Submitted:
22 September 2023
Posted:
26 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
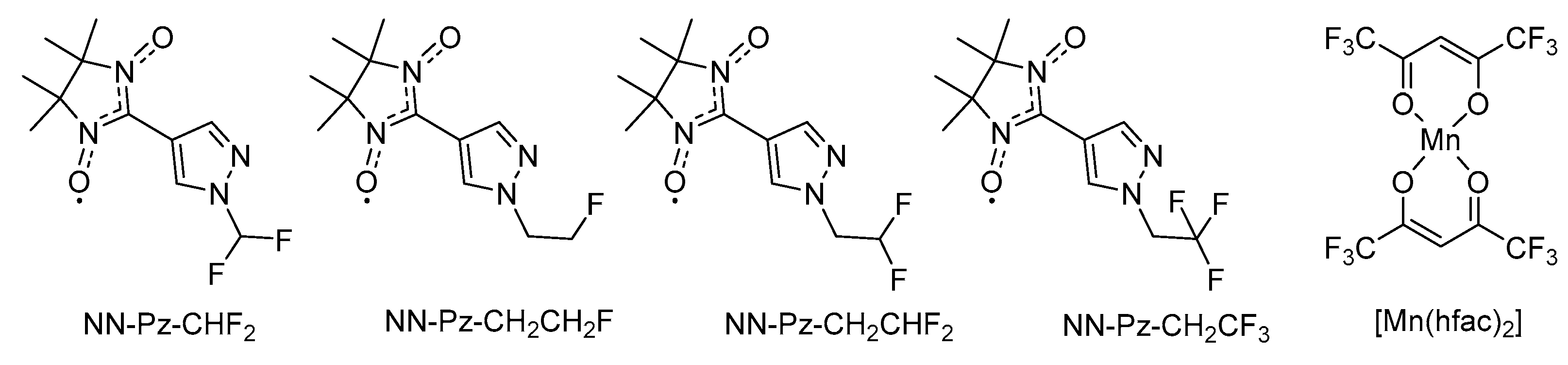
2. Materials and Methods
2.1. General procedures and materials
2.2. Synthesis of [Mn(hfac)2(NN-Pz-CHF2)]n
2.3. Synthesis of [Mn(hfac)2(NN-Pz-CH2CH2F)]n
2.4. Synthesis of [Mn(hfac)2(NN-Pz-CH2CHF2)]n⋅nC7H8 and [Mn(hfac)2(NN-Pz-CH2CHF2)]n
2.5. Synthesis of [Mn(hfac)2(NN-Pz-CH2CF3)]2⋅2C7H8
2.6. X-ray Crystallography Details
2.7. Magnetic measurements
3. Results and discussion
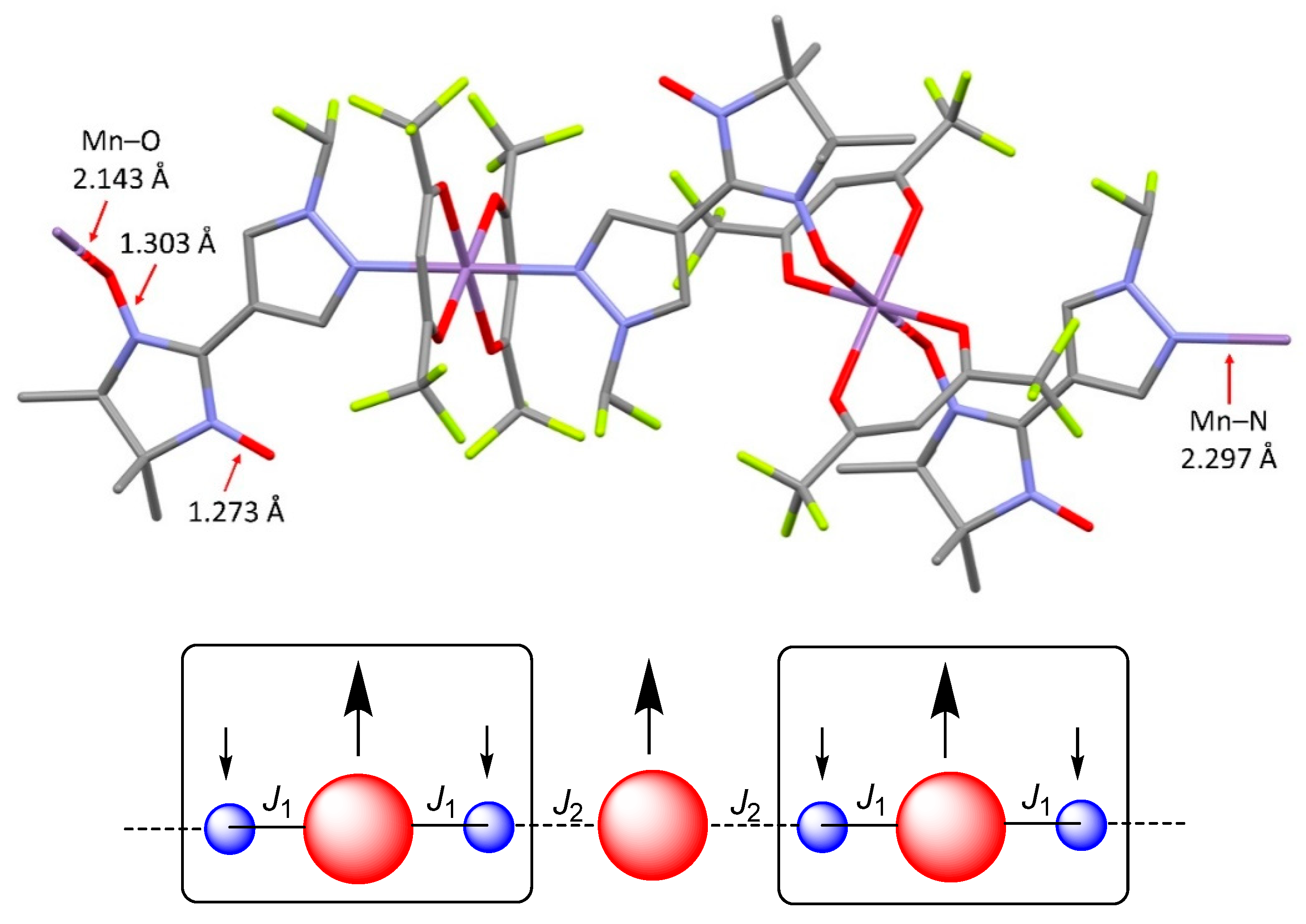
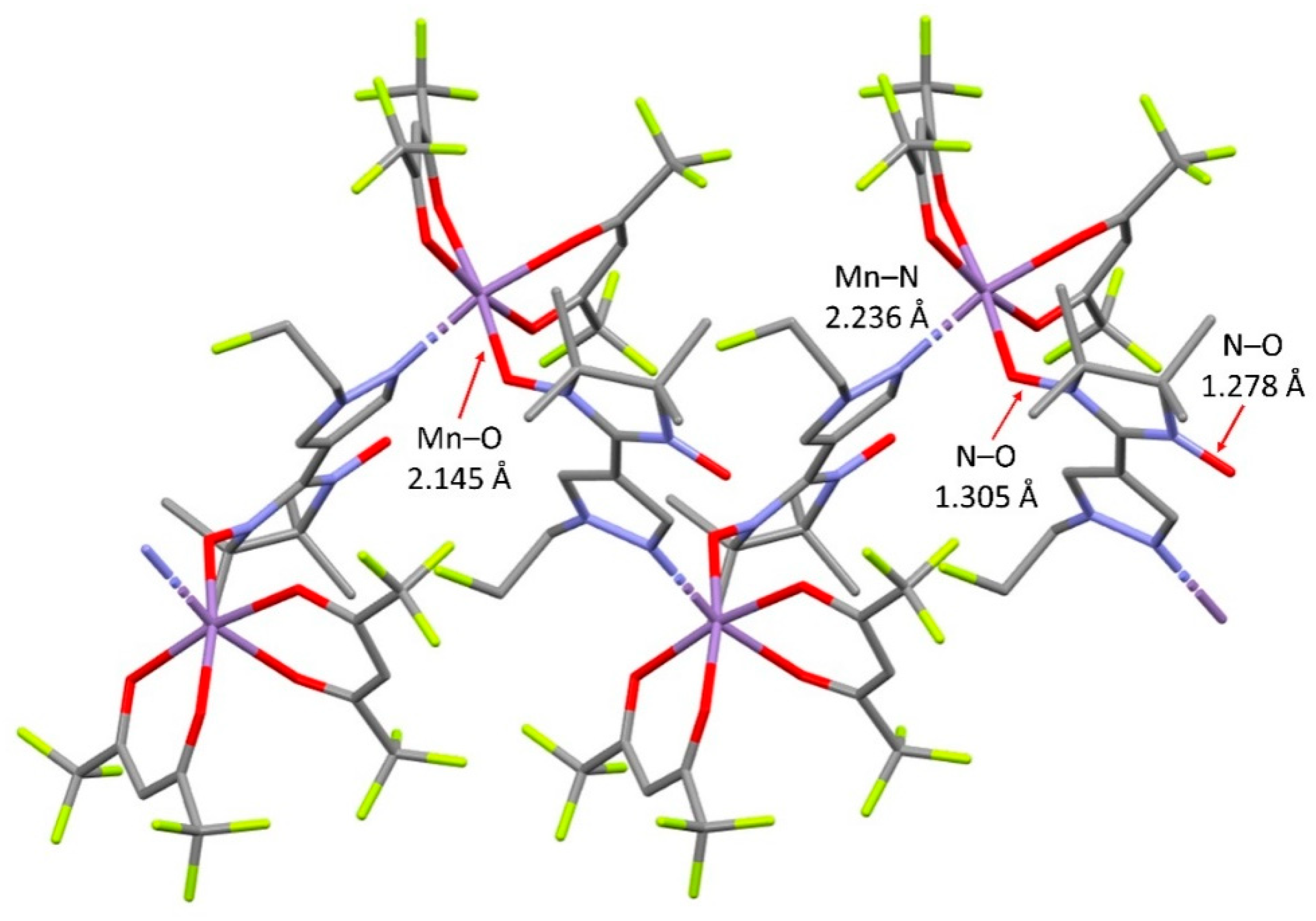
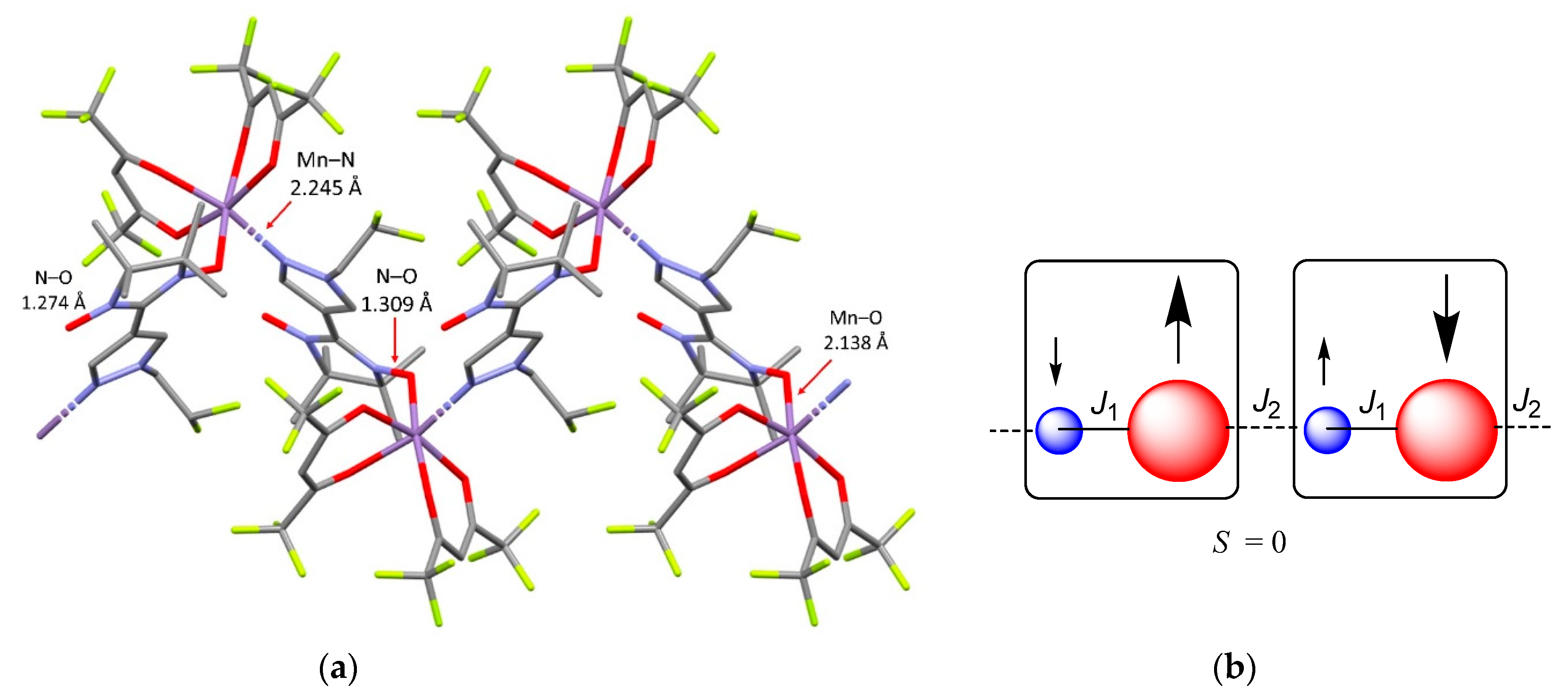
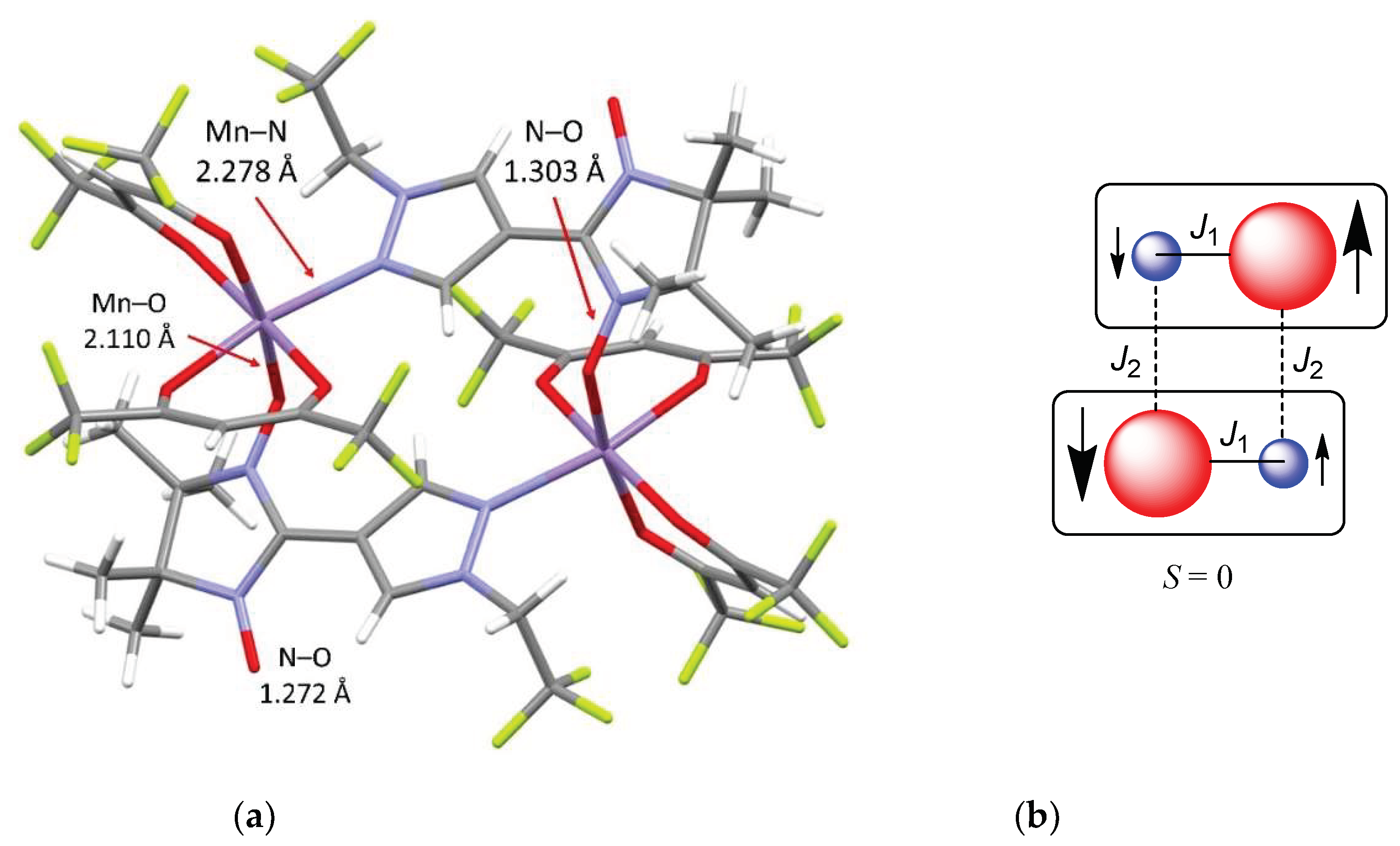
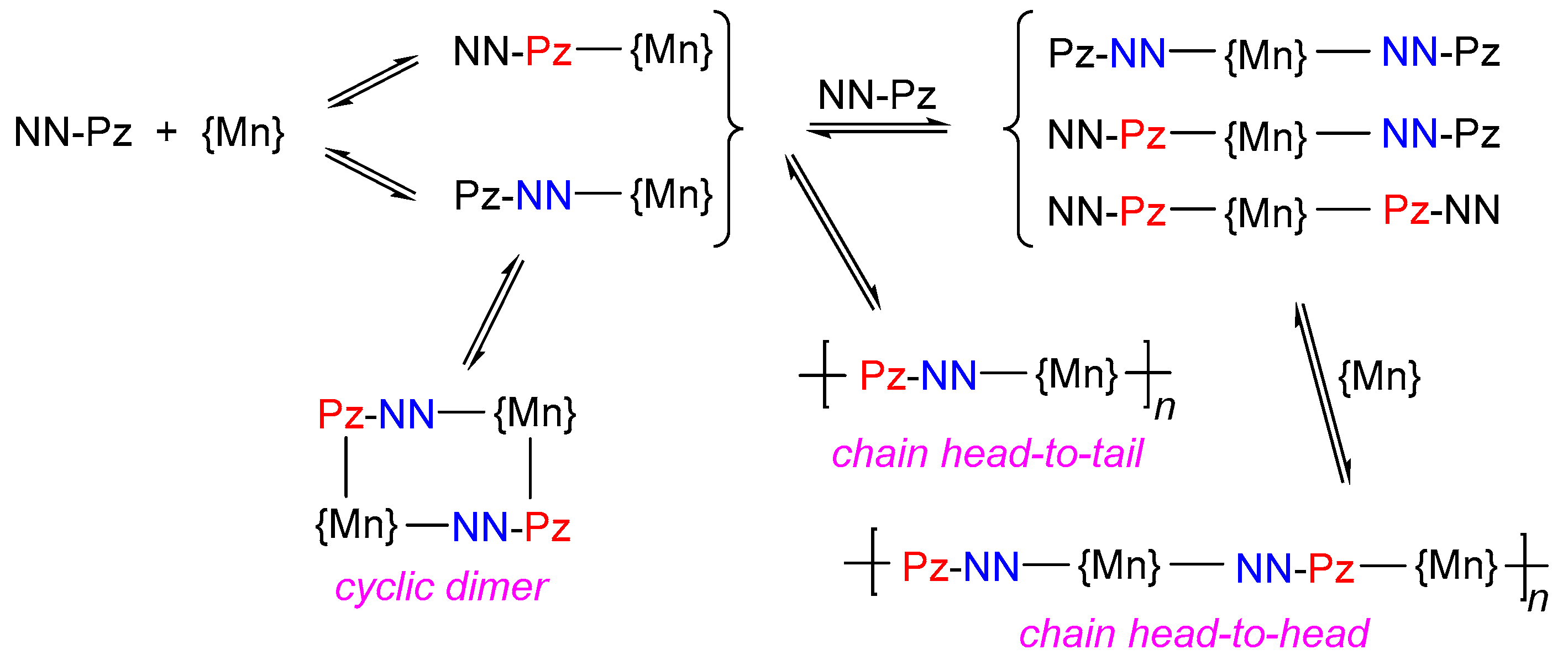
3.1. Synthesis and crystal structures of manganese–nitroxide complexes
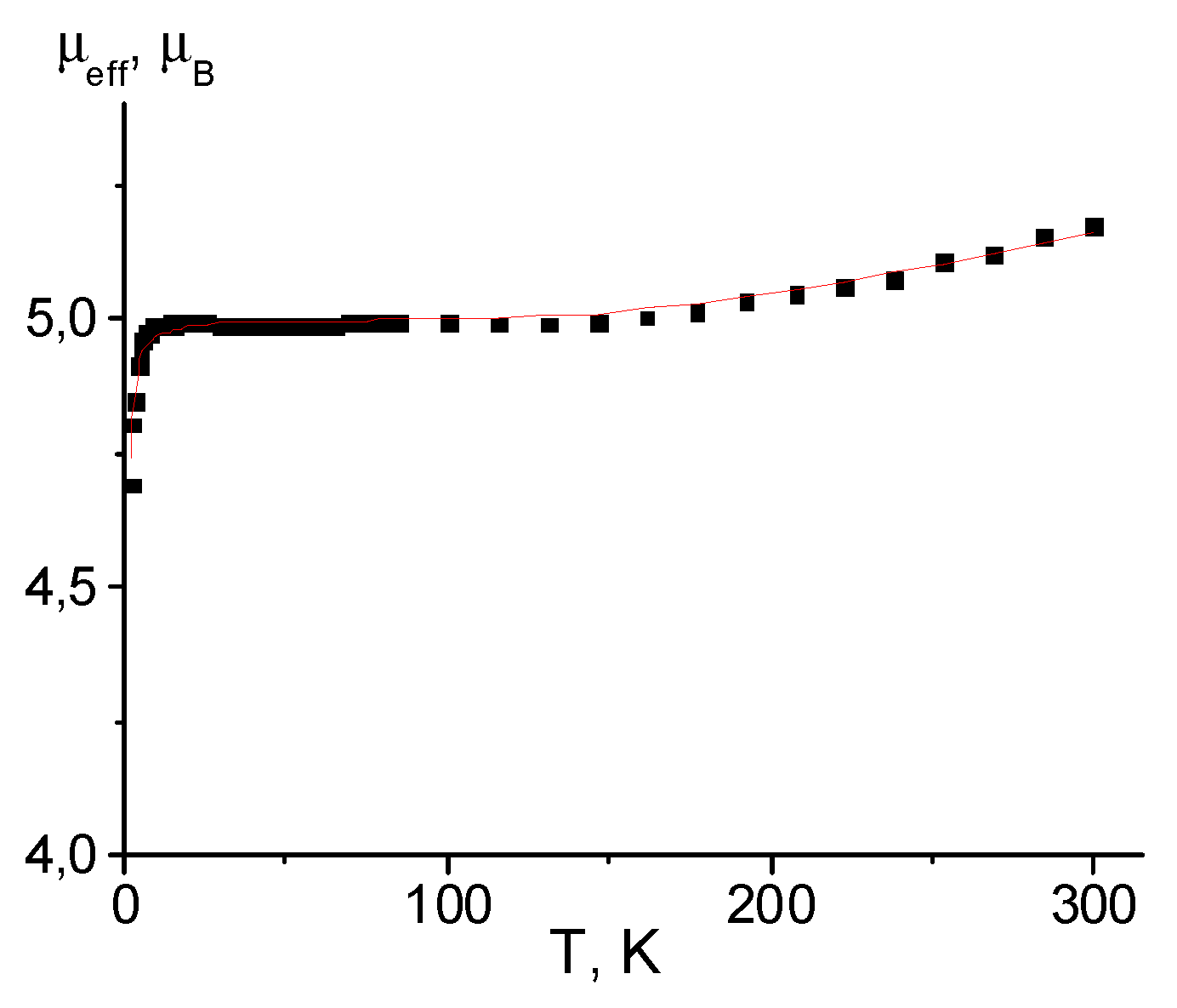
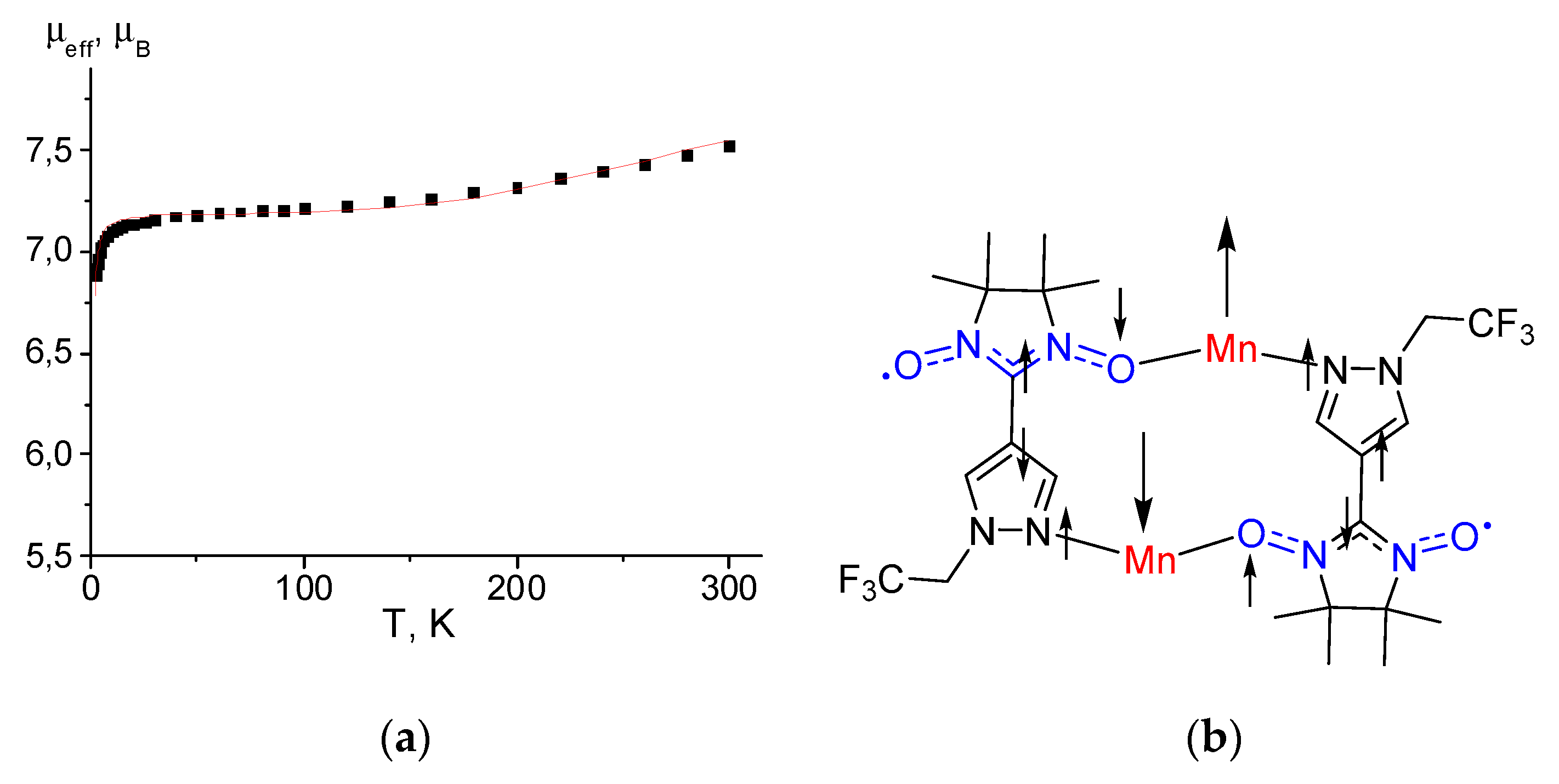
3.2. Magnetic properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benelli, C.; Gatteschi, D. Magnetism of Lanthanides in Molecular Materials with Transition-Metal Ions and Organic Radicals. Chem. Rev. 2002, 102, 2369–2388. [Google Scholar] [CrossRef] [PubMed]
- Tretyakov, E.V.; Ovcharenko, V.I.; Terent'ev, A.O.; Krylov, I.B.; Magdesieva, T.V.; Mazhukin, D.G.; Gritsan, N.P. Conjugated nitroxides. Russ. Chem. Rev. 2022, 91, RCR5025. [Google Scholar] [CrossRef]
- Ovcharenko, V. Metal-Nitroxide Complexes: Synthesis and Magnetostructural Correlations. In Stable Radicals; Hicks, R.G., Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2010; pp. 461–506. [Google Scholar] [CrossRef]
- Ferrando-Soria, J.; Vallejo, J.; Castellano, M.; Martínez-Lillo, J.; Pardo, E.; Cano, J.; Castro, I.; Lloret, F.; Ruiz-García, R.; Julve, M. Molecular magnetism, quo vadis? A historical perspective from a coordination chemist viewpoint. Coord. Chem. Rev. 2017, 339, 17–103. [Google Scholar] [CrossRef]
