Submitted:
22 September 2023
Posted:
26 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Yellow Mealworms Colony
2.2. Substrate composition/preparation
2.3. Experimental set-up
2.4. Mealworm growth perfomance
2.5. Carotenoids analysis
- 0.55 = The final hexane layer volume ratio to the volume of mixed solvents added for hexane:acetone:ethanol (2:1:1)
- W (mg) = The weight of sample analyzed
- V (ml) = The volume of mixed solvents added
- 537 = The molecular weights of lycopene and β-carotene(g/mole).
2.6. Lipid analysisin mealworms
2.7. Protein analysis
2.8. Statistical analysis
3. Results
3.1. Larval performances
3.2. Efficiency indicators
3.3. Licopene and β-carotene Quantification
3.4. Larval nutritional value
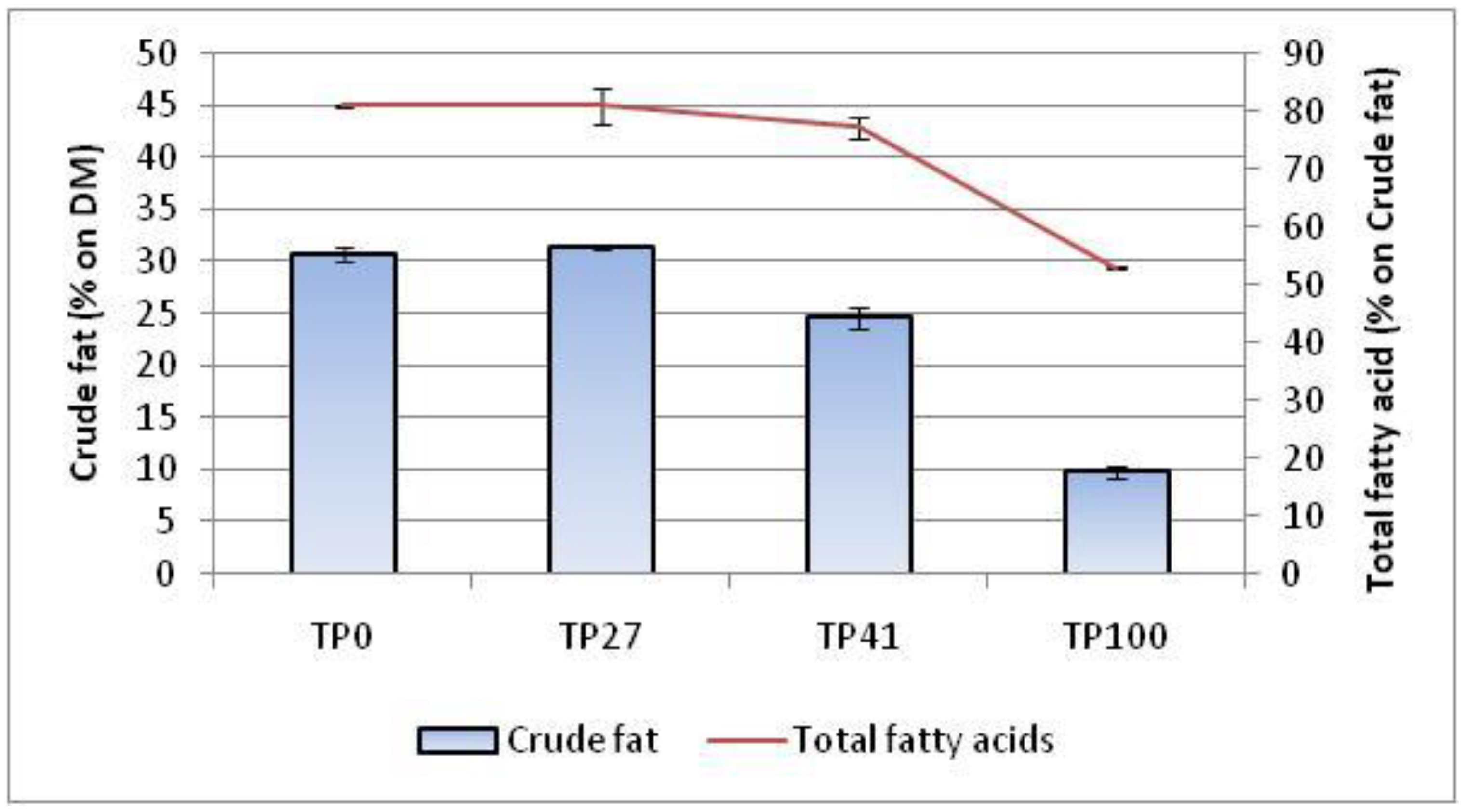
3.4.1. Lipid quality indices
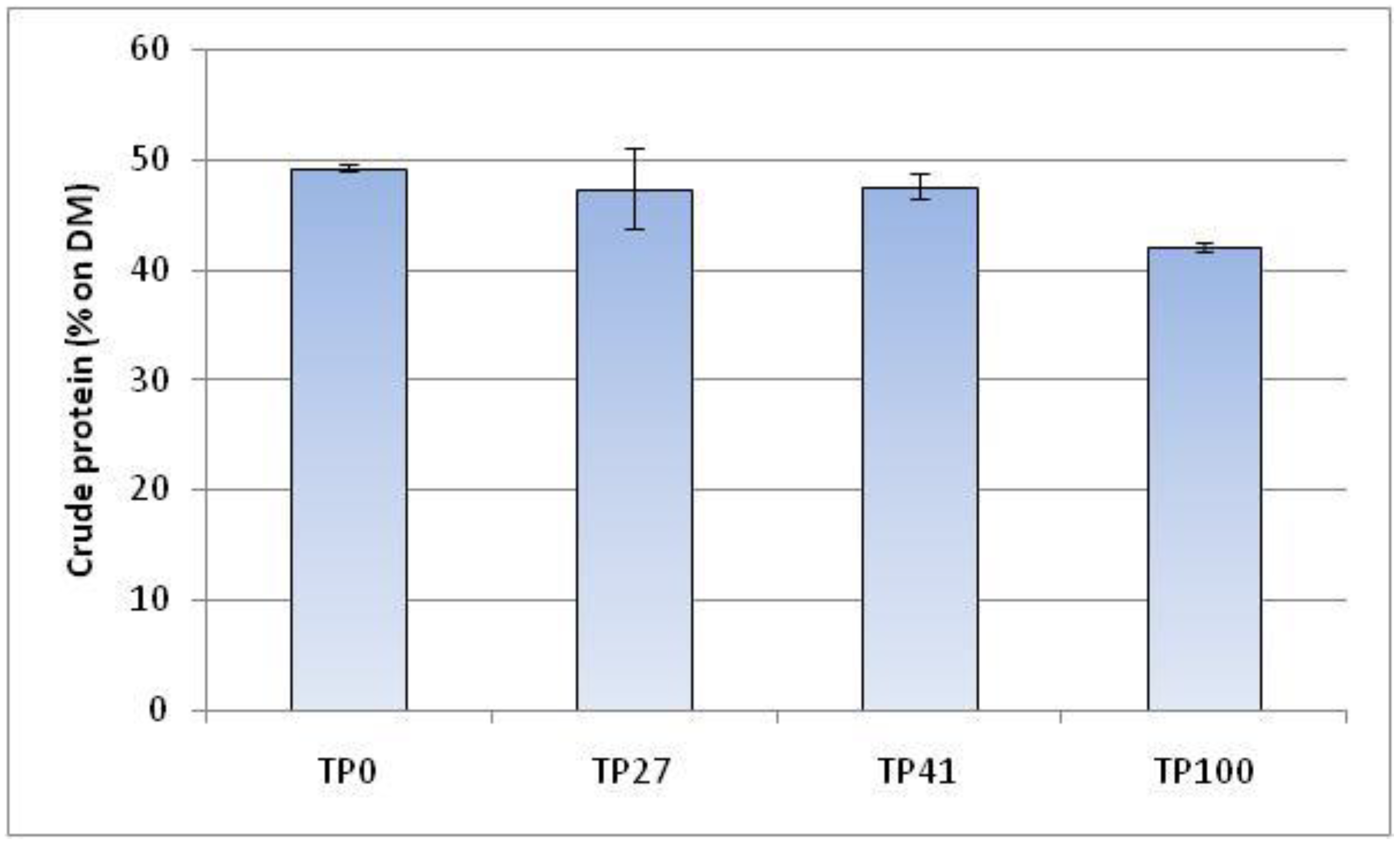
3.4.2. Crude proteine
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cadinu, L.A.; Barra, P.; Torre, F.; Delogu, F.; Madau, F.A. Insect Rearing: Potential, Challenges, and Circularity. Sustainability 2020, 12, 4567. [Google Scholar] [CrossRef]
- Jensen, H.; Elleby, C.; Domínguez, I.P.; Chatzopoulos, T.; Charlebois, P. Insect-Based Protein Feed: From Fork to Farm. J. Insects as Food Feed 2021, 7, 1219–1233. [Google Scholar] [CrossRef]
- Bordiean, A.; Krzyżaniak, M.; Aljewicz, M.; Stolarski, M.J. Influence of Different Diets on Growth and Nutritional Composition of Yellow Mealworm. Foods 2022, 11, 3075. [Google Scholar] [CrossRef] [PubMed]
- El Deen, S.N.; Lamaj, F.; Verrastro, V.; Al Bitar, L.; Baldacchino, F. Effects of Two Diets on Adults’ Survival and Productivity in Mass-Rearing of Tenebrio Molitor (Coleoptera: Tenebrionidae). J. Insects as Food Feed 2021, 7, 1149–1157. [Google Scholar] [CrossRef]
- Noyens, I.; Schoeters, F.; Van Peer, M.; Berrens, S.; Goossens, S.; Van Miert, S. The Nutritional Profile, Mineral Content and Heavy Metal Uptake of Yellow Mealworm Reared with Supplementation of Agricultural Sidestreams. Sci. Rep. 2023, 13, 11604. [Google Scholar] [CrossRef]
- Kröncke, N.; Benning, R. Influence of Dietary Protein Content on the Nutritional Composition of Mealworm Larvae (Tenebrio Molitor L.). Insects 2023, 14, 261. [Google Scholar] [CrossRef]
- Riekkinen, K.; Väkeväinen, K.; Korhonen, J. The Effect of Substrate on the Nutrient Content and Fatty Acid Composition of Edible Insects. Insects 2022, 13, 590. [Google Scholar] [CrossRef]
- Dreassi, E.; Cito, A.; Zanfini, A.; Materozzi, L.; Botta, M.; Francardi, V. Dietary Fatty Acids Influence the Growth and Fatty Acid Composition of the Yellow Mealworm Tenebrio Molitor (Coleoptera: Tenebrionidae). Lipids 2017, 52, 285–294. [Google Scholar] [CrossRef]
- FRANCARDI, V.; CITO, A.; FUSI, S.; FUSI, S.; BOTTA, M.; DREASSI, E. LINSEED TO INCREASE N-3 FATTY ACIDS IN TENEBRIO MOLITOR (COLEOPTERA TENEBRIONIDAE). Redia 2017, 73–76. [Google Scholar] [CrossRef]
- Anderson, S.J. Increasing Calcium Levels in Cultured Insects. Zoo Biol. 2000, 19, 1–9. [Google Scholar] [CrossRef]
- Latney, L. V.; Toddes, B.D.; Wyre, N.R.; Brown, D.C.; Michel, K.E.; Briscoe, J.A. Effects of Various Diets on the Calcium and Phosphorus Composition of Mealworms (Tenebrio Molitor Larvae) and Superworms (Zophobas Morio Larvae). Am. J. Vet. Res. 2017, 78, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Brai, A.; Vagaggini, C.; Pasqualini, C.; Poggialini, F.; Tarchi, F.; Francardi, V.; Dreassi, E. Use of Distillery By-Products as Tenebrio Molitor Mealworm Feed Supplement. J. Insects as Food Feed 2023, 9, 611–623. [Google Scholar] [CrossRef]
