; Zabetakis, I. Benefits of fish oil replacement by plant originated oils in compounded fish feeds. A review. LWT-Food Science and Technology 2012, 47, 217-224.
- Pasini, F.; Gómez-Caravaca, A.M.; Blasco, T.; Cvejić, J.; Caboni, M.F.; Verardo, V. Assessment of Lipid Quality in Commercial Omega-3 Supplements Sold in the French Market. Biomolecules 2022, 12, 1361.
- Albert, B.B.; Derraik, J.G.B.; Cameron-Smith, D.; Hofman, P.L.; Tumanov, S.; Villas-Boas, S.G.; Garg, M.L.; Cutfield, W.S. Fish oil supplements in New Zealand are highly oxidised and do not meet label content of n-3 PUFA. Scientific Reports 2015, 5, 7928. [CrossRef]
- Zuliani, G.; Galvani, M.; Leitersdorf, E.; Volpato, S.; Cavalieri, M.; Fellin, R. The role of polyunsaturated fatty acids (PUFA) in the treatment of dyslipidemias. Curr Pharm Des 2009, 15, 4087-4093. [CrossRef]
- Kones, R.; Howell, S.; Rumana, U. n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: Principles, Practices, Pitfalls, and Promises - A Contemporary Review. Med Princ Pract 2017, 26, 497-508. [CrossRef]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods- a review. J Food Sci Technol 2014, 51, 2289-2303. [CrossRef]
- Huang, T.-H.; Wang, P.-W.; Yang, S.-C.; Chou, W.-L.; Fang, J.-Y. Cosmetic and therapeutic applications of fish oil’s fatty acids on the skin. Marine Drugs 2018, 16, 256.
- Yamaguchi, A.; Stanger, L.; Freedman, J.C.; Prieur, A.; Thav, R.; Tena, J.; Holman, T.R.; Holinstat, M. Supplementation with omega-3 or omega-6 fatty acids attenuates platelet reactivity in postmenopausal women. Clinical and Translational Science 2022, 15, 2378-2391. [CrossRef]
- Nevigato, T.; Masci, M.; Caproni, R. Quality of Fish-Oil-Based Dietary Supplements Available on the Italian Market: A Preliminary Study. Molecules 2021, 26. [CrossRef]
- Kaur, G.; Malik, R.K.; Mishra, S.K.; Singh, T.P.; Bhardwaj, A.; Singroha, G.; Vij, S.; Kumar, N. Nisin and Class IIa Bacteriocin Resistance Among Listeria and Other Foodborne Pathogens and Spoilage Bacteria. Microbial Drug Resistance 2011, 17, 197-205. [CrossRef]
- Duda, M.K.; O'Shea, K.M.; Tintinu, A.; Xu, W.; Khairallah, R.J.; Barrows, B.R.; Chess, D.J.; Azimzadeh, A.M.; Harris, W.S.; Sharov, V.G., et al. Fish oil, but not flaxseed oil, decreases inflammation and prevents pressure overload-induced cardiac dysfunction. Cardiovasc Res 2009, 81, 319-327. [CrossRef]
- Teng, L.L.; Shao, L.; Zhao, Y.T.; Yu, X.; Zhang, D.F.; Zhang, H. The beneficial effect of n-3 polyunsaturated fatty acids on doxorubicin-induced chronic heart failure in rats. J Int Med Res 2010, 38, 940-948. [CrossRef]
- Drenjančević, I.; Pitha, J. Omega-3 Polyunsaturated Fatty Acids-Vascular and Cardiac Effects on the Cellular and Molecular Level (Narrative Review). Int J Mol Sci 2022, 23. [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687-698. [CrossRef]
- Adkins, Y.; Kelley, D.S. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem 2010, 21, 781-792. [CrossRef]
- Cottin, S.C.; Sanders, T.A.; Hall, W.L. The differential effects of EPA and DHA on cardiovascular risk factors. Proceedings of the Nutrition Society 2011, 70, 215-231. [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature reviews. Immunology 2008, 8, 349-361. [CrossRef]
- Serhan, C.N. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol 2010, 177, 1576-1591. [CrossRef]
- Skarke, C.; Alamuddin, N.; Lawson, J.A.; Li, X.; Ferguson, J.F.; Reilly, M.P.; FitzGerald, G.A. Bioactive products formed in humans from fish oils. Journal of Lipid research 2015, 56, 1808-1820.
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Platelet activation and prothrombotic mediators at the nexus of inflammation and atherosclerosis: Potential role of antiplatelet agents. Blood Reviews 2021, 45, 100694. [CrossRef]
- Schebb, N.H.; Kühn, H.; Kahnt, A.S.; Rund, K.M.; O'Donnell, V.B.; Flamand, N.; Peters-Golden, M.; Jakobsson, P.J.; Weylandt, K.H.; Rohwer, N., et al. Formation, Signaling and Occurrence of Specialized Pro-Resolving Lipid Mediators-What is the Evidence so far? Front Pharmacol 2022, 13, 838782. [CrossRef]
- O'Donnell, V.; Schebb, N.; Milne, G.; Murphy, M.; Thomas, C.; Steinhilber, D.; Wendell, S.; Kühn, H.; Jakobsson, P.; Blair, I. Failure to apply standard limit-of-detection or limit-of-quantitation criteria to specialized pro-resolving mediator analysis incorrectly characterizes their presence in biological samples. Zenodo 2021, 1, 10.5281.
- Kahnt, A.S.; Schebb, N.H.; Steinhilber, D. Formation of lipoxins and resolvins in human leukocytes. Prostaglandins Other Lipid Mediat 2023, 166, 106726. [CrossRef]
- Sinha, G. Critics Challenge Data showing key lipids can curb inflammation Science 2022. [CrossRef]
- Innes, J.K.; Calder, P.C. Marine Omega-3 (N-3) Fatty Acids for Cardiovascular Health: An Update for 2020. International Journal of Molecular Sciences 2020, 21, 1362.
