1. Introduction
During the initial stages and subsequent months, a flurry of publications emerged, with some likely being rushed, thereby overlooking the fundamental principles of scientific work, ethics, and research. During those months, the scientific community had an urgent need to understand and share what was happening with patients infected with SARS-CoV-2, and information was disseminated openly and rapidly as a top priority. This research was conducted meticulously, comprehensively, and in a multidisciplinary manner, encompassing various aspects of cutaneous manifestations in severely hospitalized COVID-19 patients with generalized rashes during the first wave. This encompassed histological examinations, immunohistochemical skin studies, blood tests, a comprehensive assessment of interleukin profiles, and long-term patient follow-up.
Reviews published about cutaneous manifestations in COVID-19 patients during 2020 mainly concur on categorizing outpatient manifestations into four groups[
1] , with a varying prevalence. These include inflammatory manifestation, exanthemas and urticarias, and vasculophatic skin lesion, such as chilblains and vasculitis [
2,
3,
4]. Nevertheless, the cutaneous manifestations observed in hospitalized COVID-19 patients likely exhibit a different clinical presentation with an estimated 11% prevalence[
5].
There have been publishedfew reports of hospitalized patients with severe COVID-19 infection and cutaneous reactions[
5,
6]. Limitations have been indentified in the study of dermatologic COVID-19 manifestation in outpatients, as also noted by other authors[
1,
3,
7].
These limitations included the selection of outpatients based solely on photographs without accompanying medical histories, the absence of confirmed SARS-Cov-2 infection, the lack of a cutaneous biopsy and eventually, the absence of an evaluation of a dermatologist. Rigorous studies were deemed necessary. However, perhaps, the most critical oversight, was the unconsideration of drug reaction as cause when describing exanthemas, being exanthemas the most reporte[
8,
9]. The occurrent of cutaneous drug reaction in COVID-19 patient was rarely reported in most published series, despite there patients are under a ,with high-risk of drug-induced exanthema, due to multiple drug treatment and viral infection. Additionally, patients were often classified without undergoing histological examination [
10,
11]. In the case of hospitalized patients, manyt of these limitations were not presented, as these patients were evaluated in a medical setting that allowed for complete examination and assessmentss by multidisciplinary medical team.
Despite the high number of reports about skin lesions in COVID-19 cutaneous manifestations were infrequent, with wide variability in estimation and in clinical presentation [
1,
11,
12]. Interestlingly, cutaneous manifestation decreased in sunsequentwaves of the pandemic, suggesting changes that occurred in the intial outbreak in relation to the cutaneous expression of SARS-Cov-2 infection[
13].
The exact mechanism underlying the expression of SARS-Cov-2 infection on the skin is unknown. Various hypothesis have been suggested, with systemic inflammation and the cytokine release being considered the main causes of cutaneous manifestations in COVID-19 patients[
7]. However other factors, as skin vascular activation should be considered depending on the type of cutaneous manifestation[
2,
9].
Not all reportedpatients underwent histological examination, and a lack of biopsies samples has been published[
7]. Nonetheless, there is sufficient data to suggest the most frequent patterns and their relationship with the clinical manifestation, being perivascular lymphocytic infiltrated being the most commonly observed in patients with maculo-papular exanthemas [
14]. Regarding immunohistochemical studies most of them yielded negative results in the detection of SARS-Cov-2 markers in the skin[
15].
Many questions persist regarding how SARS-CoV-2 interacts with the immune system, with some similarities to other viral infections in certain cases, but marked differences in others, such as vascular involvement [
16]. The immune response initiates against an external microorganism through cytokines produced by the innate immune system. Traditionally, interleukins 1 and 6 (IL-1 and IL-6) and tumor necrosis factor, particularly the alpha isoform TNFα, have been considered part of this group [
17,
18]. Macrophages are typically the primary cytokine producers in these early phases, although other cells like epithelial, endothelial, natural killer, or dendritic cells can also produce them [
19].
In contrast, coronavirus infection has shown a nearly exclusive increase in IL-6, with minimal elevation in the peripheral blood of the other two cytokines [
18]. Unlike IL-1 or TNFα, IL-6 does not act by increasing the release of other cytokines but rather by enhancing their action on immune cells. The functions of IL-6 are particularly intriguing because it exhibits both inflammatory and anti-inflammatory properties. Mainly produced by macrophages and activated T lymphocytes (LT) [
20], IL-6 influences B lymphocytes (LB) to promote antibody formation, stimulates LB and neutrophil production in the bone marrow, triggers the release of acute-phase reactants by hepatocytes, and inhibits the transformation of resting LT into regulatory LT. Meanwhile, the presence of transforming growth factor-beta (TGF-β) promotes the appearance of helper LT [
21]. On the contrary, IL-6 induces anti-inflammatory activity by promoting the release of IL-10 and inhibiting IL-1 [
17].
Subsequently, the specific immune response is driven by two subpopulations of helper T lymphocytes (Th), LTh1 and LTh17. LTh1 are releasers of gamma interferon (IFNγ), the production of which depends on IL-12 secreted by activated macrophages [
19]. Although IFNγ has limited antiviral activity, it plays a crucial role in the Th1 response by maintaining macrophage activation. IL-17 is produced by LTh17 and plays a role in defense against microorganisms, although the specific mechanism of action is not well understood [
21]. LTh17 are induced by the presence of IL-6 and TGF-β and sustained by IL-23 secreted by antigen-presenting cells (APCs) [
15].
