Submitted:
25 September 2023
Posted:
26 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
2.2. Pigments Contents
2.3. Gas Exchange Parameters
2.4. Parameters of Fluorescence
 M m-2 s-1.
M m-2 s-1.2.5. Water potential
2.6. Statistical Analysis
3. Results
3.1. Pigments Contents
3.2. Gas Exchange Parameters
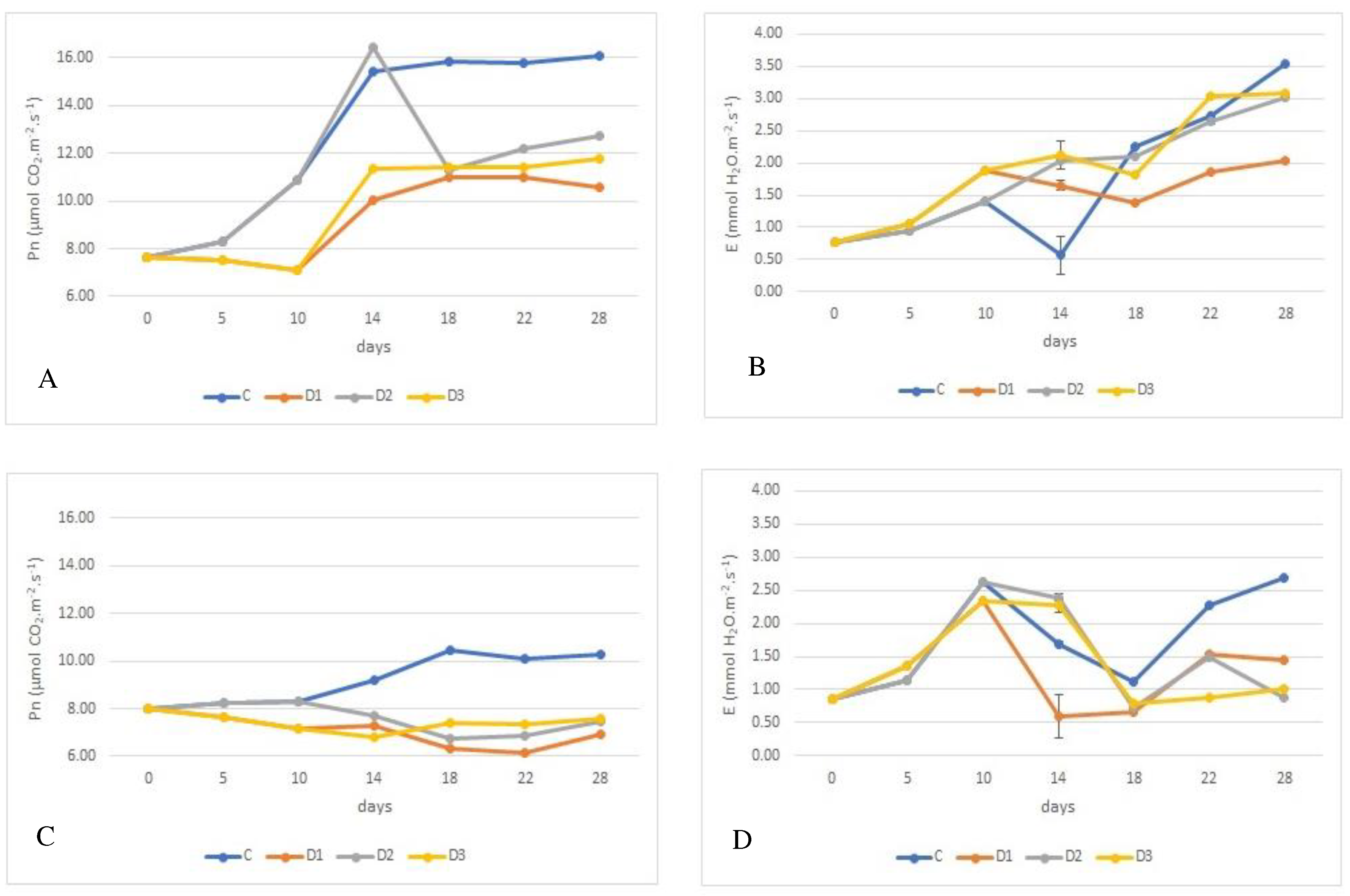
 M m-2 s-1. On the other hand, the highest rate of photosynthesis was recorded by the control plants of genotype ´29-17´ (12.213
M m-2 s-1. On the other hand, the highest rate of photosynthesis was recorded by the control plants of genotype ´29-17´ (12.213  M m-2 s-1). Photosynthesis decreased as a function of the duration of exposure drought. After rehydration, photosynthesis increased compared to the water deficit, but did not reach the values of the control plants. The highest decrease in photosynthesis due to water deficit was found in the ´Bohemia´, where rate of photosynthetic decreased by 3.243
M m-2 s-1). Photosynthesis decreased as a function of the duration of exposure drought. After rehydration, photosynthesis increased compared to the water deficit, but did not reach the values of the control plants. The highest decrease in photosynthesis due to water deficit was found in the ´Bohemia´, where rate of photosynthetic decreased by 3.243  M m-2 s-1 or 26.21% and 2.825
M m-2 s-1 or 26.21% and 2.825  M m-2 s-1 or 22.83%, respectively. Similarly, the Pn decreased in these variants in the case of the genotype ´284-17´. On the other hand, in case of genotype ´29-17´, the reduction in photosynthesis rate was conclusively the lowest. In the case of variant D2 photosynthetic rate decreased by 1.060
M m-2 s-1 or 22.83%, respectively. Similarly, the Pn decreased in these variants in the case of the genotype ´284-17´. On the other hand, in case of genotype ´29-17´, the reduction in photosynthesis rate was conclusively the lowest. In the case of variant D2 photosynthetic rate decreased by 1.060  M m-2 s-1 (3.9%) and 1.126
M m-2 s-1 (3.9%) and 1.126  M m-2 s-1 (8.74%) for variant D1.
M m-2 s-1 (8.74%) for variant D1.3.3. Parameters of Fluorescence
3.4. Water potential (ψw)
 =0.05, the lowest water potential was the lowest in the D2 variant (-1.87 MPa) and the highest in the control plants (-1.14 MPa). Lower water potential was observed for variant D1 compared to variant D3.