- AbuMweis, S.; Jew, S.; Tayyem, R.; Agraib, L. Eicosapentaenoic acid and docosahexaenoic acid containing supplements modulate risk factors for cardiovascular disease: a meta-analysis of randomised placebo-control human clinical trials. Journal of Human Nutrition and Dietetics 2018, 31, 67-84. [CrossRef]
- Grimble, R.F.; Howell, W.M.; O'Reilly, G.; Turner, S.J.; Markovic, O.; Hirrell, S.; East, J.M.; Calder, P.C. The ability of fish oil to suppress tumor necrosis factor alpha production by peripheral blood mononuclear cells in healthy men is associated with polymorphisms in genes that influence tumor necrosis factor alpha production. Am J Clin Nutr 2002, 76, 454-459. [CrossRef]
- Rundblad, A.; Sandoval, V.; Holven, K.B.; Ordovás, J.M.; Ulven, S.M. Omega-3 fatty acids and individual variability in plasma triglyceride response: A mini-review. Redox Biology 2023, 63, 102730. [CrossRef]
- Minihane, A.M. Impact of Genotype on EPA and DHA Status and Responsiveness to Increased Intakes. Nutrients 2016, 8, 123. [CrossRef]
- Troesch, B.; Eggersdorfer, M.; Laviano, A.; Rolland, Y.; Smith, A.D.; Warnke, I.; Weimann, A.; Calder, P.C. Expert Opinion on Benefits of Long-Chain Omega-3 Fatty Acids (DHA and EPA) in Aging and Clinical Nutrition. Nutrients 2020, 12. [CrossRef]
- Xin, W.; Wei, W.; Li, X.Y. Short-term effects of fish-oil supplementation on heart rate variability in humans: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2013, 97, 926-935. [CrossRef]
- Innes, K.J.; Calder, C.P. The Differential Effects of Eicosapentaenoic Acid and Docosahexaenoic Acid on Cardiometabolic Risk Factors: A Systematic Review. International Journal of Molecular Sciences 2018, 19. [CrossRef]
- Jacobson, T.A.; Glickstein, S.B.; Rowe, J.D.; Soni, P.N. Effects of eicosapentaenoic acid and docosahexaenoic acid on low-density lipoprotein cholesterol and other lipids: A review. Journal of Clinical Lipidology 2012, 6, 5-18. [CrossRef]
- Vors, C.; Joumard-Cubizolles, L.; Lecomte, M.; Combe, E.; Ouchchane, L.; Drai, J.; Raynal, K.; Joffre, F.; Meiller, L.; Le Barz, M., et al. Milk polar lipids reduce lipid cardiovascular risk factors in overweight postmenopausal women: towards a gut sphingomyelin-cholesterol interplay. Gut 2020, 69, 487-501. [CrossRef]
- Hossain, M.M.; Tovar, J.; Cloetens, L.; Florido, M.T.S.; Petersson, K.; Prothon, F.; Nilsson, A. Oat Polar Lipids Improve Cardiometabolic-Related Markers after Breakfast and a Subsequent Standardized Lunch: A Randomized Crossover Study in Healthy Young Adults. Nutrients 2021, 13, 988.
- Antonopoulou, S.; Detopoulou, M.; Fragopoulou, E.; Nomikos, T.; Mikellidi, A.; Yannakoulia, M.; Kyriacou, A.; Mitsou, E.; Panagiotakos, D.; Anastasiou, C. Consumption of yogurt enriched with polar lipids from olive oil by-products reduces platelet sensitivity against platelet activating factor and inflammatory indices: A randomized, double-blind clinical trial. Human Nutr. Metab. 2022, 28, 200145. [CrossRef]
- Le Barz, M.; Vors, C.; Combe, E.; Joumard-Cubizolles, L.; Lecomte, M.; Joffre, F.; Trauchessec, M.; Pesenti, S.; Loizon, E.; Breyton, A.-E. Milk polar lipids favorably alter circulating and intestinal ceramide and sphingomyelin species in postmenopausal women. JCI insight 2021, 6. [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of animal and marine origin: Structure, function, and anti-inflammatory properties. Molecules 2017, 22, 1964. [CrossRef]
- Anto, L.; Warykas, S.W.; Torres-Gonzalez, M.; Blesso, C.N. Milk polar lipids: Underappreciated lipids with emerging health benefits. Nutrients 2020, 12, 1001. [CrossRef]
- Lordan, R.; Blesso, C.N. Editorial: Phospholipids and sphingolipids in nutrition, metabolism, and health. Front. Nutr. 2023, 10. [CrossRef]
- Lordan, R.; Zabetakis, I.; Tsoupras, A. Inflammation and Chronic Diseases: The Polar Lipid Link. Proceedings 2021, 70, 70.
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not cholesterol, is a cause of chronic disease. Nutrients 2018, 10, 604. [CrossRef]
- Zheng, L.; Fleith, M.; Giuffrida, F.; O'Neill, B.V.; Schneider, N. Dietary Polar Lipids and Cognitive Development: A Narrative Review. Adv Nutr 2019, 10, 1163-1176. [CrossRef]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E. Benefits of the Mediterranean Diet: Insights From the PREDIMED Study. Prog Cardiovasc Dis 2015, 58, 50-60. [CrossRef]
- Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Ruiz-Gutierrez, V.; Covas, M.I.; Fiol, M.; Gomez-Gracia, E.; Lopez-Sabater, M.C.; Vinyoles, E., et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Annals of Internal Medicine 2006, 145, 1-11.