- Brai, A.; Poggialini, F.; Trivisani, C.I.; Vagaggini, C.; Tarchi, F.; Francardi, V.; Dreassi, E. Efficient Use of Agricultural Waste to Naturally Fortify Tenebrio Molitor Mealworms and Evaluation of Their Nutraceutical Properties. J. Insects as Food Feed 2023, 9, 599–610. [Google Scholar] [CrossRef]
- Kotsou, K.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Adamaki-Sotiraki, C.; Rumbos, C.I.; Athanassiou, C.G.; Lalas, S.I. Waste Orange Peels as a Feed Additive for the Enhancement of the Nutritional Value of Tenebrio Molitor. Foods 2023, 12, 783. [Google Scholar] [CrossRef] [PubMed]
- Kotsou, K.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Rumbos, C.I.; Athanassiou, C.G.; Lalas, S.I. Enhancing the Nutritional Profile of Tenebrio Molitor Using the Leaves of Moringa Oleifera. Foods 2023, 12, 2612. [Google Scholar] [CrossRef] [PubMed]
- Ruschioni, S.; Loreto, N.; Foligni, R.; Mannozzi, C.; Raffaelli, N.; Zamporlini, F.; Pasquini, M.; Roncolini, A.; Cardinali, F.; Osimani, A.; et al. Addition of Olive Pomace to Feeding Substrate Affects Growth Performance and Nutritional Value of Mealworm (Tenebrio Molitor L.) Larvae. Foods 2020, 9, 317. [Google Scholar] [CrossRef]
- Fondevila, M.; Guada, J.A.; Gasa, J.; Castrillo, C. Tomato Pomace as a Protein Supplement for Growing Lambs. Small Rumin. Res. 1994, 13, 117–126. [Google Scholar] [CrossRef]
- Heuzé V.; Tran G.; Hassoun P.; Bastianelli D.; Lebas F. Tomato Pomace, Tomato Skins and Tomato Seeds. Feed. a Program. by INRAE, CIRAD, AFZ FAO 2021.
- Del Valle, M.; Cámara, M.; Torija, M.-E. Chemical Characterization of Tomato Pomace. J. Sci. Food Agric. 2006, 86, 1232–1236. [Google Scholar] [CrossRef]
- Knoblich, M.; Anderson, B.; Latshaw, D. Analyses of Tomato Peel and Seed Byproducts and Their Use as a Source of Carotenoids. J. Sci. Food Agric. 2005, 85, 1166–1170. [Google Scholar] [CrossRef]
- Mansoori, B.; Modirsanei, M.; Radfar, M.; Kiaei, M.M.; Farkhoy, M.; Honarzad, J. Digestibility and Metabolisable Energy Values of Dried Tomato Pomace for Laying and Meat Type Cockerels. Anim. Feed Sci. Technol. 2008, 141, 384–390. [Google Scholar] [CrossRef]
- Reda, F.M.; Madkour, M.; El-Azeem, N.A.; Aboelazab, O.; Ahmed, S.Y.A.; Alagawany, M. Tomato Pomace as a Nontraditional Feedstuff: Productive and Reproductive Performance, Digestive Enzymes, Blood Metabolites, and the Deposition of Carotenoids into Egg Yolk in Quail Breeders. Poult. Sci. 2022, 101, 101730. [Google Scholar] [CrossRef] [PubMed]
- Marcos, C.N.; de Evan, T.; Molina-Alcaide, E.; Carro, M.D. Nutritive Value of Tomato Pomace for Ruminants and Its Influence on In Vitro Methane Production. Animals 2019, 9, 343. [Google Scholar] [CrossRef] [PubMed]
- Weiss, W.P.; Frobose, D.L.; Koch, M.E. Wet Tomato Pomace Ensiled with Corn Plants for Dairy Cows. J. Dairy Sci. 1997, 80, 2896–2900. [Google Scholar] [CrossRef] [PubMed]