- Bernot, K.; Pointillart, F.; Rosa, P.; Etienne, M.; Sessoli, R.; Gattesch, D. Single molecule magnet behaviour in robust dysprosium–biradical complexes. Chem. Commun. 2010, 35, 6458–6460. [Google Scholar] [CrossRef]
- Wang, J.; Miao, H.; Xiao, Z.X.; Zhou, Y.; Deng, L.D.; Zhang, Y.Q.; Wang, X.Y. Syntheses, structures and magnetic properties of the lanthanide complexes of the pyrimidyl-substituted nitronyl nitroxide radical. Dalton Trans. 2017, 31, 10452–10461. [Google Scholar] [CrossRef]
- Kadilenko, E.M.; Gritsan, N.P.; Tretyakov, E.V.; Fokin, S.V.; Romanenko, G.V.; Bogomyakov, A.S.; Gorbunov, D.E.; Schollmeyer, D.; Baumgarten, M.; Ovcharenko, V.I. A black-box approach to the construction of metal-radical multispin systems and analysis of their magnetic properties. Dalton Trans. 2020, 49, 16916–16927. [Google Scholar] [CrossRef]
- Sun, J.; Xi, L.; Xie, J.; Wang, K.; Li, L.C.; Sutter, J.P. A loop chain and a three-dimensional network assembled from a multi-dentate nitronyl nitroxide radical and M(hfac)2 (M = CoII, CuII). Dalton Trans. 2018, 47, 14630–14635. [Google Scholar] [CrossRef]
- Li, H.D.; Sun, J.; Yang, M.; Sun, Z.; Xie, J.; Ma, Y.; Li, L.C. Functionalized nitronyl nitroxide biradical bridged one-dimensional lanthanide chains: slow magnetic relaxation in the Tb and Dy analogues. New J. Chem. 2017, 41, 10181–10188. [Google Scholar] [CrossRef]
- Haraguchi, M.; Tretyakov, E.; Gritsan, N.; Romanenko, G.; Gorbunov, D.; Bogomyakov, A.; Maryunina, K.; Suzuki, S.; Kozaki, M.; Shiomi, D.; et al. (Azulene-1,3-diyl)-bis(nitronyl nitroxide) and (Azulene-1,3-diyl)-bis(iminonitroxide) and Their Copper Complexes. Chem Asian J. 2017, 12, 2929–2941. [Google Scholar] [CrossRef]