IL-10 is one of the most powerful anti-inflammatory cytokines, capable of inhibiting the production of many pro-inflammatory cytokines such as IFNγ, TNFα, IL-4, IL-6, IL-12, and growth factors [
21]. It is primarily produced by B lymphocytes (LB), monocytes-macrophages, dendritic cells, and certain subsets of regulatory T lymphocytes. IL-10's primary function is to mitigate the potential damage caused by inflammation [
22]. IL-10 production is triggered by the presence of antigens and is elevated in the blood of patients with conditions like septic shock and autoimmune diseases [
19]. Similarly, patients with coronavirus infections show a clear increase in IL-10 levels when peripheral blood IL-6 levels rise [
7,
18].
The presence of an antigen activates the immune response, but the immune system also initiates measures to regulate and control it (homeostasis) [
23]. The main idea behind conducting multidisciplinary research that spans different medical specialties is because many of the skin manifestations are likely not directly induced by the virus but by the systemic activation of the immune system and the vascular endothelium. In fact, a portion of our study [
24] demonstrates the expression of allergies to various drugs in the skin.
The goal of this study was to establish an accurate classification of cutaneous manifestations in severely hospitalized COVID-19 patients. Secondary objectives included describing generalized exanthemas, analyzing histological and immunohistochemical skin patterns, searching for the virus in the skin, evaluating interleukin profiles in peripheral blood, and tracking the long-term outcome of the patients.
3. Discussion
Initially, 33 patients were enrolled in the study, but finally, a total of 28 patients with a generalized erythematous rash were thoroughly examined to ensure sample homogeneity. Four patients with vesicular rashes were not considered due to variable histological examinations. Within the initial group, the patients had an average age of 59 years, with an almost equal representation of both sexes. All patients had confirmed SARS-CoV-2 infection with severe pneumonia that required hospitalization. The onset of cutaneous lesions after the diagnosis of COVID-19 occurred at an average of 14.9 days (standard deviation 9.88), with an average duration of 16.82 days (standard deviation 20.87). These data indicate longer durations compared to those reported in outpatient cases, where the latency and duration tend to be shorter [
25,
26]. This difference may be attributed to the severity of the patients studied and the higher prevalence of drug reactions, which typically have longer durations and latencies [
4].
Generalized exanthemas in COVID-19 are non-specific and clinically resemble other viral or inflammatory exanthemas, such as adult-onset Still's disease or lupus [
7]. A virus can trigger specific and non-specific cutaneous clinical manifestations. The significant variability in the clinical presentation of COVID-19 suggests that different mechanisms are at play in each type of presentation [
25].
The reported prevalence of cutaneous manifestations in COVID-19 varies widely, ranging from 0.1% to 20%, depending on the source consulted [
1]. Globally, it is estimated to affect approximately 1% of infected patients [
7]. However, the distribution of prevalence is not uniform, with 96.9% of published cases with skin lesions coming from Europe, compared to just 3% from Asia [
11]. The low frequency of skin involvement in coronavirus infection is still not fully understood, but it is suggested to be related to the absence of ACE2 and TMPRSS2 receptors in the skin, which serve as the virus's main entry points in other tissues such as the lungs or intestines [
9,
27].
Among the cutaneous manifestations, generalized maculopapular rash is one of the most common, ranking first or second in terms of frequency. However, the clinical presentation in outpatient settings may not be directly comparable to that in hospitalized patients. Various manifestations have been described, including chilblains (40%), maculopapular rashes (22%), urticaria (9%), livedoid lesions (2%), and others [
1]. In some studies, maculopapular rashes are the most frequently reported clinical presentation [
4,
25], potentially due to a tendency to report chilblains [
3,
7]. Nevertheless, many limitations have been observed in the published data on cutaneous manifestations in outpatients with COVID-19. Patients were often assessed through questionnaires and social media, with photographs, without involvement of dermatologists or access to medical histories [
3,
7]. A significant proportion (51.8%) of the published cases were only suspected and not confirmed, and only a minority underwent biopsies [
12]. Authors agree that many of these cases were likely misclassified, but due to patient isolation, this was the method of study. However, certain questions remain unanswered, such as why drug reactions were not considered [
3,
8,
10], and why the occurrence of cutaneous manifestations dramatically decreased in subsequent waves [
13]. Possible reasons include reduced inflammation, vaccination efforts, and more standardized treatment protocols with fewer drugs in later phases of the pandemic.
There are only a few case series regarding cutaneous manifestations in hospitalized severe COVID-19 patients [
5,
6]. Clinical experiences varied considerably, with a high prevalence of ulcers and vascular lesions, and exanthema accounted for 22% of observed lesions. While these studies were conducted in a hospital setting and thus more controlled, they still had limitations due to patient isolation, restricted ICU visiting policies, and the severity of the patients' conditions.
The primary challenge in diagnosing a generalized exanthema in the context of a viral infection is distinguishing it from a drug reaction. Clues such as a history of drug intake, pruritus, or a delayed onset suggest a drug reaction [
3]. However, a definitive diagnosis requires a compatible histological examination and further allergology studies. Interestingly, in contrast to most reports, in our patient series, 25% of the rashes were drug-induced. Analytical blood tests showed typical parameters for severe COVID-19, such as elevated neutrophils, D-Dimers, and transaminases, but our patient group exhibited higher levels of eosinophils, likely due to the presence of drug-induced reactions in 25% of cases.
Histological examination of our patient group did not significantly differ from other viral exanthemas or published data, except for patients with drug reactions. The histopathology of generalized exanthema typically revealed a superficial perivascular lymphocytic infiltrate in the skin, sometimes with perianexial involvement. Vasculitis was observed in patients with chilblains. Patients with drug reactions exhibited typical histological features, such as necrotic keratinocytes and eosinophilic infiltrates. Additional features described in the literature in COVID-19 patients with rashes include spongiosis, cell vacuolization [
28], deep vascular dermatitis [
29], lymphocytes surrounding vessels, or extravasated blood cells resembling a vasculitis pattern [
30]. Routine CD3 and CD163 immunohistochemical examinations were performed in all biopsies, and the results were similar across the board, making it challenging to distinguish mechanisms like drug reactions, viral infections, or inflammation. These findings suggest a continuous spectrum of skin involvement with varying degrees of severity. Notably, all patients in our study with chilblains, totaling only four, also had a generalized exanthema. In our group, patients with drug reactions did not exhibit vasculitis, which supports the idea that vasculitis or vascular involvement could be one of the more specific histological findings of coronavirus in the skin.
