1. Introduction
Hepatocellular carcinoma (HCC) is a major cause of cancer-related deaths worldwide and its incidence is steadily increasing.[
1] Despite progress in the treatment and surveillance of HCC, its incidence and mortality rates continue to increase.[
2] Early detection of HCC is important to reduce mortality by providing more opportunities for curative treatment. However, many patients are diagnosed with HCC at an intermediate or advanced stage, when curative treatment is not feasible. Most clinical practice guidelines recommend locoregional treatment (LRT) for patients with HCC who are ineligible for curative treatment.[3-5] Transarterial chemoembolization (TACE) is the most commonly used LRT worldwide. Despite its widespread use, TACE has several drawbacks, including the need for multiple treatments, variable effectiveness, and difficulties in implementation in advanced diseases with vascular invasion.
Transarterial radioembolization (TARE) is a type of radiation therapy that involves the selective injection of microspheres containing Yttrium-90 (Y90) into hepatic arteries. Y90 is a pure beta-emitter that enables the delivery of high radiation doses to tumor cells while preserving adjacent hepatic tissues.[
6] Additionally, Y90 treatment utilizes small-sized (20-35 μm) microspheres, which have minimal impact on hepatic arterial blood flow, thus allowing for its administration to patients with portal vein invasion. Given the aforementioned advantages, TARE is becoming an increasingly promising LRT option that can overcome the drawbacks of TACE.
The 2018 American Association for the Study of Liver Diseases guidelines for HCC stated that TARE can be used to treat patients at various stages of HCC.[
4] In early-stage HCC, TARE can be curative, whereas in the intermediate to advanced stages, it can serve as a bridge or downstaging treatment option. However, owing to the diverse stages of patients receiving TARE, treatment outcomes and prognoses can vary widely and are difficult to predict.
Sarcopenia is characterized by the progressive loss of skeletal muscle mass and function associated with aging or chronic diseases. Sarcopenia has been shown to be associated with unfavorable outcomes in various cancers, including HCC.[7-10] It can negatively impact liver function, increase inflammation throughout the body, decrease tolerance to treatments, and lower the quality of life in patients with HCC. However, the impact of sarcopenia on the survival and tumor response in patients undergoing TARE remains unclear.
This study aimed to identify prognostic factors in patients with HCC who underwent TARE and to evaluate the impact of pre-treatment sarcopenia on treatment response, overall survival (OS), and progression-free survival (PFS).
2. Materials and Methods
Study population
This retrospective, multicenter, observational study included consecutive patients with HCC who underwent TARE at five referral centers in Korea. The study was conducted in accordance with the 1975 Declaration of Helsinki and approved by the local Ethics Committees of each participating center. The study included all adult patients from each participating center who were treated with TARE between July 2009 and May 2019 and underwent an abdominal computed tomography (CT) scan capturing L3 within 1 month of treatment. The exclusion criteria for the study included a previous history of systemic chemotherapy for HCC, extrahepatic metastasis, an Eastern Cooperative Oncology Group performance status (ECOG PS) greater than 1, or a pathologically confirmed diagnosis of combined hepatocellular-cholangiocarcinoma.
Study endpoints
The primary endpoint of this study was OS, which was measured from the start of treatment until death from any cause. The secondary endpoints included PFS and treatment response at 3 months after treatment. The treatment response was assessed according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST).[
11]
Data collection
The clinical characteristics of the patients were obtained through a retrospective review of their medical records at the initiation. These characteristics encompassed demographic information, anthropometric measurements, ECOG PS, laboratory findings (liver function test, alpha-fetoprotein [AFP] and protein induced by vitamin K absence or antagonist-II [PIVKA-II]), and radiologic findings (number and size of intrahepatic lesions and macrovascular invasion). Additionally, the Barcelona Clinic Liver Cancer (BCLC) stage and hepatic function at baseline were obtained using the Child-Pugh classification or albumin-bilirubin (ALBI) grade. The ALBI score was calculated using serum albumin and total bilirubin values with the formula: ALBI score = (log10 bilirubin (µmol/L) × 0.66) + (albumin (g/L) x − 0.085). The dates of disease progression, death, or last follow-up were also collected.
TARE procedure
TARE was performed using either Y90 resin microspheres (SIR-Spheres; Sirtex Medical Ltd., Sydney, Australia) or Y90 glass microspheres (TheraSphere; BTG International Ltd., Ottawa, Canada) using standardized techniques. The procedure was administered 10-14 days after a preprocedural examination, which included an evaluation of the lung shunt fraction using 99mTc-MAA administered in the hepatic artery. For TheraSphere, a lung dose fraction of >30 Gy per treatment (or a total of 50 Gy for all estimated infusions) was the limit. For SIR-Spheres, a 20% lung shunt was the limit, with reduced activity recommended for patients with a 10-20% lung shunt. While single-photon emission CT was not essential for dosimetry, it could provide an exact distribution of 99mTc-MAA on axial images, potentially aiding in predicting the tumor response and precise dosimetry of the partition model.[
12]
Outcomes and assessments
In all centers, patients were followed up using radiological studies, including CT or magnetic resonance imaging. To evaluate the treatment response, radiological studies were performed 3 months after TARE. Laboratory tests, including liver function and tumor marker testes were regularly measured during subsequent follow-up visits. The frequency and type of follow-up tests were determined based on each patient’s individual case and treatment plans. In cases where patients did not respond to TARE, alternative therapeutic approaches were implemented and tailored to the individual patient’s circumstances.