- Barskaya, I.Y.; Veber, S.L.; Suturina, E.A.; Sherin, P.S.; Maryunina, K.Y.; Artiukhova, N.A.; Tretyakov, E.V.; Sagdeev, R.Z.; Ovcharenko, V.I.; Gritsan, N.P.; et al. Spin-state-correlated optical properties of copper(II)–nitroxide based molecular magnets. Dalton Trans. 2017, 46, 13108–13117. [Google Scholar] [CrossRef]
- Kaszub, W.; Marino, A.; Lorenc, M.; Collet, E.; Bagryanskaya, E.G.; Tretyakov, E.V.; Ovcharenko, V.I.; Fedin, M.V. Ultrafast Photoswitching in a Copper-Nitroxide-Based Molecular Magnet. Angew. Chem., Intern. Ed. 2014, 53, 10636–10640. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.D.; Fang, M.; Evans, W.J.; Long, J.R. A N23– Radical-Bridged Terbium Complex Exhibiting Magnetic Hysteresis at 14 K. J. Am. Chem. Soc. 2011, 133, 14236–14239. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.D.; Fang, M.; Evans, W.J.; Long, J.R. Strong exchange and magnetic blocking in N23−-radical-bridged lanthanide complexes. Nat. Chem. 2011, 3, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Novitchi, G.; Shova, S.; Train, C. Investigation by Chemical Substitution within 2p-3d-4f Clusters of the Cobalt(II) Role in the Magnetic Behavior of [vdCoLn]2 (vd = Verdazyl Radical). Inorg. Chem. 2022, 61, 17037–17048. [Google Scholar] [CrossRef]
- Ishii, N.; Okamura, Y.; Chiba, S.; Nogami, T.; Ishida, T. Giant coercivity in a one-dimensional cobalt-radical coordination magnet. J. Am. Chem. Soc. 2008, 130, 24–25. [Google Scholar] [CrossRef]
- Maria, G.F.V.; Cassaro, R.A.A.; Akpinar, H.; Schlueter, J.A.; Lahti, P.M.; Novak, M.A. A Cobalt Pyrenylnitronylnitroxide Single-Chain Magnet with High Coercivity and Record Blocking Temperature. Chem. Eur. J. 2014, 20, 5460–5467. [Google Scholar] [CrossRef]