At the beginning of the study, we investigated SARS-CoV-2 expression by qRT-PCR in fresh skin biopsy samples from patients with generalized exanthema. However, after the first 4 samples tested negative, we discontinued the procedure. Other studies, not focused on rashes but on conditions like chilblains [
31] or papulo-squamous eruptions [
32] in COVID-19 patients, also showed negative PCR results in skin samples. These findings suggest that the mechanism behind generalized exanthema in COVID-19 is not directly linked to the presence of the virus in the skin lesions.
To further understand whether the lesions were associated with the presence of the virus or an indirect activation of the immune response in the skin, we conducted additional immunohistochemical examinations. Our carefully selected panel included measuring the expression of interleukins on the skin (IL1, IL10, IL6, and IL12) and SARS-CoV-2 proteins (such as the Spike protein), along with selected proteins involved in potential interactions between epithelial and endothelial cells and the virus, such as VCAM, selectin, and ITGalpha5.
Severe COVID-19 infection can trigger a massive cytokine storm, primarily involving IL1, IL6, IL10, and IL12 [
15,
18]. However, we did not detect cytokine expression in the skin of patients with generalized exanthema. This supports the idea that these lesions result from mechanisms similar to other inflammatory diseases like lupus, antiphospholipid syndrome, or Still's disease [
33]. Some other findings, such as complement activation, vessel necrosis, and skin thrombosis, have been observed but are typically associated with patients who have vasculopathy [
34].
In the early days of the pandemic, some authors reported the presence of the Spike protein in skin lesions, determined by immunohistochemistry in cases of indirect cutaneous manifestations of COVID-19 [
35]. In contrast, many studies have examined viral protein expression in skin lesions. For example, in one study involving 69 patients, extensive Spike protein deposition was found in relation to vasculitis and endothelial cell damage [
36]. Recently, Marzano et al. [
37] published different findings using digital PCR, indicating a 38% positive Spike protein expression in the skin, which did not seem to correlate with clinical expression. Based on these earlier findings, it's conceivable that the use of more sensitive and specific techniques could enable the detection of viral presence in skin lesions.
The remaining biomarkers we assessed did not provide insights into the mechanism, which is consistent with other reports. VCAM1 and Selectin have been considered markers of high mortality risk in COVID-19 patients with cytokine storms affecting the nervous system or lungs but not in the skin [
38,
39]. Both markers indicate endothelial damage and platelet interaction, typical phenomena in severe SARS-CoV-2 infections. Galpha is a protein involved in various physiological functions, including integrin activation and urotenin and ECA function, which have been found to be part of the SARS-CoV-2 virus interaction in some systems but were not demonstrated in the skin [
40].
In our blood samples, we exclusively studied the systemic levels of cytokines involved in the Th1 and Th17 inflammatory responses, along with IL-10. These were the cytokines used to guide the treatment of hospitalized patients during the initial wave of the coronavirus pandemic [
41]. We did not evaluate other cytokines, which could be considered a limitation. We also did not analyze classic Th2 responses, as they did not offer significant benefits or prognostic information in COVID-19 [
42]. Additionally, an important limitation is the quantification of cytokines in hospitalized and severe patients, as the cytokines were not measured precisely when the skin lesions appeared, but rather with a time interval of several days. The number of patients ultimately included in the study could also influence the results. Despite these limitations, no other published studies have conducted a comprehensive analysis and correlation between skin manifestations, including histological classification, and the interleukin profile. This data could potentially yield interesting findings.
It has been generally hypothesized that skin rashes occurring in the context of viral infections are exclusively caused by the inflammatory response [
9]. However, not all COVID-19 patients with a high inflammatory state develop skin lesions. Other factors may be at play, such as genetic predisposition or the virus itself. Not all skin manifestations of COVID-19 can be solely attributed to endothelial activation or vessel inflammatory damage. The interaction with drug intake should also be considered, especially since many patients, particularly in the early stages of the pandemic, received various drugs simultaneously. In fact, this group of patients with generalized rashes during severe COVID-19 infection underwent a thorough investigation for potential drug reactions, and 25% had biopsy results compatible with this diagnosis. After recovery, some of them tested positive in clinical and in vitro tests for drug allergies [
24].
No correlation was found between the levels of interleukins, the interleukin profile, and the systemic or vascular damage or skin manifestations in the studied patients. No specific risk factors or situations were identified as enhancers, whether from the blood analysis, demographic data, or histological staining.
