Submitted:
22 September 2023
Posted:
25 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and discussion
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Determination of size, charge, and colloidal stability of formed complexes
4.3. Isothermal titration calorimetry
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aranganathan, L.; Radhika Rajasree, S.R.; Govindaraju, K.; Sivarathna Kumar, S.; Gayathri, S.; Remya, R.R.; Suman, T.Y. Spectral and microscopic analysis of fulvic acids isolated from marine fish waste and sugarcane bagasse co-compost. Biocatal. Agric. Biotechnol. 2020, 29, 101762. [Google Scholar] [CrossRef]
- Liu, S.; Benedetti, M.F.; Han, W.; Korshin, G.V. Comparison of the properties of standard soil and aquatic fulvic and humic acids based on the data of differential absorbance and fluorescence spectroscopy. Chemosphere 2020, 261, 128189. [Google Scholar] [CrossRef] [PubMed]
- Laborda, F.; Bolea, E.; Gorriz, M.P.; Martın-Ruiz, M.P.; Ruiz-Beguerıa, S.; Castillo, J.R. A speciation methodology to study the contributions of humic-like and fulvic-like acids to the mobilization of metals from compost using size exclusion chromatography–ultraviolet absorption–inductively coupled plasma mass spectrometry and deconvolution analysis. Anal. Chim. Acta 2008, 606, 1–8. [Google Scholar] [PubMed]
- Bai, Y.C.; Wu, F.C.; Liu, C.Q.; Li, W.; Guo, J.Y.; Fu, P.Q.; Xing, B.S.; Zheng, J. Ultraviolet absorbance titration for determining stability constants of humic substances with Cu(II) and Hg(II). Anal. Chim. Acta 2008, 616, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Takahashi, Z. Effect of complexation with humic substances on diffusion of metal ions in water. Chemosphere 2008, 73, 1272–1278. [Google Scholar] [CrossRef]
- Evangelou, V.P.; Marsi, M.; Chappell, M.A. Potentiometric–spectroscopic evaluation of metal-ion complexes by humic fractions extracted from corn tissue. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2002, 58, 2159–2175. [Google Scholar] [CrossRef]
- Li, J.; Ding, Y.; Shi, Z. Binding properties of fulvic acid before and after fractionation on ferrihydrite: Effects of phosphate. ACS Earth Space Chem. 2021, 5, 1535–1543. [Google Scholar] [CrossRef]
- Gondar, D.; López, R.; Fiol, S.; Antelo, J.M.; Arce, F. Cadmium, lead, and copper binding to humic acid and fulvic acid extracted from an ombrotrophic peat bog. Geoderma 2006, 135, 196–203. [Google Scholar] [CrossRef]
- Gondar, D.; Iglesias, A.; López, R.; Fiol, S.; Antelo, J.M.; Arce, F. Copper binding by peat fulvic and humic acids extracted from two horizons of an ombrotrophic peat bog. Chemosphere 2006, 63, 82–88. [Google Scholar] [CrossRef]
- Christl, I,; Milne, C. J.; Kinniburgh, D.G.; Kretzschmar, R. Relating ion binding by fulvic and humic acids to chemical composition and molecular size. 2. Metal binding. Environ. Sci. Technol. 2001, 35, 2512–2517. [Google Scholar] [CrossRef]
- Garcia-Mina, J.M. Stability, solubility and maximum metal binding capacity in metal–humic complexes involving humic substances extracted from peat and organic compost. Org. Geochem. 2006, 37, 1960–1972. [Google Scholar] [CrossRef]
- Bertoli, A.C.; Garcia, J.S.; Trevisan, M.G.; Ramalho, T.C.; Freitas, M.P. Interactions fulvate-metal (Zn2+, Cu2+ and Fe2+): theoretical investigation of thermodynamic, structural and spectroscopic properties. BioMetals 2016, 29, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Nantis, E.A.; Carper, W.R. Molecular structure of divalent metal ion–fulvic acid complexes. J. Mol. Struct. 1998, 423, 203–212. [Google Scholar] [CrossRef]