Measurement of skeletal muscle mass
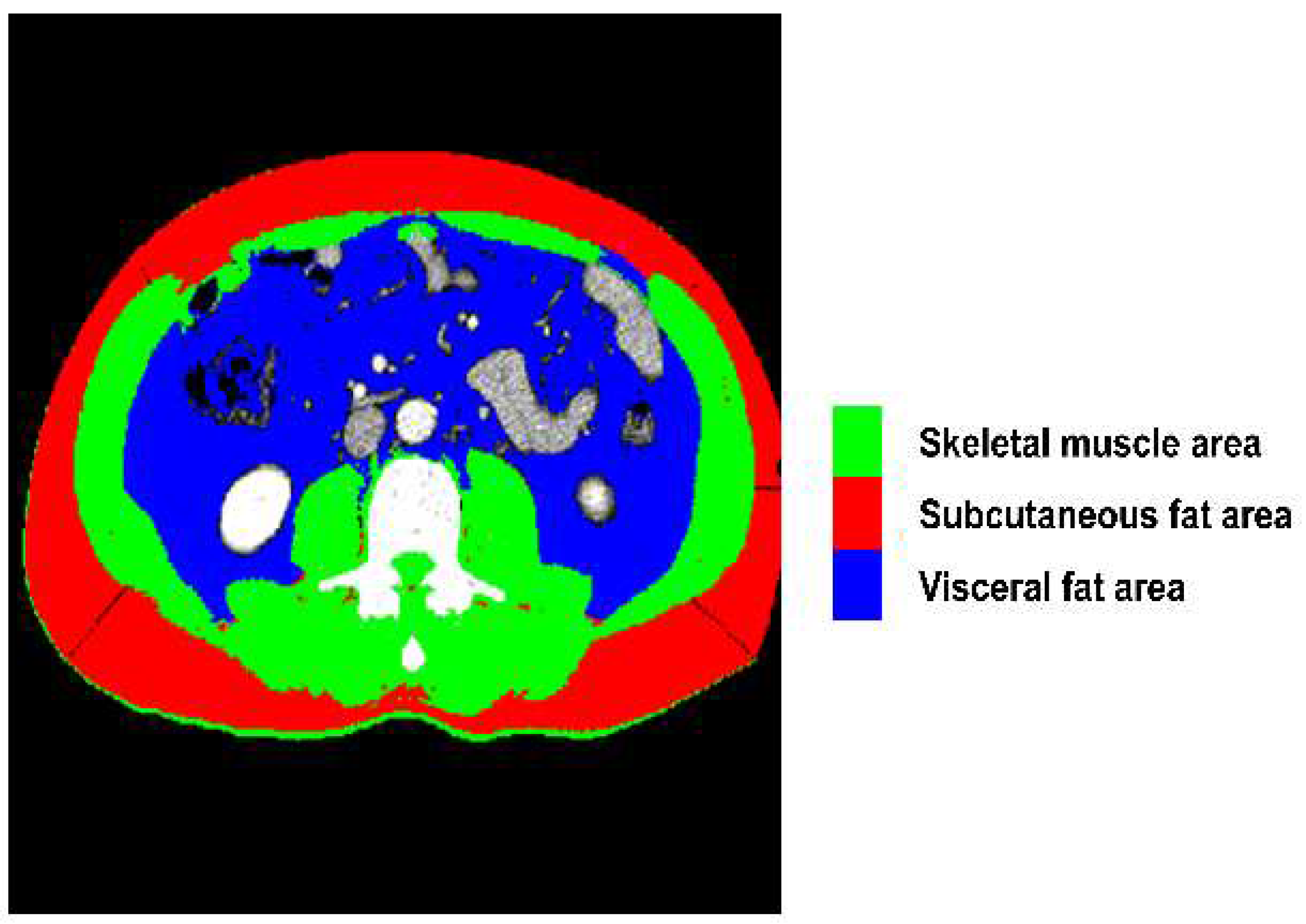
We utilized a semi-automated open-source software (BMI measurement tools, version 1.0;
https://sourceforge.net/projects/muscle-fat-area-measurement) to measure the cross-sectional area (cm2) of the skeletal muscles based on attenuation segmentation. The Hounsfield unit (HU) thresholds ranged from -29 to 150 HU for skeletal muscle mass, -190 to -30 HU for subcutaneous fat, and -150 to -50 HU for visceral fat, as shown in
Figure 1. Measurements were obtained from CT scans performed within 1 month prior to TARE at the level of the transverse processes of the third lumbar (L3) vertebra. The cross-sectional area of the muscle was segmented by a single trained researcher (HY) who was blinded to subject identification and outcomes.
