Submitted:
22 September 2023
Posted:
22 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
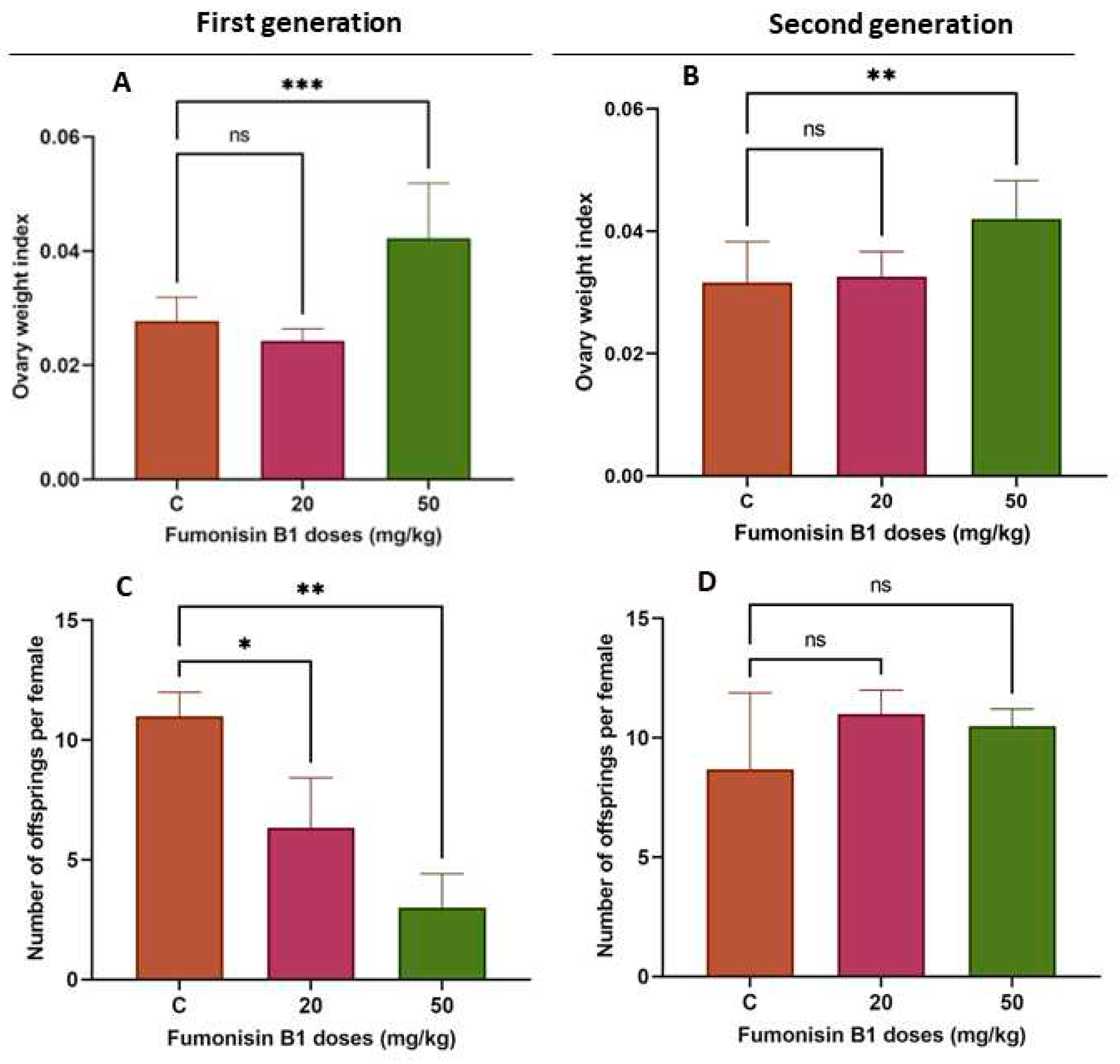
2.1. Fumonisin B1 Changes Weight and Fertility Rate
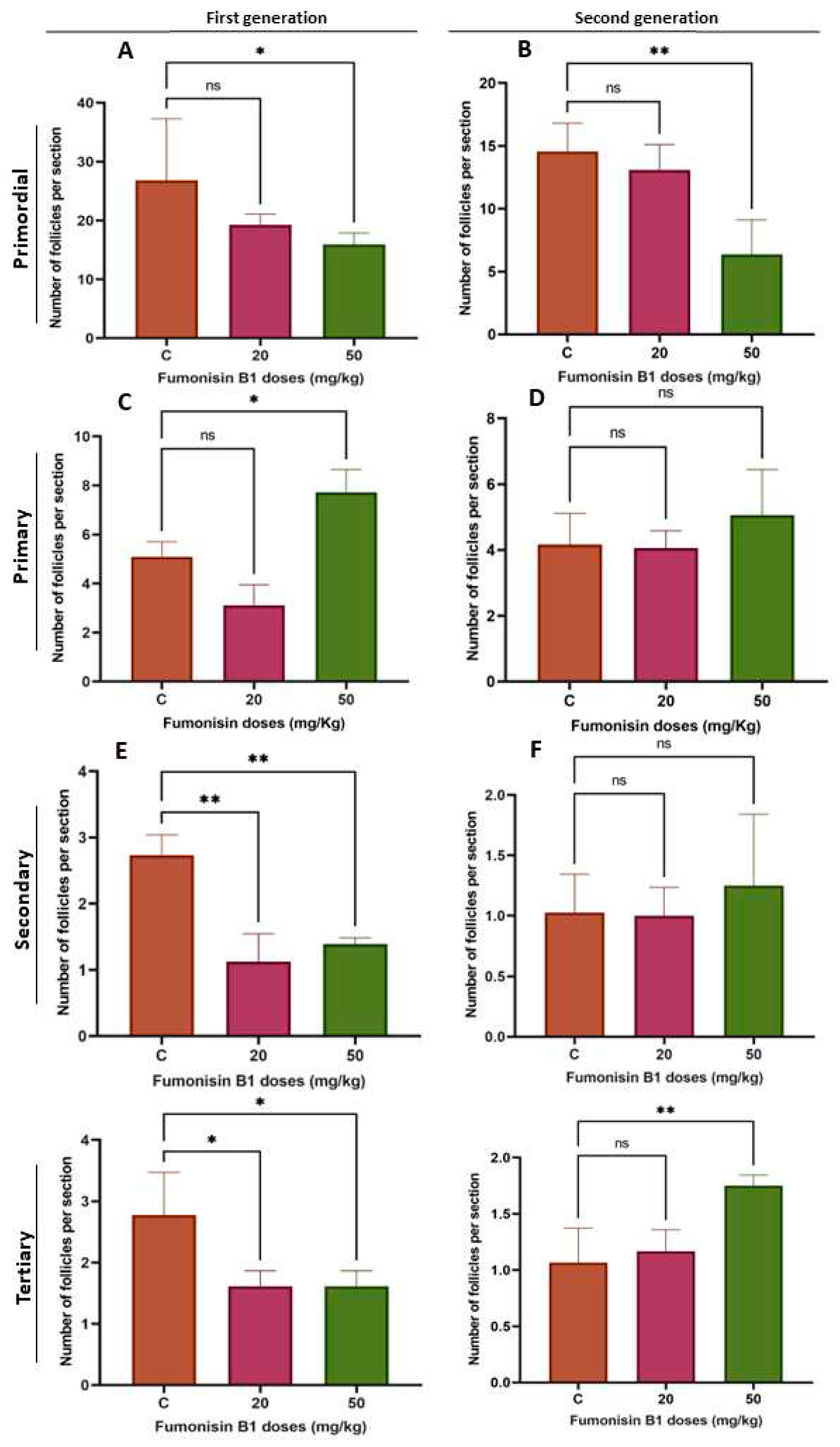
2.2. Fumonisin B1 Alters the Number of Follicles
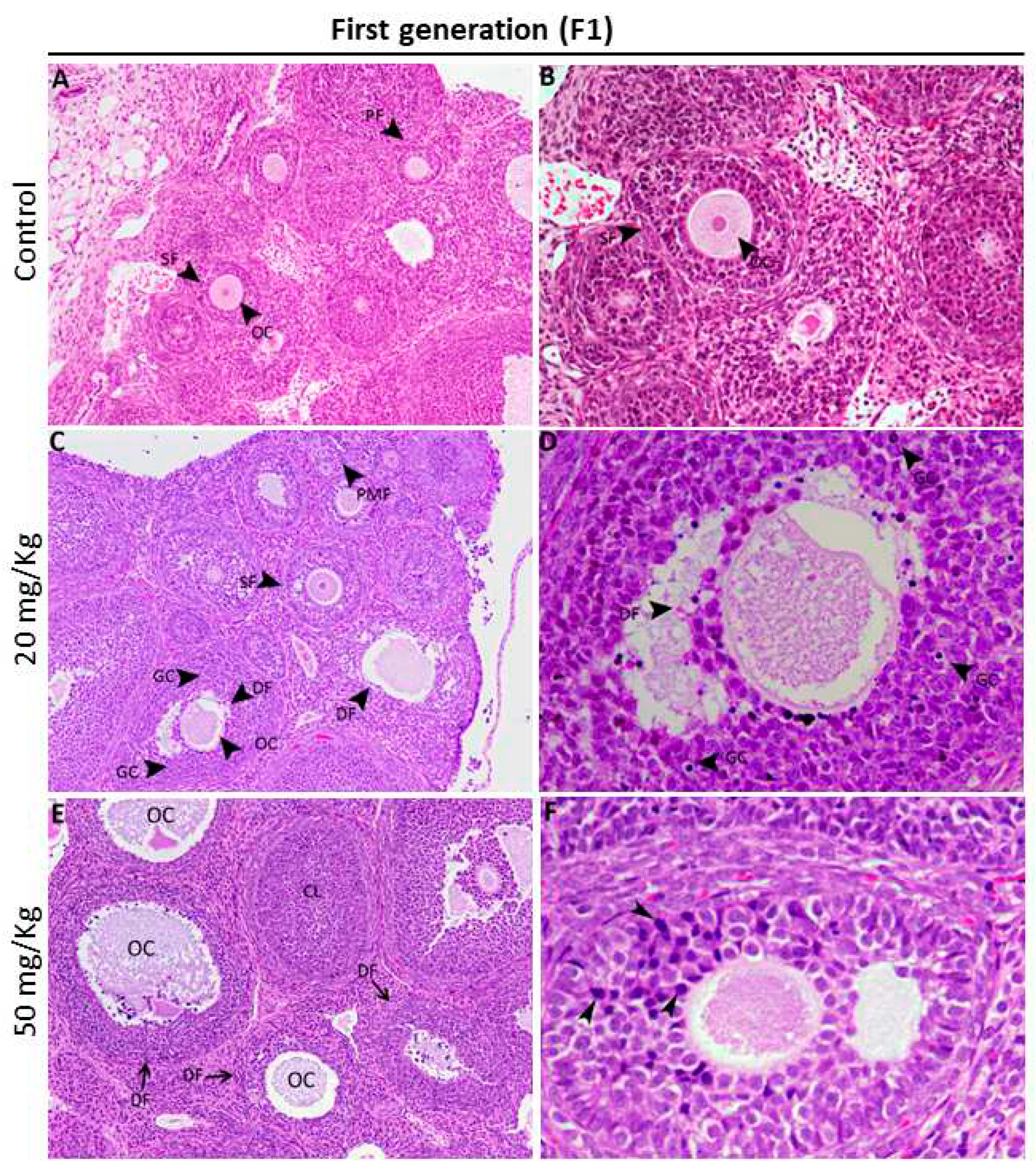
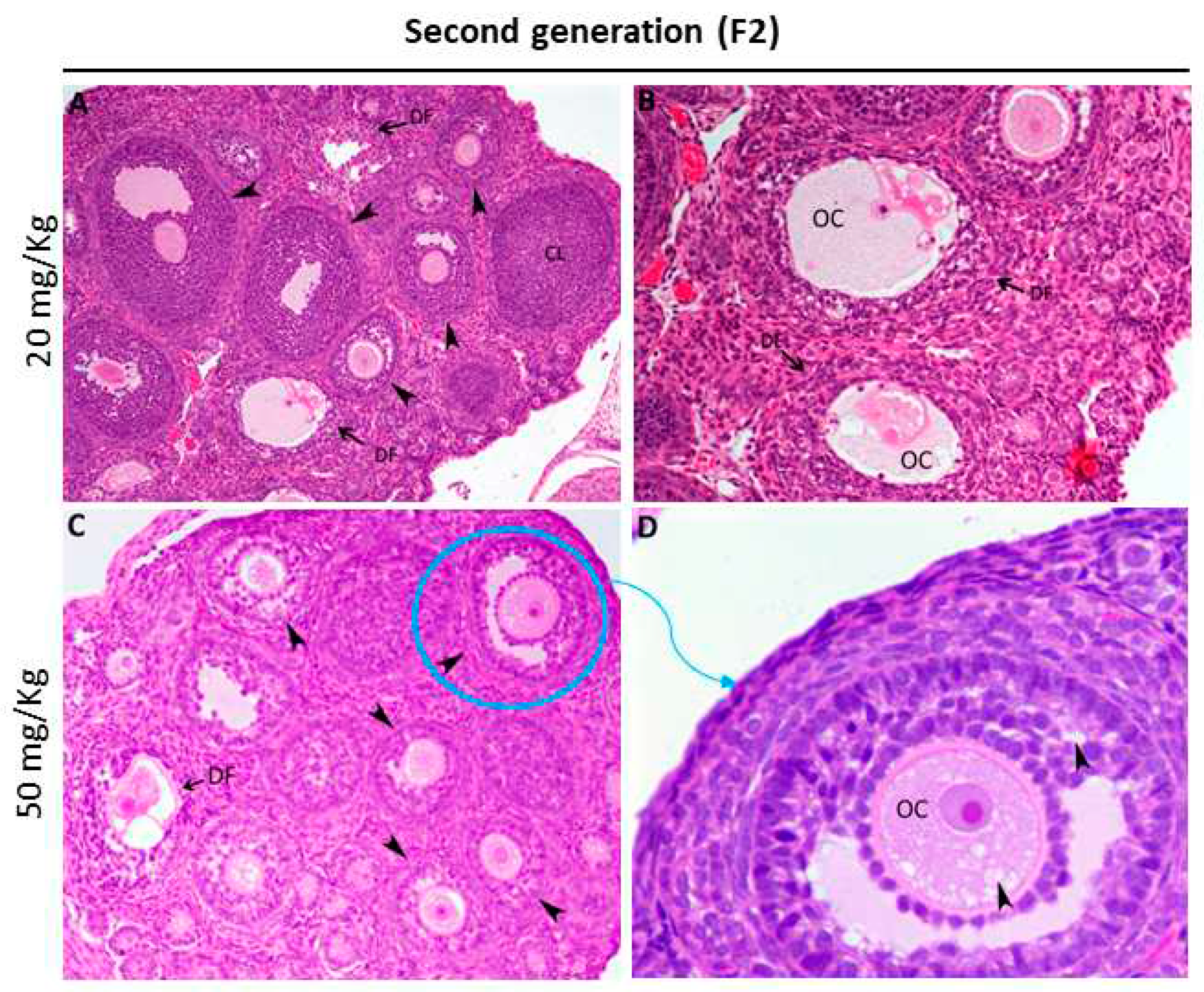
2.3. Fumonisin B1 Alters Oocyte Structure and Follicle Growth Histology
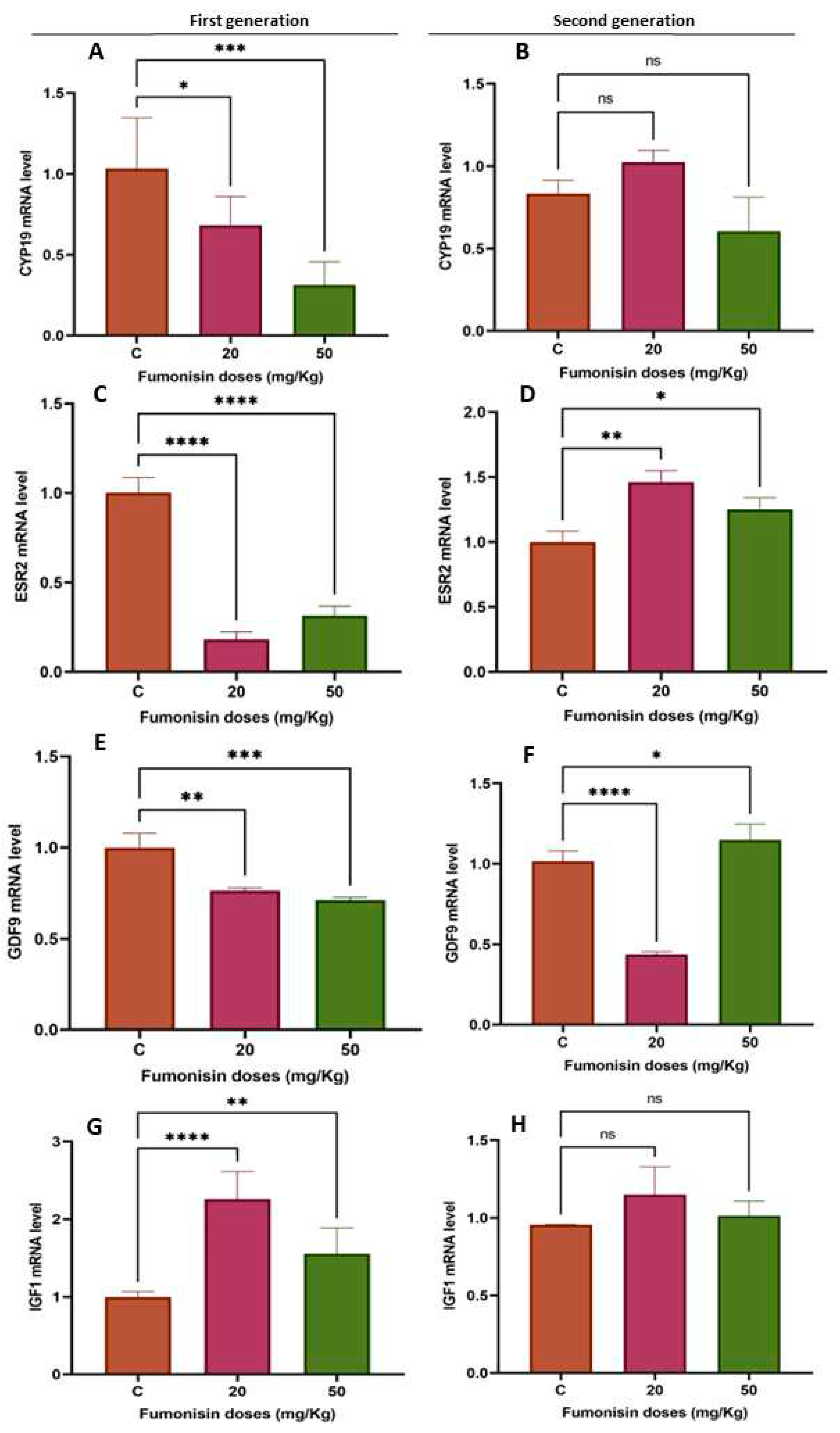
2.4. Fumonisin B1 affects Folliculogenesis- and Steroidogenesis-Related Gene Expression
2.5. The Role of Autophagy in Fumonisin B1 Treatment
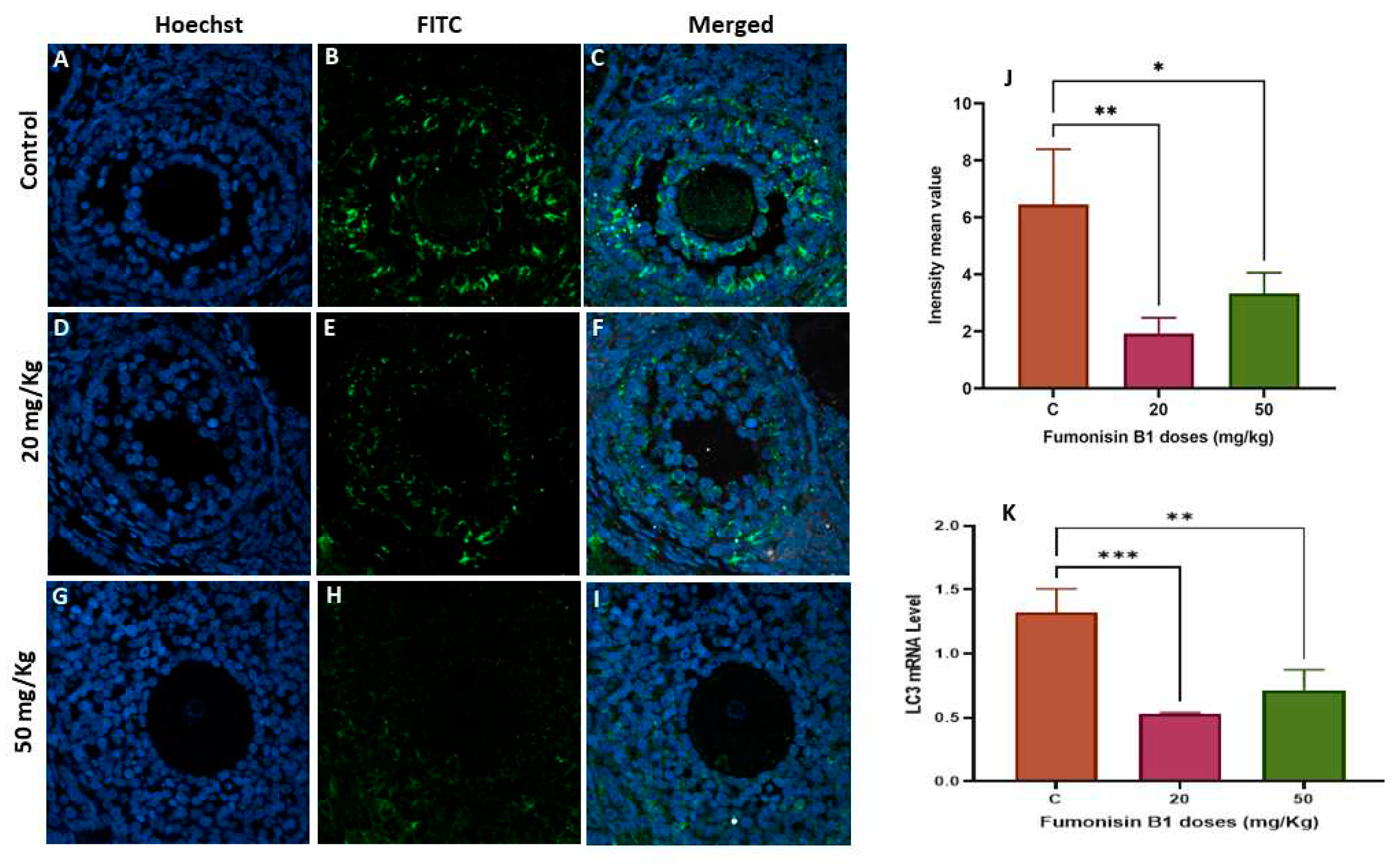
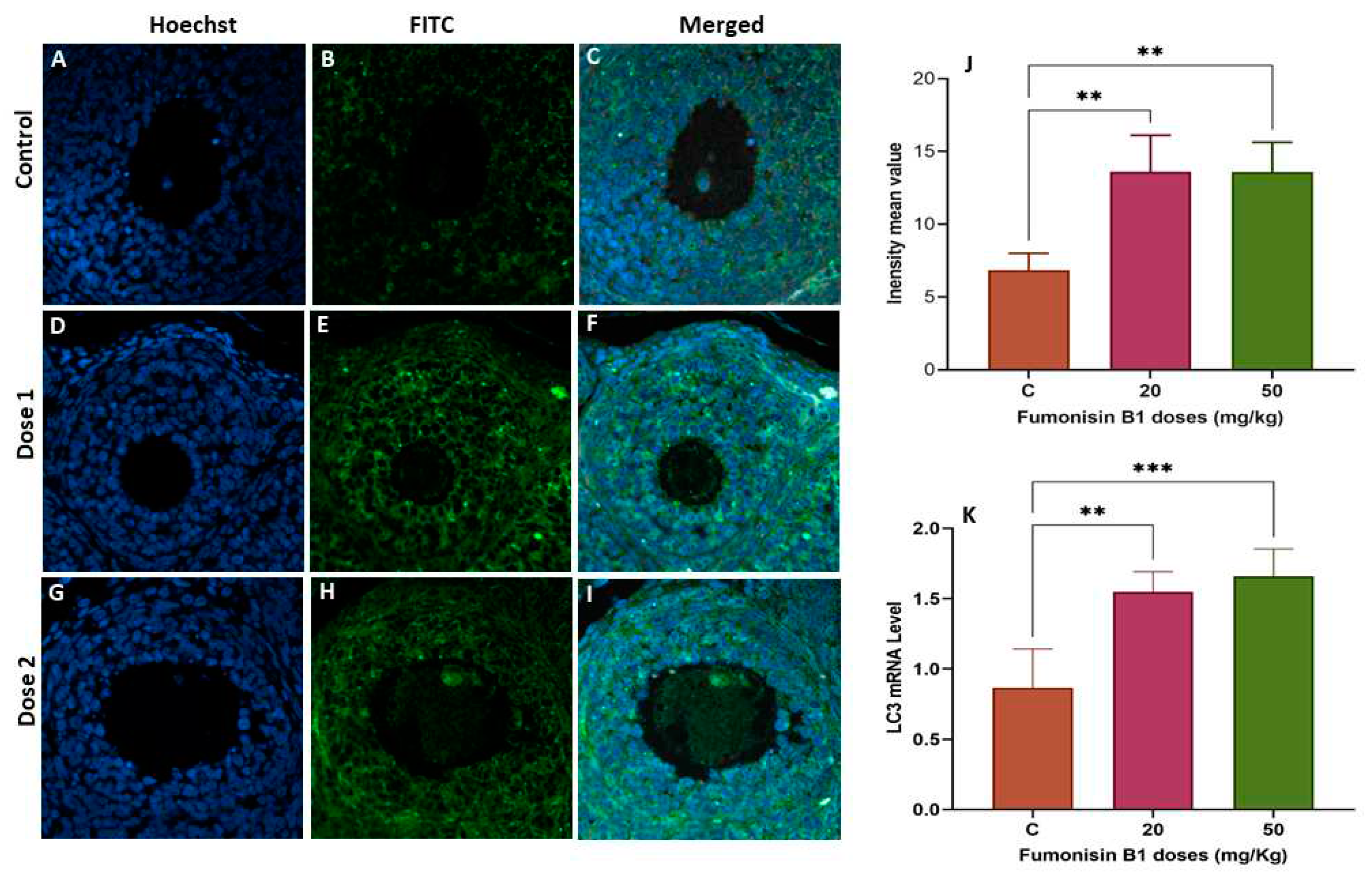
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Study Design and Sampling