- Nesci, S.; Spagnoletta, A.; Oppedisano, F. Inflammation, Mitochondria and Natural Compounds Together in the Circle of Trust. Int. J. Mol. Sci. 2023, 24, 6106. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.P.; Ross, A.C.; Stephensen, C.B.; Bohn, T.; Tanumihardjo, S.A. Metabolic Effects of Inflammation on Vitamin A and Carotenoids in Humans and Animal Models. Adv. Nutr. 2017, 8, 197–212. [Google Scholar] [CrossRef]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.; Biesalski, H.K. β-Carotene Is an Important Vitamin A Source for Humans. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar] [CrossRef]
- de Almeida Torres, R.J.; dos Anjos Ferreira, A.L.; Luchini, A.; de Almeida Torres, R.J.; Correa, C.R. The Role of Non-Enzymatic Antioxidants on Age-Related Macular Degeneration. Front. Drug Chem. Clin. Res. 2022, 5, 1–19. [Google Scholar]
- Heinrich, U.; Wiebusch, M.; Tronnier, H.; Gärtner, C.; Eichler, O.; Sies, H.; Stahl, W. Supplementation with β-Carotene or a Similar Amount of Mixed Carotenoids Protects Humans from UV-Induced Erythema. J. Nutr. 2003, 133, 98–101. [Google Scholar] [CrossRef]
- Palozza, P.; Simone, R.E.; Catalano, A.; Mele, M.C. Tomato Lycopene and Lung Cancer Prevention: From Experimental to Human Studies. Cancers (Basel) 2011, 3, 2333–2357. [Google Scholar] [CrossRef]
- Jacques, P.F.; Lyass, A.; Massaro, J.M.; Vasan, R.S.; D’Agostino Sr, R.B. Relationship of Lycopene Intake and Consumption of Tomato Products to Incident CVD. Br. J. Nutr. 2013, 110, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Leni, G.; Maistrello, L.; Pinotti, G.; Sforza, S.; Caligiani, A. Production of Carotenoid-Rich Hermetia Illucens Larvae Using Specific Agri-Food by-Products. J. Insects as Food Feed 2023, 9, 171–181. [Google Scholar] [CrossRef]
- NASER EL DEEN, S.; SPRANGHERS, T.; BALDACCHINO, F.; DERUYTTER, D. The Effects of the Particle Size of Four Different Feeds on the Larval Growth of Tenebrio Molitor (Coleoptera: Tenebrionidae). Eur. J. Entomol. 2022, 119, 242–249. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists) No Title. Off. Methods Anal. 1990, Virginia, Arlington.
- Morales-Ramos, J.A.; Rojas, M.G.; Shapiro-llan, D.I.; Tedders, W.L. Use of Nutrient Self-Selection as a Diet Refining Tool in Tenebrio Molitor (Coleoptera: Tenebrionidae). J. Entomol. Sci. 2013, 48, 206–221. [Google Scholar] [CrossRef]
- RHO, M.S.; Pum LEE, K. Nutrient-Specific Food Selection Buffers the Effect of Nutritional Imbalance in the Mealworm Beetle, Tenebrio Molitor (Coleoptera: Tenebrionidae). Eur. J. Entomol. 2015, 112, 251–258. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Bliamplias, D.; Gourgouta, M.; Michail, V.; Athanassiou, C.G. Rearing Tenebrio Molitor and Alphitobius Diaperinus Larvae on Seed Cleaning Process Byproducts. Insects 2021, 12, 293. [Google Scholar] [CrossRef] [PubMed]
- Melgar-Lalanne, G.; Hernández-Álvarez, A.; Salinas-Castro, A. Edible Insects Processing: Traditional and Innovative Technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1166–1191. [Google Scholar] [CrossRef]
- Waldbauer, G.B. The Consumption and Utilization of Food by Insects. Adv. Insect Physiolgy 1968, 5, 229–288. [Google Scholar]
- Anthon, G.; Barrett, D.M. STANDARDIZATION OF A RAPID SPECTROPHOTOMETRIC METHOD FOR LYCOPENE ANALYSIS. Acta Hortic. 2007, 111–128. [Google Scholar] [CrossRef]
- Gkinali, A.-A.; Matsakidou, A.; Paraskevopoulou, A. Characterization of Tenebrio Molitor Larvae Protein Preparations Obtained by Different Extraction Approaches. Foods 2022, 11, 3852. [Google Scholar] [CrossRef] [PubMed]