Definition of sarcopenia
The cross-sectional muscle area was normalized by height squared (in meters) to calculate the skeletal muscle index (SMI) (cm2/m2). We applied optimal stratification to determine the body mass index (BMI) and sex-specific cutoffs for SMI to identify sarcopenia.[
7] For men, the SMI cut-offs were < 43 cm2/m2 for underweight or normal-weight patients (BMI < 25 kg/m2) and < 53 cm2/m2 for overweight (BMI ≥ 25 kg/m2) or obese patients (BMI ≥ 30 kg/m2). For women, the cut-off value was < 41 cm2/m2 regardless of weight.
Statistical analysis
Categorical variables are presented as counts and percentages [n (%)]. Continuous variables are expressed as either the mean ± standard deviation or median with interquartile range and were transformed into two-level categorical data based on their median values. Appropriate statistical tests, including Chi-square test, Fisher’s exact test, Student’s t-test, and Mann-Whitney U-test, were used for the analysis. The Kaplan-Meier method was used to estimate the cumulative incidence of events over time. Differences between groups were analysed using the log-rank test, and the Bonferroni correction was applied to account for multiple comparisons. Univariate and multivariate Cox proportional hazards regression models were used to assess the risk factors for OS and PFS. Statistical significance was set at p < 0.05. All statistical analyses were performed using STATA software 17 (StataCorp LLC, TX, USA).
3. Results
3.1. Baseline characteristics
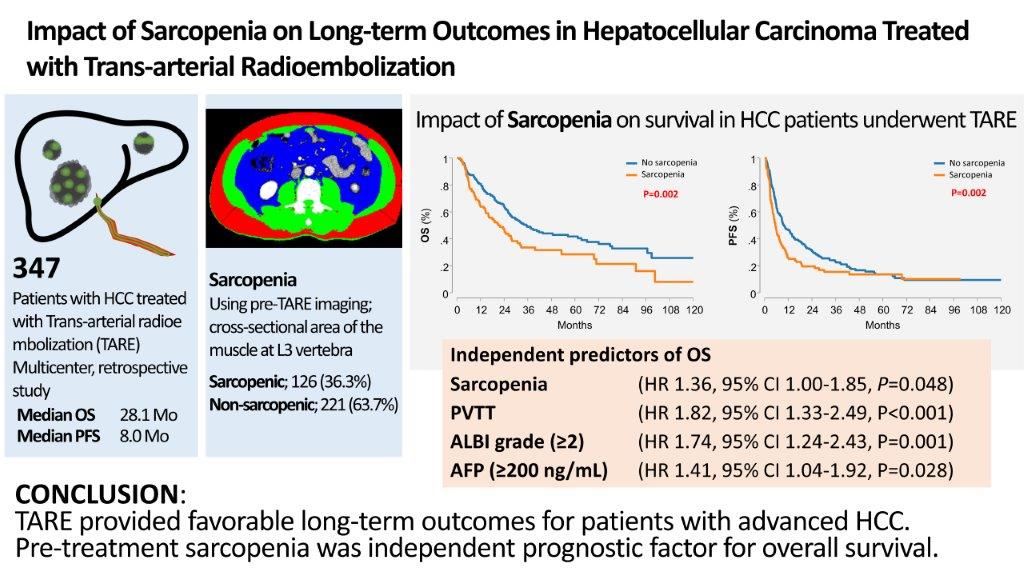
A total of 347 patients who underwent TARE were included in this study. The baseline patient characteristics are presented in
Table 1. The median age of the patients was 65 years, 81.8% were male patients, and the mean BMI was 23.9 kg/m2. Hepatitis B virus (HBV) infection was the major cause of liver disease (57.3%). Nearly all patients (94.5%) were classified as Child-Pugh class A, and almost half (42.9%) were classified as ALBI grade 1. The median tumor diameter (largest) was 8.4 (6.2-10.6) cm and 53.6% of patients had multifocal tumors. Portal vein tumor thrombus (PVTT) was found in 46.0% of the patients. According to BCLC staging, 18.7% of the patients had BCLC stage A, 41.5% had BCLC stage B, and 39.8% had BCLC stage C. Mean follow-up time was 28.6 months ± 25 (95% confidence interval [CI]; 4.3-112.2).
The patients were divided into non-sarcopenic (n = 221) and sarcopenic (n = 126) groups. The baseline characteristics revealed significant differences between the two groups in terms of sarcopenia. The sarcopenic group was older, had a lower BMI, and had a higher proportion of female patients and patients with alcoholic liver disease than the non-sarcopenic group. Albumin levels also showed significant differences between the two groups, resulting in a better ALBI grade in the non-sarcopenic group. However, tumor characteristics, such as median tumor diameter, proportion of multifocal tumors, presence of PVTT, and tumor markers (AFP and PIVKA-II) did not show significant differences between the two groups.
3.2. Response to TARE
During the study, none of the patients underwent repeated TARE of the same target lesion, even in cases of local tumor progression. The median administered radiation activity was 2.5 GBq, and the median hepatopulmonary shunt was 6.7%. Additional detailed information on the TARE procedure is provided in
Supplementary Table S1. Response evaluations 3 months after TARE were available for 333 patients, representing 96.0% of the study population. According to the mRECIST criteria, complete response, partial response and stable disease were observed in 26 (7.8%), 121 (36.3%), and 106 (31.8%) patients, respectively. The overall response rate was 44.1%, and the disease control rate was 76.0% (
Table 2). The non-sarcopenic group demonstrated significantly higher objective response (p = 0.031) and disease control (p < 0.001) rates than the sarcopenic group.