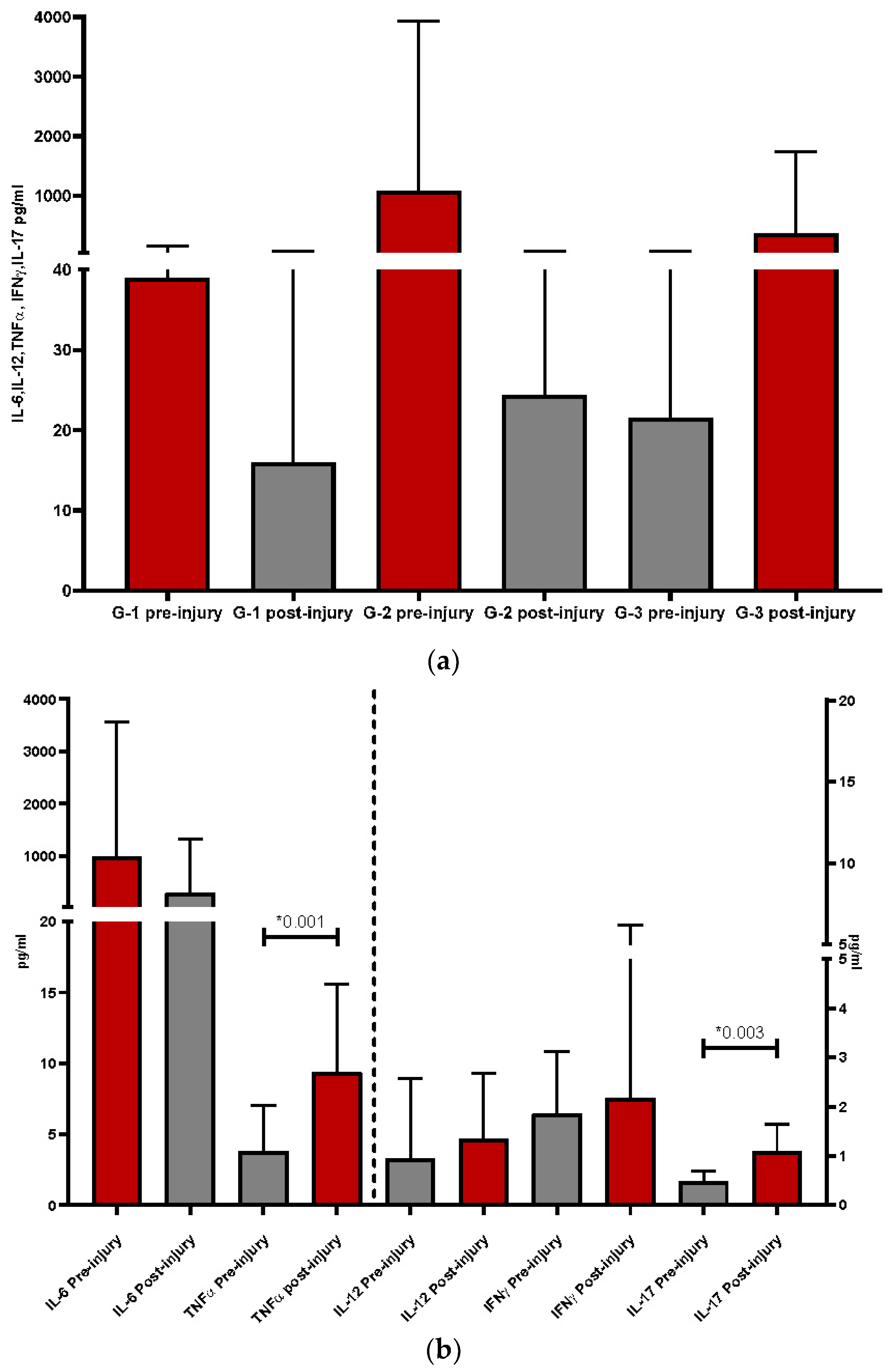
This multidisciplinary study allowed us to classify patients referred to the Dermatology department into three groups based on histological alterations observed in skin biopsies by pathologists, with the aim of describing different pathogenic patterns. The histological classification served as the "gold standard" and resulted in three groups: group 1 (G1) with dermatitis, group 2 (G2) with drug reactions, and group 3 (G3) with vasculitis. Interleukin profiles were studied in these three groups, both before and after the appearance of skin lesions, to assess differences. Interestingly, G1 and G2 exhibited higher proinflammatory levels before the onset of skin lesions, while G3 showed higher values after the appearance of cutaneous lesions.
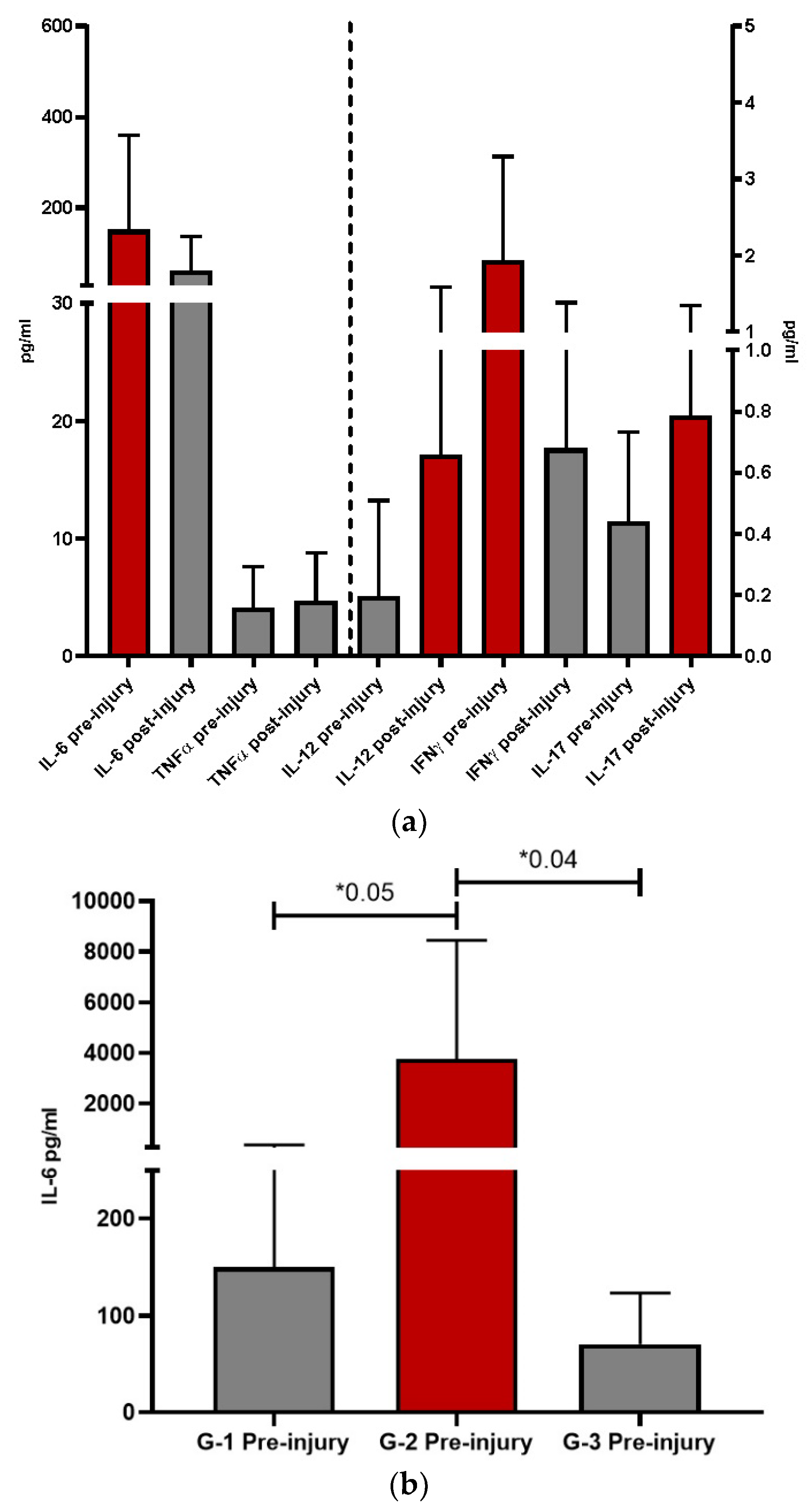
When we analyzed each pro-inflammatory interleukin separately, we observed different behavior patterns among them and between the groups. IL-6 consistently appeared as the most elevated interleukin in all groups, as is typically found in patients with COVID-19 infection [
9], regardless of whether they had skin lesions or not [
15]. As cutaneous lesions progressed, similar to the systemic immune response, the pattern of interleukins changed, with TNFα (p<0.04) and IL-17 (p<0.03) showing the greatest increases post-injury. Both TNFα and IL-17 were potentiated by the presence of IL-6, which is closely related to them, except in G3 patients, where IL-6 also increased after the cutaneous lesions.