- Sheng, G.; Li, J.; Shao, D.; Hu, J.; Chen, C.; Chen, Y.; Wang, X. Adsorption of copper(II) on multiwalled carbon nanotubes in the absence and presence of humic or fulvic acids. J. Hazadr. Mater. 2010, 178, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Manceau, A.; Matynia, A. The nature of Cu bonding to natural organic matter. Geochim. Cosmochim. Acta 2010, 74, 2556–2580. [Google Scholar] [CrossRef]
- Shvartseva, O.; Skripkina, T.; Gaskova, O.; Podgorbunskikh, E. Modification of natural peat for removal of copper ions from aqueous solutions. Water 2022, 14, 2114. [Google Scholar] [CrossRef]
- Fomin, V.N.; Gogol, D.B.; Rozhkovoy, I.E.; Ponomarev, D.L. Quantum chemical and thermodynamic calculations of fulvic and humic copper complexes in reactions of malachite and azurite formation. J. Appl. Geochem. 2017, 79, 9–16. [Google Scholar] [CrossRef]
- Klučáková, M.; Pelikán, P.; Lapčík, L.; Lapčíková, B.; Kučerík, J.; Kaláb, M. Study of structure and properties of humic and fulvic acids. I. Properties and reactivity of humic and fulvic acids. J. Polym. Mater. 2000, 17, 337–356. [Google Scholar]
- Bryan, N.D.; Hesketh, N.; Livens, F.R.; Tipping, E.; M. N. Jones, M.N. Metal ion–humic substance interaction. A thermodynamic study. J. Chem. Soc., Faraday Trans. 1998, 94, 95–100. [Google Scholar] [CrossRef]
- Antonelli, M.L.; Calace, N.; Fortini, C.; Petronio, B.M.; Pietroletti, M. Complexing capacity of different molecular weight fractions of sedimentary humic substances. Anal. Lett. 2001, 34, 989–1002. [Google Scholar] [CrossRef]
- Antonelli, M.L.; Calace, N.; Centioli, D.; Petronio, B.M.; Pietroletti, M.; Pusceddu, P. Calorimetric investigation of complex formation of some humic compounds. J. Therm. Anal. Calorim. 2002, 70, 291–297. [Google Scholar] [CrossRef]
- Qi, Y.; Zhu, J.; Fu, Q.; Hu, H.; Huang, Q.; Violante, A. Sorption of Cu by organic matter from the decomposition of rice straw. J Soils Sediments 2016, 16, 2203–2210. [Google Scholar] [CrossRef]
- Sheng, G.P.; Xu, J.; Luo, H.W.; Li, W.W.; Li, W.H.; Yu, H.Q.; Xie, Z.; Wei, S.Q.; Hu, F.C. Thermodynamic analysis on the binding of heavy metals onto extracellular polymeric substances (EPS) of activated sludge. Water Res. 2013, 47, 607–614. [Google Scholar] [CrossRef]
- Taraba, B. Immobilisation of lead(II) ions from aqueous solutions on natural coal. J. Therm. Anal. Calorim. 2013, 112, 1559–1563. [Google Scholar] [CrossRef]
- Du, H.H.; Lin, Y.P.; Chen, W.L.; Cai, P.; Rong, X.M.; Shi, Z. H.; Huang, Q.Y. Copper adsorption on composites of goethite, cells of Pseudomonas putida and humic acid. Eur. J. Soil Sci. 2017, 68, 514–523. [Google Scholar] [CrossRef]
- Kimuro, S.; Kirishima, A.; Kitatsuji, Y.; Miyakawa, K.; Akiyama, D.; Sato, N. Thermodynamic study of the complexation of humic acid by calorimetry. J. Chem. Thermodyn. 2019, 132, 352–362. [Google Scholar] [CrossRef]
- Hilal, N.; Al-Abri, M.; Moran, A.; Al-Hinai, H. Effects of heavy metals and polyelectrolytes in humic substance coagulation under saline conditions. Desalination 2008, 220, 85–95. [Google Scholar] [CrossRef]
- Zhang, Z.; Buffle, J.; Alemani, D. Metal flux and dynamic speciation at (bio)interfaces. Part II: Evaluation and compilation of physicochemical parameters for complexes with particles and aggregates. Environ. Sci. Technol. 2007, 41, 7621–7631. [Google Scholar] [CrossRef]
- Baigorri, R.; Fuentes, M.; Gonzalez-Gaitano, G.; Garcia-Mina, J.M.; Almendros, G.; Gonzalez-Vila, F.J. Complementary multianalytical approach to study the distinctive structural features of the main humic fractions in solution: Gray humic acid, brown humic acid, and fulvic acid. J. Agric. Food Chem. 2009, 57, 3266–3272. [Google Scholar] [CrossRef]