3.3. Factors associated with overall survival
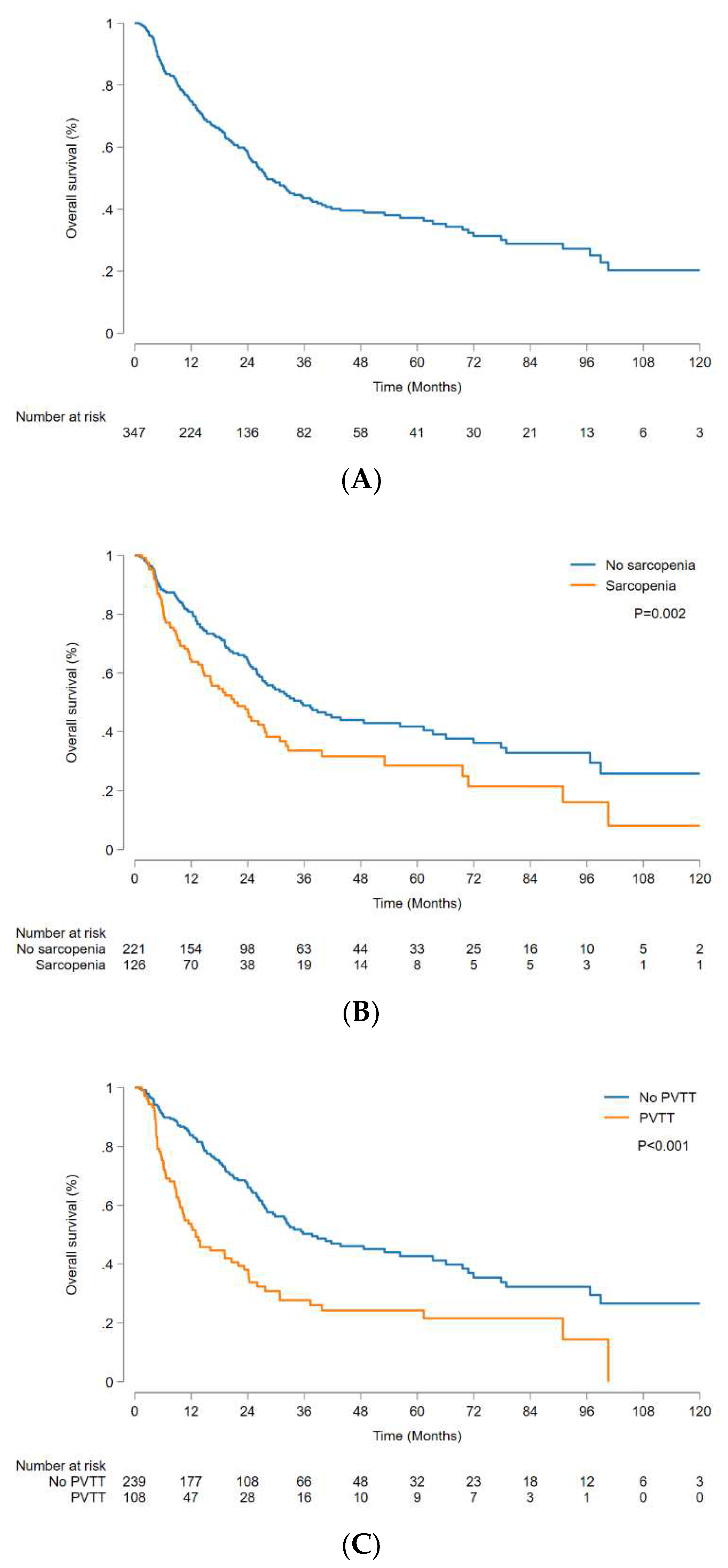
The median OS was 28.1 (95% CI, 24.8-35.7) months, and 1-, 3-, and 5-year OS rates were 74.9% (95% CI, 69.8-79.2%), 43.5% (95% CI, 37.2-49.6%), and 37.2% (95% CI, 30.7-43.6%), respectively (
Figure 2A). The non-sarcopenic group had a significantly higher OS than the sarcopenic group, with a median OS of 35.3 (95% CI, 27.1-56.9) months and 21.1 (95% CI, 14.8-27.6) months, respectively (p = 0.002) (
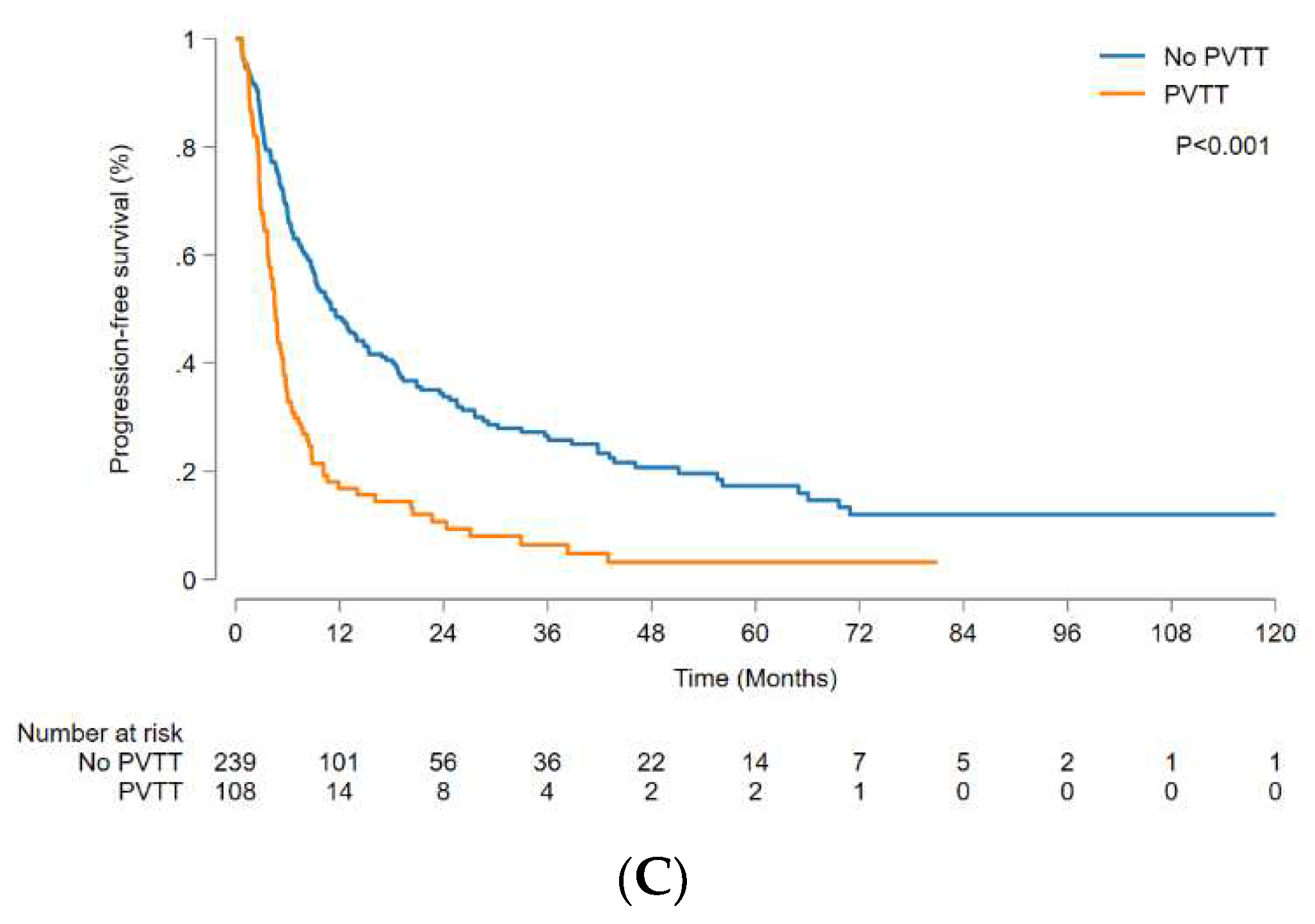
Figure 2B). The OS was significantly higher in patients without PVTT than in those with PVTT, with a median OS of 37.7 (95% CI, 29.4-63.3) months and 13.0 (95% CI, 9.6-22.0) months, respectively (p < 0.001) (
Figure 2C). Sarcopenia, PVTT, AFP level (≥200 ng/mL), ALBI grade (2-3), tumor number (≥2), and largest tumor diameter (>8 cm) were identified as significant factors in univariate analysis (
Table 3). Multivariate analysis revealed that sarcopenia (hazard ratio [HR], 1.36; 95% CI, 1.00-1.85; p = 0.048), PVTT (HR, 1.82; 95% CI, 1.33-2.49; p < 0.001), AFP (≥200 ng/mL) (HR 1.41; 95% CI, 1.04-1.92; p = 0.028), and ALBI grade (2-3) (HR 1.74; 95% CI, 1.24-2.43; p = 0.001) were independently associated with OS.