- Laroche; Perreault; Marciniak; Gravel; Chamberland; Doyen Comparison of Conventional and Sustainable Lipid Extraction Methods for the Production of Oil and Protein Isolate from Edible Insect Meal. Foods 2019, 8, 572. [CrossRef] [PubMed]
- Tasselli, G.; Filippucci, S.; Borsella, E.; D’Antonio, S.; Gelosia, M.; Cavalaglio, G.; Turchetti, B.; Sannino, C.; Onofri, A.; Mastrolitti, S.; et al. Yeast Lipids from Cardoon Stalks, Stranded Driftwood and Olive Tree Pruning Residues as Possible Extra Sources of Oils for Producing Biofuels and Biochemicals. Biotechnol. Biofuels 2018, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Fidio, N. Di; Liuzzi, F.; Mastrolitti, S.; Albergo, R.; Bari, I. De Single Cell Oil Production from Undetoxified Arundo Donax L. Hydrolysate by Cutaneotrichosporon Curvatus. J. Microbiol. Biotechnol. 2019, 29, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Hammond, E.G. Analysis of Oleate, Linoleate and Linolenate Hydroperoxides in Oxidized Ester Mixtures. Lipids 1980, 15, 379–385. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.; Santos-Silva, F. Effect of Genotype, Feeding System and Slaughter Weight on the Quality of Light Lambs. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Zhao, X.; Vázquez-Gutiérrez, J.L.; Johansson, D.P.; Landberg, R.; Langton, M. Yellow Mealworm Protein for Food Purposes - Extraction and Functional Properties. PLoS One 2016, 11, e0147791. [Google Scholar] [CrossRef]
- NISHA, R.; RAJAVEL, D.S. Effect of Tomatine on Termites Odontotermes Wallonensis (Wasmann) Vis-a-Vis Antifeedant and Repellent Activity. Int. J. Plant Prot. 2016, 9, 97–101. [Google Scholar] [CrossRef]
- Lu, F.M.; Chu, Y.I. Antifeeding Effects Of-Tomatine on Larvae of the Diamondback Moth (Plutella Xylostella L. CHINESE J. Entomol. 1992. [Google Scholar]
- Friedman, M. Analysis of Biologically Active Compounds in Potatoes (Solanum Tuberosum), Tomatoes (Lycopersicon Esculentum), and Jimson Weed (Datura Stramonium) Seeds. J. Chromatogr. A 2004, 1054, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Zanfini, A.; Franchi, G.; Massarelli, P.; Corbini, G.; Dreassi, E. PHENOLIC COMPOUNDS, CAROTENOIDS AND ANTIOXIDANT ACTIVITY IN FIVE TOMATO (LYCOPESICON ESCULENTUM MILL.) CULTIVARS. Ital. J. FOOD Sci. 2017, 29. [Google Scholar]
- Pavela, R. The Feeding Effect of Polyphenolic Compounds on the Colorado Potato Beetle [Leptinotarsa Decemlineata (Say)]. Pest Technol. 2007, 1, 81–84. [Google Scholar]
- Kumar, M.; Umesh, K.P.; Pandey, P.P.; Firake, D.M.; Pandit, S.S. Eggplant{\textquoteright}s Foliar Chlorogenic Acid Provides Resistance against the Tropical Armyworm. bioRxiv 2023. [Google Scholar] [CrossRef]
- Diaz Napal, G.N.; Palacios, S.M. Bioinsecticidal Effect of the Flavonoids Pinocembrin and Quercetin against Spodoptera Frugiperda. J. Pest Sci. (2004) 2015, 88, 629–635. [Google Scholar] [CrossRef]
- Rho, M.S.; Lee, K.P. Geometric Analysis of Nutrient Balancing in the Mealworm Beetle, Tenebrio Molitor L. (Coleoptera: Tenebrionidae). J. Insect Physiol. 2014, 71, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Rho, M.S.; Lee, K.P. Balanced Intake of Protein and Carbohydrate Maximizes Lifetime Reproductive Success in the Mealworm Beetle, Tenebrio Molitor (Coleoptera: Tenebrionidae). J. Insect Physiol. 2016, 91–92, 93–99. [Google Scholar] [CrossRef]