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J., et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. New England Journal of Medicine 2018, 0, null. [CrossRef]
- Tognon, G.; Nilsson, L.M.; Lissner, L.; Johansson, I.; Hallmans, G.; Lindahl, B.; Winkvist, A. The Mediterranean diet score and mortality are inversely associated in adults living in the subarctic region. Journal of Nutrition 2012, 142, 1547-1553. [CrossRef]
- Mayr, H.L.; Itsiopoulos, C.; Tierney, A.C.; Ruiz-Canela, M.; Hebert, J.R.; Shivappa, N.; Thomas, C.J. Improvement in dietary inflammatory index score after 6-month dietary intervention is associated with reduction in interleukin-6 in patients with coronary heart disease: The AUSMED heart trial. Nutrition Research 2018, 55, 108-121. [CrossRef]
- Mayr, H.L.; Tierney, A.C.; Kucianski, T.; Thomas, C.J.; Itsiopoulos, C. Australian patients with coronary heart disease achieve high adherence to 6-month Mediterranean diet intervention: preliminary results of the AUSMED Heart Trial. Nutrition 2018. [CrossRef]
- Lordan, R.; Nasopoulou, C.; Tsoupras, A.; Zabetakis, I. The anti-inflammatory properties of food polar lipids. In Bioactive Molecules in Food, Mérillon, J.M., Ramawat, K.G., Eds. Springer International Publishing: Cham, Switzerland, 2018; pp. 1-34. [CrossRef]
- Nomikos, T.; Fragopoulou, E.; Antonopoulou, S.; Panagiotakos, D.B. Mediterranean diet and platelet-activating factor; a systematic review. Clinical Biochemistry 2018. [CrossRef]
- Detopoulou, P.; Demopoulos, C.; Karantonis, H.; Antonopoulou, S. Mediterranean diet and its protective mechanisms against cardiovascular disease: An insight into Platelet Activating Factor (PAF) and diet interplay. Ann Nutr Disord Ther 2015, 2, 1-10.
- Demopoulos, C.A.; Karantonis, H.C.; Antonopoulou, S. Platelet-activating factor — a molecular link between atherosclerosis theories. European Journal of Lipid Science and Technology 2003, 105, 705-716. [CrossRef]
- Harishkumar, R.; Hans, S.; Stanton, J.E.; Grabrucker, A.M.; Lordan, R.; Zabetakis, I. Targeting the Platelet-Activating Factor Receptor (PAF-R): Antithrombotic and Anti-Atherosclerotic Nutrients. Nutrients 2022, 14, 4414. [CrossRef]
- English, C.J.; Mayr, H.L.; Lohning, A.E.; Reidlinger, D.P. The association between dietary patterns and the novel inflammatory markers platelet-activating factor and lipoprotein-associated phospholipase A2: a systematic review. Nutr Rev 2022, 80, 1371-1391. [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I.; Demopoulos, A.C. Forty years since the structural elucidation of platelet-activating factor (PAF): Historical, current, and future research perspectives. Molecules 2019, 24. [CrossRef]
- English, C.J.; Mayr, H.L.; Lohning, A.E.; Reidlinger, D.P. The association between dietary patterns and the novel inflammatory markers platelet-activating factor and lipoprotein-associated phospholipase A2: a systematic review. Nutrition Reviews 2022, 80, 1371-1391. [CrossRef]
- Palur Ramakrishnan, A.V.K.; Varghese, T.P.; Vanapalli, S.; Nair, N.K.; Mingate, M.D. Platelet activating factor: A potential biomarker in acute coronary syndrome? Cardiovascular Therapeutics 2017, 35, 64-70. [CrossRef]
- Detopoulou, M.; Ntzouvani, A.; Petsini, F.; Gavriil, L.; Fragopoulou, E.; Antonopoulou, S. Consumption of Enriched Yogurt with PAF Inhibitors from Olive Pomace Affects the Major Enzymes of PAF Metabolism: A Randomized, Double Blind, Three Arm Trial. Biomolecules 2021, 11. [CrossRef]
- Argyrou, C.; Vlachogianni, I.; Stamatakis, G.; Demopoulos, C.A.; Antonopoulou, S.; Fragopoulou, E. Postprandial effects of wine consumption on Platelet Activating Factor metabolic enzymes. Prostaglandins & Other Lipid Mediators 2017. [CrossRef]
- Meyer, B.; Groot, R. Effects of Omega-3 Long Chain Polyunsaturated Fatty Acid Supplementation on Cardiovascular Mortality: The Importance of the Dose of DHA. Nutrients 2017, 9, 1305.
- Chaddha, A.; Eagle, K.A. Omega-3 Fatty Acids and Heart Health. Circulation 2015, 132, e350-e352. [CrossRef]
- Stone, N.J. Fish Consumption, Fish Oil, Lipids, and Coronary Heart Disease. Circulation 1996, 94, 2337-2340. [CrossRef]
- Weichselbaum, E.; Coe, S.; Buttriss, J.; Stanner, S. Fish in the diet: A review. Nutrition Bulletin 2013, 38, 128-177. [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Horn, L.V.; Wylie-Rosett, J. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e472-e487. [CrossRef]
- Rimm, E.B.; Appel, L.J.; Chiuve, S.E.; Djoussé, L.; Engler, M.B.; Kris-Etherton, P.M.; Mozaffarian, D.; Siscovick, D.S.; Lichtenstein, A.H. Seafood Long-Chain n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 2018, 138, e35-e47. [CrossRef]
- Kwak, S.; Myung, S.; Lee, Y.; Seo, H.; Korean Meta-analysis Study Group, f. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: A meta-analysis of randomized, double-blind, placebo-controlled trials. Archives of Internal Medicine 2012, 172, 686-694. [CrossRef]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.O.; AlAbdulghafoor, F.K.; Summerbell, C.D.; Worthington, H.V., et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2018, 10.1002/14651858.CD003177.pub4. [CrossRef]
- Bowen, K.J.; Harris, W.S.; Kris-Etherton, P.M. Omega-3 Fatty Acids and Cardiovascular Disease: Are There Benefits? Current Treatment Options in Cardiovascular Medicine 2016, 18, 69. [CrossRef]
- Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447-455. [CrossRef]
- De Lorgeril, M.; Renaud, S.; Salen, P.; Monjaud, I.; Mamelle, N.; Martin, J.; Guidollet, J.; Touboul, P.; Delaye, J. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. The Lancet 1994, 343, 1454-1459.