=0.05, the lowest water potential was the lowest in the D2 variant (-1.87 MPa) and the highest in the control plants (-1.14 MPa). Lower water potential was observed for variant D1 compared to variant D3. 3.5. Statistical analysis
| coefficient | transpiration | photosynthesis | Fv/Fm | Fv/F0 | total chlorophylls | carotenoids | water potential |
|---|---|---|---|---|---|---|---|
| constant | 1.705 | 11.005 | 0.796 | 3.98 | 7.811 | 1.256 | 1,089 |
| D1 (d1) | -0.244 | -1.678 | 0.0002 | 0.0017 | -1.553 | 0.143 | -0,416 |
| D2 (d2) | -0.086 | -0.785 | -0.0017 | -0.0540 | -1.608 | -0.072 | -0,725 |
| D3 (d3) | -0.017 | -1.655 | 0.0007 | 0.0050 | -0.462 | -0.109 | -0,282 |
| Genotype ́´284-17́ (v1) |
0.021 | -2.095 | 0.0025 | 0.0720 | 8.030 | 1.242 | -0,099 |
| genotype ́´29-17́ (v2) |
0.183 | 1.499 | 0.0039 | 0.0201 | 4.137 | 0.638 | -0,063 |
 M m-2 s-1, 0.79
M m-2 s-1, 0.79
 M m-2 s-1 and 1.65
M m-2 s-1 and 1.65  M m-2 s-1 lower than the control, respectively. Similarly, it was shown effect of genotype on changes in photosynthetic rate. Differences were found between genotypes ´284-17´ and ´29-17´ compared to the cv. ´Bohemia´, where both new cultivars the rate of photosynthetic was on average 2.09
M m-2 s-1 lower than the control, respectively. Similarly, it was shown effect of genotype on changes in photosynthetic rate. Differences were found between genotypes ´284-17´ and ´29-17´ compared to the cv. ´Bohemia´, where both new cultivars the rate of photosynthetic was on average 2.09  M m-2 s-1 and 1.5
M m-2 s-1 and 1.5  M m-2 s-1 lower.
M m-2 s-1 lower. 4. Discusion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lipiec, J.; Doussan, C.; Nosalewicz, A.; Kondracka, K. Effect of drought and heat stresses on plant growth and yield: a review. Int. Agrophys. 2013, 27(4), 463–477. [Google Scholar] [CrossRef]
- Khan, M.A.; Iqbal, M.; Akram, M.; Ahmad, M.; Hassan, M.W.; Jamil, M. Recent advances in molecular tool development for drought tolerance breeding in cereal crops: A review. Zemdirb. Agric. 2013, 100, 325–334. [Google Scholar] [CrossRef]
- Asfaw, A.; Blair, M.W. Quantification of Drought Tolerance in Ethiopian Common Bean Varieties. Agric. Sci. 2014, 5, 124–139. [Google Scholar] [CrossRef]
- Sekhon, H.S., Singh, G., Sharma, P., Bains, T.S. Water use efficiency under stress environments. In.: Yadav, S.S.; Redden, R. (eds.): Climate change and management of cool season grain legume crops. Springer. 2010, 207-227.
- Tian, L.; Li, J.; Bi, W.; Zuo, S.; Li, L.; Li, W.; Sun, L. Effects of waterlogging stress at different growth stages on the photosynthetic characteristics and grain yield of spring maize (Zea mays L.) under field conditions. Agric. Water Manag. 2019, 218, 250–258. [Google Scholar] [CrossRef]
- Bettaieb, I.; Zakhama, N.; Wannes, W.A.; Kchouk, M. , Marzouk; B. Water deficit effects on Salvia officinalis fatty acids and essential oils composition. Sci. Hortic. 2009, 120, 271–275. [Google Scholar] [CrossRef]
- Mladenov, P.; Aziz, S.; Topalova, E.; Renaut, J.; Planchon, S.; Raina, A.; Tomlekova, N. Physiological responses of common bean genotypes to drought stress. Agron. 2023, 13(4), 1022. [Google Scholar] [CrossRef]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Salami, S.A.; Babalar, M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9(1), e19250. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae. 2021, 7(3), 1–36. [Google Scholar] [CrossRef]
- Keerthi Sree, Y.; Lakra, N.; Manorama, K.; Ahlawat, Y.; Zaid, A.; Elansary, H.O.; Saved, S.R.M.; Mahmoud, E.A. Drought-Induced Morpho-Physiological, Biochemical, Metabolite Responses and Protein Profiling of Chickpea (Cicer arietinum L.). Agron. 2023, 13(7), 1814.
- Majeed, S.; Nawaz, F.; Naeem, M.; Ashraf, M.Y.; Ejaz, S.; Ahmad, K.S.; Saba, T.; Farid, G.; Khalid, I.; Mehmood, K. Nitric oxide regulates water status and associated enzymatic pathways to inhibit nutrients imbalance in maize (Zea mays L.) under drought stress. Plant Physiol. Biochem. 2020, 155, 147–160. [Google Scholar] [CrossRef]
- Ding, Y.; Nie, Y.; Chen, H.; Wang, K.; Querejeta, J.I. Water uptake depth is coordinated with leaf water potential, water-use efficiency and drought vulnerability in karst vegetation. New Phytol. 2021, 229(3), 1339–1353. [Google Scholar] [CrossRef] [PubMed]
- Hura, T.; Hura, K.; Ostrowska, A.; Urban, K. Non-rolling flag leaves use an effective mechanism to reduce water loss and light-induced damage under drought stress. Ann. Bot. 2022, 130(3), 393–408. [Google Scholar] [CrossRef] [PubMed]
- Sewore, B.M.; Abe, A.; Nigussie, M. Evaluation of bread wheat (Triticum aestivum L.) genotypes for drought tolerance using morpho-physiological traits under drought-stressed and well-watered conditions. Plos one 2023, 18(5), e0283347. [Google Scholar] [CrossRef]
- Liu, H.; Song, S.; Zhang, H.; Li, Y.; Niu, L.; Zhang, J.; Wang, W. Signaling transduction of ABA, ROS, and Ca2+ in plant stomatal closure in response to drought. Int. J. Mol. Sci. 2022, 23(23), 14824. [Google Scholar] [CrossRef]
- Onyemaobi, O.; Sangma, H.; Garg, G.; Wallace, X.; Kleven, S.; Suwanchaikasem, P.; Roessner, U.; Dolferus, R. Reproductive stage drought tolerance in wheat: Importance of stomatal conductance and plant growth regulators. Genes 2021, 12(11), 1742. [Google Scholar] [CrossRef] [PubMed]
- Khalvandi, M.; Siosemardeh, A.; Roohi, E.; Keramati, S. Salicylic acid alleviated the effect of drought stress on photosynthetic characteristics and leaf protein pattern in winter wheat. Heliyon. 2021, 7(1), e05908. [Google Scholar] [CrossRef]
- Liang, G.; Liu, J.; Zhang, J.; Guo, J. Effects of drought stress on photosynthetic and physiological parameters of tomato. J. Am. Soc. Hortic. Sci. 2020, 145(1), 12–17. [Google Scholar] [CrossRef]
- Parry, M.A.; Andralojc, P.J.; Khan, S.; Lea, P.J.; Keys, A.J. Rubisco activity: effects of drought stress. Ann. Bot. 2002, 89(7), 833–839. [Google Scholar] [CrossRef]