- Li, L.; Stasiak, M.; Li, L.; Xie, B.; Fu, Y.; Gidzinski, D.; Dixon, M.; Liu, H. Rearing Tenebrio Molitor in BLSS: Dietary Fiber Affects Larval Growth, Development, and Respiration Characteristics. Acta Astronaut. 2016, 118, 130–136. [Google Scholar] [CrossRef]
- Morales Ramos, J.; Rojas, M.-G.; Shapiro Ilan, D.; TEDDERS, W. Developmental Plasticity in Tenebrio Molitor (Coleoptera: Tenebrionidae): Analysis of Instar Variation in Number and Development Time under Different Diets. J. Entomol. Sci. 2010, 45, 75–90. [Google Scholar] [CrossRef]
- van Broekhoven, S.; Oonincx, D.G.A.B.; van Huis, A.; van Loon, J.J.A. Growth Performance and Feed Conversion Efficiency of Three Edible Mealworm Species (Coleoptera: Tenebrionidae) on Diets Composed of Organic by-Products. J. Insect Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ramos, J.A.; Rojas, M.G.; Kelstrup, H.C.; Emery, V. Self-Selection of Agricultural By-Products and Food Ingredients by Tenebrio Molitor (Coleoptera: Tenebrionidae) and Impact on Food Utilization and Nutrient Intake. Insects 2020, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ramos, J.A.; Rojas, M.G. Effect of Larval Density on Food Utilization Efficiency of Tenebrio Molitor (Coleoptera: Tenebrionidae). J. Econ. Entomol. 2015, 108, 2259–2267. [Google Scholar] [CrossRef]
- Mattioli, S.; Paci, G.; Fratini, F.; Dal Bosco, A.; Tuccinardi, T.; Mancini, S. Former Foodstuff in Mealworm Farming: Effects on Fatty Acids Profile, Lipid Metabolism and Antioxidant Molecules. LWT 2021, 147, 111644. [Google Scholar] [CrossRef]
- Finke, M.D. Complete Nutrient Content of Four Species of Commercially Available Feeder Insects Fed Enhanced Diets during Growth. Zoo Biol. 2015, 34, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Rovai, D.; Ortgies, M.; Amin, S.; Kuwahara, S.; Schwartz, G.; Lesniauskas, R.; Garza, J.; Lammert, A. Utilization of Carrot Pomace to Grow Mealworm Larvae (Tenebrio Molitor). Sustainability 2021, 13, 9341. [Google Scholar] [CrossRef]
- Cobbs, C.; Heath, J.; Stireman, J.O.; Abbot, P. Carotenoids in Unexpected Places: Gall Midges, Lateral Gene Transfer, and Carotenoid Biosynthesis in Animals. Mol. Phylogenet. Evol. 2013, 68, 221–228. [Google Scholar] [CrossRef]
- Mein, J.R.; Dolnikowski, G.G.; Ernst, H.; Russell, R.M.; Wang, X.-D. Enzymatic Formation of Apo-Carotenoids from the Xanthophyll Carotenoids Lutein, Zeaxanthin and β-Cryptoxanthin by Ferret Carotene-9′,10′-Monooxygenase. Arch. Biochem. Biophys. 2011, 506, 109–121. [Google Scholar] [CrossRef]
- Poveda, J. Insect Frass in the Development of Sustainable Agriculture. A Review. Agron. Sustain. Dev. 2021, 41, 5. [Google Scholar] [CrossRef]
- Errico, S.; Spagnoletta, A.; Verardi, A.; Moliterni, S.; Dimatteo, S.; Sangiorgio, P. Tenebrio Molitor as a Source of Interesting Natural Compounds, Their Recovery Processes, Biological Effects, and Safety Aspects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 148–197. [Google Scholar] [CrossRef]
- Ravzanaadii, N.; Kim, S.-H.; Choi, W.-H.; Hong, S.-J.; Kim, N.-J. Nutritional Value of Mealworm, Tenebrio Molitor as Food Source. Int. J. Ind. Entomol. 2012, 25, 93–98. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.-J.; Barroso, F.G.; Manzano-Agugliaro, F. Insect Meal as Renewable Source of Food for Animal Feeding: A Review. J. Clean. Prod. 2014, 65, 16–27. [Google Scholar] [CrossRef]