3.4. Factors associated with progression free survival
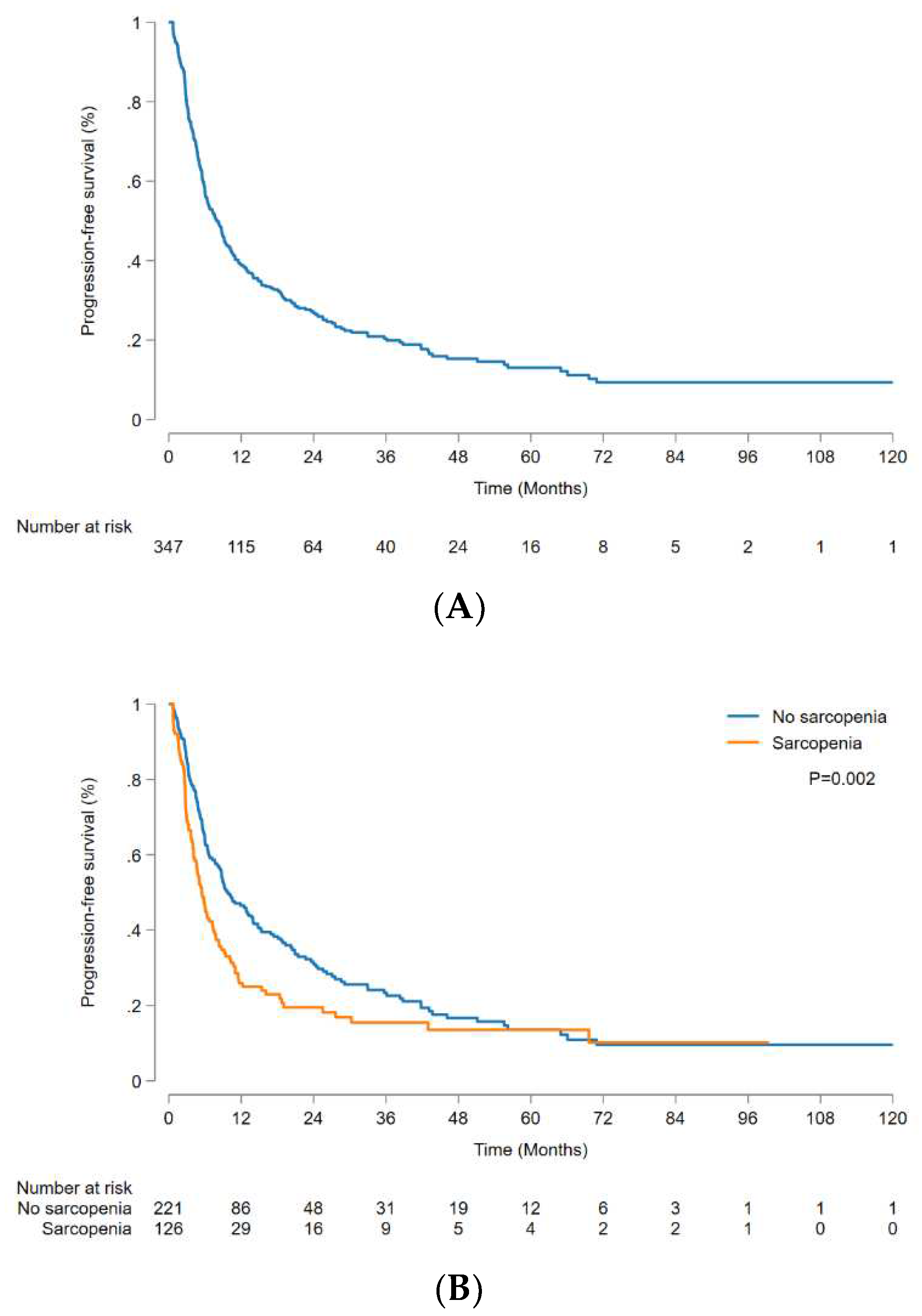
The median PFS was 8.0 (95% CI, 6.4-9.4) months, and 1-, 3-, and 5-year PFS rates were 38.9% (95% CI, 33.7-44.2%), 20.4% (95% CI, 15.8-25.4%), and 13.1% (95% CI, 8.9-18.1%), respectively (
Figure 3A). Analysis of factors affecting PFS yielded findings consistent with the aforementioned OS analysis results. The non-sarcopenic group demonstrated significantly better outcomes than the sarcopenic group (p = 0.002) (
Figure 3B), and the patients without PVTT had significantly better outcomes than those with PVTT (p < 0.001) (
Figure 3C). Sarcopenia (HR 1.54; 95% CI, 1.19-1.98; p = 0.001), PVTT (HR 2.08; 95% CI, 1.59-2.72; p < 0.001), AFP (≥200 ng/mL) (HR 1.38; 95% CI, 1.07-1.77; p = 0.013), ALBI grade (2-3) (HR 1.49; 95% CI, 1.15-1.93; p = 0.002), and tumour number (≥2) (HR 1.55; 95% CI, 1.20-2.01; p = 0.001) were independently correlated with PFS (
Table 3).
4. Discussion
TARE is a safe and effective treatment for patients with locally advanced HCC who are not amenable to curative-intent treatments such as surgical resection or ablative therapies or for those who do not respond to other therapies. TACE is the most commonly used LRT for HCC; however, it often requires repeated procedures, and the rate of TACE refractoriness is considerable.[
13] Some institutions have adopted TARE as the first-line transarterial LRT for HCC.[
14] However, the widespread clinical application of TARE is often constrained by its comparatively high cost in comparison to other LRT, the limited availability of facilities and expertise, the incapability to administer repeat treatment for recurrent or progressive disease, and the absence of a consensus on patient selection criteria. Therefore, the identification of predictive biomarkers or clinical characteristics that can identify patients likely to respond to TARE would be invaluable for optimizing treatment outcomes.