- Grzesiak, M.T.; Rzepka, A.; Hura, T.; Hura, K.; Skoczowski, A. Changes in response to drought stress of triticale and maize genotypes differing in drought tolerance. Photosynthetica, 2007, 45, 280–287. [Google Scholar] [CrossRef]
- Sabagh, A.E.; Hossain, A.; Islam, M.S.; Barutcular, C.; Hussain, S.; Hasanuzzaman, M.; Akram, T.; Mubeen, M.; Nasim, W.; Fahad, S.; Kumar, N.; Meena, R.S.; Ferhat, K.; Fwerhat, K.; Mehmet, Y.; Ratnasekera, D.; Saneoka, H. Drought and salinity stresses in barley: consequences and mitigation strategies. Aust. J. Crop Sci. 2019, 13(6), 810–820. [Google Scholar] [CrossRef]
- Rahim, F.P.; María Alejandra, T.T.; Víctor Manuel, Z.V.; José Elías, T.R.; Maginot, N.H. Stomatal traits and barley (Hordeum vulgare L.) forage yield in drought conditions of Northeastern Mexico. Plants 2021, 10(7), 1318. [Google Scholar] [CrossRef]
- Farooq, M.; Rizwan, M.; Nawaz, A.; Rehman, A.; Ahmad, R. Application of natural plant extracts improves the tolerance against combined terminal heat and drought stresses in bread wheat. J. Agron. Crop Sci. 2017, 203, 528–538. [Google Scholar] [CrossRef]
- Popko, M.; Michalak, I.; Wilk, R.; Gramza, M.; Chojnacka, K.; Górecki, H. Effect of the new plant growth biostimulants based on amino acids on yield and grain quality of winter wheat. Molecules. 2018, 23(2), 470. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Moustaka, J. Early drought stress warning in plants: Color pictures of photosystem II photochemistry. Climate. 2022, 10(11), 179. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, Y.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants. 2021, 10(2), 259. [Google Scholar] [CrossRef]
- Aliyeva, D.R.; Aydinli, L.M.; Pashayeva, A.N.; Zulfugarov, I.S.; Huseynova, I.M. Photosynthetic machinery and antioxidant status of wheat genotypes under drought stress followed by rewatering. Photosynthetica. 2020, 58(5), 1217–1225. [Google Scholar] [CrossRef]
- Mwadzingeni, L.; Shimelis, H.; Dube, E.; Laing, M. D.; Tsilo, T.J. Breeding wheat for drought tolerance: Progress and technologies. J. Integr. Agric. 2016, 15(5), 935–943. [Google Scholar] [CrossRef]
- Rizwan, M.; Mahboob, W.; Faheem, M.; Shimelis, H.; Hameed, A.; Sial, M.A.; Shokat, S. Can we exploit supernumerary spikelet and spike branching traits to boost bread wheat (Triticum aestivum L.) yield? Appl. Ecol. Environ. Res. 2020, 18(5), 6243–6258. [Google Scholar] [CrossRef]
- Wang, Y.; Du, F.; Wang, J.; Wang, K.; Tian, C.; Qi, X.; Lu, F.; Liz, X.; Jiao, Y. (2022). Improving bread wheat yield through modulating an unselected AP2/ERF gene. Nat. Plants. 2022, 8(8), 930–939. [Google Scholar] [CrossRef]
- Zhang, X.; Qiao, L.; Li, X.; Yang, Z.; Liu, C.; Guo, H.; Chang, Z.; Zheng, J.; Zhangm, S.; Chang, S.; Chang, L.; Chen, F.; Jia, J.; Yan, L.; Chang, Z. Genetic incorporation of the favorable alleles for three genes associated with spikelet development in wheat. Front. Plant Sci., 2022, 13, e892642. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Zhao, M.; Guo, L.; Guo, X.; Zhao, D.; Batool, A.; Dong, B.; Xu, H.; Cui, S.; Zhang, A.; Fu, X.; Jing, R.; Liu, X. Wheat FRIZZY PANICLE activates VERNALIZATION1-A and HOMEOBOX4-A to regulate spike development in wheat. Plant Biotechnol. J. 2021, 19(6), 1141–1154. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Zhang, D.; Yuan, J.; Feng, M.; Li, Z.; Wang, Z.; Zhang, Z.; Li, X.; Ke, W.; Li, R.; Chen, Z.; Chai, L.; Hu, Z.; Guo, W.; Ni, Z. FRIZZY PANICLE defines a regulatory hub for simultaneously controlling spikelet formation and awn elongation in bread wheat. New Phytol. 2021, 231(2), 814–833. [Google Scholar] [CrossRef]
- Bienkowska, T.; Suchowilska, E.; Wiwart, M. Triticum polonicum L. as promising source material for breeding new wheat cultivars. J. Elem. 2020, 25(1), 237–248. [Google Scholar]
- Romanov, B.V.; Pimonov, K.I.; Lipskij, D.D. Produkcionnye ocobennosti pšenicy Triticum petropavlovskyi. Izvestiya Niznevolzskogo agrouniversitetskogo kompleksa: nauka i vyssee professionalnoe obrazovanie. 2020, 4(60), 172–182. [only Russian].
- Porra, R.; Thompson, W.; Kriedemann, P. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta (BBA)-Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Kuklova, M.; Hnilickova, H.; Kukla, J.; Hnilicka, F. Environmental impact of the Al smelter on physiology and macronutrient contents in plants and Cambisols. Plant Soil Environ. 2015, 61, 72–78. [Google Scholar] [CrossRef]
- Banks, J.M.; Hirons, A.D. Alternative methods of estimating the water potential at turgor loss point in Acer genotypes. Plant Methods. 2019, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Radzikowska, D.; Sulewska, H.; Bandurska, H.; Ratajczak, K.; Szymańska, G.; Kowalczewski, P.Ł.; Głowicka-Wołoszyn, R. Analysis of physiological status in response to water deficit of spelt (Triticum aestivum ssp. spelta) cultivars in reference to common wheat (Triticum aestivum ssp. vulgare). Agron. 2022, 12(8), 1822. [Google Scholar] [CrossRef]
- Sayed, O.H. Chlorophyll fluorescence as a tool in cereal crop research. Photosynthetica. 2003, 41, 321–330. [Google Scholar] [CrossRef]
- Zhang, R.R.; Wang, Y.H.; Li, T.; Tan, G.F.; Tao, J.P.; Su, X.J.; Xu, S.H.; Tian, Y.S.; Xiong, A.S. Effects of simulated drought stress on carotenoid contents and expression of related genes in carrot taproots. Protoplasma, 2021, 258, 379–390. [Google Scholar] [CrossRef]
- Hussain, I.; Rasheed, R.; Ashraf, M.; Mohsin, M.; Ali, S.; Rashid, A.; Akram, M.; Nisar, J.; Riaz, M. Foliar Applied acetylsalicylic acid induced growth and key-biochemical changes in chickpea (Cicer arietinum L.) under drought stress. Dose-Response 2020, 18(4), 1–13. [Google Scholar] [CrossRef]
- Shafiq, S.; Akram, N.A.; Ashraf, M.; García-Caparrós, P.; Ali, O.M.; Latef, A.A.H.A. Influence of glycine betaine (natural and synthetic) on growth, metabolism and yield production of drought-stressed maize (Zea mays L.) plants. Plants, 2021, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Talebi, R.; Ensafi, M.H.; Baghebani, N.; Karami, E.; Mohammadi, K. Physiological responses of chickpea (Cicer arietinum) genotypes to drought stress. Environ. Exp. Biol. 2013, 11, 9–15. [Google Scholar]