- Zheng, T.; Li, H.; Han, N.; Wang, S.; Hackney Price, J.; Wang, M.; Zhang, D. Functional Characterization of Two Elongases of Very Long-Chain Fatty Acid from Tenebrio Molitor L. (Coleoptera: Tenebrionidae). Sci. Rep. 2017, 7, 10990. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.G.A.B.; Finke, M.D. Nutritional Value of Insects and Ways to Manipulate Their Composition. J. Insects as Food Feed 2021, 7, 639–659. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional Composition and Safety Aspects of Edible Insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security. FAO For. Pap. FAO, Rome, Italy. 2013, 171. Available online: http//www.fao.Org/3/i3253e/i3253e.pdf.
- Bjørge, J.D.; Overgaard, J.; Malte, H.; Gianotten, N.; Heckmann, L.-H. Role of Temperature on Growth and Metabolic Rate in the Tenebrionid Beetles Alphitobius Diaperinus and Tenebrio Molitor. J. Insect Physiol. 2018, 107, 89–96. [Google Scholar] [CrossRef]
- Hoc, B.; Genva, M.; Fauconnier, M.-L.; Lognay, G.; Francis, F.; Caparros Megido, R. About Lipid Metabolism in Hermetia Illucens (L. 1758): On the Origin of Fatty Acids in Prepupae. Sci. Rep. 2020, 10, 11916. [Google Scholar] [CrossRef]
- van Dooremalen, C.; Ellers, J. A Moderate Change in Temperature Induces Changes in Fatty Acid Composition of Storage and Membrane Lipids in a Soil Arthropod. J. Insect Physiol. 2010, 56, 178–184. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- GEORGESCU, B.; STRUȚI, D.; PĂPUC, T.; CIGHI, V.; BOARU, A. Effect of the Energy Content of Diets on the Development and Quality of the Fat Reserves of Larvae and Reproduction of Adults of the Black Soldier Fly, Hermetia Illucens (Diptera: Stratiomyidae). Eur. J. Entomol. 2021, 118, 297–306. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Z.; Ma, Y.; Qu, Y.; Lu, X.; Guo, H.; Luo, H. Effect of Dietary Lycopene Supplementation on Growth Performance, Meat Quality, Fatty Acid Profile and Meat Lipid Oxidation in Lambs in Summer Conditions. Small Rumin. Res. 2015, 131, 99–106. [Google Scholar] [CrossRef]
- Hernández-López, S.H.; Rodríguez-Carpena, J.G.; Lemus-Flores, C.; Grageola-Nuñez, F.; Estévez, M. Avocado Waste for Finishing Pigs: Impact on Muscle Composition and Oxidative Stability during Chilled Storage. Meat Sci. 2016, 116, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Biondi, L.; Luciano, G.; Cutello, D.; Natalello, A.; Mattioli, S.; Priolo, A.; Lanza, M.; Morbidini, L.; Gallo, A.; Valenti, B. Meat Quality from Pigs Fed Tomato Processing Waste. Meat Sci. 2020, 159, 107940. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Pedro, S.; Lourenço, H.; Batista, I.; Teixeira, B.; Bandarra, N.M.; Murta, D.; Nunes, R.; Pires, C. Evaluation of Tenebrio Molitor Larvae as an Alternative Food Source. NFS J. 2020, 21, 57–64. [Google Scholar] [CrossRef]
- Mercola, J.; D’Adamo, C.R. Linoleic Acid: A Narrative Review of the Effects of Increased Intake in the Standard American Diet and Associations with Chronic Disease. Nutrients 2023, 15, 3129. [Google Scholar] [CrossRef] [PubMed]
- Fasel, N.J.; Mène-Saffrané, L.; Ruczyński, I.; Komar, E.; Christe, P. Diet Induced Modifications of Fatty-Acid Composition in Mealworm Larvae (Tenebrio Molitor). J. Food Res. 2017, 6, 22–31. [Google Scholar] [CrossRef]
- Rossi, G.; Mattioli, S.; Rondoni, G.; Bosco, A.D.; Servili, M.; Castellini, C.; Conti, E. Characterisation of Fatty Acid Profiles of Tenebrio Molitor Larvae Reared on Diets Enriched with Edible Oils. J. Insects as Food Feed 2022, 8, 901–912. [Google Scholar] [CrossRef]
