1. Introduction
Lactic acid bacteria (LAB) are Gram-positive organisms that produce lactic acid as a key fermentation product by utilizing carbohydrates during fermentation (Ayivi et al., 2020). The bacteria included in the group are gram-positive, non-sporing, non-respiring cocci or rods that produce lactic acid as the major product during the fermentation of carbohydrates and other organic substances. This fermentation contributes to the flavor, texture and aroma that result in unique organoleptic characteristics as the product of carbohydrate catabolism (Quinto et al., 2014; Hayek et al., 2019; Hayek and Ibrahim, 2013). LAB is important in food, agriculture and clinical applications and is responsible for the degradation of proteins and lipids as well as the production of various alcohols, aldehydes, acids, esters and sulphur compounds that contribute to specific flavor development in different fermented food products (Bintsis, 2018; Krastonov et al., 2023). The importance of LAB is primarily associated with their safe metabolic activity during proliferation in foods and utilizing available sugar to produce organic acids and other metabolites. The common occurrence of LAB in foods along with their daily usage contributes to their classification as GRAS (Generally Recognized as Safe) for human consumption. For example, LAB has been classified based on the following factors: glucose fermentation characteristics, cell morphology, capacity to utilize sugars, and optimum growth temperature range (Axelsson, 2004; Yeboah et al., 2023). These four classification systems thus recognized only four lactic acid bacteria genera: Lactobacillus, Pediococcus, Leucononstoc, and Streptococcus (Axelsson, 2004). Some LAB are used as starter cultures in the dairy industry. For example, one of the most common LAB used in the dairy industry is Lactobacillus delbrueckii ssp. bulgaricus (L. bulgaricus).
L. bulgaricus is a probiotic and an important member of the uniquely diverse LAB group with many industrial applications (Gyawali et al., 2020; Ibrahim et al., 2023). L. bulgaricus is generally used as a starter culture to produce fermented dairy products such as yogurt and cheese and is also considered a probiotic due to its significant human health benefits (Aponte et al., 2020, Ibrahim et al., 2023). In addition, L. bulgaricus is typically used as a synergistic with Streptococcus thermophilus (S. thermophilus) on an industrial scale to produce yogurt. During yogurt production, this LAB plays a significant role in the development of the organoleptic and probiotic properties of yogurt (Hayek et al., 2019; Ayivi et al., 2020). Research has shown L. bulgaricus to be a safe probiotic with several health benefits when administered in adequate amounts. Consequently, the flavor, texture, and organoleptic properties of yogurt are generally a result of the synergistic and symbiotic interaction of L. bulgaricus and S. thermophilus. Therefore, an imbalance in the level/proportion of these two (2) bacteria will affect the sensorial characteristics and qualities of the fermented dairy product (Sieuwerts, 2016). L. bulgaricus as a starter culture is vital in many fermentation and bio-processing operations. Ayivi et al. (2020) reported that the fermentation performance and functionality of most strains of L. bulgaricus are enhanced by nutritional requirements and the overall enrichment of the fermentation medium. Thus, the food and dairy industries continually look for probiotic bacteria applications with cost-effective fermentation processes including growth medium requirements (Hayek and Ibrahim, 2013; Atilola et al., 2015). Generally, the nutritional quality and performance of the fermentation medium are enhanced by the supplementation of casein hydrolysates and yeast extract, which are key nitrogen sources that act as growth promoters to boost bacterial cell growth (Hayek and Ibrahim, 2013). Significant biomass levels and high volumes of lactic acid production by LAB species have also been attributed to the presence of amino acids, vitamins and yeast extract in the fermentation medium (Manzoor et al., 2017; Ayad et al., 2020). However, the limiting factor for supplementing fermentation media with high levels of nitrogen sources is the exorbitant cost (Manzoor et al., 2017; Hayek et al., 2019). Moreover, the standard LAB fermentation medium, de Man, Rogosa, and Sharpe (MRS), is more expensive due to its nitrogen sources (meat, peptone, and yeast extract) and does not support all growth LAB and probiotic cultures. This makes the media significantly expensive to prepare as it includes the addition of animal-based nitrogen sources. The available literature also suggests that significant research is directed at finding alternative cost-effective ingredients for LAB growth media and obtaining higher cell densities and biomass levels. Additionally, as consumer preferences shift from animal-based products to plant-based products, it is prudent for researchers to investigate the development of a lower-cost, plant-based growth medium (GM) to produce LAB for fermented food products. Therefore, the aim of the present study was to investigate the growth and viability of L. bulgaricus cultivated with alternative plant-based nitrogen sources.
2. Materials and Method
2.1. Source of Lactobacillus bulgaricus
Different bacteria strains were used for the preliminary work, a total of 8
L. bulgaricus strains were used and tested during the preliminary study. However, depending on their different growth behavior and acidification rates, two strains (LB6 and S22) were selected and used for the study. Two of the strains were isolated from commercial yogurt products obtained from the market (United States) and six stains were obtained from Albert Krastonov, Department of Biotechnology, University of Food Technologies, Bulgaria. All samples were kept at -80℃ until further use.
Table 1 shows the probiotic strains used for the study and their source.
2.2. Chemicals and Reagents
All chemicals and reagents were acquired from Fisher Scientific (Pittsburgh, PA) for this study.
2.3. Standard media preparation and bacteria culture activation
2.3.1. L. bulgaricus culture broth/agar preparation and bacteria activation
De Man, Rogosa and Sharpe (MRS) media broth was prepared according to the manufacturer’s instruction with slight modifications by completely dissolving 55g of MRS (Neogen Co, Michigan, USA), 1g of L-Cysteine, 1g of yeast extract, 2g of beef extract and 2 mL of tween 80 in 1 L of distilled water (DDW). The mixture was stirred for 30 minutes and the resultant solution was pipetted into test tubes (6 mL each), autoclaved at 121⁰C for 15 min and then cooled at room temperature. In the case of the agar media, 15 g of agar powder (Neogen) was added before sterilizing for 15 min at 121℃, and all freshly prepared agar media in the study were poured into sterile Petri dishes and stored at 4 ⁰C until further needed. The two selected L. bulgaricus strains (LB6 and S22) were activated in the freshly prepared MRS broth and were incubated anaerobically at 42⁰C overnight. This study did not involve the use of animals, making ethical approval for animal testing unnecessary.
2.3.3. Modified reinforced clostridial medium-pyruvate (mRCM-PYR)
To ensure selectivity and accurate enumeration of L. bulgaricus, a reinforced clostridial medium was prepared by dissolving 10g peptone #3, 10g beef extract, 5g yeast extract, 5g sodium chloride, 3g sodium acetate, 2g K2HPO4, 0.1g uracil, 0.25g calcium chloride, 5g dextrose, 5g fructose, 10g maltose, 2g sodium pyruvate, 0.2% Tween 80, and 0.5g L- Cysteine in 1 L of DW. The pH of the solution was adjusted to 6.0 ± 0.2 using 6N HCl before adding 0.008% aniline blue and 15g agar. After sterilizing the medium at 121 ⁰C for 15 minutes, it was cooled in a water bath and poured into sterile Petri dishes. All freshly prepared media were stored at 4⁰C until ready to use.