- Mahdavi Mashaki, K.; Garg, V.; Nasrollahnezhad Ghomi, A.A.; Kudapa, H.; Chitikineni, A.; Zaynali Nezhad, K.; Yamchi, A.; Soltanloo, H.; Varshney, R.K.; Thudi, M. RNA-Seq analysis revealed genes associated with drought stress response in kabuli chickpea (Cicer arietinum L.). PLoS ONE. 2018, 13, e0199774. [Google Scholar] [CrossRef]
- Egert, M.; Tevini, M. Influence of drought on some physiological parameters symptomatic for oxidative stress in leaves of chives (Allium schoenoprasum). Environ. Exp. Bot. 2002, 48, 43–49. [Google Scholar] [CrossRef]
- Barboričová, M.; Filaček, A.; Vysoká, D.M.; Gašparovič, K.; Živčák, M.; Brestic, M. Sensitivity of fast chlorophyll fluorescence parameters to combined heat and drought stress in wheat genotypes. Plant, Soil and Environ. 2022, 68(7), 309–316. [Google Scholar] [CrossRef]
- Wasaya, A.; Manzoor, S.; Yasir, T.A.; Sarwar, N.; Mubeen, K.; Ismail, I.A.; Raza, A.; Rehman, A.; Hossain, A.; EL Sabagh, A. Evaluation of fourteen bread wheat (Triticum aestivum L.) genotypes by observing gas exchange parameters, relative water and chlorophyll content, and yield attributes under drought stress. Sustainability 2021, 13(9), 4799. [Google Scholar] [CrossRef]
- Ababaf, M.; Omidi, H.; Bakhshandeh, A. Changes in antioxidant enzymes activities and alkaloid amount of Catharanthus roseus in response to plant growth regulators under drought condition. Ind. Crops Prod. 2021, 167, 113505. [Google Scholar] [CrossRef]
- Ramel, F.; Mialoundama, A.S.; Havaux, M. Nonenzymic carotenoid oxidation and photooxidative stress signalling in plants. J. Exp. Bot. 2013, 64(3), 799–805. [Google Scholar] [CrossRef]
- Khalilzadeh, R.; Seyed Sharifi, R.; Jalilian, J. Antioxidant status and physiological responses of wheat (Triticum aestivum L.) to cycocel application and bio fertilizers under water limitation condition. J. Plant Interact. 2016, 11(1), 130–137. [Google Scholar] [CrossRef]
- Mohammadkhani, N.; Heidari, R. Effects of water stress on respiration, photosynthetic pigments and water. Pak. J. Biol. Sci. 2007, 10(22), 4022–4028. [Google Scholar] [CrossRef]
- Rys, M.; Szaleniec, M.; Skoczowski, A.; Stawoska, I.; Janeczko, A. FT-Raman spectroscopy as a tool in evaluation the response of plants to drought stress. Open Chem. 2015, 13(1), 1091–1100. [Google Scholar] [CrossRef]
- Taheri, Z.; Vatankhah, E.; Jafarian, V. Methyl jasmonate improves physiologicalical responses of Anchusa italica under salinity stress. South Afr. J. Bot. 2020, 0, 375–382. [Google Scholar] [CrossRef]
- Kollist, H.; Zandalinas, S.I.; Sengupta, S.; Nuhkat, M.; Kangasjärvi, J.; Mittler, R. Rapid responses to abiotic stress: priming the landscape for the signal transduction network. Trends Plant Sci. 2019, 24, 25–37. [Google Scholar] [CrossRef]
- Onyemaobi, O.; Sangma, H.; Garg, G.; Wallace, X.; Kleven, S.; Suwanchaikasem, P.; Roessner, U.; Dolferus, R. Reproductive stage drought tolerance in wheat: Importance of stomatal conductance and plant growth regulators. Genes. 2021, 12(11), 1742. [Google Scholar] [CrossRef] [PubMed]
- Dib, T.A.; Monneveux, P.; Acevedo, E.; Nachit, M.M. Evaluation of proline analysis and chlorophyll fluorescence quenching measurements as drought tolerance indicators in durum wheat (Triticum turgidum L. var. durum). Euphytica. 1994, 79, 65–73. [Google Scholar] [CrossRef]
- Kandel, S. Wheat responses, defence mechanisms and tolerance to drought stress: a review article. Int. J. Appl. Sci. Biotechnol. 2021, 8, 99–109. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Han, Z.; Feng, H.; Wang, Y.; Kang, J.; Han, X.; Wang, L.; Wang, C.; Li, H.; Ma, G. Analysis of physiological indicators associated with drought tolerance in wheat under drought and re-watering conditions. Antioxidants. 2022, 11(11), 2266. [Google Scholar] [CrossRef] [PubMed]
- Badr, A.; Brüggemann, W. Comparative analysis of drought stress response of maize genotypes using chlorophyll fluorescence measurements and leaf relative water content. Photosynthetica. 2020, 58(2), 38–645. [Google Scholar] [CrossRef]
- Pinheriro, C.; Passarinho, J.A.; Ricardo, C.P. Effect of drought and rewatering on the metabolism of Lupinus albus organs. J. Plant Physiol. 2004, 161, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef]
- Qi, M.; Liu, X.; Li, Y.; Song, H.; Yin, Z.; Zhang, F.; He, Q.; Xu, Z.; Zhou, G. Photosynthetic resistance and resilience under drought, fooding and rewatering in maize plants. Photosynth. Res. 2021, 148, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Nisa, W.; Nisa, V.; Nagoo, S.A.; Dar, Z.A. Drought tolerance mechanism in wheat: a review. Pharma Innovation, 2019, 2, 714–724. [Google Scholar]
- Qayyum, A.; Al Ayoubi, S.; Sher, A.; Bibi, Y.; Ahmad, S.; Shen, Z.; Jenks, M. Improvement in drought tolerance in bread wheat is related to an improvement in osmolyte production, antioxidant enzyme activities, and gaseous exchange. Saudi J. Biol. Sci. 2021, 28(9), 5238–5249. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Cernusak, L.A.; Song, X. Dynamic responses of gas exchange and photochemistry to heat interference during drought in wheat and sorghum. Funct. Plant Biol. 2020, 47(7), 611–627. [Google Scholar] [CrossRef]
- Katam, R.; Shokri, S.; Murthy, N.; Singh, S.K.; Suravajhala, P.; Khan, M.N.; Bahmani, M.; Sakata, K.; Reddy, K.R. Proteomics, physiological, and biochemical analysis of cross tolerance mechanisms in response to heat and water stresses in soybean. PLoS ONE. 2020, 15, e0233905. [Google Scholar] [CrossRef]
- Bakhshandeh, S.; Corneo, P.E.; Yin, L.; Dijkstra, F.A. Drought and heat stress reduce yield and alter carbon rhizodeposition of different wheat genotypes. J. Agron. Crop Sci. 2019, 205(2), 157–167. [Google Scholar] [CrossRef]
- Poudel, M.R.; Ghimire, S.; Pandey, M.P.; Dhakal, K.H.; Thapa, D.B.; Poudel, H.K. Evaluation of wheat genotypes under irrigated, heat stress and drought conditions. J Biol Today's World. 2020, 9(1), 1–12.