- Brandstetter, B.; Ruther, J. An Insect with a Delta-12 Desaturase, the Jewel Wasp Nasonia Vitripennis, Benefits from Nutritional Supply with Linoleic Acid. Sci. Nat. 2016, 103, 40. [Google Scholar] [CrossRef] [PubMed]
- Amoedo, N.D.; Punzi, G.; Obre, E.; Lacombe, D.; De Grassi, A.; Pierri, C.L.; Rossignol, R. AGC1/2, the Mitochondrial Aspartate-Glutamate Carriers. Biochim. Biophys. Acta - Mol. Cell Res. 2016, 1863, 2394–2412. [Google Scholar] [CrossRef] [PubMed]
- Romero-Lorente, M.-Á.; Fabrikov, D.; Montes, J.; Morote, E.; Barroso, F.G.; Vargas-García, M. del C.; Varga, Á.T.; Sánchez-Muros, M.-J. Pre-Treatment of Fish By-Products to Optimize Feeding of Tenebrio Molitor L. Larvae. Insects 2022, 13, 125. [Google Scholar] [CrossRef]
- Lawal, K.G.; Kavle, R.R.; Akanbi, T.O.; Mirosa, M.; Agyei, D. Enrichment in Specific Fatty Acids Profile of Tenebrio Molitor and Hermetia Illucens Larvae through Feeding. Futur. Foods 2021, 3, 100016. [Google Scholar] [CrossRef]
- Otero, P.; Gutierrez-Docio, A.; Navarro del Hierro, J.; Reglero, G.; Martin, D. Extracts from the Edible Insects Acheta Domesticus and Tenebrio Molitor with Improved Fatty Acid Profile Due to Ultrasound Assisted or Pressurized Liquid Extraction. Food Chem. 2020, 314, 126200. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.-H.; Son, Y.-J.; Kim, S.-H.; Yun, E.-Y.; Kang, H.-J.; Hwang, I.-K. Physicochemical Properties and Oxidative Stabilities of Mealworm (Tenebrio Molitor) Oils under Different Roasting Conditions. Food Sci. Biotechnol. 2016, 25, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Oppedisano, F.; Mollace, R.; Tavernese, A.; Gliozzi, M.; Musolino, V.; Macrì, R.; Carresi, C.; Maiuolo, J.; Serra, M.; Cardamone, A.; et al. PUFA Supplementation and Heart Failure: Effects on Fibrosis and Cardiac Remodeling. Nutrients 2021, 13, 2965. [Google Scholar] [CrossRef] [PubMed]
- Fedor, D.; Kelley, D.S. Prevention of Insulin Resistance by N-3 Polyunsaturated Fatty Acids. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Oppedisano, F.; Bulotta, R.M.; Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Ilari, S.; Serra, M.; Muscoli, C.; Gratteri, S.; et al. The Role of Nutraceuticals in Osteoarthritis Prevention and Treatment: Focus on n-3 PUFAs. Oxid. Med. Cell. Longev. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Hashimoto, K. Role of Soluble Epoxide Hydrolase in Metabolism of PUFAs in Psychiatric and Neurological Disorders. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Marcone, M.; Baptista, J. Health-Promoting Ingredients from Four Selected Azorean Macroalgae. Food Res. Int. 2016, 89, 432–438. [Google Scholar] [CrossRef]
- Son, Y.-J.; Choi, S.Y.; Hwang, I.-K.; Nho, C.W.; Kim, S.H. Could Defatted Mealworm (Tenebrio Molitor) and Mealworm Oil Be Used as Food Ingredients? Foods 2020, 9, 40. [Google Scholar] [CrossRef]
| By-product | Dry Matter | Crude protein | Crude Fat | Crude fiber | Ash | Carbohydrate |
|---|---|---|---|---|---|---|
| Bran | 91.2 | 16.7 | 6.5 | 36.1 | 4.2 | 30.2 |
| Tomato pomace | 92.1 | 9.5 | 3.2 | 67.1 | 3.9 | 8.9 |
| Brewer’s spent grain | 93.4 | 24.7 | 4.8 | 42 | 2.6 | 24 |
| Yeast | 93.0 | 47.6 | 2.4 | 6.8 | 8.0 | 13.8 |
| Diet | Bran (%) |
Tomato pomace (%) |
Brewer’s spent grain (%) |
Yeast (%) |
Protein value (%) |
Carbohydrate (%) |