- Maehre, H.K.; Jensen, I.J.; Elvevoll, E.O.; Eilertsen, K.E. ω-3 Fatty Acids and Cardiovascular Diseases: Effects, Mechanisms and Dietary Relevance. Int J Mol Sci 2015, 16, 22636-22661. [CrossRef]
- Messori, A.; Fadda, V.; Maratea, D.; Trippoli, S. ω-3 Fatty Acid Supplements for Secondary Prevention of Cardiovascular Disease: From “No Proof of Effectiveness” to “Proof of No Effectiveness”. JAMA Internal Medicine 2013, 173, 1466-1468. [CrossRef]
- Curfman, G.; Shehada, E. Icosapent ethyl: scientific and legal controversies. Open Heart 2021, 8, e001616. [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Brinton, E.A.; Jacobson, T.A.; Miller, M.; Tardif, J.-C.; Ketchum, S.B.; Doyle Jr, R.T.; Murphy, S.A.; Soni, P.N., et al. Rationale and design of REDUCE-IT: Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial. Clinical Cardiology 2017, 40, 138-148. [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C., et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. New England Journal of Medicine 2019, 380, 11-22. [CrossRef]
- Bäck, M.; Hansson, G.K. Omega-3 fatty acids, cardiovascular risk, and the resolution of inflammation. The FASEB Journal 2019, 33, 1536-1539. [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Jiao, L.; Tardif, J.-C.; Gregson, J.; Pocock, S.J.; Ballantyne, C.M. Reduction in First and Total Ischemic Events With Icosapent Ethyl Across Baseline Triglyceride Tertiles. Journal of the American College of Cardiology 2019, 74, 1159-1161. [CrossRef]
- Baum, S.J.; Scholz, K.P. Rounding the corner on residual risk: Implications of REDUCE-IT for omega-3 polyunsaturated fatty acids treatment in secondary prevention of atherosclerotic cardiovascular disease. Clinical Cardiology 2019, 42, 829-838. [CrossRef]
- Ballantyne, C.M.; Bays, H.E.; Kastelein, J.J.; Stein, E.; Isaacsohn, J.L.; Braeckman, R.A.; Soni, P.N. The Effect of Two Doses of AMR101 on Fasting Serum Triglycerides and Other Lipid Parameters in Statin-Treated Patients with Persistent High Triglycerides (≥ 200 and< 500 mg/dL): The ANCHOR Study. Journal of Clinical Lipidology 2012, 6, 279-280.
- Bays, H.E.; Ballantyne, C.M.; Kastelein, J.J.; Isaacsohn, J.L.; Braeckman, R.A.; Soni, P.N. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). The American journal of cardiology 2011, 108, 682-690.
- Olshansky, B.; Chung, M.K.; Budoff, M.J.; Philip, S.; Jiao, L.; Doyle, J., Ralph T; Copland, C.; Giaquinto, A.; Juliano, R.A.; Bhatt, D.L. Mineral oil: safety and use as placebo in REDUCE-IT and other clinical studies. European Heart Journal Supplements 2020, 22, J34-J48. [CrossRef]
- Hu, Y.; Hu, F.B.; Manson, J.E. Marine Omega-3 Supplementation and Cardiovascular Disease: An Updated Meta-Analysis of 13 Randomized Controlled Trials Involving 127 477 Participants. J Am Heart Assoc 2019, 8, e013543. [CrossRef]
- Shen, S.; Gong, C.; Jin, K.; Zhou, L.; Xiao, Y.; Ma, L. Omega-3 Fatty Acid Supplementation and Coronary Heart Disease Risks: A Meta-Analysis of Randomized Controlled Clinical Trials. Front Nutr 2022, 9, 809311. [CrossRef]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090-1098.
- Tavazzi, L.; Maggioni, A.P.; Marchioli, R.; Barlera, S.; Franzosi, M.G.; Latini, R.; Lucci, D.; Nicolosi, G.L.; Porcu, M.; Tognoni, G. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1223-1230. [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D., et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. New England Journal of Medicine 2019, 380, 33-44. [CrossRef]
- The Origin Trial Investigators. n–3 Fatty Acids and Cardiovascular Outcomes in Patients with Dysglycemia. New England Journal of Medicine 2012, 367, 309-318. [CrossRef]
- The ASCEND Study Collaborative Group. Effects of n−3 fatty acid supplements in diabetes mellitus. New England Journal of Medicine 2018, 0, null. [CrossRef]
- Budoff, M.J.; Bhatt, D.L.; Kinninger, A.; Lakshmanan, S.; Muhlestein, J.B.; Le, V.T.; May, H.T.; Shaikh, K.; Shekar, C.; Roy, S.K., et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. European Heart Journal 2020, 41, 3925-3932. [CrossRef]
- Watanabe, T.; Ando, K.; Daidoji, H.; Otaki, Y.; Sugawara, S.; Matsui, M.; Ikeno, E.; Hirono, O.; Miyawaki, H.; Yashiro, Y., et al. A randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J Cardiol 2017, 70, 537-544. [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K., et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020, 324, 2268-2280. [CrossRef]
- Kalstad, A.A.; Myhre, P.L.; Laake, K.; Tveit, S.H.; Schmidt, E.B.; Smith, P.; Nilsen, D.W.T.; Tveit, A.; Fagerland, M.W.; Solheim, S., et al. Effects of n-3 Fatty Acid Supplements in Elderly Patients After Myocardial Infarction. Circulation 2021, 143, 528-539. [CrossRef]
- Kataoka, Y.; Uno, K.; Puri, R.; Nicholls, S.J. Epanova® and hypertriglyceridemia: pharmacological mechanisms and clinical efficacy. Future Cardiology 2013, 9, 177-186. [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; Mozaffarian, D., et al. Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: Rationale and design of the STRENGTH trial. Clinical Cardiology 2018, 41, 1281-1288. [CrossRef]