Sarcopenia and cancer cachexia are complex syndromes characterized by a gradual loss of skeletal muscle mass and are widely recognized as predictors of poor survival in various types of cancer.[
7] Sarcopenia can result in decreased functional reserves and reduced ability to carry out daily activities. Additionally, it is often accompanied by conditions such as insulin resistance, vitamin D deficiency, and increased levels of inflammatory cytokines, all of which are associated with the progression of liver fibrosis and HCC.[
8] Consequently, sarcopenia can significantly affect the prognosis of HCC, and several studies have explored its association with patients undergoing various treatments.[15-17] Accurate assessment of sarcopenia is crucial to identify patients who are at risk. To achieve this, it is important to use the most reliable and consistent method among the various methods available for measuring muscle mass. CT attenuation-based segmentation is a commonly used method for distinguishing fat from other soft tissues, and various software programs have been used to quantify body composition. This study utilized a semi-automated program that has been validated in multiple studies and demonstrated high reliability.[18-20]
This study investigated the association between sarcopenia and the long-term outcomes of TARE in patients with HCC. Although the exact mechanisms underlying this relationship are not fully understood, one possible explanation is that the immune response has been shown to have a significant impact on TARE outcomes. Valerie et al. reported a correlation between immune activation and sustained response to TARE.[
21] Their study found that the robust activation of CD8+ T cells within both the tumor and the systemic environment was significantly associated with positive TARE outcomes. Given that sarcopenia is closely linked to host immunity, this could provide an insight into our findings. Chronic inflammation in cancer can contribute to the development of sarcopenia, partly due to immune dysfunction such as T cell exhaustion.[
22] Skeletal muscle tissue produces myokines such as interleukin (IL)-6, IL-15, tumour necrosis factor-α, and transforming growth factor-β and altered myokine activity can induce immune senescence in sarcopenia. Therefore, patients with sarcopenia who receive TARE may have a poor prognosis.
Compared to TACE or sorafenib, TARE has various advantages as a treatment for locally advanced HCC, in terms of quality of life, treatment tolerability, and time to progression.[23-25] Despite these advantages, TARE did not demonstrate improved survival compared to sorafenib as a first-line treatment for unresectable HCC patients in recent randomized controlled trials.[
26,
27] However, a more recent randomized trial (DOSISPHERE-01) showed significantly improved OS with a personalized dosimetry model compared to a standard dose calculation model.[
28] The unique features of TARE, including its intricate relationship with immunity and the requirement for personalized dosimetry models, underscore the importance of refining patient selection and individualizing treatment applications to enhance survival outcomes. According to our findings, no sarcopenia, no PVTT, an ALBI grade of 1, and low AFP levels (<200 ng/mL) were independent predictors of favorable OS following TARE. These findings highlight the importance of patient stratification and personalized treatment for optimizing treatment outcomes.
PVTT is a notable prognostic factor for TARE. Unlike TACE, TARE is considered a relatively safe and effective LRT that can be used even in cases accompanied by PVTT. Some studies have reported the therapeutic efficacy of TARE in patients with PVTT, and a predictive model for outcomes has been developed for this population.[
14,
29] However, our results indicate that both OS and PFS were significantly superior in patients without PVTT. Although TARE can still be used in cases with PVTT, it appears to show better outcomes in patients without PVTT. Along these lines, some studies have shown that TARE has comparable effects to surgery in treating large HCC without PVTT and can be applied as a potential curative therapy for early-stage HCC.[
30,
31] There is an emerging opinion that TARE should be used in the early or intermediate stages rather than in the advanced stages based on such evidence.[
32] The findings of our study corroborate this perspective, as we observed superior long-term OS and PFS in patients without PVTT. However, further investigation is required to fully understand the implications of these results.
This study had several limitations that should be acknowledged. First, the lack of a validation set may have affected the generalisability and reliability of our findings. Nevertheless, our study is one of the largest retrospective studies to date that investigate the impact of sarcopenia on long-term outcomes of TARE. Obtaining a significant number of patients underwent TARE can be challenging, as it requires experienced medical professionals and is relatively costly. Future studies should include a validation set to further corroborate our results. Second, this study was conducted in a single country and in patients with the same ethnicity. Korea is an HBV-endemic area with a relatively small proportion of obese patients. Therefore, the results of our study may not be applicable to populations with different etiologies and body composition. Third, many patients underwent additional treatments after TARE that may have influenced their prognoses. However, sarcopenia affects the efficacy and tolerability of subsequent treatments and long-term prognosis, underscoring its importance.
5. Conclusions
In conclusion, our analysis demonstrated that TARE is an effective therapeutic option for patients with advanced HCC. Notably, pre-treatment sarcopenia is identified as an independent prognostic factor for both long-term OS and PFS. Additionally, PVTT, ALBI grade, and the level of AFP were significant prognostic factors for outcomes. These findings underscore the importance of incorporating sarcopenia as a useful biomarker in implementing TARE. To optimize survival outcomes of TARE, further studies are warranted to refine patient selection and personalize treatment strategies.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Details of TARE procedure.
Author Contributions
H Nam: study concept and design, writing of the manuscript, data collection, statistical analysis and interpretation of the data. H Yang: measurement of skeletal muscle mass. HS Chun, HA Lee: data collection, statistical analysis and interpretation of the data. YS Seo, DY Kim, YJ Kim: patient management and critical revision of the manuscript. SH Bae: study concept and design, critical revision of the manuscript for important intellectual content.