2.4. Nitrogen sources validation, growth media optimization and bacterial culture propagation in optimized growth media
2.4.1. Validation of nitrogen sources as a growth medium
The 4 yeast samples (X-Seed Nucleo Max, NuCel 780MG, NuCel 532MG and NuCel 585MG) were tested in different blends and concentrations for growth efficiency in a preliminary study. The preliminary study thus proved that X-Seed Nucleo Max (X-Seed), NuCel 780MG (N7) and Nucel 532MG (N5) yeast samples have higher growth and thus superior alternative nitrogen sources for the growth of bacteria.
Table 2 shows the overall protein and nitrogen quantities for X-Seed, N7, and N5.
2.4.2. Optimized L. bulgaricus growth medium
The optimized GM was prepared by dissolving 20g Dextrose, 2g Potassium phosphate dibasic, 5g Sodium acetate, 2g Ammonium citrate, 0.2g Magnesium sulphate, 0.05g Manganese sulphate, 0.2% Tween 80, 0.35g calcium chloride, 1g L-Cysteine, 2g X-Seed Nucleo Max in 1 L of DDW. This medium was blended with optimized proportions of N7 and N5 (3g and 1.5g) yeast extracts. The final pH value of the medium was adjusted to 6.2 ± 0.2, using 6N HCl and 6 mL of the resultant solution was then pipetted into test tubes and autoclaved at 121⁰C for 15 min. After sterilization, the tubes were cooled at room temperature and stored at 4℃ until further needed. The difference in composition between the optimized GM and MRS is highlighted in Table 3.
Table 3.
Composition per liter of MRS medium and the optimized growth medium.
Table 3.
Composition per liter of MRS medium and the optimized growth medium.
| MRS (Neogen) g/L |
Optimized Growth Media g/L |
| 20g Glucose |
20g Dextrose |
| 5g Sodium Acetate |
5g Sodium Acetate |
| 0.2g Magnesium Sulphate |
0.2g Magnesium Sulphate |
| 0.05g Manganese Sulphate |
0.05g Manganese Sulphate |
| 1.08ml Tween 80 |
2ml Tween 80 |
| 2g Ammonium Citrate |
2g Ammonium Citrate |
| * 5g Yeast |
0.35g Calcium Chloride |
| * 10g Meat Extract |
2g Potassium Phosphate Dibasic |
| *10g Peptone |
1g L – Cysteine |
| |
*2g X-Seed |
| |
* 3g/1.5g (N7) |
| |
* 3g/1.5g (N5) |
2.4.3. Culture propagation in optimized growth medium with different nitrogen concentrations
After overnight activation of bacteria strains in MRS broth in the incubator at 42℃, 100 µL of the culture was picked into fresh MRS broth and incubated for 8 hours to reach an optical density (OD) between 0.7 and 0.9. The strains were centrifuged afterward at 5000 rpm for 20 minutes at 4℃ using a Thermo Scientific Sorvall ST 16R centrifuge. The resulting supernatant was decanted, and cells were washed with 0.1M phosphate-buffered saline (PBS) solution. Washed cells were then ten-fold serially diluted in PBS solution. From the third-fold dilution, 200µl was inoculated into each batch of test tubes containing different concentration blends of the GM as found in
Table 4. All test tubes with the different batches of nitrogen sources were vortexed, covered with parafilm and anaerobically incubated at 42℃ for 12 hours and the initial and final growth was monitored at different time intervals of 0, 6, 10 and 12 hours using Thermo Fisher Scientific Evolution 201 UV-visible spectrophotometer at optical density (OD610nm) (Thermo Fisher Scientific, Inc. Waltham, MA, USA). The initial bacterial population was determined using the bacterial enumeration method.
2.5. Bacterial Growth
2.5.1. Bacterial enumeration
The bacterial growth of the strains (LB6 and S22) was monitored using spectrophotometric measurement of their optical densities (OD610) at different time intervals (0, 6, 10 and 12 hours) using a Thermo Fisher Scientific Evolution 201 UV visible spectrophotometer (Thermo Fisher Scientific, Inc. Waltham, MA, USA). The uncultured media were used as blanks during the spectrophotometric measurement and the initial and final bacterial populations were determined. At the end of the fermentation period (12 h), a 1 mL sample was drawn from each inoculated sample and serially diluted in a 9 mL PBS solution. Appropriate dilutions were then surface plated (100 µl) onto duplicate MRS and mRCM-PYR agar plates. All plates were anaerobically incubated for 48 h at 42 °C and plates with 25 – 250 colonies were counted. The bacterial populations were then expressed in Log CFU/mL.
2.5.2. Determination of pH values
The pH values of each test tube containing the media sample were measured in duplicates starting from (0 h) and the end (12 h) of the fermentation period using a pH meter (Accumet AB 150, Fisher Scientific, Pittsburgh, PA). The pH meter was first calibrated with standard pH buffers 10, 7 and 4. After calibration, the pH readings for the fermented media were measured. The pH electrode was completely rinsed with deionized water and cleaned with Kim wipes between the different samples.
2.6. Statistical analysis
Each experiment was conducted with three technical replicates. SAS version 9.4 was used to analyze study data and results are displayed as means of with standard errors (SE). ANOVA was employed to determine significant differences between groups, and Tukey's test was used for comparisons (P < 0.05) between treatment means. Bacterial population counts were expressed in log10 before analysis.
3. Results
3.1. Preliminary study (selection of L. bulgaricus strains and validation of the nitrogen source)
During the preliminary studies, we assessed the growth of different strains of L. bulgaricus in MRS and the various nitrogen sources. Typically, bacterial growth in a fermentation medium is specific to each strain (strain-dependent), however, we observed that certain strains of L. bulgaricus had significantly higher growth rates (measured by OD610mn) than others (with a significance of P < 0.05). Therefore, the LB6 and S22 L. bulgaricus were chosen for the study based on the growth and acidification rates from the initial study.
Additionally, the different nitrogen sources (X-Seed, N7, N5 and N8) were tested in different concentrations and blends to assess which nitrogens could serve as the best complex nitrogen source combinations to support the growth of the L. bulgaricus strains. Several formulation trials were carried out with the nitrogens, however, the results demonstrated that the nitrogen source; N585 did not have a significant growth impact on the bacteria strains and therefore was not included in the study. Therefore, X-Seed, N7 and N5 were selected for the study based on their superior outcome and were therefore blended with different concentrations for the final optimized GM. The X-Seed was selected based on a recommendation from Ayivi et al. (2022), however, it also had a great growth impact on the L. bulgaricus during the preliminary study. Nevertheless, the selected nitrogen sources have a high protein content of 62.5%, 70% and 71% for N7, N5 and X-Seed respectively compared to N8. The GM was formulated using different blends of nitrogen sources, inspired by Ayivi et al.'s (2022) study design. Numerous trials were conducted with different strains of L. bulgaricus until a formulation was achieved that produced comparable results to MRS.