- Barskaya, I. Yu.; Veber, S.L.; Fokin, S.V.; Tretyakov, E.V.; Bagryanskaya, E.G.; Ovcharenko, V.I.; Fedin, M.V. Structural specifics of light-induced metastable states in copper(II)–nitroxide molecular magnets. Dalton Trans. 2015, 44, 20883–20888. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Romanenko, G.V.; Veber, S.L.; Fedin, M.V.; Polushkin, A.V.; Tkacheva, A.O.; Ovcharenko, V.I. Cu(hfac)2 Complexes with Nitronyl Ketones Structurally Mimicking Nitronyl Nitroxides in Breathing Crystals. Aust. J. Chem. 2015, 68, 970–980. [Google Scholar] [CrossRef]
- Furui, T.; Suzuki, S.; Kozaki, M.; Shiomi, D.; Sato, K.; Takui, T.; Okada, K.; Tretyakov, E.V.; Tolstikov, S.E.; Romanenko, G.V.; et al. Preparation and Magnetic Properties of Metal-Complexes from N-t-Butyl-N-oxidanyl-2-amino-(nitronyl nitroxide). Inorg. Chem. 2014, 53, 802–809. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Fedyushin, P.A.; Panteleeva, E.V.; Stass, D.V.; Bagryanskaya, I. Yu.; Beregovaya, I.V.; Bogomyakov, A.S. Substitution of a Fluorine Atom in Perfluorobenzonitrile by a Lithiated Nitronyl Nitroxide. J. Org. Chem. 2017, 82, 4179–4185. [Google Scholar] [CrossRef]
- Tretyakov, E.; Fedyushin, P.; Panteleeva, E.; Gurskaya, L.; Rybalova, T.; Bogomyakov, A.; Zaytseva, E.; Kazantsev, M.; Shundrina, I.; Ovcharenko, V. Aromatic SNF-Approach to Fluorinated Phenyl tert-Butyl Nitroxides. Molecules 2019, 24, 4493. [Google Scholar] [CrossRef]
- Politanskaya, L.V.; Fedyushin, P.A.; Rybalova, T.V.; Bogomyakov, A.S.; Asanbaeva, N.B.; Tretyakov, E.V. Fluorinated Organic Paramagnetic Building Blocks for Cross-Coupling Reactions. Molecules 2020, 25, 5427. [Google Scholar] [CrossRef] [PubMed]