4.3. Histological Preparation
4.4. Immunofluorescence Staining and Confocal Microscopy
4.5. Analysis of Gene Expression
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
References
- Bennett, J.W. , Klich, M. Mycotoxins. Clin Microbiol Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Park, D.L. , Njapau, H., Boutrif, E. Minimizing risks posed by mycotoxins utilizing the HACCP concept. Food Nutr Agr. 1999, 49–56. [Google Scholar]
- Magan, N. , Medina, A., Aldred, D. Possible climate-change effects on mycotoxin contamination of food crops pre- and postharvest. Plant Pathol. 2011, 60, 150–163. [Google Scholar] [CrossRef]
- Miraglia, M. , Marvin, H., Kleter, G., Battilani, P., Brera, C., Coni, E., Cubadda, F., Croci, L., De Santis, B., Dekkers, S. Climate change and food safety: an emerging issue with special focus on Europe. Food Chem Toxicol. 2009, 47, 1009–1021. [Google Scholar] [CrossRef]
- Coppock, R.W. , Jacobsen, B.J. Mycotoxins in animal and human patients. Toxicol Ind Health. 2009, 25, 637–655. [Google Scholar] [CrossRef] [PubMed]
- Voss, K. , Gelineau-van Waes, J., Riley, R. Fumonisins: current research trends in developmental toxicology. Mycotoxin research. 2006, 22, 61–69. [Google Scholar] [CrossRef]
- Rheeder, J. , Van der Westhuizen, L., Imrie, G., Shephard, G.S. Fusarium species and fumonisins in subsistence maize in the former Transkei region, South Africa: a multi-year study in rural villages. Food Additives & Contaminants: Part B. 2016, 9, 176–184. [Google Scholar]