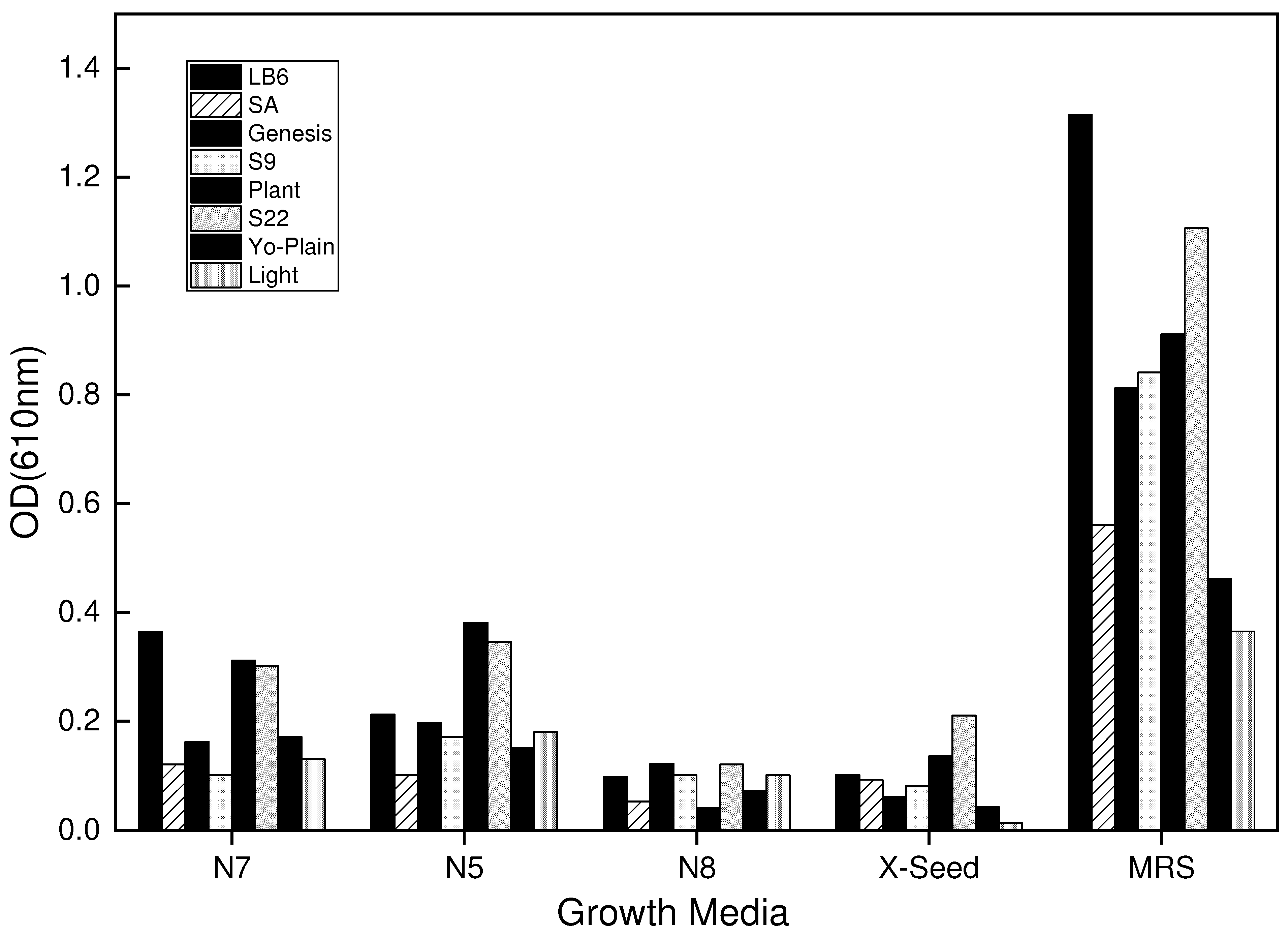
The initial formulation utilized a combination of 0.1% nitrogen sources and various
L. bulgaricus strains (LB6, GS, Plant, S9, SA, S22, YP, and LT). This study assessed the growth rates of different
L. bulgaricus strains in the MRS medium along with various nitrogen sources. Interestingly, certain strains exhibited significantly higher growth rates compared to others. The MRS was included in all preliminary studies to serve as a control/standard medium. The growth of the bacteria was monitored for 14 hours with an incubation temperature of 42°C. Although the optical density (OD610nm) results of the strains in the first growth medium were not promising, MRS (the standard control) displayed good growth, confirming the viability of the strains, as shown in
Figure 1. This observation is in line with the strain-dependent nature of bacterial growth. Based on their superior growth and acidification rates, strains LB6 and S22 were chosen for further study.
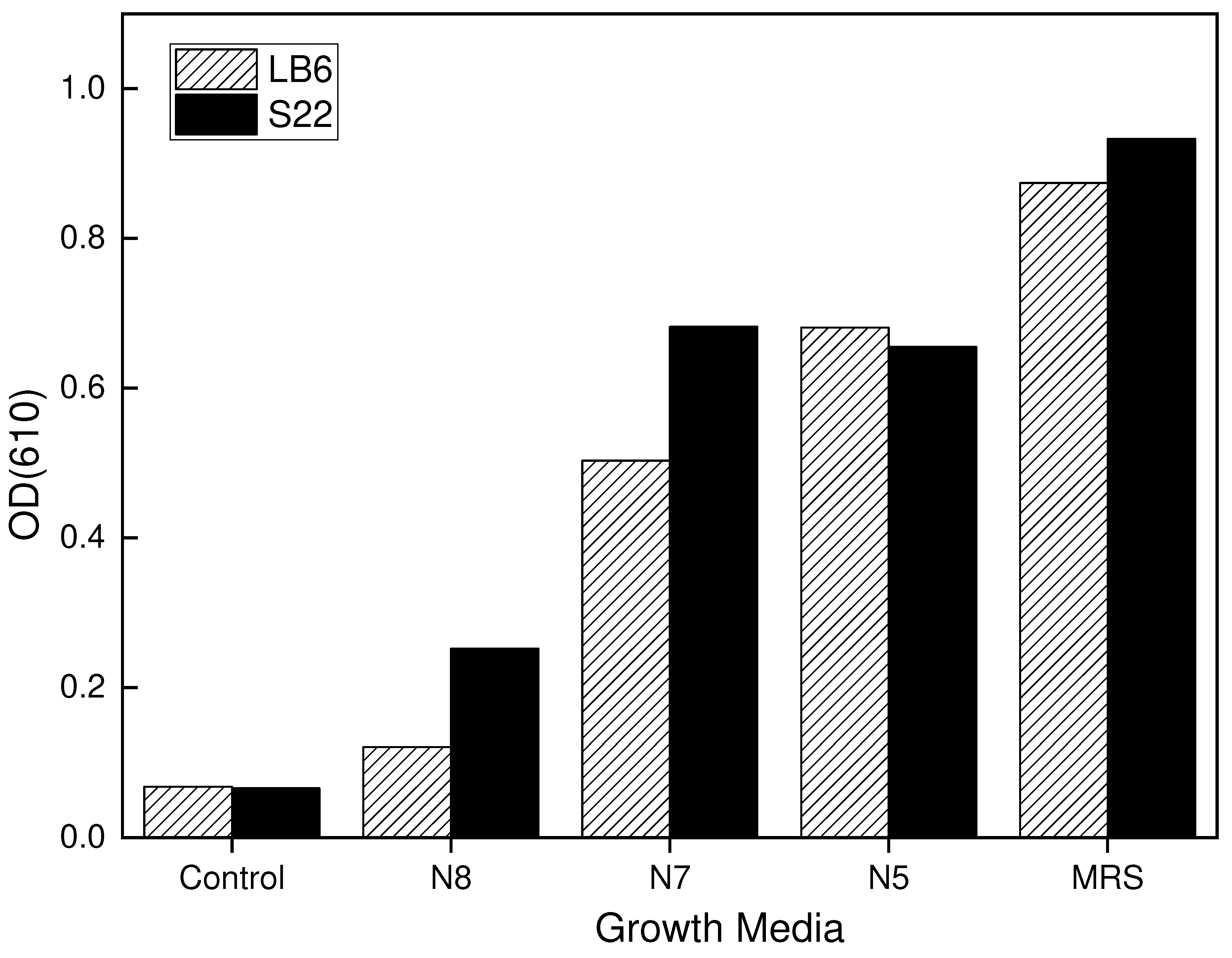
In the second formulation, the basal medium was supplemented with 2g/L of X-Seed combined with 1% of different nitrogen sources and incubated for 12 hours. The growth of the
L. bulgaricus in this formulation was inspiring compared to the first formulation with 0.1% nitrogen sources. This inspiring growth could be attributed to the recommended 2g/L X-Seed, which significantly affected the formulation as deployed by Ayivi et al. (2022). However, the growth in the MRS still superseded the 2
nd formulation and therefore needs to be improved with more nutrients for effective and maximum growth of the
L. bulgaricus strains. Therefore, a third formulation option was proposed that would increase the amount of nitrogen (protein) used.