- Fedyushin, P.; Rybalova, T.; Asanbaeva, N.; Bagryanskaya, E.; Dmitriev, A.; Gritsan, N.; Kazantsev, M.; Tretyakov, E. Synthesis of Nitroxide Diradical Using a New Approach. Molecules 2020, 25, 2701. [Google Scholar] [CrossRef] [PubMed]
- Serykh, A.; Tretyakov, E.; Fedyushin, P.; Ugrak, B.; Dutova, T.; Lalov, A.; Korlyukov, A.; Akyeva, A.; Syroeshkin, M.; Bogomyakov, A.; et al. N-Fluoroalkylpyrazolyl-substituted Nitronyl Nitroxides. J. Mol. Struct. 2022, 1269, 133739. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Fedyushin, P.A. Polyfluorinated organic paramagnets. Russ. Chem. Bull. 2021, 70, 2298–2314. [Google Scholar] [CrossRef]
- Zayakin, I.A.; Korlyukov, A.A.; Gorbunov, D.E.; Gritsan, N.P.; Akyeva, A. Ya.; Syroeshkin, M.A.; Stass, D.V.; Tretyakov, E.V.; Egorov, M.P. Au–Au Chemical Bonding in Nitronyl Nitroxide Gold(I) Derivatives. Organometallics 2022, 41, 1710–1720. [Google Scholar] [CrossRef]
- Tretyakov, E.; Fedyushin, P.; Bakuleva, N.; Korlyukov, A.; Dorovatovskii, P.; Gritsan, N.; Dmitriev, A.; Akyeva, A.; Syroeshkin, M.; Stass, D.; et al. Series of Fluorinated Benzimidazole-Substituted Nitronyl Nitroxides: Synthesis, Structure, Acidity, Redox Properties, and Magnetostructural Correlations. J. Org. Chem. 2023, 88, 10355–10370. [Google Scholar] [CrossRef]
- Reichenbächer, K.; Süss, H.I.; Hulliger, J. Fluorine in crystal engineering—“the little atom that could”. Chem. Soc. Rev. 2005, 34, 22–30. [Google Scholar] [CrossRef]
- Schwarzer, A.; Weber, E. Influence of Fluorine Substitution on the Crystal Packing of N-Phenylmaleimides and Corresponding Phthalimides. Cryst. Growth Des. 2008, 8, 2862–2874. [Google Scholar] [CrossRef]
- Singla, L.; Yadav, H.R.; Choudhury, A.R. Structural and Computational Analysis of Organic Fluorine-Mediated Interactions in Controlling the Crystal Packing of Tetrafluorinated Secondary Amides in the Presence of Weak C–H···O=C Hydrogen Bonds. Cryst. Growth Des. 2022, 22, 1604–1622. [Google Scholar] [CrossRef]