- Van der Westhuizen, L. , Shephard, G.S., Burger, H.M., Rheeder, J.P., Gelderblom, W.C., Wild, C.P., Gong, Y.Y. Fumonisin B1 as a urinary biomarker of exposure in a maize intervention study among South African subsistence farmers. Cancer Epidemiol Biomarkers Prev. 2011, 20, 483–489. [Google Scholar] [CrossRef]
- Gong, Y.Y. , Torres-Sanchez, L., Lopez-Carrillo, L., Peng, J.H., Sutcliffe, A.E., White, K.L., Humpf, H.-U., Turner, P.C., Wild, C.P. Association between tortilla consumption and human urinary fumonisin B1 levels in a Mexican population. Cancer Epidemiology Biomarkers & Prevention. 2008, 17, 688–694. [Google Scholar]
- Misihairabgwi, J. , Ezekiel, C., Sulyok, M., Shephard, G., Krska, R. Mycotoxin contamination of foods in Southern Africa: A 10-year review (2007–2016). Crit Rev Food Sci Nutr. 2019, 59, 43–58. [Google Scholar] [CrossRef]
- Rheeder, J.P. , Marasas, W. F., Thiel, P.G., Sydenham, E.W., Shephard, G.S., Van Schalkwyk, D.J. Fusarium moniliforme and fumonisins in corn in relation to human esophageal cancer in Transkei. 1992. [Google Scholar]
- Haschek, W.M. , Gumprecht, L.A., Smith, G., Tumbleson, M.E., Constable, P.D. Fumonisin toxicosis in swine: an overview of porcine pulmonary edema and current perspectives. Environ Health Perspect. 2001, 109 (suppl 2), 251-257.
- Harrison, L.R. , Colvin, B.M., Greene, J.T., Newman, L.E., Cole Jr, J.R. Pulmonary edema and hydrothorax in swine produced by fumonisin B1, a toxic metabolite of Fusarium moniliforme. J Vet Diagn Invest. 1990, 2, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Voss, K.A. , Riley, R.T., Norred, W., Bacon, C.W., Meredith, F.I., Howard, P.C., Plattner, R.D., Collins, T., Hansen, D.K., Porter, J.K. An overview of rodent toxicities: liver and kidney effects of fumonisins and Fusarium moniliforme. Environ Health Perspect. 2001, 109 (suppl 2), 259-266.
- Bolger, M. , Safety evaluation of certain mycotoxins in food, Prepared by the 56th Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 2001, Geneva, Switzerland, WHO, 2001.
- Howard, P.C. , Eppley, R.M., Stack, M.E., Warbritton, A., Voss, K.A., Lorentzen, R.J., Kovach, R.M., Bucci, T.J. Fumonisin b1 carcinogenicity in a two-year feeding study using F344 rats and B6C3F1 mice. Environ Health Perspect. 2001, 109 (suppl 2), 277-282.