Figure 2 shows the results for formulation 2, media without nitrogen sources was used as a control while MRS media was used as a standard.
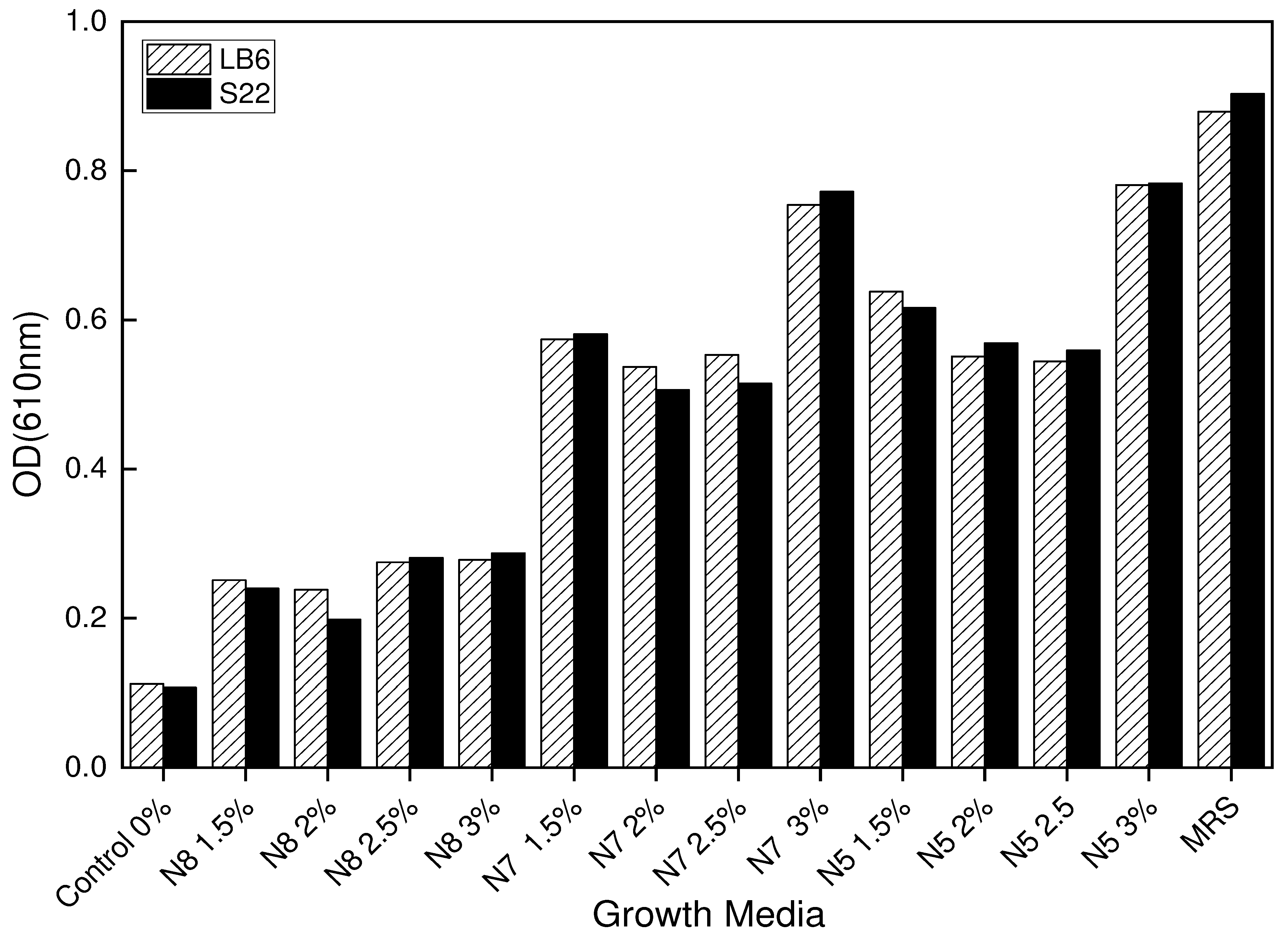
In the third formulation, the X-Seed was again used as a basal medium at a concentration of 2g/L, combined with different blends of nitrogen sources of 1.5%, 2%, 2.5% and 3% of N8, N7 and N5 nitrogen sources media. This combination produced satisfactory results, especially the 1.5% and the 3% leading to the development of the final optimized growth media considering all three 3 formulations (formulations 1, 2 and 3) from the preliminary study. The N8 nitrogen source did not give positive results in all three formulations and therefore was not included in the final optimized complex media formulations.
Figure 3 shows the results for formulation 2.