G1 was clearly the least inflammatory group, with the lowest values of leukocytes, sub-populations, and pro-inflammatory interleukins. In this group, genetic influence or other factors may have predisposed individuals to cutaneous reactions.
G2 was the most inflammatory, particularly before the injury, although many interleukins maintained elevated levels post-injury compared to the other groups. However, most of them, except for TNFα, tended to decrease once the lesion had developed. In these patients, the presence of inflammation itself may play a more important role in the pathogenesis of cutaneous manifestations, influenced by factors such as drugs or genetic predisposition. In any case, high levels of interleukins were more intensely related to this group, likely originating from neutrophils even before the appearance of skin lesions.
G3 presented a different scenario from the other groups. Notably, IL-6 levels increased after the onset of lesions (p=0.04), possibly contributing to the increase in neutrophils observed post-injury. Another interesting finding was the increase in IL-12 after the appearance of cutaneous lesions, another interleukin produced by innate immune system cells in response to viral antigens. IL-12 primarily promotes differentiation towards IFN-producing Th1 cells in the presence of viruses. Based on this, it can be inferred that in G3, skin involvement is directly related to local activation of the vascular endothelium due to the presence of various factors, including the virus. When the virus is eliminated, it is no longer found in the lesions, but the result of the activation persists. Additionally, G3 showed a significant increase in the number of neutrophils after the appearance of lesions compared to its previous values, indicating acute inflammation and potential cell recruitment by the endothelium [
43]. This observation aligns with the vascular lesions found in the histological study.
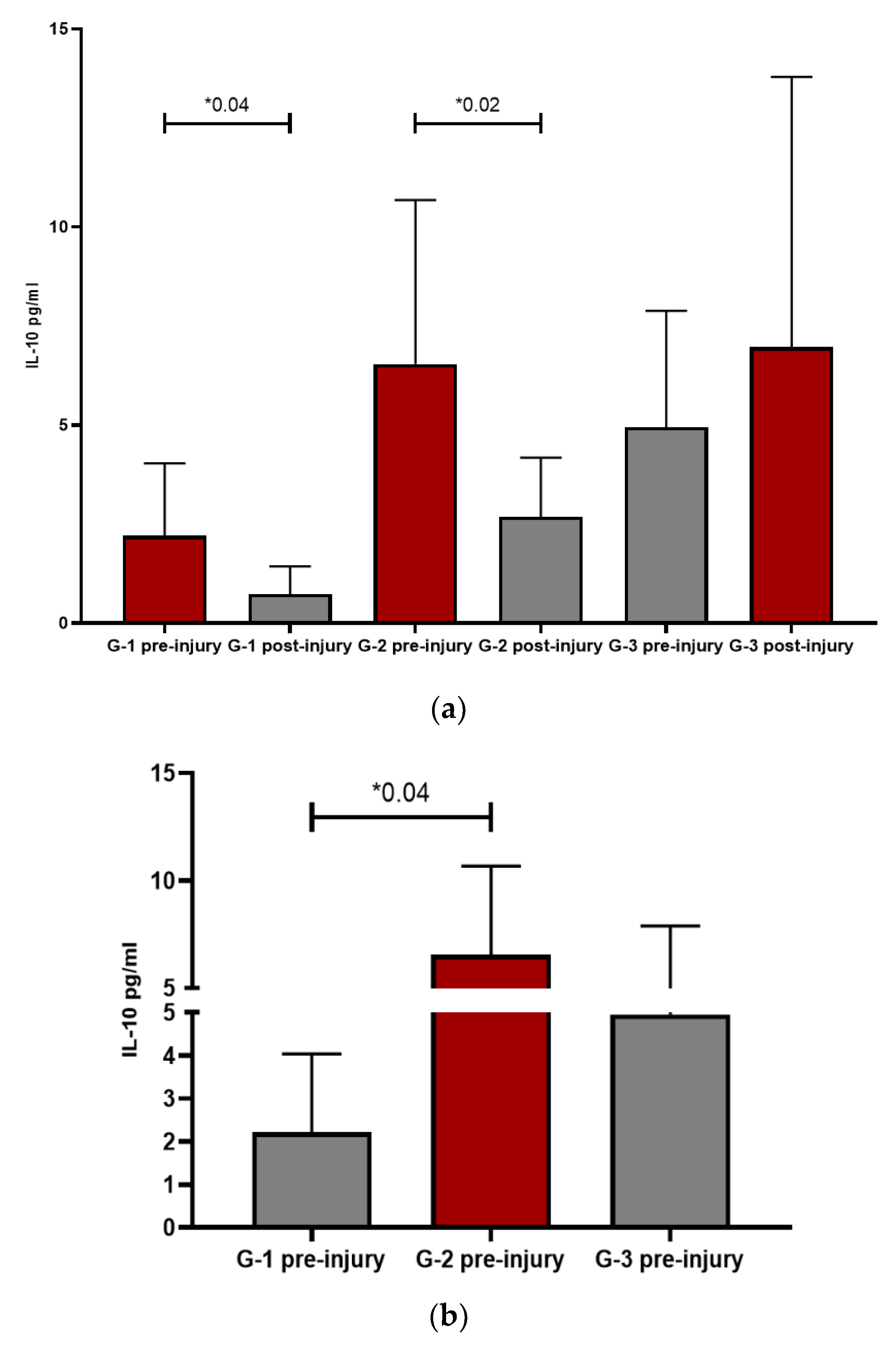
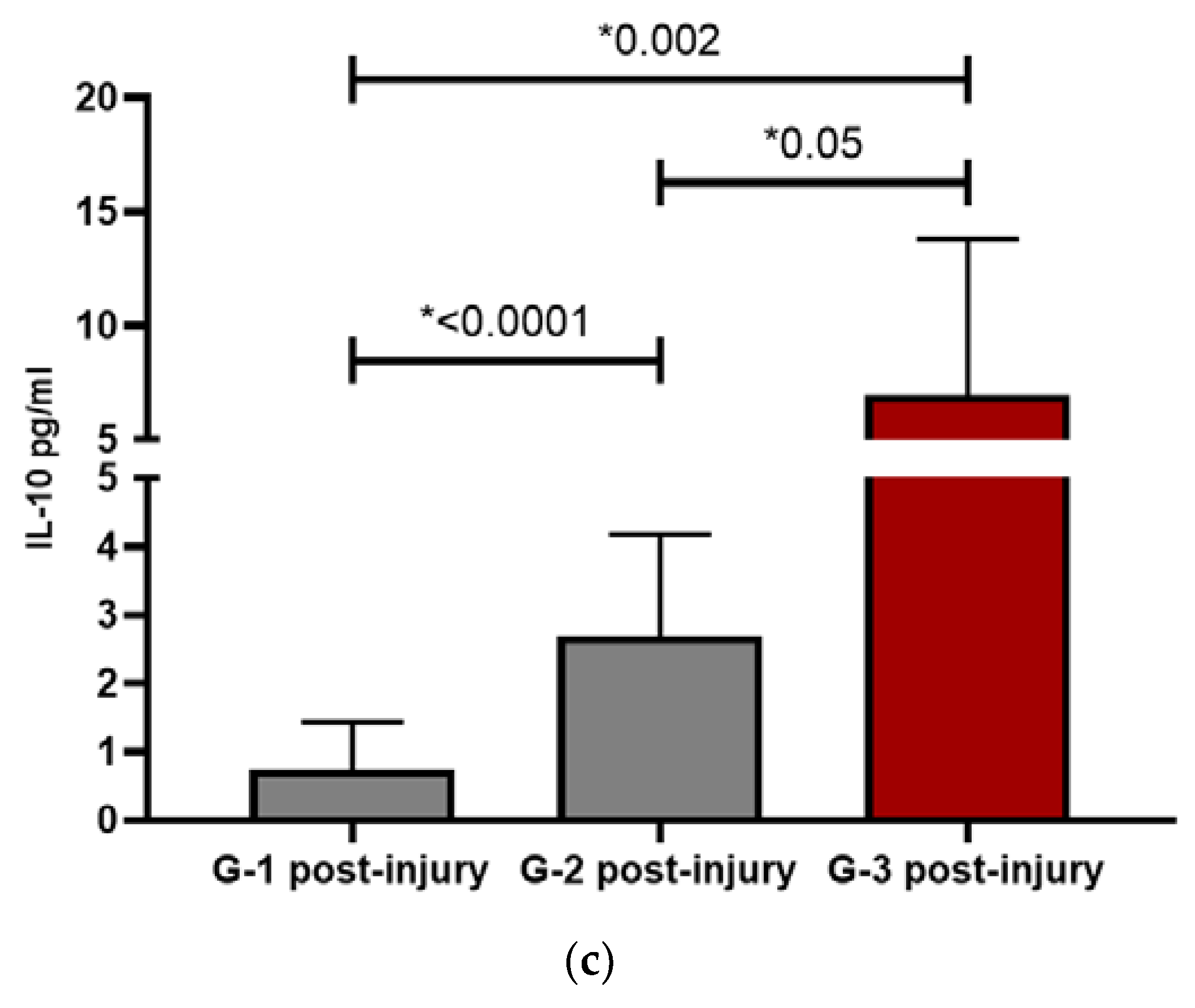
IL-10 is an anti-inflammatory interleukine and showed the same pattern between the groups: G1 and G2 presented a decrease after the start of the lesion, statistically significant in both groups (p=0.04 and p= 0.02 respectively), while in G3, IL10 raised after lesions appearance. Once again, the highest values were presented by G2 except at the post-injury level which, as occurred with IL-6, spikes in G3 patients. In G1 and G2, this data could support the direct relationship of inflammation with the pathogenesis of skin involvement. In G3, this great elevation of IL-6 could be responsible for the elevation of IL-10 itself and be part of the physiological response to the immunological stimulus. After facing intense systemic inflammation, an increase in IL-10 counteracts the harmful effects. This fact was also observed in patients with COVID-19 [
44].