- Papageorgiou, G.C., Stamatakis, K.2004). Water and solute transport in cyanobacteria as probed by chlorophyll fluorescence. In Chlorophyll a fluorescence: a signature of photosynthesis (pp. 663–678). Dordrecht: Springer Netherlands.
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Peterson, R. , Havir, E. Photosynthetic properties of an Arabidopsis thaliana mutant possessing a defective PsbS gene. Planta. 2001, 214, 142–152. [Google Scholar] [CrossRef]
- Afshar Mohamadian, M.; Omidipour, M.; Jamal Omidi, F. Effect of different drought stress levels on chlorophyll fluorescence indices of two bean cultivars. J. Plant Res. (Iran. J. Biol.). 2018, 31, 511–525. [Google Scholar]
- Wu, X.; Tang, Y.; Li, C.; Wu, C.; Huang, G. Chlorophyll fluorescence and yield responses of winter wheat to waterlogging at different growth stages. Plant Prod. Sci. 2015, 18, 284–294. [Google Scholar] [CrossRef]
- Sun, Z.W.; Ren, L.K.; Fan, J.W.; Li, Q.; Wang, K.J.; Guo, M.M.; Li, J.; Zhang, G.X.; Yang, Z.Y.; Chen, F.; Li, X.N. Salt response of photosynthetic electron transport system in wheat cultivars with contrasting tolerance. Plant, Soil and Environ. 2016, 62(11), 515–521. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, J. Effects of water stress on photosynthesis, chlorophyll fluorescence and photoinhibition in wheat plants. Funct. Plant Biol. 1998, 25, 883–892. [Google Scholar] [CrossRef]
- Gilmore, A.; Björkman, O. Temperature-sensitive coupling and uncoupling of ATPase-mediated, nonradiative energy dissipation: Similarities between chloroplasts and leaves. Planta. 1995, 197, 646–654. [Google Scholar] [CrossRef]
- Zlatev, Z. Drought-induced changes in chlorophyll fluorescence of young wheat plants. Biotechnol. Biotechnol. Equip. 2009, 23, 438–441. [Google Scholar] [CrossRef]
- Simova-Stoilova, L.; Pecheva, D.; Kirova, E. Drought stress response in winter wheat varieties –changes in leaf proteins and proteolytic activities. Acta Bot. Croat. 2020, 79, 121–130. [Google Scholar] [CrossRef]
- Larouk, C.; Gabon, F.; Kehel, Z.; Djekoun, A.; Nachit, M.; Amri, A. Chlorophyll fluorescence and drought tolerance in a mapping population of durum wheat. Curr. Agric. Res. J 2021, 70(3-4), 123–134. [Google Scholar] [CrossRef]
- Zlatev, Z.S. Drought-induced changes and recovery of photosynthesis in two bean cultivars (Phaseolus vulgaris L.). Emir. J. Food Agric. 2013, 25, 1014–1023. [Google Scholar] [CrossRef]
 M m-2 s-1 and rate of transpiration (E) - mM m-2 s-1, depending on wheat genotype, with photosynthetic rates shown in graphs A (´Bohemia´), C (´284-17´), and E (´ 29-17´). Transpiration rates are shown in graphs B (´Bohemia´), D (´284-17´), and F (´29-17´). The lines in the graphs indicate the standard error (S.E.) values at the
M m-2 s-1 and rate of transpiration (E) - mM m-2 s-1, depending on wheat genotype, with photosynthetic rates shown in graphs A (´Bohemia´), C (´284-17´), and E (´ 29-17´). Transpiration rates are shown in graphs B (´Bohemia´), D (´284-17´), and F (´29-17´). The lines in the graphs indicate the standard error (S.E.) values at the  = 0.05 significance level.
= 0.05 significance level.
 M m-2 s-1 and rate of transpiration (E) - mM m-2 s-1, depending on wheat genotype, with photosynthetic rates shown in graphs A (´Bohemia´), C (´284-17´), and E (´ 29-17´). Transpiration rates are shown in graphs B (´Bohemia´), D (´284-17´), and F (´29-17´). The lines in the graphs indicate the standard error (S.E.) values at the
M m-2 s-1 and rate of transpiration (E) - mM m-2 s-1, depending on wheat genotype, with photosynthetic rates shown in graphs A (´Bohemia´), C (´284-17´), and E (´ 29-17´). Transpiration rates are shown in graphs B (´Bohemia´), D (´284-17´), and F (´29-17´). The lines in the graphs indicate the standard error (S.E.) values at the  = 0.05 significance level.
= 0.05 significance level.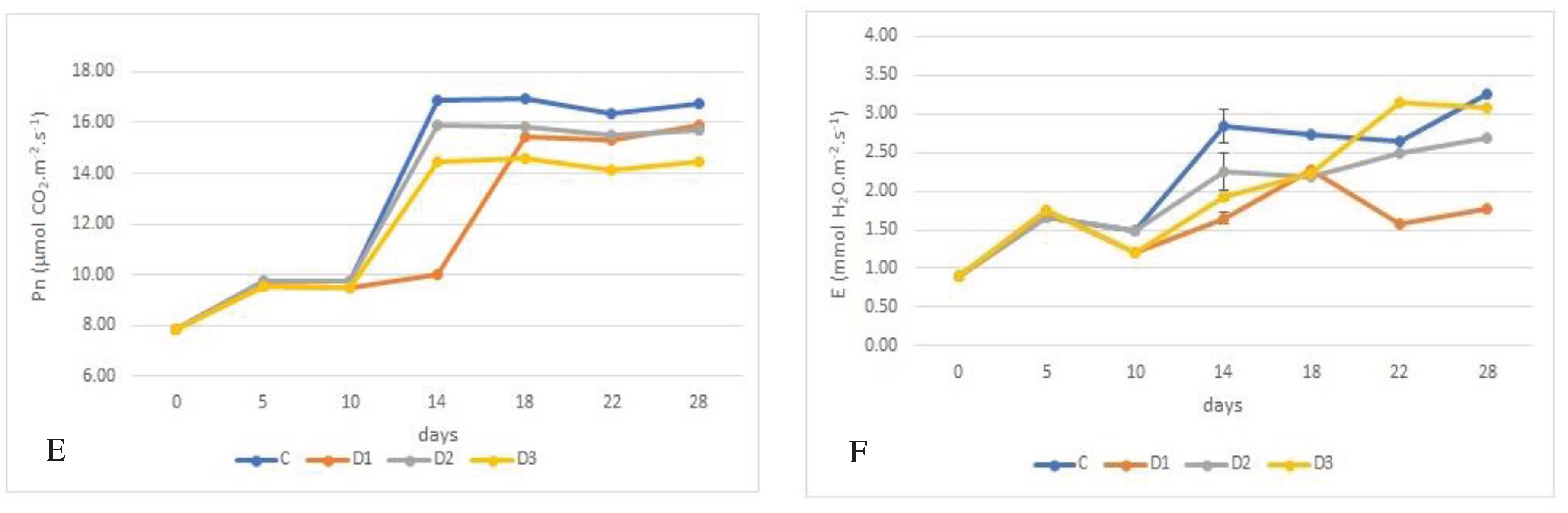
 = 0.05 significance level.