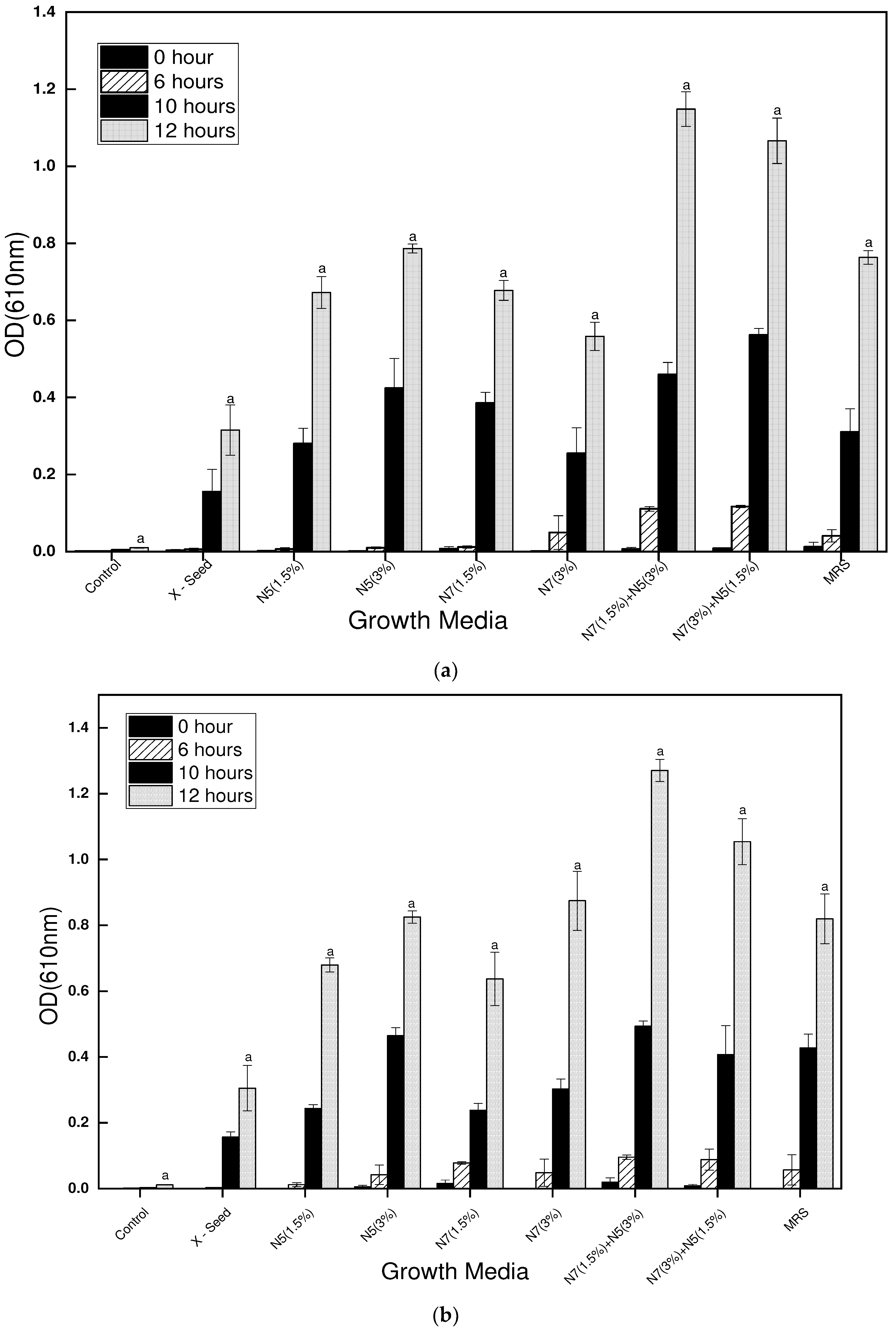
After seeing promising results from previous trials, the study was therefore settled on formulation 4 as the final GM based on preliminary formulation results. This formulation, therefore, combines 1.5% (1.5g/L) and 3% (3g/L) of the verified nitrogen sources (N7 and N5) with a basal medium that includes 2 g/L of X-Seed. Different combinations of nitrogen media complexes were therefore developed, including N7 (1.5%), N7 (3%), N5 (1.5%), N5 (3%), N7+N5 (3/1.5) %, and N7+N5 (1.5/3) %. These optimized media were supplemented with a basal medium of 2g/L of X-Seed. For this fermentation study, MRS served as the standard medium in all cases and the medium without any nitrogen source served as the control. The nitrogen source blends N7+N5 (3/1.5) % contained 3g/L of N780MG yeast extract and 1.5g/L of N532MG yeast extract, while the opposite blend N7+N5 (1.5/3) % consisted of 1.5g/L of 780MG and 3g/L of 532MG.
3.2. Growth and cell density of the bacteria (LB6 and S22) in the optimized growth media
During the 12-hour incubation period at 42°C, the study observed varying growth patterns in the
L. bulgaricus strains (LB6 and S22) used. The strains were confirmed viable in the standard growth medium MRS, as they efficiently utilized its nutrients for their cellular activity. However, the blends of nitrogen sources in the growth media performed better than MRS after 12 hours of fermentation, as demonstrated by the OD610nm.
Figure 4 depicts the growth pattern (OD610mn) of the LB6 and S22 strains in the complex nitrogen-supplemented media during 12 hours of fermentation at 42°C. The results of the OD tests showed that there was no significant difference between the blends of N7 and N5 media when compared to other growth media. The growth of LB6 and S22 in different media indicated that the availability of complex nitrogen in the formulated medium was crucial for microbial growth. The basal medium (X-Seed) alone was not enough to support the metabolic activities of the Lb. bulgaricus strains, leading to poor growth. Therefore, bacterial growth in MRS, N7 and N5 media suggested that the nitrogen content in the media had a substantial impact on growth.
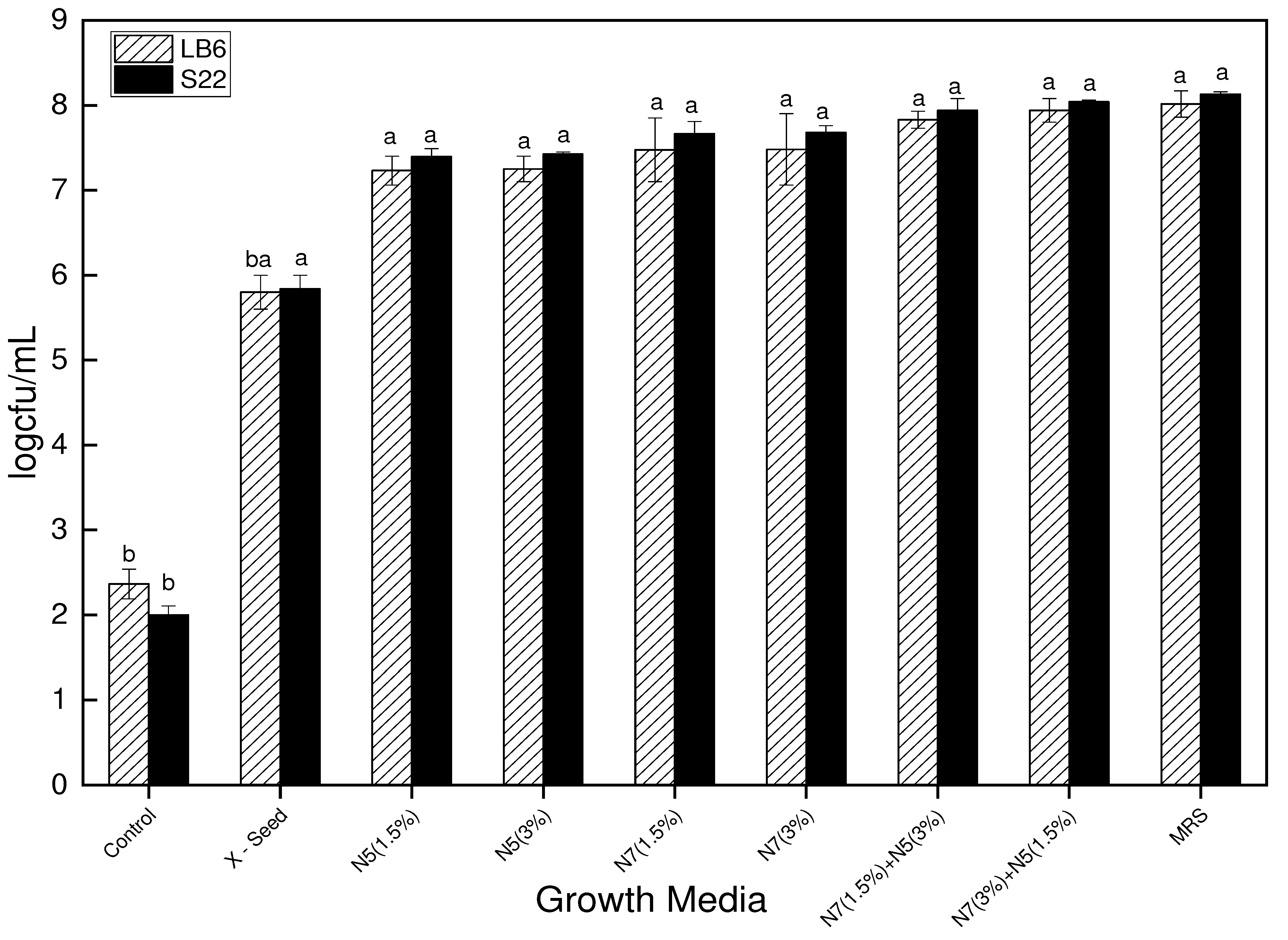
3.3. L. bulgaricus (LB6 and S22) strains enumeration
The results of the bacterial count for the
L. bulgaricus strains indicate that the standard MRS growth media yielded slightly more growth than the complex optimized growth media. However, there was no significant difference in the bacterial count between the standard MRS and the complex optimized growth media (N7 1.5%, N7 3%, N5 1.5%, N5 3% N7+N5 1.5/3% and N7+N5 3/1.5%). Nonetheless, there was a significant difference (P < 0.05) between the control and the other media (MRS and optimized media). The observed trend in the bacterial count could be attributed to the complex media having a slightly lower buffering capacity, causing the strains to grow to some extent and then die due to acid injury during fermentation. Although bacterial growth performance varies by strain, the bacterial counts, particularly from the blends of nitrogen sources N7 and N5, suggest that these media could be used as an alternative to MRS for supporting the growth of Lb. bulgaricus. The initial CFU/mL of the bacteria growth was determined to be 2.8 log CFU/mL.