- Levina, E.O.; Chernyshov, I.Y.; Voronin, A.P.; Alekseiko, L.N.; Stash, A.I.; Vener, M.V. Solving the enigma of weak fluorine contacts in the solid state: a periodic DFT study of fluorinated organic crystals. RSC Adv. 2019, 9, 12520–12537. [Google Scholar] [CrossRef] [PubMed]
- Hebeisen, P.; Weiss, U.; Alker, A.; Kuhn, B.; Müller, K.; Wang, F.; Prakash, G.K.S. Molecular Structure and Crystal Packing of Monofluoromethoxyarenes. Eur. J. Org. Chem. 2018, 3724–3734. [Google Scholar] [CrossRef]
- Berger, R.; Resnati, G.; Metrangolo, P.; Weberd, E.; Hulliger, J. Organic fluorine compounds: a great opportunity for enhanced materials properties. Chem. Soc. Rev. 2011, 40, 3496–3508. [Google Scholar] [CrossRef]
- Chopra, D.; Guru Row, T.N. Role of organic fluorine in crystal engineering. CrystEngComm 2011, 13, 2175–2186. [Google Scholar] [CrossRef]
- Cole, J.C.; Taylor, R. Intermolecular Interactions of Organic Fluorine Seen in Perspective. Cryst. Growth Des. 2022, 22, 1352–1364. [Google Scholar] [CrossRef]
- Dunitz, J.D. Organic Fluorine: Odd Man Out. ChemBioChem. 2004, 5, 614–621. [Google Scholar] [CrossRef]
- Murray, J.S.; Seybold, P.G.; Politzer, P. The Many Faces of Fluorine: Some Noncovalent Interactions of Fluorine Compounds. J. Chem. Thermodyn. 2021, 156, 106382. [Google Scholar] [CrossRef]
- Varadwaj, A.; Marques, H.M.; Varadwaj, P.R. Nature of Halogen-Centered Intermolecular Interactions in Crystal Growth and Design: Fluorine-Centered Interactions in Dimers in Crystalline Hexafluoropropylene as a Prototype. J. Comput. Chem. 2019, 40, 1836–1860. [Google Scholar] [CrossRef]
- CrysAlisPro. Version 1.171.41.106a. Rigaku Oxford Diffraction, 2021.