- Adusei-Gyamfi, J.; Ouddane, B.; Rietveld, L.; Cornard, J.P.; Criquet, J. Natural organic matter-cations complexation and its impact on water treatment: A critical review. Water Res. 2019, 160, 130–147. [Google Scholar] [CrossRef]
- Guo, J.; Ma, J. AFM study on the sorbed NOM and its fractions isolated from River Songhua. Water Res. 2006, 40, 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- Shiu, R.F.; Lee, C.L.; Chin, W.C. Reduction in the exchange of coastal dissolved organic matter and microgels by inputs of extra riverine organic matter. Water Res. 2018, 131, 161–166. [Google Scholar] [PubMed]
- Zhou, Z.; Zhang, C.; Xi, M.; Ma, H.; Jia, H. Multi-scale modeling of natural organic matter–heavy metal cations interactions: Aggregation and stabilization mechanisms. Water Res. 2023, 238, 120007. [Google Scholar] [PubMed]
- Klučáková, M. Size and charge evaluation of standard humic and fulvic acids as crucial factors to determine their environmental behavior and impact. Front. Chem. 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Klučáková, M.; Věžníková, K. The role of concentration and solvent character in the molecular organization of humic acids. Molecules 2016, 21, 1410. [Google Scholar] [CrossRef]
- Klučáková, M.; Věžníková, K. Micro-organization of humic acids in aqueous solutions. J. Mol. Struct. 2017, 1144, 33–40. [Google Scholar] [CrossRef]
- Duan, J.; Wang, J.; Graham, N.; Wilson, F. Coagulation of humic acid by aluminium sulphate in saline water conditions. Desalination 2002, 150, 1–14. [Google Scholar]
- Weng, L.; Fest, E.P.M.V; Fillius, J.; Temminghoff, E.J.M.; Van Riemsdijk, W.H. Transport of humic and fulvic acids in relation to metal mobility in a copper-contaminated acid sandy soil. Environ. Sci. Technol. 2002, 36, 1699–1704. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Y.; Liu, L.; He, Z.; Giesy, J.P.; Bai, Y.; Sun, F.; Wu, F. Cation-induced coagulation of aquatic plant-derived dissolved organic matter: Investigation by EEM-PARAFAC and FT-IR spectroscopy. Environ. Pollut. 2018, 234, 726–734. [Google Scholar]
- Tamamura, S.; Ohashi, R.; Nagao, S.; Yamamoto, M.; Mizuno, M. Molecular-size-distribution-dependent aggregation of humic substances by Na(I), Ag(I), Ca(II), and Eu(III). Colloid. Surface. A 2013, 434, 9–15. [Google Scholar] [CrossRef]
- Christl, I. , Kretzschmar, R. C-1s NEXAFS spectroscopy reveals chemical fractionation of humic acid by cation-induced coagulation. Environ. Sci. Technol. 2007, 41, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Klučáková, M.; Kalina, M. Composition, particle size, charge and colloidal stability of pH-fractionated humic acids. J. Soils Sediments 2015, 15, 1900–1908. [Google Scholar] [CrossRef]
- Klučáková, M. Characterization of pH-fractionated humic acids with respect to their dissociation behaviour. Environ. Sci. Pollut. Res. 2016, 23, 7722–7731. [Google Scholar]
- Pranzas, P.K.; Willumeit, R.; Gehrke, R.; Thieme, J.; Knöchel, A. Characterisation of structure and aggregation processes of aquatic humic substances using small-angle scattering and X-ray microscopy. Anal. Bioanal. Chem. 2003, 376, 618–625. [Google Scholar] [CrossRef]
- Klučáková, M.; Kalina, M. Diffusivity of Cu(II) ions in humic gels - influence of reactive functional groups of humic acids. Colloid. Surface. A 2015, 483, 162–170. [Google Scholar]
- Klučáková, M. Complexation of metal ions with solid humic acids, humic colloidal solutions, and humic hydrogel. Environ. Eng. Sci. 2014, 31, 612–620. [Google Scholar]
- Hume, S.; Caron, F.; Siemann, S. Binding of Cu, Co, and Cs to fluorescent components of natural organic matter (NOM) from three contrasting sites. Environ. Sci. Pollut. Res. 2018, 25, 20141–20153. [Google Scholar]