- Casula, M.; Soranna, D.; Catapano, A.L.; Corrao, G. Long-term effect of high dose omega-3 fatty acid supplementation for secondary prevention of cardiovascular outcomes: A meta-analysis of randomized, double blind, placebo controlled trials. Atherosclerosis Supplements 2013, 14, 243-251. [CrossRef]
- Goff, Z.D.; Nissen, S.E. N-3 polyunsaturated fatty acids for cardiovascular risk. Curr Opin Cardiol 2022, 37, 356-363. [CrossRef]
- Nissen, S.E.; Lincoff, A.M.; Wolski, K.; Ballantyne, C.M.; Kastelein, J.J.P.; Ridker, P.M.; Ray, K.K.; McGuire, D.K.; Mozaffarian, D.; Koenig, W., et al. Association Between Achieved ω-3 Fatty Acid Levels and Major Adverse Cardiovascular Outcomes in Patients With High Cardiovascular Risk: A Secondary Analysis of the STRENGTH Trial. JAMA Cardiology 2021, 6, 910-917. [CrossRef]
- Doi, T.; Langsted, A.; Nordestgaard, B.G. A possible explanation for the contrasting results of REDUCE-IT vs. STRENGTH: cohort study mimicking trial designs. European Heart Journal 2021, 42, 4807-4817. [CrossRef]
- Ahmmed, M.K.; Ahmmed, F.; Tian, H.; Carne, A.; Bekhit, A.E.-D. Marine omega-3 (n-3) phospholipids: A comprehensive review of their properties, sources, bioavailability, and relation to brain health. Comprehensive Reviews in Food Science and Food Safety 2020, 19, 64-123. [CrossRef]
- Konagai, C.; Yanagimoto, K.; Hayamizu, K.; Han, L.; Tsuji, T.; Koga, Y. Effects of krill oil containing n-3 polyunsaturated fatty acids in phospholipid form on human brain function: a randomized controlled trial in healthy elderly volunteers. Clinical Interventions in Aging 2013, 8, 1247-1257. [CrossRef]
- Schuchardt, J.P.; Hahn, A. Bioavailability of long-chain omega-3 fatty acids. Prostaglandins Other Lipid Mediat. 2013, 89, 1-8. [CrossRef]
- Köhler, A.; Sarkkinen, E.; Tapola, N.; Niskanen, T.; Bruheim, I. Bioavailability of fatty acids from krill oil, krill meal and fish oil in healthy subjects--a randomized, single-dose, cross-over trial. Lipids Health Dis 2015, 14, 19. [CrossRef]
- Schuchardt, J.P.; Schneider, I.; Meyer, H.; Neubronner, J.; von Schacky, C.; Hahn, A. Incorporation of EPA and DHA into plasma phospholipids in response to different omega-3 fatty acid formulations - a comparative bioavailability study of fish oil vs. krill oil. Lipids in Health and Disease 2011, 10, 145. [CrossRef]
- Lapointe, J.-F.; Harvey, L.; Aziz, S.; Jordan, H.; Hegele, R.A.; Lemieux, P. A single-dose, comparative bioavailability study of a formulation containing OM3 as phospholipid and free fatty acid to an ethyl ester formulation in the fasting and fed states. Clinical Therapeutics 2019, 41, 426-444. [CrossRef]
- Akram, W.; Rihan, M.; Ahmed, S.; Arora, S.; Ahmad, S.; Vashishth, R. Marine-Derived Compounds Applied in Cardiovascular Diseases: Submerged Medicinal Industry. Marine Drugs 2023, 21, 193.
- Nomikos, T.; Karantonis, H.C.; Skarvelis, C.; Demopoulos, C.A.; Zabetakis, I. Antiatherogenic properties of lipid fractions of raw and fried fish. Food Chemistry 2006, 96, 29-35.
- Tsoupras, A.; O’Keeffe, E.; Lordan, R.; Redfern, S.; Zabetakis, I. Bioprospecting for antithrombotic polar lipids from salmon, herring, and boarfish by-products. Foods 2019, 8, 416. [CrossRef]
- Tsoupras, A.; Lordan, R.; Demuru, M.; Shiels, K.; Saha, S.K.; Nasopoulou, C.; Zabetakis, I. Structural elucidation of Irish organic farmed salmon (Salmo salar) polar lipids with antithrombotic activities. Marine Drugs 2018, 16, 176. [CrossRef]
- Tsoupras, A.; Lordan, R.; Shiels, K.; Saha, S.K.; Nasopoulou, C.; Zabetakis, I. In vitro antithrombotic properties of salmon (Salmo salar) phospholipids in a novel food-grade extract. Marine Drugs 2019, 17, 62. [CrossRef]
- Fountoulaki, E.; Vasilaki, A.; Hurtado, R.; Grigorakis, K.; Karacostas, I.; Nengas, I.; Rigos, G.; Kotzamanis, Y.; Venou, B.; Alexis, M.N. Fish oil substitution by vegetable oils in commercial diets for gilthead sea bream (Sparus aurata L.); effects on growth performance, flesh quality and fillet fatty acid profile: Recovery of fatty acid profiles by a fish oil finishing diet under fluctuating water temperatures. Aquaculture 2009, 289, 317-326. [CrossRef]
- Bell, J.G.; Tocher, D.R.; Henderson, R.J.; Dick, J.R.; Crampton, V.O. Altered fatty acid compositions in atlantic salmon (Salmo salar) fed diets containing linseed and rapeseed oils can be partially restored by a subsequent fish oil finishing diet. J Nutr 2003, 133, 2793-2801. [CrossRef]
- Montero, D.; Robaina, L.; Caballero, M.J.; Ginés, R.; Izquierdo, M.S. Growth, feed utilization and flesh quality of European sea bass (Dicentrarchus labrax) fed diets containing vegetable oils: A time-course study on the effect of a re-feeding period with a 100% fish oil diet. Aquaculture 2005, 248, 121-134. [CrossRef]
- Tsantila, N.; Karantonis, H.C.; Perrea, D.N.; Theocharis, S.E.; Iliopoulos, D.G.; Antonopoulou, S.; Demopoulos, C.A. Antithrombotic and antiatherosclerotic properties of olive oil and olive pomace polar extracts in rabbits. Mediators of Inflammation 2007, 2007.