- Sheldrick, G.M. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 229–341. [Google Scholar] [CrossRef]
- Bruker APEX3, RLATT, CELL_NOW, TWINABS, SAINT-Plus and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA, 2016.
- Carlin, R.L. Magnetochemistry; Springer: Berlin, Germany, 1986. [Google Scholar]
- Romanenko, G.V.; Fokin, S.V.; Vasilevskii, S.F.; Tretyakov, E.V.; Shvedenkov, Yu. G.; Ovcharenko, V.I. Dimeric Complexes of Manganese(II) and Nickel(II) Hexafluoroacetylacetonates with Pyrazole-Containing Nitronylnitroxyl Radicals. Russ. J. Coord. Chem. 2001, 27, 360–367. [Google Scholar] [CrossRef]
- Maryunina, K.; Fokin, S.; Ovcharenko, V.; Romanenko, G.; Ikorskii, V. Solid solutions: An efficient way to control the temperature of spin transition in heterospin crystals MxCu1 − x(hfac)2L (M = Mn, Ni, Co; L = nitronyl nitroxide). Polyhedron 2005, 24, 2094–2101. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Sessoli, R. Magnetic properties of a layered molecular material comprising manganese hexafluoroacetylacetonate and nitronyl nitroxide radicals. Inorg. Chem. 1993, 32, 4612–4616. [Google Scholar] [CrossRef]
- Okada, K.; Nagao, O.; Mori, H.; Kozaki, M.; Shiomi, D.; Sato, K.; Takui, T.; Kitagawa, Y.; Yamaguchi, K. Preparation and Magnetic Properties of Mn(hfac)2-Complexes of 2-(5-Pyrimidinyl)- and 2-(3-Pyridyl)-Substituted Nitronyl Nitroxides. Inorg. Chem. 2003, 42, 3221–3228. [Google Scholar] [CrossRef]
- Zhu, M.; Lou, D.; Deng, X.; Zhang, L.; Zhang, W.; Lü, Y. A functional nitroxide ligand builds up two 2p–3d complexes with different spin ground states and a 2p–3d–4f chain of rings. CrystEngComm 2018, 20, 2583–2592. [Google Scholar] [CrossRef]
- Okada, K.; Beppu, S.; Tanaka, K.; Kuratsu, M.; Furuichi, K.; Kozaki, M.; Suzuki, S.; Shiomi, D.; Sato, K.; Takui, T.; et al. Preparation, structure, and magnetic interaction of a Mn(hfac)2-bridged [2-(3-pyridyl)(nitronyl nitroxide)–Mn(hfac)2]2 chain complex. Chem. Commun. 2007, 2485–2487. [Google Scholar] [CrossRef]
- Field, L.M.; Lahti, P.M.; Palacio, F. 1∶1 Complexes of 5-(4-[N-tert-butyl-N-aminoxyl]phenyl)pyrimidine with manganese(II) and copper(II) hexafluoroacetonylacetonate. Chem. Commun. 2002, 636–637. [Google Scholar] [CrossRef]
- Wang, H.-M.; Liu, Z.-L.; Liu, C.-M.; Zhang, D.-Q.; Lu, Z.-L.; Geng, H.; Shuai, Z.-G.; Zhu, D.-B. Coordination Complexes of 2-(4-Quinolyl)nitronyl Nitroxide with M(hfac)2 [M = Mn(II), Co(II), and Cu(II)]: Syntheses, Crystal Structures, and Magnetic Characterization. Inorg. Chem. 2004, 43, 4091–4098. [Google Scholar] [CrossRef]
- Zhou, N.; Wang, Y.-L.; Wang, H.-L.; Li, W.-J.; Guo, Z.; Ma, Y.; Li, L.-C.; Wang, Q.-L.; Cheng, P.; Liao, D.-Z. Structural design and magnetic properties study on two nitronyl nitroxide radicals–MnII complexes with hetero chain or mononuclear tri-spin structures. Polyhedron 2015, 89, 96–100. [Google Scholar] [CrossRef]
- Liu, R.-N.; Li, L.-C.; Xing, X.-Y.; Liao, D.-Z. Cyclic metal–radical complexes based on 2-[4-(1-imidazole)phenyl]-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide: Syntheses, crystal structures and magnetic properties. Inorganica Chim. Acta 2009, 362, 2253–2258. [Google Scholar] [CrossRef]