Figure 5 shows the bacterial counts of the LB6 and S22 strains.stylefix
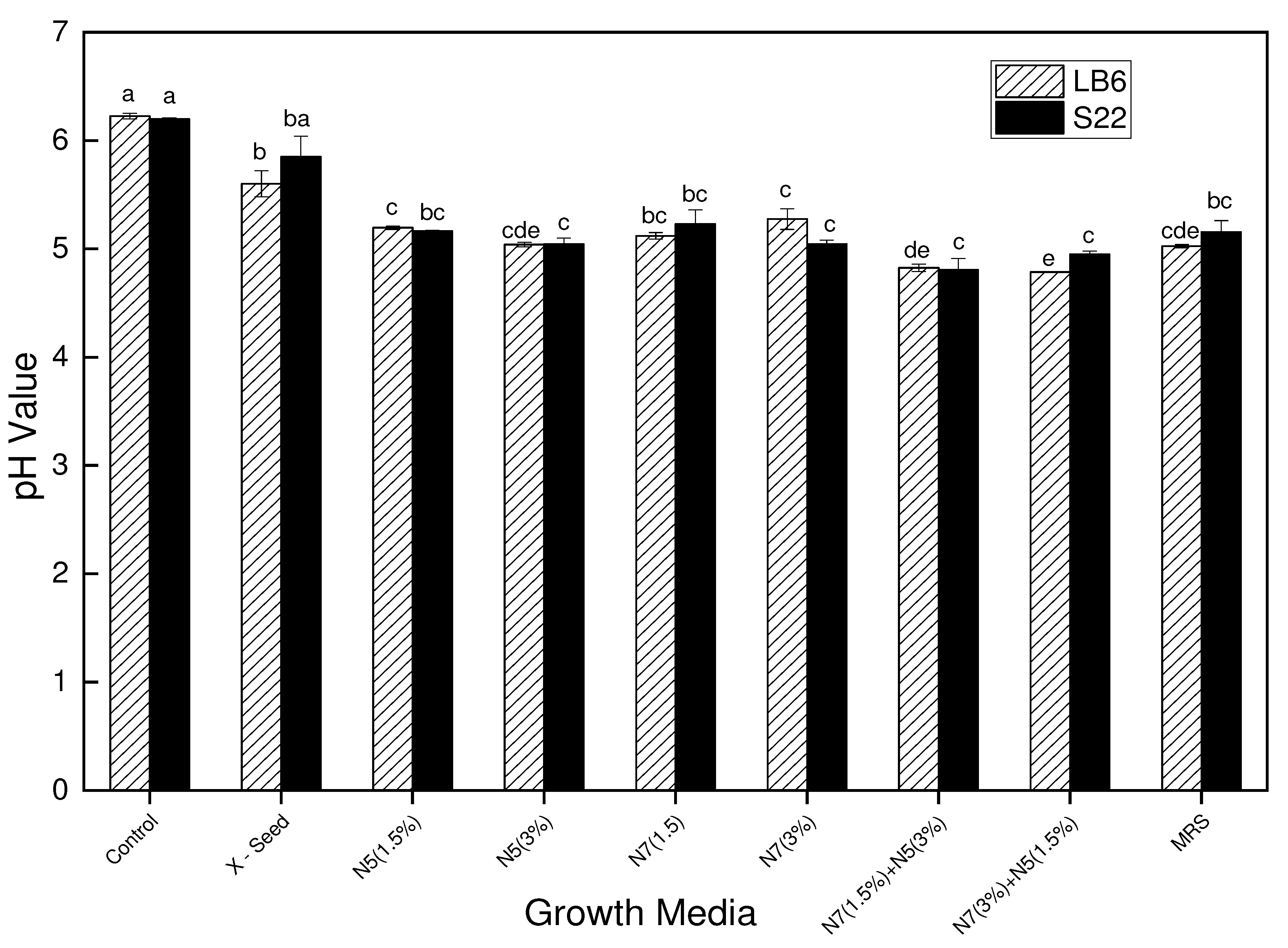
3.4. Changes in the pH values in the different growth media
The initial pH value of all the media was adjusted to 6.5 before the start of the fermentation process using the LB6 and S22 strains. However, at the end of the 12-h fermentation period, there was a rapid decline of the pH values in the various growth media for both strains (LB6 and S22). For LB6, there was a decline in pH value from 6.5 to the range of 4.5 and 6.3 with control having little change in the pH (6.23) and the nitrogen sources blend having a greater pH drop (4.83 and 4.79). This rapid decline in pH was similarly observed for the S22 strain whereby the final pH values after fermentation were in the range of 4.79 to 6.20 for the growth media. Therefore, a significant difference (P < 0.05) was observed for the formulated media with different complex nitrogen sources in comparison to the MRS (standard) and the control medium.
Changes in the pH of the various media are depicted in
Figure 6. The observed differences in pH values could be attributed to the presence of alternative protein sources in the various media, which resulted in a buffering potential of the growth media, especially the complex optimized media which shows low buffering capacity.