= 0.05 significance level.
 = 0.05 significance level.
= 0.05 significance level.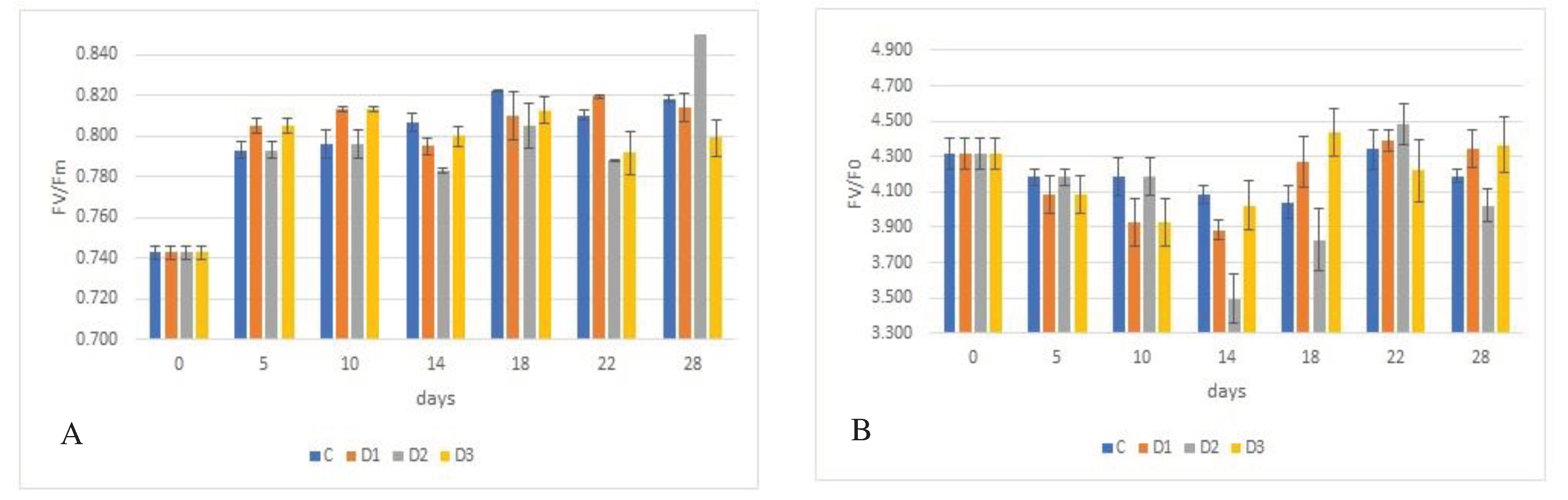
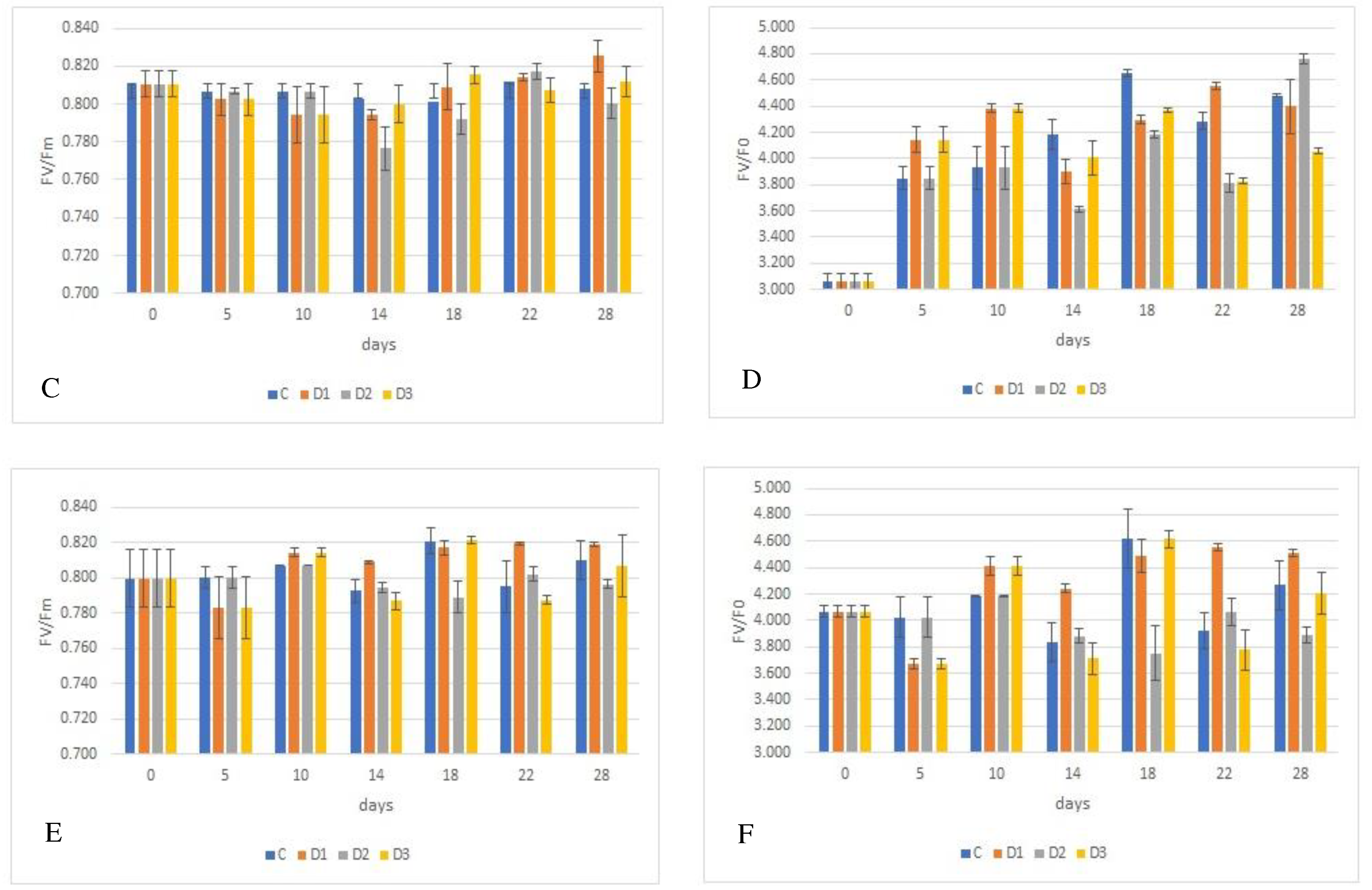
 = 0.05.
= 0.05.
 = 0.05.