- Gelderblom, W.C. , Kriek, N., Marasas, W., Thiel, P. Toxicity and carcinogenicity of the Fusanum monilzforine metabolite, fumonisin B1, in rats. Carcinogenesis. 1991, 12, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Voss, K.A. , Riley, R.T. Fumonisin toxicity and mechanism of action: overview and current perspectives. Food Safety. 2013, 1, 2013006–2013006. [Google Scholar] [CrossRef]
- Gbore, F.A. , Owolawi, T.J., Erhunwunsee, M., Akele, O., Gabriel-Ajobiewe, R.A. Evaluation of the Reproductive Toxicity of Dietary Fumonisin B 1 in Rats. Jordan Journal of Biological Sciences. 2012, 5. [Google Scholar]
- Cortinovis, C. , Caloni, F., Schreiber, N.B., Spicer, L.J. Effects of fumonisin B1 alone and combined with deoxynivalenol or zearalenone on porcine granulosa cell proliferation and steroid production. Theriogenology. 2014, 81, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Harrath, A.H. , Alrezaki, A., Mansour, L., Alwasel, S.H., Palomba, S. Food restriction during pregnancy and female offspring fertility: adverse effects of reprogrammed reproductive lifespan. Journal of Ovarian Research. 2017, 10, 1–9. [Google Scholar] [CrossRef]
- Jalouli, M. , Mofti, A., Elnakady, Y.A., Nahdi, S., Feriani, A., Alrezaki, A., Sebei, K., Bizzarri, M., Alwasel, S., Harrath, A.H. Allethrin promotes apoptosis and autophagy associated with the oxidative stress-related PI3K/AKT/mTOR signaling pathway in developing rat ovaries. Int J Mol Sci. 2022, 23, 6397. [Google Scholar]
- Pereira, V. , Fernandes, J., Cunha, S. Mycotoxins in cereals and related foodstuffs: A review on occurrence and recent methods of analysis. Trends Food Sci Technol. 2014, 36, 96–136. [Google Scholar] [CrossRef]
- Sun, X.D. , Su, P., Shan, H. Mycotoxin contamination of rice in China. J Food Sci. 2017, 82, 573–584. [Google Scholar] [CrossRef]
- Rodrigues, I. , Naehrer, K., A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins 4, 663–675, 2012.
- Wagacha, J. , Muthomi, J. Mycotoxin problem in Africa: current status, implications to food safety and health and possible management strategies. Int J Food Microbiol. 2008, 124, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J. , Ryu, D. Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: Public health perspectives of their co-occurrence. J Agric Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef] [PubMed]
- Udomkun, P. , Wiredu, A.N., Nagle, M., Bandyopadhyay, R., Müller, J., Vanlauwe, B. Mycotoxins in Sub-Saharan Africa: Present situation, socio-economic impact, awareness, and outlook. Food Control. 2017, 72, 110–122. [Google Scholar] [CrossRef]
- Marasas, W.F.O. , Kellerman, T.S., Gelderblom, W.C., Thiel, P., Van der Lugt, J.J. Leukoencephalomalacia in a horse induced by fumonisin B₁isolated from Fusarium moniliforme. 1988. [Google Scholar]
- Karuna, R. , Rao, B.S. Lack of micronuclei induction by fumonisin B 1 mycotoxin in BALB/c mice. Mycotoxin research. 2013, 29, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Murashiki, T.C. , Chidewe, C., Benhura, M.A., Maringe, D.T., Dembedza, M.P., Manema, L.R., Mvumi, B.M., Nyanga, L.K. Levels and daily intake estimates of aflatoxin B1 and fumonisin B1 in maize consumed by rural households in Shamva and Makoni districts of Zimbabwe. Food Control. 2017, 72, 105–109. [Google Scholar] [CrossRef]
- Hanson, B. , Johnstone, E., Dorais, J., Silver, B., Peterson, C.M., Hotaling, J. Female infertility, infertility-associated diagnoses, and comorbidities: a review. J Assist Reprod Genet. 2017, 34, 167–177. [Google Scholar] [CrossRef]
- Aldawood, N. , Alrezaki, A., Alanazi, S., Amor, N., Alwasel, S., Sirotkin, A., Harrath, A.H. Acrylamide impairs ovarian function by promoting apoptosis and affecting reproductive hormone release, steroidogenesis and autophagy-related genes: An in vivo study. Ecotoxicol Environ Saf. 2020, 197, 110595. [Google Scholar] [CrossRef]
- Hartshorne, G.M. , Lyrakou, S., Hamoda, H., Oloto, E., Ghafari, F. Oogenesis and cell death in human prenatal ovaries: what are the criteria for oocyte selection? Mol Human Reprod. 2009, 15, 805–819. [Google Scholar] [CrossRef]
- Perez, G.I. , Robles, R., Knudson, C.M., Flaws, J.A., Korsmeyer, S.J., Tilly, J.L. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet. 1999, 21, 200–203. [Google Scholar] [CrossRef]
- Brehm, E. , Flaws, J.A. Prenatal exposure to a mixture of phthalates accelerates the age-related decline in reproductive capacity but may not affect direct biomarkers of ovarian aging in the F1 generation of female mice. Environmental Epigenetics. 2021, 7, dvab010. [Google Scholar] [CrossRef]
- Bernal, A.B. , Vickers, M.H., Hampton, M.B., Poynton, R.A., Sloboda, D.M. Maternal undernutrition significantly impacts ovarian follicle number and increases ovarian oxidative stress in adult rat offspring. PLoS One. 2010, 5, e15558. [Google Scholar] [CrossRef]