- Guo, X.; Zhu, N.; Chen, L.; Yuan, D.; He, L. Characterizing the fluorescent properties and copper complexation of dissolved organic matter in saline-alkali soils using fluorescence excitation-emission matrix and parallel factor analysis. J. Soils Sediments 2015, 15, 1473–1482. [Google Scholar]
- Yan, M.; Korshin, G.V. Comparative examination of effects of binding of different metals on chromophores of dissolved organic matter. Environ. Sci. Technol. 2014, 48, 3177–3185. [Google Scholar] [CrossRef]
- Klučáková, M.; Pekař, M. Study of structure and properties of humic and fulvic acids. III. Study of complexation of Cu2+ ions with humic acid in cols. J. Polym. Mater. 2003, 20, 145–154. [Google Scholar]
- Liu, S.; Benedetti, M.F.; Han, W.; Korshin, G.V. Comparison of the properties of standard soil and aquatic fulvic and humic acids based on the data of differential absorbance and fluorescence spectroscopy. Chemosphere 2020, 261, 128189. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Fasfous, I.I.; Murimboh, J.; Chakrabarty, C.L. Simultaneous determination of speciation parameters of Cu, Pb, Cd and Zn in model solutions of Suwannee River fulvic acid by pseudopolarography. Anal. Bioanal. Chem. 2007, 388, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Jaffé, R. Characterizing the interactions between trace metals and dissolved organic matter using excitation-emission matrix and parallel factor analysis. Environ. Sci. Technol. 2008, 42, 7374–7379. [Google Scholar] [CrossRef]
- Klučáková, M.; Pekař, M. New model for equilibrium sorption of metal ions on solid humic acids. Colloid. Surface. A 2006, 286, 126–133. [Google Scholar] [CrossRef]
- Riedel, T.; Biester, H.; Dittmar, T. Molecular fractionation of dissolved organic matter with metal salts. Environ. Sci. Technol. 2012, 46, 4419–4426. [Google Scholar] [CrossRef] [PubMed]
- Milne, J.C.; Kinninburgh, D.G.; Van Riemsdijk, W.H.; Tipping, E. Generic NICA−Donnan model parameters for metal-ion binding by humic substances. Environ. Sci. Technol. 2003, 37, 958–971. [Google Scholar] [CrossRef]
- Martyniuk, H.; Więckowska, J. Adsorption of metal ions on humic acids extracted from brown coals. Fuel Process. Technol. 2003, 84, 23–36. [Google Scholar] [CrossRef]
- Dinu, M.I. Interaction between metal ions in waters with humic acids in gley–podzolic soils. Geochem. Int. 2015, 53, 265–276. [Google Scholar] [CrossRef]
- Rey-Castro, C.; Mongin, S.; Huidobro, C.; David, C.; Salvador, J.; Garcés, J.L.; Galceran, J.; Mas, F.; Puy, J. Effective distribution for the binding of metal ions to a generic fulvic acid in natural waters. Environ. Sci. Technol. 2009, 43, 7184–7191. [Google Scholar] [CrossRef]
- Wu, Y.; Hendershot, W.H. Effect of calcium and pH on copper binding and rhizotoxicity to pea (Pisum sativum L.) root: Empirical relationships and modeling. Arch. Environ. Contam. Toxicol. 2010, 59, 109–119. [Google Scholar] [CrossRef]
- Christl, I.; Metzger, A.; Heidmann, I.; Kretzschmar, R. Effect of humic and fulvic acid concentrations and ionic strength on copper and lead binding. Environ. Sci. Technol. 2005, 39, 5319–5326. [Google Scholar] [CrossRef]
- Kogut, M.B.; Voelker, B.M. Strong copper-binding behavior of terrestrial humic substances in seawater. Environ. Sci. Technol. 2001, 35, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yan, M.; Korshin, G. Effects of calcium on the chromophores of dissolved organic matter and their interactions with copper. Water. Res. 2015, 81, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.M. Handbook of Chemistry and Physics, 93rd ed.; CRC Press, Boca Raton, 2012.