= 0.05.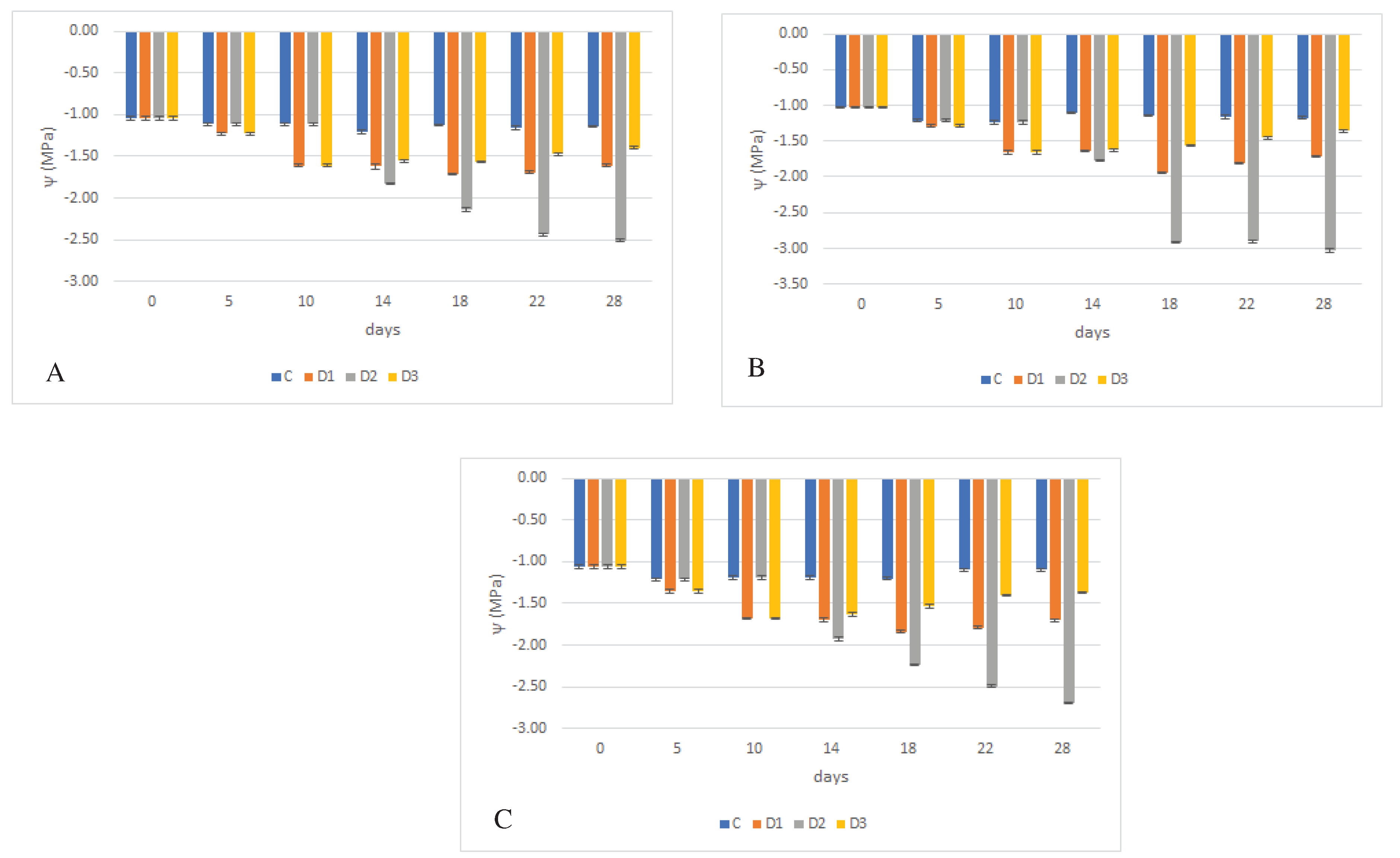
| Variant | Day of experiment | |||||
|---|---|---|---|---|---|---|
| 0 – 5th | 6th – 10th | 10th – 14th | 14th – 19th | 19th – 24th | 24th – 28th | |
| Control (C) | irrigation | irrigation | irrigation | irrigation | irrigation | irrigation |
| Drought 1 (D1) | drought | drought | irrigation | drought | drought | irrigation |
| Drought 2 (D2) | irrigation | irrigation | drought | drought | drought | drought |
| Drought 3 (D3) | drought | drought | irrigation | irrigation | irrigation | irrigation |
|
Variant |
Days |
´Bohemia´ | ´287-17´ | ´29-17´ | |||
| Chltot | Car | Chltot | Car | Chltot | Car | ||
|
C |
0 | 5.368 ± 0.133 | 0.889 ± 0.030 | 15.118 ± 0.059 | 2.271 ± 0.031 | 9.027 ± 0.067 | 1.330 ± 0.005 |
| 5 | 5.860 ± 0.480 | 1.001 ± 0.084 | 15.509 ± 0.926 | 2.102 ± 0.130 | 11.277 ± 0.837 | 1.558 ± 0.153 a, b | |
| 10 | 7.027 ± 0.064 | 1.201 ± 0.014 | 15.279 ± 1.541 | 2.636 ± 0.223 | 11.801 ± 0.325 | 1.934 ± 0.041 | |
| 14 | 7.596 ± 0.738 | 1.223 ± 0.124 | 16.698 ± 5.123 | 2.571 ±0.637 d | 11.944 ± 1.376 | 1.912 ± 0.268 a, b, c | |
| 18 | 7.962 ± 0.246 | 1.343 ± 0.042 | 17.232 ± 0.596 | 2.789 ± 0.102 | 12.511 ± 0.549 | 1.923 ± 0.072 | |
| 22 | 8.881 ± 0.483 c, d | 1.471 ± 0.057 | 17.490 ± 0.795 | 2.862 ± 0.344 | 13.635 ± 0.317 | 2.085 ± 0.079 | |
| 28 | 9.468 ± 0.434 a | 1.524 ± 0.077 a, b, c | 19.948 ± 6.642 f | 3.288 ± 0.207 f | 13.703 ± 0.618 | 2.230 ± 0.021 b, c, d | |
|
D1 |
0 | 5.368 ± 0.133 | 0.889 ± 0.030 | 15.118 ± 0.059 c | 2.271 ± 0.031 | 9.027 ± 0.067 d | 1.330 ± 0.005 a |
| 5 | 5.266 ± 0.448 | 0.890 ± 0.048 | 14.496 ± 0.295 | 2.355 ± 0.076 | 11.012 ± 0.081 | 1.791 ± 0.034 | |
| 10 | 5.086 ± 0.377 a | 0.979 ± 0.080 b | 14.065 ± 1.361 | 2.181 ± 0.226 | 10.995 ± 1.589 | 1.797 ± 0.239 | |
| 14 | 7.599 ± 0.740 | 1.181 ± 0.102 | 15.400 ± 0.026 | 3.306 ± 0.077 | 11.674 ± 0.021 | 3.214 ± 0.044 | |
| 18 | 7.487 ± 0.180 | 1.148 ± 0.036 | 13.141 ± 0.001 | 3.190 ± 0.162 | 11.666 ± 0.027 | 3.213 ± 0.042 e | |
| 22 | 7.293 ± 0.786 a, b, c | 1.182 ± .0132 | 12.351 ± 0.015 | 3.130 ± 0.126 | 10.388 ± 0.004 | 2.902 ± 0.017 d, e | |
| 28 | 8.638 ± 0.378 b | 1.425 ± 0.076 | 11.597 ± 0.011 | 2.580 ± 1.002 | 9.683 ± 0.012 | 2.745 ± 052 c, d, e | |
|
D2 |
0 | 5.369 ± 0.133 | 0.889 ± 0.030 | 15.118 ± 0.059 | 2.271 ± 0.031 | 9.027 ± 0.067 | 1.330 ± 0.005 |
| 5 | 5.860 ± 0.480 a, b, c | 1.001 ± 0.084 c | 15.509 ± 0.926 | 2.102 ± 0.130 | 11.277 ± 0.837 | 1.556 ± 0.153 | |
| 10 | 7.027 ± 0.064 | 1.201 ± 0.014 | 15.279 ± 1.541 | 2.636 ± 0.223 | 11.801 ± 0.325 | 1.934 ± 0.041 | |
| 14 | 8.090 ± 1.618 a | 1.279 ± 0.318 | 13.923 ± 0.863 b | 2.201 ± 0.132 | 11.232 ± 0.481 | 1.792 ± 0.078 | |
| 18 | 6.960 ± 0.010 | 2.472 ± 0.206 a, d | 13.366 ± 0.032 | 2.191 ± 0.398 | 10.693 ± 1.018 e | 1.646 ± 0.160 | |
| 22 | 6.898 ± 0.031 a, b, c, d | 2.576 ± 0.335 a | 10.915 ± 0.004 | 2.962 ± 0.221 e | 10.307 ± 0.532 | 1.591 ± 0.114 | |