4. Discussion
The preliminary study aimed to select suitable strains of L. bulgaricus and identify optimal nitrogen sources to formulate a growth medium that could support their growth and metabolic activities. This study therefore modified the composition of MRS and tested the impact of different nitrogen sources (X-Seed, N7 and N5) in an optimized medium on the growth and performance of L bulgaricus strains. Previous studies have examined the effects of commercial nitrogen sources in various selective growth media to promote the growth of LAB cultures (Ayivi et al., 2022; Atilola et al., 2015). Yeast extracts have been reported as excellent sources of vitamins, amino acids, and peptides that have been shown to enhance the growth of LAB cultures when added to the culture medium (Hayek and Ibrahim, 2013; (Gobbetti, 1998). Additionally, the nitrogen component of the culture medium used to produce dairy starter cultures has been identified as a major factor in achieving high yields and viability of any bacteria culture. This nitrogen source has been noted to contain the main components needed for this LAB viability and growth (Hayek et al., 2019).
The nutritional requirements of different strains of lactobacillus species vary, requiring various optimization techniques to achieve high cell density (Hernández-Macias et al., 2021). In this regard, alternative yeast extracts like X-Seed, N7, and N5 have proven to be highly efficient and promising compared to the standard MRS medium. The use of yeast extract in promoting the growth and viability of LAB cultures has also been validated by Ayivi et al. (2022), who enhanced a modified MRS medium with alternative yeast extract to promote the growth of Lactobacillus bulgaricus. Similarly, Abbasiliasi et al. (2011) also established that yeast extract serves as an excellent nitrogen source, significantly enhancing the production of bacteriocin-like inhibitory substances in Lactobacillus paracasei LAB's developed growth medium. It was therefore observed from the obtained results that, during the studies, using a combination of N7 and N5 (1.5/3) % nitrogen sources in a basal medium containing L. bulgaricus strains produced better results than using the standard MRS medium. The improvement in these results could be attributed to the presence and bioavailability of the high rate of free nucleotides, amino acids, and peptides in the optimized medium (Somani et al., 2020b). The combination of yeast extracts also contributed to the enhanced growth of LAB strains, as the optimized medium without these nitrogen sources showed little or no growth. Therefore, selecting and blending appropriate nutrient-rich yeast extracts for growth medium supplementation can potentially boost the growth and cellular activity of LAB cultures, as this has also been supported in other studies such as Ayivi et al. (2022) and Xiao et al. (2020) who conducted similar research. During the 12-hour incubation at 42°C, growth patterns of strains LB6 and S22 were monitored in various growth media. Notably, the blends of nitrogen sources in the growth media outperformed the standard MRS medium in terms of growth, as demonstrated by optical density (OD610nm) measurements. This suggests that complex nitrogen sources were crucial for supporting microbial growth. The basal medium (X-Seed) alone was insufficient to support metabolic activities, indicating the importance of nitrogen supplementation. Despite variations in growth performance among strains, the nitrogen-rich media, particularly N7 and N5 blends, displayed potential as alternatives to MRS for L. bulgaricus growth. The enhanced superior growth at OD610nm and the cell counts for both S9 and LB6 strains in the optimized medium were also confirmed by Somani et al. (2020b) in their study when similar yeast extracts significantly affected the growth performance of LAB cultures.
Notwithstanding, the cell count of L. bulgaricus strains revealed slightly higher growth in the standard MRS medium compared to the complex optimized growth media. However, this difference was not statistically significant. The bacterial counts indicated that the optimized media, particularly those containing N7 and N5 blends, could serve as viable alternatives to support L. bulgaricus growth. It's noteworthy that while the optimized media showed promising growth, the buffering capacity of these media appeared slightly lower leading to the stains growing at lower pH, and thus potentially contributing to some acid-induced oxidative stress leading to strain mortality during fermentation (Tian et al., 2020). Buffering capacity and pH are thus very critical factors in media development as they ensure a stable pH without comprising the enhanced cellular activities of LAB cultures (Hayek et al., 2019; Hayek and Ibrahim, 2013). This was also confirmed by Azatian et al. (2019) whereby increasing a growth medium's buffering capacity led to higher protein yields in Escherichia coli. The bacterial counts of the L. bulgaricus strains were measured in different growth media. While the standard MRS medium yielded slightly more growth than the complex optimized growth media, there was no significant difference in bacterial count between them. However, all formulated media (optimized GM and MRS) showed a significant difference in bacterial count compared to the control medium without any nitrogen source. This suggests that the complex media supported bacterial growth, although with some variation among the strains.
During the 12-hour fermentation period, there was a significant decrease in pH observed across all growth media for both LB6 and S22 strains. The initial pH of the growth medium was standardized at 6.5. The decrease in pH was more noticeable in the media with complex nitrogen sources (N7 and N5) blends, indicating that more metabolic activities were taking place (Chen et al., 2003; Yang et al., 2018). The decrease in pH levels was a result of the presence of different protein sources in the media, which affected the buffering capacity, and this led to acid injury and a lower count of bacteria (Charalampopoulos et al., 2003) The optimized media experienced a more significant drop in pH levels due to its potentially lower buffering capacity, indicating a higher metabolic activity of L. bulgaricus strains (Mennah-Govela, 2019; Garcia-Gonzalez, 2007).
5. Conclusion
In this study, an efficient nitrogen source growth medium was developed to facilitate the growth and metabolic functions of L. bulgaricus using plant-based nitrogen sources. Our results revealed that the optimized growth media, particularly those containing a combination of N7 and N5 nitrogen sources, showed higher or comparable performance to the MRS medium in terms of bacterial growth and cell density. This would indicate that the selected nitrogen sources, especially when blended, could serve as effective alternatives to MRS for supporting the growth of L. bulgaricus strains. Complex nitrogen sources, rich in free nucleotides, amino acids, and peptides, substantially enhanced the growth of L. bulgaricus strains. Overall, this research contributes to the development of more cost-effective and plant-based growth media for LAB cultures, with relevance for industrial applications. Moreover, our results align with the increasing consumer preference for plant-based products. Additional investigations and optimizations in this same direction could lead to the development of more sustainable and economically viable growth media for probiotic bacteria such as L. bulgaricus, with potential benefits for the food and dairy industries. Further research is also warranted to improve the buffering capacity of the optimized media for higher cell counts.
Funding
This research was funded by Grant Number NC.X308-5-18-170-1 from the National Institute of Food and Agriculture (NIFA). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIFA.
Acknowledgments
Authors would also like to acknowledge the support of the Department of Family and Consumer Sciences and the Agricultural Research Station at North Carolina Agricultural and Technical State University (Greensboro, NC 27411, USA) and the entire research team in the Food Microbiology and Biotechnology Laboratory.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ayivi, R. D., Gyawali, R., Krastanov, A., Aljaloud, S. O., Worku, M., Tahergorabi, R., ... & Ibrahim, S. A. (2020). Lactic acid bacteria: Food safety and human health applications. Dairy, 1(3), 202-232. [CrossRef]
- Quinto, E.J.; Jimenez, P.; Caro, I.; Tejero, J.; Mateo, J.; Girbes, T. Probiotic Lactic Acid Bacteria: A Review. Food Nutr. Sci. 2014, 5, 1765–1775. [CrossRef]
- Hayek, S.A.; Gyawali, R.; Aljaloud, S.O.; Krastanov, A.; Ibrahim, S.A. Cultivation media for lactic acid bacteria used in dairy products. J. Dairy Res. 2019, 86, 490–502. [CrossRef]
- Hayek SA, Ibrahim SA. Current limitations and challenges with lactic acid bacteria: a review. Food and Nutrition Sciences. 2013; 4:73–87. [CrossRef]
- Bintsis, T. (2018). Lactic acid bacteria: their applications in foods. J. Bacteriol. Mycol, 6(2), 89-94. [CrossRef]
- Krastanov, A., Yeboah, P. J., Wijemanna, N. D., Eddin, A. S., Ayivi, R. D., & Ibrahim, S. A. Volatile Aromatic Flavor Compounds in Yogurt: A Review. In Dairy Processing - From Basics to Advances, 2023, IntechOpen, London, United Kingdom.