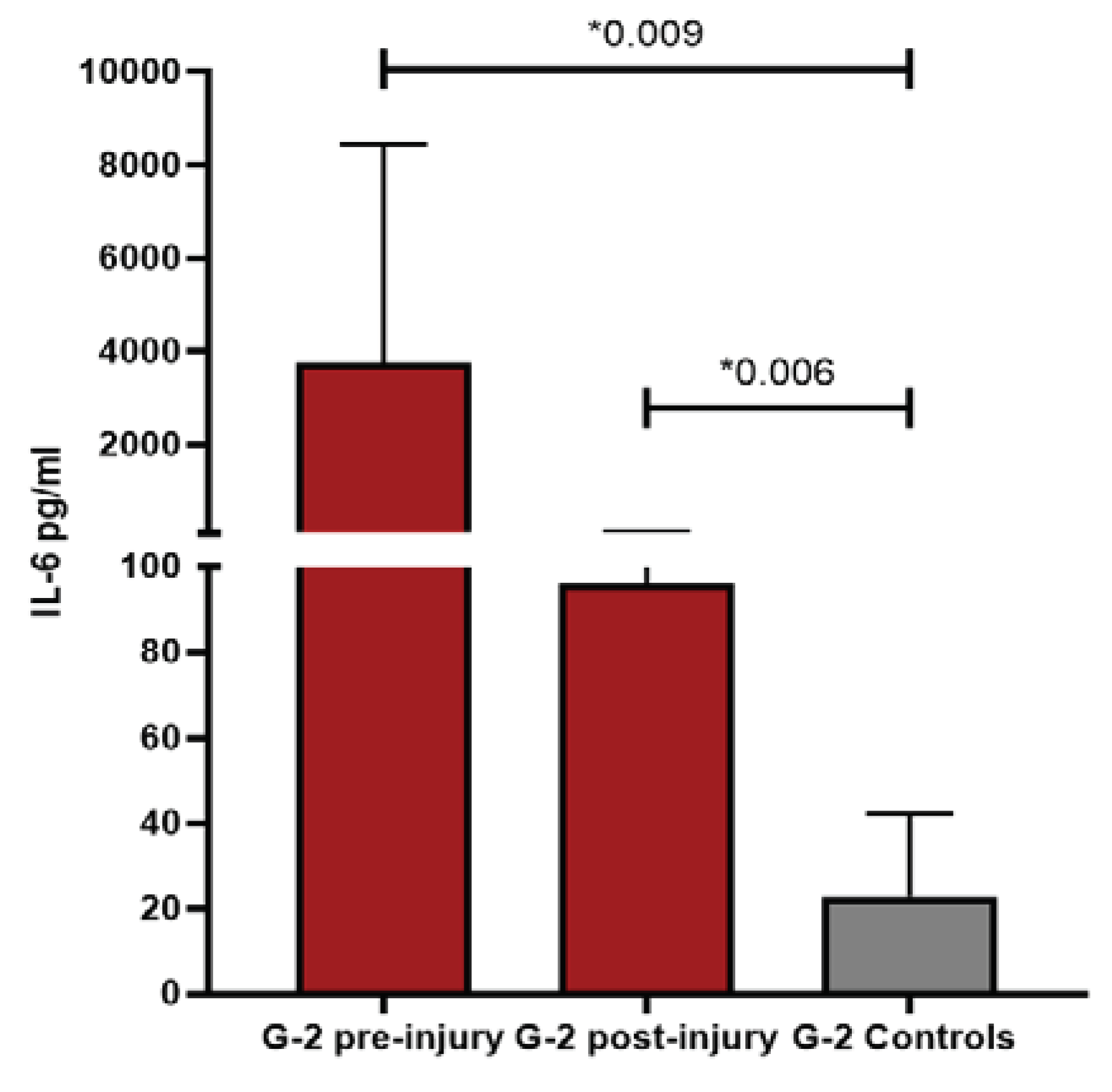
Patients with severe COVID-19 infection and cutaneous lesions were compared with those without cutaneous lesions, given that many of the alterations observed in the pattern of ILs were seen in patients without cutaneous manifestations. COVID-19 positive control patients were selected among severe coronavirus infection, regardless of the skin lesion, and once again, G1 and G2 patients behaved differently from G3 and had higher IL-6 values than couplets COVID-19 controls, being statistically significant in G2 (p=0.009). Suggesting again that inflammation plays a very important role, especially IL-6. At the post-injury level, the only one that persists in clearly higher values was G2 (p=0.006) with respect to controls, showing a clear inflammatory predominance. On the other hand, G3 maintained values like controls, possibly because it was closer to the viral pathogenesis itself than to an effect influenced by other factors. The difference between the controls and G3 resides in IL-10, since its values were significantly increased compared to controls, both at the pre-injury (p=0.05) and post-injury (p=0.016) levels. For years, the activity of pro-inflammatory ILs has been related to the pathogenesis of vascular damage, with special emphasis on the role of IL-10 as a protector [
22,
23]. Maybe that in G3 patients, due to the activation of the vascular endothelium, the values of this IL rise much more than in the rest.