- Yan, M.; Dryer, D.; Korshin, G.V.; Benedetti, M.F. In situ study of binding of copper by fulvic acid: Comparison of differential absorbance data and model predictions. Water Res. 2013, 47, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, J.D.; Perdue, E.M. Proton-binding study of standard and reference fulvic acids, humic acids, and natural organic matter. Geochim. Cosmochim. Acta 2003, 67, 85–96. [Google Scholar] [CrossRef]
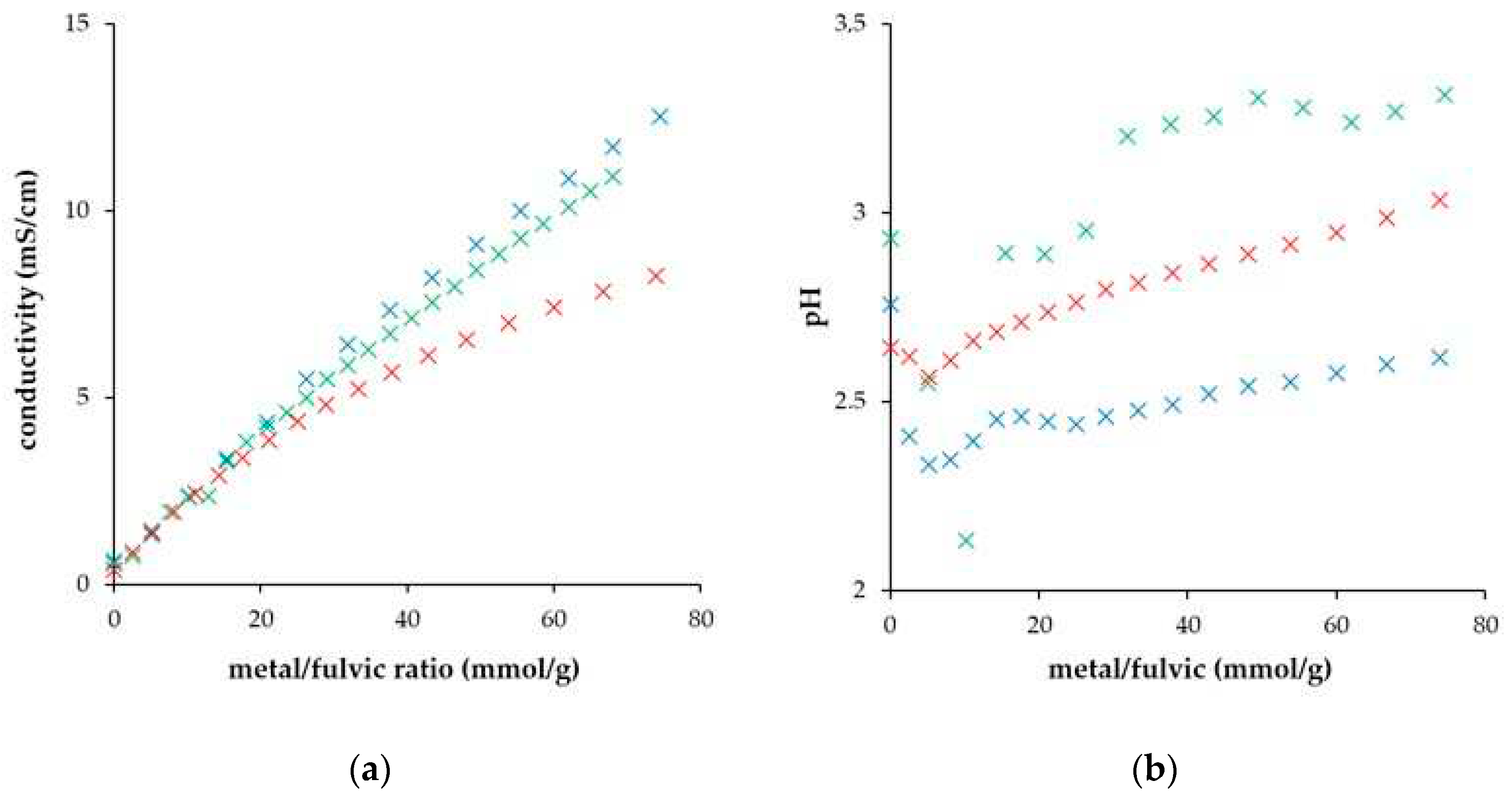
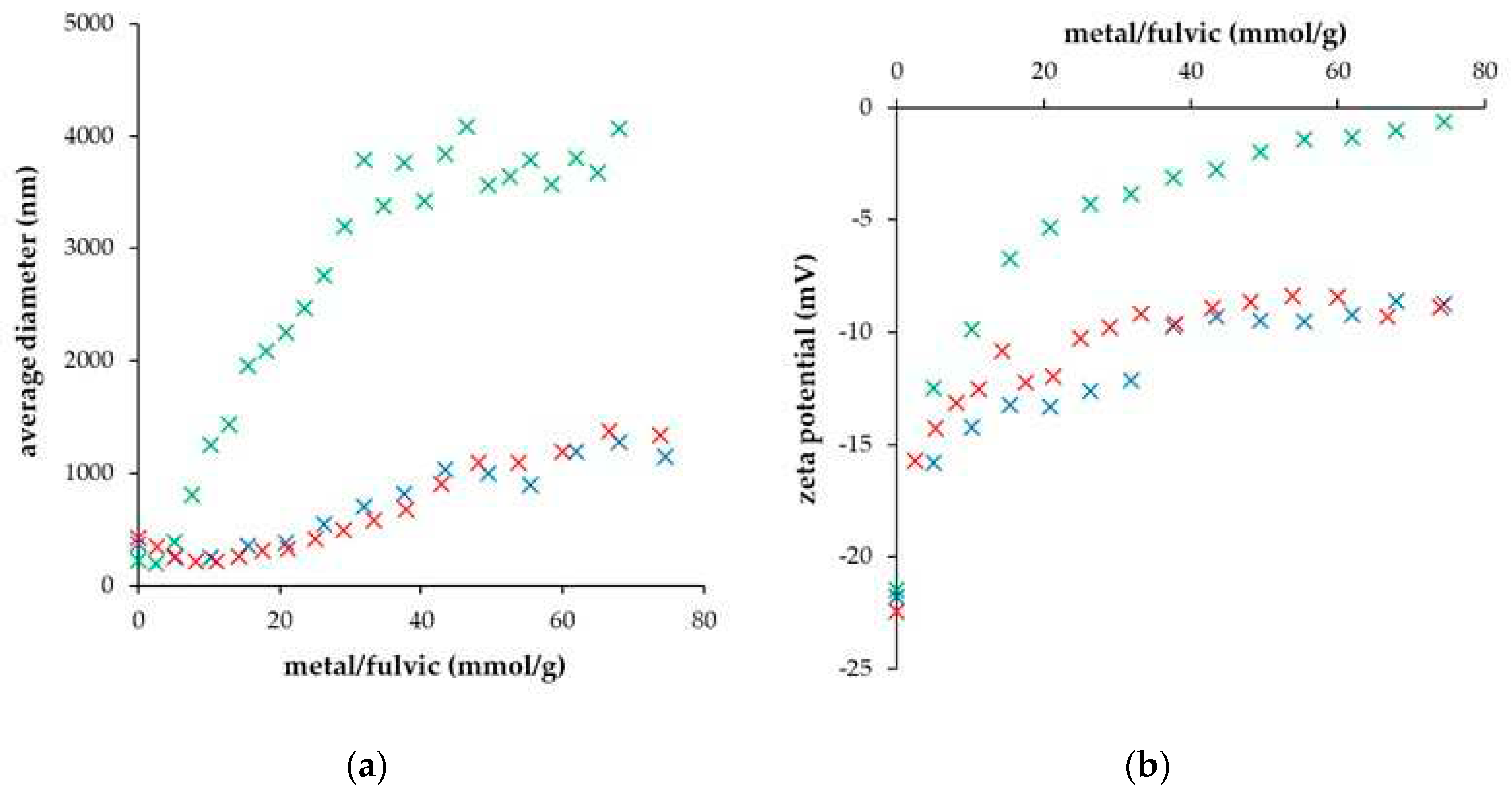
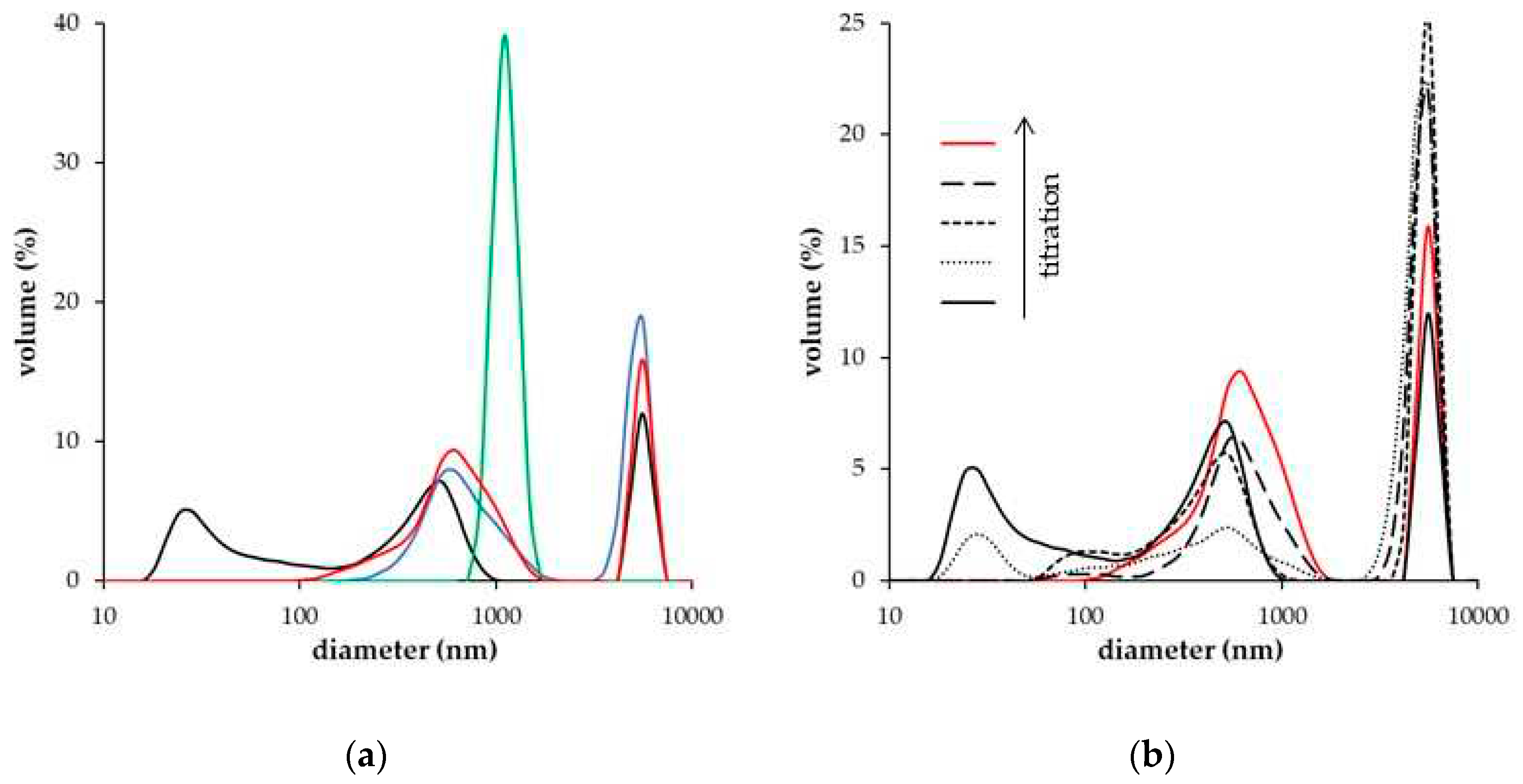

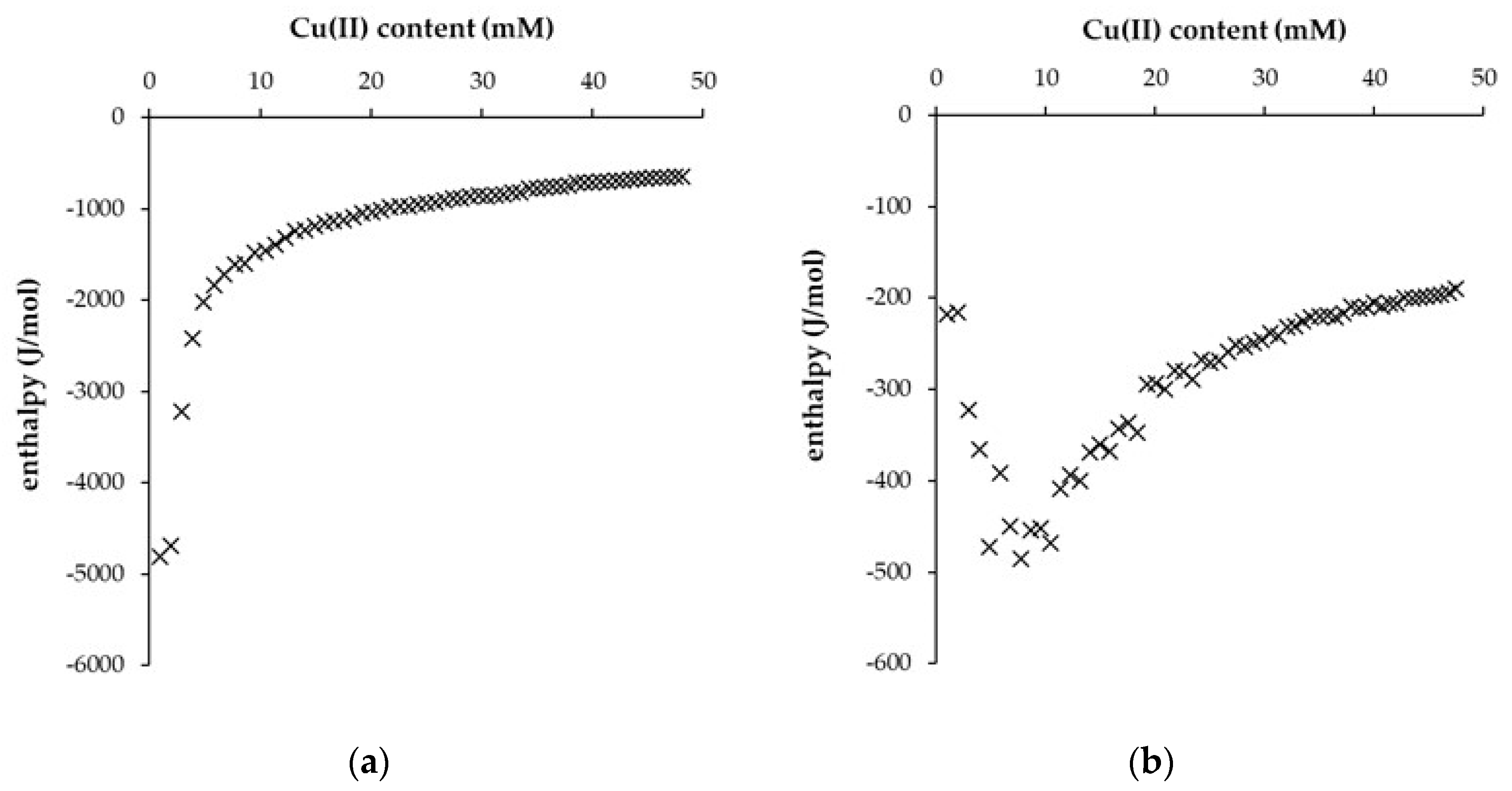
| DH° (J/mol) | DG° (kJ/mol) | DS° (J/mol.K) | |
| calcium | 524 ± 46 | -16.3 ± 0.1 | 56.3 ± 0.4 |
| copper | 687 ± 30 | -15.0 ± 0.3 | 52.4 ± 0.9 |
| magnesium | 672 ± 47 | -17.5 ± 0.3 | 62.4 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