- Axelsson, L. (2004). Lactic acid bacteria: Classification and physiology. Food Science and Technology-New York-Marcel Dekker-, 139, 1-66.
- Bourdichon F, Berger B, Casaregola S, et al. A Safety assessment of microbial food cultures with a history of use in fermented dairy products. Bullet IDF. 2012; 455:2–12.
- Yeboah, P. J., Wijemanna, N. D., Eddin, A. S., Williams, L. L., Ibrahim, S. A. Lactic Acid Bacteria: Review on the Potential Delivery System as an Effective Probiotic. In Dairy Processing - From Basics to Advances, 2023, IntechOpen, London, United Kingdom.
- Ayivi, R. D., Ibrahim, S. A., Krastanov, A., Somani, A., & Siddiqui, S. A. (2022). The impact of alternative nitrogen sources on the growth and viability of lactobacillus delbrueckii ssp. bulgaricus. Journal of Dairy Science, 105(10), 7986-7997. [CrossRef]
- Gyawali, R., Oyeniran, A., Zimmerman, T., Aljaloud, S. O., Krastanov, A., & Ibrahim, S. A. (2020). A comparative study of extraction techniques for maximum recovery of β-galactosidase from the yogurt bacterium Lactobacillus delbrueckii ssp. bulgaricus. Journal of Dairy Research, 87(1), 123-126.
- Aponte, M., Murru, N., & Shoukat, M. (2020). Therapeutic, prophylactic, and functional use of probiotics: a current perspective. Frontiers in Microbiology, 11, 562048. [CrossRef]
- Ibrahim, S. A., Yeboah, P. J., Ayivi, R. D., Eddin, A. S., Wijemanna, N. D., Paidari, S., & Bakhshayesh, R. V. (2023). A review and comparative perspective on health benefits of probiotic and fermented foods. International Journal of Food Science & Technology, n/a . [CrossRef]
- Sieuwerts, S. (2016). Microbial interactions in the yoghurt consortium: current status and product implications. SOJ Microbiology & Infectious Diseases, 4(2), 1-5. [CrossRef]
- Atilola, O. A., Gyawali, R., Aljaloud, S. O., & Ibrahim, S. A. (2015). Use of phytone peptone to optimize growth and cell density of Lactobacillus reuteri. Foods, 4(3), 318-327. [CrossRef]
- Manzoor, A., Qazi, J. I., Mukhtar, H., & Rasool, A. (2017). Significantly enhanced biomass production of a novel bio-therapeutic strain Lactobacillus plantarum (AS-14) by developing a low-cost media cultivation strategy. Journal of Biological Engineering, 11(1), 1-10. [CrossRef]
- Ayad, A. A., Gad El-Rab, D. A., Ibrahim, S. A., & Williams, L. L. (2020). Nitrogen sources effect Lactobacillus reuteri growth and performance cultivated in date palm (Phoenix dactylifera L.) by-products. Fermentation, 6(3), 64.
- Oyeniran, A., Ibrahim, S. A., Gyawali, R., Tahergorabi, R., Zimmerman, T., & Krastanov, A. (2020). A modified reinforced clostridial medium for the isolation and enumeration of Lactobacillus delbrueckii ssp. bulgaricus in a mixed culture. Journal of Dairy Science, 103(6), 5030-5042. [CrossRef]
- Gobbetti, M. (1998). The sourdough microflora: Interactions of lactic acid bacteria and yeasts. Trends in Food Science & Technology, 9(7), 267-274. [CrossRef]
- Hernández-Macias, Salvador, Oriol Comas-Basté, Anna Jofré, Sara Bover-Cid, M. Luz Latorre-Moratalla, and M. Carmen Vidal-Carou. "Growth-promoting effect of cava lees on lactic acid bacteria strains: A potential revalorization strategy of a winery by-product." Foods 10, no. 7 (2021): 1636. [CrossRef]
- Abbasiliasi, S., R. N. Ramanan, T. A. T. Ibrahim, S. Mustafa, R. Mo hamad, H. H. M. Daud, and A. B. Ariff. 2011. Effect of medium composition and culture condition on the production of bacteriocin-like inhibitory substances (BLIS) by Lactobacillus paracasei LA07, a strain isolated from Buclu. Biotechnol. Equip. 25:2652-2657.
- Somani, S. A., B. Boran, and K. Bekers. 2020b. Impact of nucleotides on L. bulgaricus growth, viability and performance. Ohly vVhite Paper, Ohly GmbH.
- Xiao, S., M. K. Bekers, and M. J. van der Werf. 2020. Using DoE to optimize yeast extract composition for lactic acid bacteria. Ohly White Paper, Ohly GmbH.
- Tian, Tiantian, Dianhui Wu, Chan-Tat Ng, Hua Yang, Junyong Sun, Jianming Liu, and Jian Lu. "A multiple-step strategy for screening Saccharomyces cerevisiae strains with improved acid tolerance and aroma profiles." Applied microbiology and biotechnology 104 (2020): 3097-3107. [CrossRef]
- Azatian, S. B., . Kaur, and M. P. Latham. 2019. Increasing the buffering capacity of minimal media leads to higher protein yield. J. Biomol. NMR 73:11-17.
- Chen, Kuo-Cheng, Jane-Yii Wu, Dar-Jen Liou, and Sz-Chwun John Hwang. "Decolorization of the textile dyes by newly isolated bacterial strains." Journal of Biotechnology 101, no. 1 (2003): 57-68. [CrossRef]
- Yang, En, Lihua Fan, Jinping Yan, Yueming Jiang, Craig Doucette, Sherry Fillmore, and Bradley Walker. "Influence of culture media, pH and temperature on growth and bacteriocin production of bacteriocinogenic lactic acid bacteria." AMB express 8, no. 1 (2018): 1-14. [CrossRef]
- Charalampopoulos, D., Pandiella, S. S., & Webb, C. (2003). Evaluation of the effect of malt, wheat and barley extracts on the viability of potentially probiotic lactic acid bacteria under acidic conditions. International Journal of Food Microbiology, 82(2), 133-141. [CrossRef]
- Mennah-Govela, Yamile A., R. Paul Singh, and Gail M. Bornhorst. "Buffering capacity of protein-based model food systems in the context of gastric digestion." Food & Function 10, no. 9 (2019): 6074-6087. [CrossRef]
- Garcia-Gonzalez, Linsey, A. H. Geeraerd, Sara Spilimbergo, K. Elst, L. Van Ginneken, Johan Debevere, J. F. Van Impe, and Frank Devlieghere. "High-pressure carbon dioxide inactivation of microorganisms in foods: the past, the present and the future." International journal of food microbiology 117, no. 1 (2007): 1-28.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).