Long-term follow-up was conducted 24 months after the generalized rash through phone interviews. A total of 20 patients were contacted, and no specific symptoms were reported, except for 50% of the patients who mentioned tiredness without a diagnosis of post-COVID syndrome [
20] and thus without establishing causality.
In conclusion, all these findings described and characterized for the first time skin lesions associated to SARS-Cov2 infection, pointing out new factors contributing to the appearance and the resolution of the lesions, related and unrelated to the viral presence.
4. Materials and Methods
Hospitalized patients with severe pulmonary confirmed COVID-19 infectious with a biopsied cutaneous generalized rash were selected between March and May of 2020 (33 patients). Generalized rash was considered disseminated lesions affecting two different body areas, including the trunk plus limbs or plus arms. The primary cutaneous lesions were classified as macules and papules, vesicles, and nodules in the hands (chilblains). Days between the broke up of the lesions and the start of COVID-19symptoms were measured (latency) and the duration of the cutaneous manifestations. Day 0 was considered the day of the spring up of the lesions. This study was approved by the Ethics Committee (Number 197-20) and all patients gave informant consent for undergoing the biopsy and been included. The demographic data of the patients, the description of the cutaneous rash, hemogram, transaminases, reactive C protein (RCP), creatin phosphokinase (CPK) and D-dimmers were compiled (day 0±3 days). A history of drug intake before the reaction was assessed, considering treatment included in the 3 weeks before the broke up of the skin lesions.
Vesicular rashes were finally excluded of interleukin blood levels and immunohistochemical analysis because of their internal variability and small number of cases. The histological pattern of the erythematous rashes were analyzed by an expert dermatopathologist and classified into perivascular dermatitis with or without perianexial lymphocytic infiltrates, cutaneous small vessels vasculitis and drug reaction with interphase necrosis, necrotic keratinocytes and eosinophilic infiltrated. The distribution and immunohistochemical patterns were analyzed and classified as positive (+) or negative (-) and by distribution in interstitial, perivascular, and not-evaluable/technique failure.Skin biopsies were processed in conventional hematoxylin-eosin and immunohistochemical investigation
4.1. Levels of ILS in Skin Biopsies
In Fresh skin biopsies ILs were determined by qRT-PCR. Briefly, total RNA was isolated with TriPure Isolation Reagent (1166716500, Roche Diagnostics). RNA concentration was determined using NanoDrop (Thermo Fisher Scientific), 2 µg of RNA was used to obtain cDNA using Transcriptor First Strand cDNA Synthesis Kit (04897030001, Roche Diagnostics). 1ul of cDNA was used as the template for a quantitative PCR reaction with SYBR Green (11066420, SYBR Green I Master, Roche Diagnostics). PCRs were carried out in Lightcycler 480 equipment (Roche). For each sample and experiment, triplicates were made, and Ribosomal 28s mRNA levels were used as housekeeping for data normalization.
4.2. Immunohistochemistry for Markers Detection in Skin Biopsies
The markers selected for assessing activation and inflammation were assessed by immunohistochemistry. For this approach, 4 μm paraffin-embedded skin sections were treated with peroxidase blocking solution (Dako) to inhibit endogenously peroxidase activity, blocked in 1% BSA-PBS, followed by incubation with CD3 (Abcam® Ref: ab16669), CD86(Abcam® Ref: ab243887), CD163 (Abcam® Ref: ab74604), VCAM (CD106) (LsBio® Ref:LS-C 172657), ITGalpha5 (Abcam® Ref: ab15036), and E-Selectina(Novus® Ref: NBP1-45545SS). Antibodies of cellular signaling of SARS-CoV2 were also tested, and finally SARS-CoV2 (alpha Rabbit) (Sino Biological®, ref: 40592-T62) and Nucleocapsid antibody (Rabbit Mab) (Sino Biological, ref:40143-R001) were performed. Secondary antibody conjugated with HRP (1:100, DAKO) was used and finally developed using DAB solution (DAKO).
4.3. Serum ILs Detection
Interleukins measured in peripheral blood includedIL-6, IL-12, TNFα, INFγ, IL-17 and IL10. Controls were patients hospitalized with severe COVID-19 which did not develop skin lesions and matched in sex and age. Patients included were analyzed considering each control with the same patient, at the same time pre- and post-lesions.
Blood systemic levels of interleukins were assessed throughout flow cytometry, using the BD TM Cytometric Bead array (Human inflammatory cytokine CBA kit). Levels of IL6, IL12, TNF-alpha, IL10, IL-1B, IL8, INFγ and IL17 were measured at the start of the cutaneous lesions (day 0 ± 3 days) and days after the cutaneous lesions flare-up. From the Immunology data base of interleukins control patients without skin lesions were compiled. The inclusion criteria were SARS-COV-2infection, same age, gender, date of blood analysis (day 0 ± 3 days) and disease outcome (alive or exitus). Patients who had previously received treatment with systemic corticosteroids or/and Tocilizumab were excluded as Tocilizumab is a monoclonal antibody that competitively inhibits the binding of IL6 to its receptor, causing secondary elevated levels of the interleukin.
4.4. Statistical Analyses
The data was analyzed with ANOVA and U Mann-Whitney statistics tests. An analysis for each interleukin, leukocyte and the D Dimer was made between the different groups, including levels before and after the cutaneous lesion. Additionally, each group was compared with the COVID-19 control patients. A significant value of P <0.05 was considered. In the result we analyzed which group had the predominant profile of inflammatory (IL6, IL12, TNF-alpha, IL-1B, IL8, INFγ and IL17) or tolerogenic (IL10) interleukin and when, before or after the cutaneous lesion. All analyses were conducted using Graph Pad Prism® (GraphPad software, Inc).