- Treloar, S. , Sadrzadeh, S., Do, K.-A., G. Martin, N., B. Lambalk, C. Birth weight and age at menopause in Australian female twin pairs: exploration of the fetal origin hypothesis. Hum Reprod. 2000, 15, 55–59. [Google Scholar] [CrossRef]
- Aldawood, N. , Jalouli, M., Alrezaki, A., Nahdi, S., Alamri, A., Alanazi, M., Manoharadas, S., Alwasel, S., Harrath, A.H. Fetal programming: in utero exposure to acrylamide leads to intergenerational disrupted ovarian function and accelerated ovarian aging. Aging (Albany NY). 2022, 14, 6887. [Google Scholar] [CrossRef]
- Zaid, S.S.M. , Othman, S., Kassim, N.M. Potential protective effect of Tualang honey on BPA-induced ovarian toxicity in prepubertal rat. BMC Complement Altern Med. 2014, 14, 1–12. [Google Scholar] [CrossRef]
- Al-Hiyasat, A.S. , Elbetieha, A.M., Darmani, H. Reproductive toxic effects of ingestion of sodium fluoride in female rats. Fluoride. 2000, 33, 79–84. [Google Scholar]
- Kimura, N. , Kimura, T., Suzuki, M., Totsukawa, K. Effect of gestational exposure to nonylphenol on the development and fertility of mouse offspring. J. Reprod. Dev. 2006, 52, 789–795. [Google Scholar] [CrossRef]
- Hamilton, M.P. , Fleming, R., Coutts, J.R., Macnaughton, M.C., Whitfield, C.R. Luteal cysts and unexplained infertility: biochemical and ultrasonic evaluation. Fertil Steril. 1990, 54, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Micu, M.C. , Micu, R., Ostensen, M. Luteinized unruptured follicle syndrome increased by inactive disease and selective cyclooxygenase 2 inhibitors in women with inflammatory arthropathies. Arthritis Care Res (Hoboken). 2011, 63, 1334–1338. [Google Scholar] [CrossRef]
- Eissa, M.K. , Sawers, R.S., Docker, M.F., Lynch, S.S., Newton, J.R. Characteristics and incidence of dysfunctional ovulation patterns detected by ultrasound. Fertil Steril. 1987, 47, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Kopera, D. , Wehr, E., Obermayer-Pietsch, B. Endocrinology of hirsutism. International journal of trichology. 2010, 2, 30. [Google Scholar] [CrossRef]
- Kafali, H. , Iriadam, M., Ozardalı, I., Demir, N. Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res. 2004, 35, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Shi, D. , Vine, D.F. Animal models of polycystic ovary syndrome: a focused review of rodent models in relationship to clinical phenotypes and cardiometabolic risk. Fertil Steril. 2012, 98, 185–193.e82. [Google Scholar] [CrossRef]
- Zurvarra, F.M. , Salvetti, N.R., Mason, J.I., Velazquez, M.M., Alfaro, N.S., Ortega, H.H. Disruption in the expression and immunolocalisation of steroid receptors and steroidogenic enzymes in letrozole-induced polycystic ovaries in rat. Reprod Fertil Dev. 2009, 21, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Gerez, J.R. , Camacho, T., Marutani, V.H.B., de Matos, R.L.N., Hohmann, M.S., Júnior, W.A.V., Bracarense, A.P.F. Ovarian toxicity by fusariotoxins in pigs: does it imply in oxidative stress? Theriogenology. 2021, 165, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Harrath, A.H. , Alrezaki, A., Jalouli, M., Aldawood, N., Aldahmash, W., Mansour, L., Alwasel, S. Ethylbenzene exposure disrupts ovarian function in Wistar rats via altering folliculogenesis and steroidogenesis-related markers and activating autophagy and apoptosis. Ecotoxicol Environ Saf. 2022, 229, 113081. [Google Scholar] [CrossRef]
- Zhang, C. , Hu, J., Wang, W., Sun, Y., Sun, K. HMGB1-induced aberrant autophagy contributes to insulin resistance in granulosa cells in PCOS. The FASEB Journal. 2020, 34, 9563–9574. [Google Scholar] [CrossRef]
- Liu, G.-y. , Jiang, X.-x., Zhu, X., He, W.-y., Kuang, Y.-l., Ren, K., Lin, Y., Gou, X. ROS activates JNK-mediated autophagy to counteract apoptosis in mouse mesenchymal stem cells in vitro. Acta Pharmacol Sin. 2015, 36, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C. , Zalckvar, E., Kimchi, A., Kroemer, G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nature reviews Molecular cell biology. 2007, 8, 741–752. [Google Scholar] [CrossRef]
- White, E. Deconvoluting the context-dependent role for autophagy in cancer. Nature reviews cancer. 2012, 12, 401–410. [Google Scholar] [CrossRef]
- Mulcahy Levy, J.M. , Thorburn, A. Autophagy in cancer: moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ. 2020, 27, 843–857. [Google Scholar] [CrossRef]
- Kumariya, S. , Ubba, V., Jha, R.K., Gayen, J.R. Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective. Autophagy. 2021, 17, 2706–2733. [Google Scholar] [CrossRef] [PubMed]
- Li, X. , Qi, J., Zhu, Q., He, Y., Wang, Y., Lu, Y., Wu, H., Sun, Y. The role of androgen in autophagy of granulosa cells from PCOS. Gynecol Endocrinol. 2019, 35, 669–672. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