- Fokin, S.V.; Tolstikov, S.E.; Tret’yakov, E.V.; Romanenko, G.V.; Bogomyakov, A.S.; Veber, S.L.; Sagdeev, R.Z.; Ovcharenko, V.I. Molecular magnets based on chain polymer complexes of copper(II) bis(hexafluoroacetylacetonate) with isoxazolylsubstituted nitronyl nitroxides. Russ. Chem. Bull. 2011, 60, 2470–2484. [Google Scholar] [CrossRef]
- Yang, M.; Xie, S.; Liang, X.; Zhang, Y.; Dong, W. A novel functional nitronyl nitroxide and its manganese and cobalt complexes: Synthesis, structures and magnetic properties. Polyhedron 2019, 161, 132–136. [Google Scholar] [CrossRef]
- Wang, C.; Ma, Y.; Wang, Y.; Wang, Q.; Li, L.; Cheng, P.; Liao, D. A New Quinoxalinyl-Substituted Nitronyl Nitroxide Radical and its Five-Spin CuII and Four-Spin MnII Complexes: Syntheses, Crystal Structures, and Magnetic Properties. Aust. J. Chem. 2012, 65, 672–679. [Google Scholar] [CrossRef]
- Mei, Z.; Dingshuo, L.; Xiaochun, D.; Li, Z.; Wei, Z.; Yaohong, L. A functional nitroxide ligand builds up two 2p–3d complexes with different spin ground states and a 2p–3d–4f chain of rings. CrystEngComm 2018, 20, 2583–2592. [Google Scholar] [CrossRef]
- Maryunina, K.; Fokin, S.; Ovcharenko, V.; Romanenko, G.; Ikorskii, V. Solid solutions: An efficient way to control the temperature of spin transition in heterospin crystals MxCu1−x(hfac)2L (M = Mn, Ni, Co; L = nitronyl nitroxide). Polyhedron 2005, 24, 2094–2101. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Liu, C.-M.; Zhang, D.-Q.; Gao, S.; Zhu, D.-B. Syntheses, crystal structures, and magnetic properties of two cyclic dimer M2L2 complexes constructed from a new nitronyl nitroxide ligand and M(hfac)2 (M = Cu2+, Mn2+). Inorganica Chim. Acta 2007, 360, 3553–3559. [Google Scholar] [CrossRef]
- Borra´s-Almenar, J.J.; Clemente-Juan, J.M.; Coronado, E.; Tsukerblat, B.S. MAGPACK1 A package to calculate the energy levels, bulk magnetic properties, and inelastic neutron scattering spectra of high nuclearity spin clusters. J. Comput. Chem. 2001, 22, 985–991. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Sessoli, R.; Rey, P. Toward molecular magnets: the metal-radical approach. Acc. Chem. Res. 1989, 22, 392–398. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Sessoli, R.; Rey, P. Structure and magnetic properties of a ring of four spins formed by manganese(II) and a pyridine substituted nitronyl nitroxide. Inorganica Chim. Acta 1991, 184, 67–71. [Google Scholar] [CrossRef]
- Guo, J.-N.; Wang, J.-J.; Sun, G.-F.; Li, H.-D.; Li, L.-C. A novel nitronyl nitroxide radical containing thiophene and pyridine rings and its manganese(II) complex: synthesis, structure, and magnetic properties. J. Coord. Chem. 2017, 70, 1926–1935. [Google Scholar] [CrossRef]
- Rancurel, C.; Leznoff, D.B.; Sutter, J.-P.; Golhen, S.; Ouahab, L.; Kliava, J.; Kahn, O. Synthesis, Structure, and Magnetism of Mono- and Binuclear Manganese(II) Compounds of Nitronyl Nitroxide Substituted Phosphine Oxides. Inorg. Chem. 1999, 38, 4753–47538. [Google Scholar] [CrossRef] [PubMed]
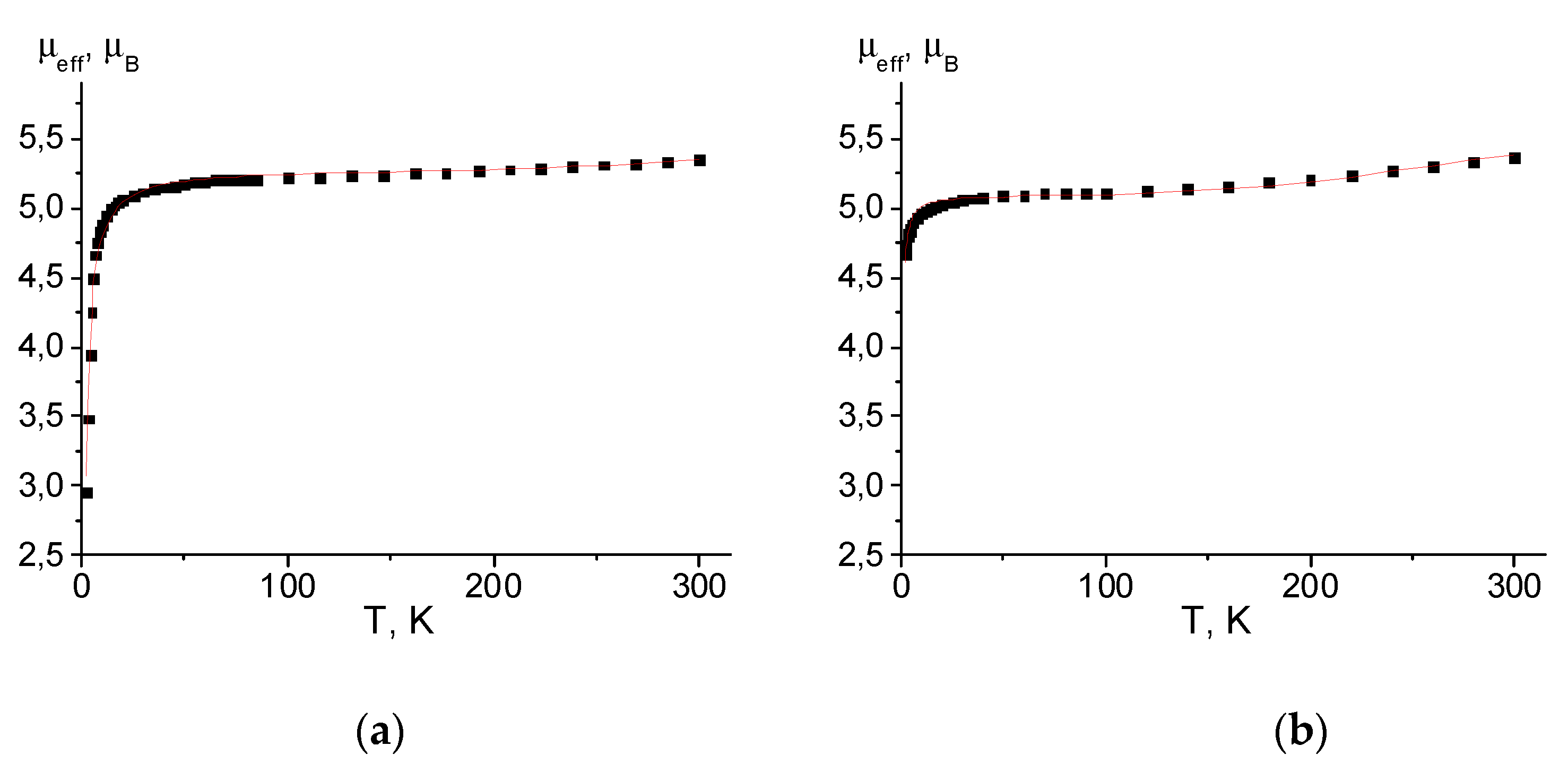
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