| 28 | 6.061 ± 0.173 | 1.097 ± 0.007 | 10.570 ± 1.227 a | 1.803 ± 0.031 a | 9.676 ± 1.158 | 1.597 ± 0.118 | |
|
D3 |
0 | 5.368 ± 0.133 a, b | 0.889 ± 0.030 | 15.118 ± 0.059 | 2.271 ± 0.076 | 9.027 ± 0.067 | 1.330 ± 0.005 |
| 5 | 5.266 ± 0.448 | 0.890 ± 0.048 | 14.496 ± 0.295 | 2.355 ± 0.226 | 11.012 ± 0.081 f | 1.791 ± 0.034 | |
| 10 | 5.086 ± 0.377 | 0.979 ± 0.080 b | 14.065 ± 1.361 | 2.181 ± 0.180 b | 10.995 ± 1.589 | 1.797 ± 0.239 | |
| 14 | 8.605 ± 0.986 b, c, d | 1.465 ± 0.124 | 14.380 ± 1.005 | 2.256 ± 0.015 | 11.224 ± 2.353 | 1.796 ± 0.446 | |
| 18 | 8.711 ± 1.313 c, d | 1.456 ± 0.149 | 16.456 ± 2.241 e | 2.352 ± 0.042 c | 11.860 ± 0.929 | 1.714 ± 0.101 | |
| 22 | 9.151 ± 0.356 a | 1.493 ± 0.052 a, b, c | 16.959 ± 0.012 | 2.360 ± 0.184 | 12.916 ± 1.963 b | 2.021 ± 0.310 | |
| 28 | 9.788 ± 1.784 a | 1.617 ± 0.257 a, c | 17.361 ± 2.708 d | 2.568 ± 0.327 | 13.443 ± 2.366 a | 2.046 ± 0.320 | |
| variant | days | ´Bohemia´ | ´284-17´ | ´29-17´ |
|---|---|---|---|---|
| 0 | 0.010 ± 0 a | 0.010 ± 0 a | 0.010 ± 0 a | |
| 5 | 0.138 ± 0.004 f, g | 0.142 ± 0.003 c, d | 0.220 ± 0.005 d | |
| 10 | 0.140 ± 0.003 f, g | 0.146 ± 0.006 c, d | 0.282 ± 0.005 f | |
| C | 14 | 0.097 ± 0.008 d | 0.152 ± 0.010 c, d | 0.258 ± 0.015 e. f |
| 18 | 0.097 ± 0.005 d, e | 0.126 ± 0.009 b, c, d | 0.131 ± 0.007 c | |
| 22 | 0.050 ± 0.004 a, b, c | 0.031 ± 0.002 a, b | 0.041 ± 0.002 a. b | |
| 28 | 0.087 ± 0.003 c, d, e | 0.019 ± 0.002 a | 0.070 ± 0.004 a. b | |
| 0 | 0.010 ± 0 a | 0.010 ± 0 a | 0.010 ± 0 a | |
| 5 | 0.123 ± 0.006 f, g | 0.164 ± 0.030 d | 0.231 ± 0.005 d. e | |
| 10 | 0.147 ± 0.009 e, f | 0.158 ± 0.005 c, d | 0.270 ± 0.006 f | |
| D1 | 14 | 0.015 ± 0.001 a | 0.015 ± 0.001 a | 0.015 ± 0.001 a |
| 18 | 0.160 ± 0.009 g | 0.119 ± 0.006 b, c, d | 0.217 ± 0.009 d | |
| 22 | 0.025 ± 0.002 a | 0.071 ± 0.004 a, b, c | 0.028 ± 0.002 a. b | |
| 28 | 0.042 ± 0.002 a, b, c | 0.050 ± 0.003 a, b | 0.035 ± 0.003 a. b | |
| 0 | 0.010 ± 0 a | 0.010 ± 0 a | 0.010 ± 0 a | |
| 5 | 0.138 ± 0.004 f, g | 0.142 ± 0.003 c, d | 0.220 ± 0.005 d | |
| 10 | 0.140 ± 0.003 f, g | 0.146 ± 0.006 c, d | 0.282 ± 0.005 f | |
| D2 | 14 | 0.020 ± 0 a | 0.028 ± 0.001 a | 0.024 ± 0.001 a |
| 18 | 0.251 ± 0.005 h | 0.202 ± 0.035 | 0.026 ± 0.001 a | |
| 22 | 0.036 ± 0.002 a, b | 0.031 ± 0.003 a, b | 0.026 ± 0.003 a. b | |
| 28 | 0.047 ± 0.003 a, b, c | 0.047 ± 0.003 a, b | 0.033 ± 0.003 a. b | |
| 0 | 0.010 ± 0 a | 0.010 ± 0 a | 0.010 ± 0 a | |
| 5 | 0.123 ± 0.006 e, f | 0.164 ± 0.030 d | 0.231 ± 0.005 f | |
| 10 | 0.147 ± 0.009 f, g | 0.158 ± 0.005 d | 0.270 ± 0.006 d. e | |
| D3 | 14 | 0.026 ± 0.001 a | 0.026 ± 0.001 a | 0.020 ± 0 a |
| 18 | 0.023 ± 0.002 a | 0.018 ± 0.001 a | 0.020 ± 0 a | |
| 22 | 0.042 ± 0.003 a, b, c | 0.031 ± 0.002 a, b | 0.040 ± 0.004 a. b | |
| 28 | 0.075 ± 0.003 b, c, d | 0.020 ± 0.002 a | 0.050 ± 0.003 a. b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