- Karantonis, H.C.; Tsantila, N.; Stamatakis, G.; Samiotaki, M.; Panayotou, G.; Antonopoulou, S.; Demopoulos, C.A. Bioactive polar lipids in olive oil, pomace and waste byproducts. J Food Biochem 2008, 32, 443-459. [CrossRef]
- Nasopoulou, C.; Karantonis, H.C.; Perrea, D.N.; Theocharis, S.E.; Iliopoulos, D.G.; Demopoulos, C.A.; Zabetakis, I. In vivo anti-atherogenic properties of cultured gilthead sea bream (Sparus aurata) polar lipid extracts in hypercholesterolaemic rabbits. Food Chemistry 2010, 120, 831-836. [CrossRef]
- Nasopoulou, C.; Smith, T.; Detopoulou, M.; Tsikrika, C.; Papaharisis, L.; Barkas, D.; Zabetakis, I. Structural elucidation of olive pomace fed sea bass (Dicentrarchus labrax) polar lipids with cardioprotective activities. Food Chemistry 2014, 145, 1097-1105. [CrossRef]
- Nasopoulou, C.; Tsoupras, A.B.; Karantonis, H.C.; Demopoulos, C.A.; Zabetakis, I. Fish polar lipids retard atherosclerosis in rabbits by down-regulating PAF biosynthesis and up-regulating PAF catabolism. Lipids in Health and Disease 2011, 10, 1-18. [CrossRef]
- Tsoupras, A.B.; Fragopoulou, E.; Iatrou, C.; Demopoulos, C.A. In vitro protective effects of olive pomace polar lipids towards platelet activating factor metabolism in human renal cells. Curr Top Nutraceutical Res 2011, 9, 105.
- Petsini, F.; Ntzouvani, A.; Detopoulou, M.; Papakonstantinou, V.D.; Kalogeropoulos, N.; Fragopoulou, E.; Nomikos, T.; Kontogianni, M.D.; Antonopoulou, S. Consumption of Farmed Fish, Fed with an Olive-Pomace Enriched Diet, and Its Effect on the Inflammatory, Redox, and Platelet-Activating Factor Enzyme Profile of Apparently Healthy Adults: A Double-Blind Randomized Crossover Trial. Foods 2022, 11, 2105.
- Sioriki, E.; Smith, T.K.; Demopoulos, C.A.; Zabetakis, I. Structure and cardioprotective activities of polar lipids of olive pomace, olive pomace-enriched fish feed and olive pomace fed gilthead sea bream (Sparus aurata). Food Res. Int. 2016, 83, 143-151. [CrossRef]
- Morphis, G.; Kyriazopoulou, A.; Nasopoulou, C.; Sioriki, E.; Demopoulos, C.A.; Zabetakis, I. Assessment of the in Vitro antithrombotic properties of sardine (Sardina pilchardus) fillet lipids and cod liver oil. Fishes 2016, 1, 1-15.
- Nasopoulou, C.; Gogaki, V.; Stamatakis, G.; Papaharisis, L.; Demopoulos, C.; Zabetakis, I. Evaluation of the in Vitro Anti-Atherogenic Properties of Lipid Fractions of Olive Pomace, Olive Pomace Enriched Fish Feed and Gilthead Sea Bream (Sparus aurata) Fed with Olive Pomace Enriched Fish Feed. Marine Drugs 2013, 11, 3676.
- Xie, D.; Li, P.; Zhu, Y.; He, J.; Zhang, M.; Liu, K.; Lin, H.; Zhai, H.; Li, X.; Ma, Y. Comparative bioactivity profile of phospholipids from three marine byproducts based on the zebrafish model. Journal of Food Biochemistry 2022, 46, e14229. [CrossRef]
- Lordan, R.; Walsh, A.M.; Crispie, F.; Finnegan, L.; Cotter, P.D.; Zabetakis, I. The effect of ovine milk fermentation on the antithrombotic properties of polar lipids. J. Funct. Foods 2019, 54, 289-300. [CrossRef]
- Poutzalis, S.; Lordan, R.; Nasopoulou, C.; Zabetakis, I. Phospholipids of goat and sheep origin: Structural and functional studies. Small Ruminant Res. 2018, 167, 39-47. [CrossRef]
- Antonopoulou, S.; Semidalas, C.E.; Koussissis, S.; Demopoulos, C.A. Platelet-activating factor (PAF) antagonists in foods: a study of lipids with PAF or anti-PAF-like activity in cow's milk and yogurt. Journal of Agriculture and Food Chemistry 1996, 44, 3047-3051. [CrossRef]
- Falk-Petersen, S.; Sargent, J.R.; Henderson, J.; Hegseth, E.N.; Hop, H.; Okolodkov, Y.B. Lipids and fatty acids in ice algae and phytoplankton from the Marginal Ice Zone in the Barents Sea. Polar Biology 1998, 20, 41-47. [CrossRef]
- Moreira, A.S.P.; Gonçalves, J.; Conde, T.A.; Couto, D.; Melo, T.; Maia, I.B.; Pereira, H.; Silva, J.; Domingues, M.R.; Nunes, C. Chrysotila pseudoroscoffensis as a source of high-value polar lipids with antioxidant activity: A lipidomic approach. Algal Research 2022, 66, 102756. [CrossRef]
- Robertson, R.; Guihéneuf, F.; Bahar, B.; Schmid, M.; Stengel, D.; Fitzgerald, G.; Ross, R.; Stanton, C. The Anti-Inflammatory Effect of Algae-Derived Lipid Extracts on Lipopolysaccharide (LPS)-Stimulated Human THP-1 Macrophages. Marine Drugs 2015, 13, 5402. [CrossRef]
- Jesionowska, M.; Ovadia, J.; Hockemeyer, K.; Clews, A.C.; Xu, Y. EPA and DHA in microalgae: Health benefits, biosynthesis, and metabolic engineering advances. Journal of the American Oil Chemists' Society n/a. [CrossRef]
- da Costa, E.; Amaro, H.M.; Melo, T.; Guedes, A.C.; Domingues, M.R. Screening for polar lipids, antioxidant, and anti-inflammatory activities of Gloeothece sp. lipid extracts pursuing new phytochemicals from cyanobacteria. Journal of Applied Phycology 2020, 32, 3015-3030. [CrossRef]
- Novik, G.I.; Astapovich, N.I.; Pasciak, M.; Gamian, A. [Biological activity of polar lipids from bifidobacteria]. Mikrobiologiia 2005, 74, 781-787.