P:C | Crude fiber (%) |
Fat (%) | Energy (kcal (100 g) |
|---|---|---|---|---|---|---|---|---|---|---|
| TP0 | 100 | - | - | - | 16.7 | 30.2 | 1:1.8 | 36.1 | 6.5 | 318.3 |
| TP27 | 50 | 27 | 23 | - | 16.6 | 23.0 | 1:1.4 | 45.8 | 5.21 | 296.9 |
| TP41 | 50 | 41 | - | 9 | 16.5 | 20.0 | 1:1.2 | 46.2 | 4.78 | 281.4 |
| TP100 | - | 100 | - | - | 9.5 | 8.9 | 1:0.9 | 67.1 | 3.2 | 236.6 |
| Diet | Survival (%) |
Growth time (d) | Larval Weight (mg) | Pupal Weight (mg) |
|---|---|---|---|---|
| TP0 | 99.5±1.6 | 32.0±5.5 a | 100.0±14.1 | 117.5±14.0 ab |
| TP27 | 100.0±0.0 | 38.5±3.9 a | 104.0±9.7 | 114.0±13.5 ab |
| TP41 | 99.5±1.6 | 37.7±5.0 a | 109.0±11.0 | 127.0±20.2 b |
| TP100 | 99.5±1.6 | 63.4±18.5 b | 91.0±17.9 | 101.0±9.9 a |
| Diet | FCR | SRG (% day-1) |
ECI (%) |
ECD (%) |
|---|---|---|---|---|
| TP0 | 2.7±0.2 a | 4.9±0.7 a | 15.4±1.1 a | 34. 6±3.3 a |
| TP27 | 3.2±0.1 b | 4.1±0.4 b | 13.1±0.5 b | 42.8±4.5 b |
| TP41 | 3.8±0.3 c | 4.3±0.4 b | 10.8±0.7 c | 30.0±6.3 a |
| TP100 | 4.3±0.4 d | 2.5±0.7 c | 9.8±1.0 d | 65.9±12.7 c |
| Diet | Feed | Mealworm | Frass | |||
|---|---|---|---|---|---|---|
| Lycopene (ug/g) |
β-Carotene (ug/g) | Lycopene (ug/g) |
β-Carotene (ug/g) | Lycopene (ug/g) |
β-Carotene (ug/g) | |
| TP0 | 2.66±0.24 | 0.30±0.07 | 0.08±0.06 | 1.43±0.99 | 0.70±0.04 | 12.09±0.26 |
| TP27 | 22.68±0.79 | 45.30±1.95 | 0.08±0.01 | 1.11±0.16 | 12.44±0.35 | 50.99±0.57 |
| TP41 | 52.43±1.71 | 95.09±0.70 | 0.61±0.33 | 2.56±0.76 | 24.10±0.32 | 76.32±1.24 |
| TP100 | 179.75±2.74 | 241.47±2.53 | 1.19±0.27 | 7.28±0.06 | 39.67±1.56 | 147.46±4.61 |
| Fatty Acid (%) | Diets | |||||
|---|---|---|---|---|---|---|
| Common Name | Lipid number | TP0 | TP27 | TP41 | TP100 | |
| Caprilic acid | C8:0 | 0 | 0 | 0 | 0 | |
| Capric acid | C10:0 | 0 | 0 | 0 | 0 | |
| Lauric acid | C12:0 | 0 | 0 | 0 | 0 | |
| Myristic acid | C14:0 | 3.68±0.04 | 3.81±0.03 | 3.42±0.12 | 2.66±0.06 | |
| Palmitic acid | C16:0 | 15.60±0.04 | 14.8±0.09 | 15.28±1.13 | 13.80±0.09 | |
| Palmitoleic acid | C16:1 | 1.56±0.27 | 4.19±0.03 | 1.44±0.29 | 1.00±0.01 | |
| Stearic acid | C18:0 | 2.89±0.20 | 3.16±0.08 | 3.38±0.17 | 5.59±0.05 | |
| Oleic acid | C18:1 | 50.20±0.20 | 44.7±0.28 | 42.87±0.229 | 26.20±1.46 | |
| α-Linoleic acid | C18:2n-6 | 25.70±0.31 | 28.9±0.32 | 32.61±1.24 | 48.10±1.27 | |
| α-Linolenic acid | C18:3n3 | 0.42±0.04 | 0.51±0.09 | 0.99±0.11 | 2.68±0.08 | |
| Arachidic acid | C20:0 | 0 | 0 | 0 | 0 | |
| Behenic acid | C22:0 | 0 | 0 | 0 | 0 | |
| Erucic acid | C22:1 | 0 | 0 | 0 | 0 | |
| Lignoceric acid | C24:0 | 0 | 0 | 0 | 0 | |
| Σ SFA | 22.14±0.07 | 21.76±0.19 | 18.71±1.25 | 22.02±0.19 | ||
| Σ MUFA | 51.75±0.19 | 48.84±0.32 | 44.32±0.52 | 27.22±1.46 | ||
| Σ PUFA | 26.10±0.19 | 29.40±0.40 | 33.60±1.35 | 50.8±1.4 | ||
| Σ UFA | 77,9±0,08 | 78,2±0,27 | 77,9±0,39 | 78±0,20 | ||
| PUFA : SFA ratio | 1.18 | 1.35 | 1.79 | 2.30 | ||
| MUFA:PUFA ratio | 1.98 | 1.66 | 1.32 | 0.53 | ||
| ω6 : ω3 ratio | 61.47 | 56.13 | 32.99 | 17.95 | ||
| Index | Diets | |||
|---|---|---|---|---|
| TP0 | TP27 | TP41 | TP100 | |
| COX | 3.2±0.43 | 3.53±0.18 | 4.01±0.3 | 5.79±0.14 |
| IT | 0.55±0.05 | 0.54±0.03 | 0.53±0.05 | 0.48±0.01 |
| IA | 0.39±0.06 | 0.38±0.01 | 0.37±0.02 | 0.31±0.02 |
| HH | 3.96±0.21 | 3.98±0.55 | 4.09±0.31 | 4.68±0.24 |
| UI | 104.4±0.78 | 108.1±0.92 | 112.5±0.15 | 131.4±0.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