- Shiels, K.; Tsoupras, A.; Lordan, R.; Zabetakis, I.; Murray, P.; Kumar Saha, S. Anti-inflammatory and antithrombotic properties of polar lipid extracts, rich in unsaturated fatty acids, from the Irish marine cyanobacterium Spirulina subsalsa. Journal of Functional Foods 2022, 94, 105124. [CrossRef]
- Shiels, K.; Tsoupras, A.; Lordan, R.; Nasopoulou, C.; Zabetakis, I.; Murray, P.; Saha, S.K. Bioactive Lipids of Marine Microalga Chlorococcum sp. SABC 012504 with Anti-Inflammatory and Anti-Thrombotic Activities. Marine Drugs 2021, 19, 28.
- Lopes, D.; Melo, T.; Meneses, J.; Abreu, M.H.; Pereira, R.; Domingues, P.; Lillebø, A.I.; Calado, R.; Domingues, M.R. A New Look for the Red Macroalga Palmaria palmata: A Seafood with Polar Lipids Rich in EPA and with Antioxidant Properties. Mar Drugs 2019, 17. [CrossRef]
- da Costa, E.; Melo, T.; Reis, M.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Polar Lipids Composition, Antioxidant and Anti-Inflammatory Activities of the Atlantic Red Seaweed Grateloupia turuturu. Mar Drugs 2021, 19. [CrossRef]
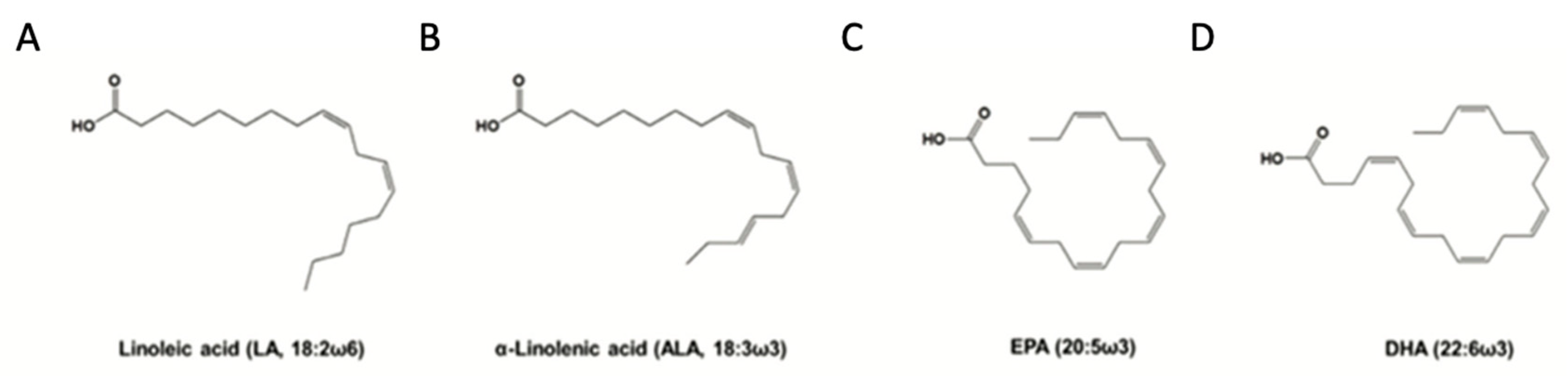
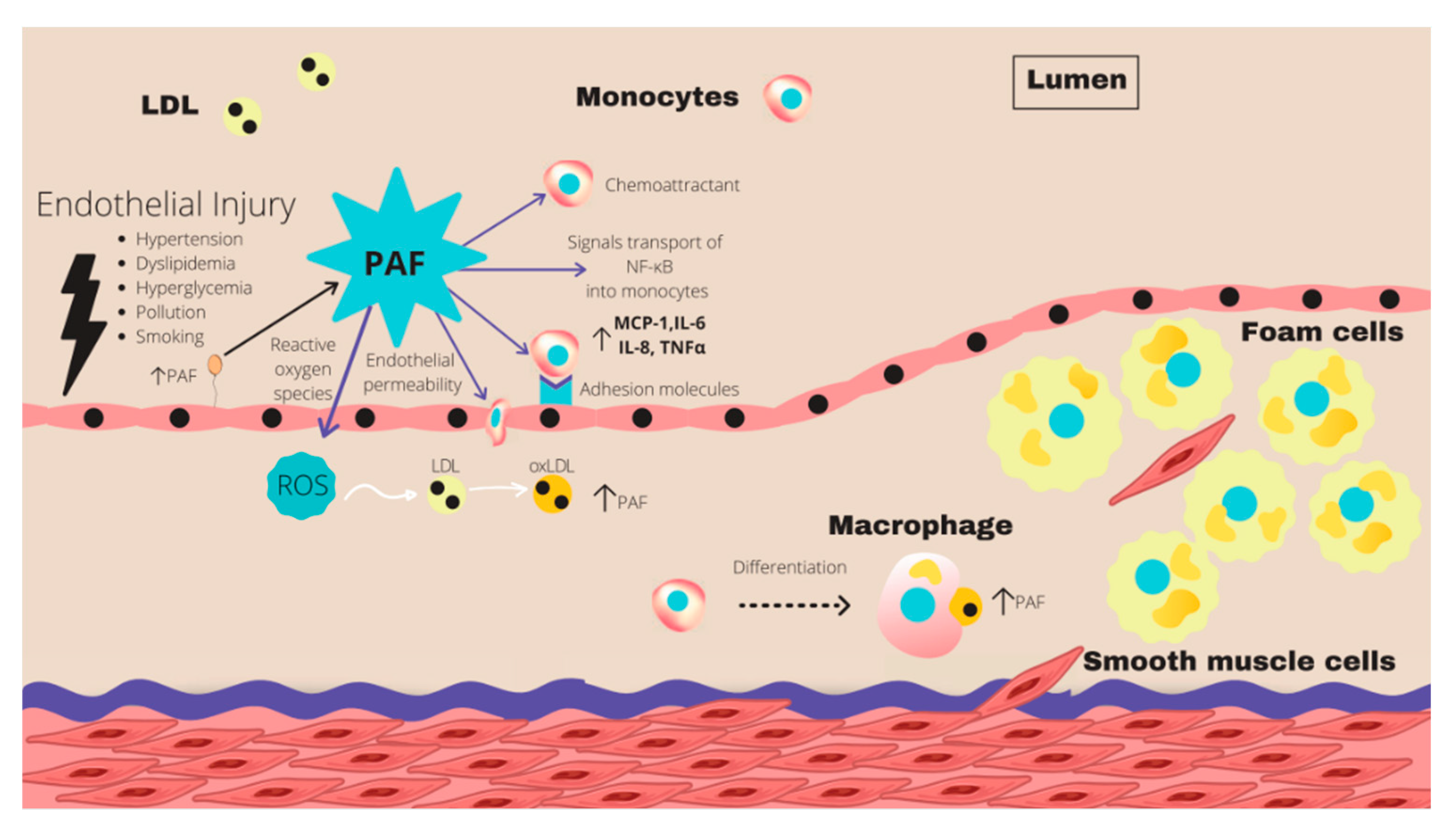
| Supplements | n-3 PUFA content per gram of oil |
|---|---|
| Krill oil | 205 mg |
| Tuna oil | 460 mg |
| Fish oil (standard) | 300 mg |
| Cod liver oil | 200 mg |
| Algal oil | 400 mg |
| Pharmaceuticals | EPA/DHA content per gram of oil |
| Omacor® (ethyl esters) | 460mg (EPA) & 380mg (DHA) |
| Epanova® (carboxylic acids) | 550mg (EPA) & 200mg (DHA) |
| Vascepa® (ethyl ester) | 900 mg EPA |
| Trial | N | Age | Formulation & Dose | Inclusion criteria/ cohort characteristics |
Duration (Years) |
Placebo |
| Successful – Primary endpoint reached* | ||||||
|
REDUCE-IT [103] |
8,179 | 45 with CVD or 50 with DM | IPE 4 g |
Patients with established CVD or DM on statin therapy with increased TG levels | 4.9 | Mineral oil |
|
EVAPORATE [117] |
80 | 30-85 | IPE 4 g |
Patients with confirmed coronary artery stenosis on statin therapy with increased TG levels. | 1.5 | Mineral oil |
|
JELIS [112] |
18,645 | Men 40-75 Women up to 75 years |
EPA 1.8 g + pravastatin or simvastatin |
Patients with previous MI or PCI or with confirmed angina pectoris or without CVD. | 4.6 | No placebo |
|
CHERRY [118] |
193 | 67 10 | Pivastatin + EPA 4 mg + 1800 mg |
Patients with CHD after PCI | 6-8 months | Pitavastatin 4 mg/day |
| Unsuccessful – Failed to reach primary endpoint* | ||||||
|
STRENGTH [119] |
13,078 | 18-99 (>40 for men 50 for women if with DM) | EPA + DHA carboxylic acids 4 g |
LDL-C < 100 mg/dL, on statins, TG levels 180-499 mg/dL, HDL-C < 42 mg/dL in men, <47mg/dL in women, patients with CVD or diabetes with risk factors. | 5 | Corn oil |
|
VITAL [114] |
25,871 | Men > 50 Women > 55 |
EPA + DHA 1 g |
Healthy men >50 and healthy women >55. TG levels not specified. | 5.3 | Not specified |
|
ASCEND [116] |
15,480 | >40 | EPA + DHA 1g |
Persons older than 40 years with DM without CVD | 7.4 | Olive oil 1 g |
|
ORIGIN [115] |
12,536 | 50 | EPA + DHA 465 mg + 375 mg |
High risk of CVD + impaired fasting glucose/glucose intolerance/DM. | 6.2 | Olive oil 1 g |
|
OMEMI [120] |
1027 | 70-82 + Recent (2-8 weeks) MI |
EPA + DHA 930 mg + 660 mg |
Recent acute MI | 2 | Corn oil |
| Clinical Trial | STRENGTH | REDUCE- IT |
| Number of participants | 13078 | 8179 |
| Population | High CVR, elevated TG levels, low HDL levels | High CVR, elevated TG levels, Diabetes |
| Treatment | DHA/EPA carboxylic acids (4g/d) (Epanova®) | Icosapent-ethyl ester (4g/d) |
| Placebo | Corn oil | Mineral oil |
| Follow-up Median | 3.5 years | 4.9 years |
| Primary Endpoint | Non-fatal stroke and MI, cardiovascular death, non-fatal MI, coronary revascularization or unstable angina | Non-fatal stroke and MI, cardiovascular death, coronary revascularization or unstable angina |
| 95% CI of Primary Endpoint | 0.99,0.90-1.09 | 0.75, 0.68-0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