Funding
This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIT) (RS-2023-00208767).
Institutional Review Board Statement
This study was approved by the Institutional Review Board of the Catholic University of Korea (approved number: KC20RISI0475).
Informed Consent Statement
Patient consent was waived due to retrospective nature of the study and the analysis used anonymous clinical data.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplemental Material; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Global Burden of Disease Cancer, C.; Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2019, 5, 1749–1768. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Kelly, S.P.; Altekruse, S.F.; McGlynn, K.A.; Rosenberg, P.S. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol 2016, 34, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Electronic address, e.e.e.; European Association for the Study of the, L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017, 11, 317–370. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Kim, H.C. Radioembolization for hepatocellular carcinoma: what clinicians need to know. J Liver Cancer 2022, 22, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Fujiwara, N.; Nakagawa, H.; Kudo, Y.; Tateishi, R.; Taguri, M.; Watadani, T.; Nakagomi, R.; Kondo, M.; Nakatsuka, T.; Minami, T.; et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 2015, 63, 131–140. [Google Scholar] [CrossRef]
- Oura, K.; Morishita, A.; Manabe, T.; Takuma, K.; Nakahara, M.; Tadokoro, T.; Fujita, K.; Mimura, S.; Tani, J.; Ono, M.; et al. Relationship between Accurate Diagnosis of Sarcopenia and Prognosis in Patients with Hepatocellular Carcinoma Treated with Atezolizumab plus Bevacizumab Combination Therapy. Cancers (Basel) 2023, 15, 3243. [Google Scholar] [CrossRef]
- Imai, K.; Takai, K.; Unome, S.; Miwa, T.; Hanai, T.; Suetsugu, A.; Shimizu, M. Lenvatinib or Sorafenib Treatment Causing a Decrease in Skeletal Muscle Mass, an Independent Prognostic Factor in Hepatocellular Carcinoma: A Survival Analysis Using Time-Varying Covariates. Cancers (Basel) 2023, 15, 4223. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C. Radioembolization for the treatment of hepatocellular carcinoma. Clin Mol Hepatol 2017, 23, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, B.K.; Kim, S.U.; Park, J.Y.; Ahn, S.H.; Seong, J.S.; Han, K.H.; Kim, D.Y. A survey on transarterial chemoembolization refractoriness and a real-world treatment pattern for hepatocellular carcinoma in Korea. Clin Mol Hepatol 2020, 26, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Gabr, A.; Riaz, A.; Mora, R.; Ali, R.; Abecassis, M.; Hickey, R.; Kulik, L.; Ganger, D.; Flamm, S.; et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology 2018, 68, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ren, Y.; Zhu, L.; Yang, L.; Zheng, C. Association between sarcopenia and clinical outcomes in patients with hepatocellular carcinoma: an updated meta-analysis. Sci Rep 2023, 13, 934. [Google Scholar] [CrossRef]
- Lee, J.; Cho, Y.; Park, S.; Kim, J.W.; Lee, I.J. Skeletal Muscle Depletion Predicts the Prognosis of Patients With Hepatocellular Carcinoma Treated With Radiotherapy. Front Oncol 2019, 9, 1075. [Google Scholar] [CrossRef]
- Fujita, M.; Takahashi, A.; Hayashi, M.; Okai, K.; Abe, K.; Ohira, H. Skeletal muscle volume loss during transarterial chemoembolization predicts poor prognosis in patients with hepatocellular carcinoma. Hepatol Res 2019, 49, 778–786. [Google Scholar] [CrossRef]
- Kang, S.H.; Jeong, W.K.; Baik, S.K.; Cha, S.H.; Kim, M.Y. Impact of sarcopenia on prognostic value of cirrhosis: going beyond the hepatic venous pressure gradient and MELD score. J Cachexia Sarcopenia Muscle 2018, 9, 860–870. [Google Scholar] [CrossRef]
- Goh, M.J.; Kang, W.; Jeong, W.K.; Sinn, D.H.; Gwak, G.Y.; Paik, Y.H.; Choi, M.S.; Lee, J.H.; Koh, K.C.; Paik, S.W. Prognostic significance of cachexia index in patients with advanced hepatocellular carcinoma treated with systemic chemotherapy. Sci Rep 2022, 12, 7647. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.H.; Cho, E.S.; Lee, H.S.; Shin, S.J.; Park, E.J.; Baik, S.H.; Lee, K.Y.; Kang, J. Albumin-myosteatosis gauge as a novel prognostic risk factor in patients with non-metastatic colorectal cancer. J Cachexia Sarcopenia Muscle 2023, 14, 860–868. [Google Scholar] [CrossRef]
- Chew, V.; Lee, Y.H.; Pan, L.; Nasir, N.J.M.; Lim, C.J.; Chua, C.; Lai, L.; Hazirah, S.N.; Lim, T.K.H.; Goh, B.K.P.; et al. Immune activation underlies a sustained clinical response to Yttrium-90 radioembolisation in hepatocellular carcinoma. Gut 2019, 68, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, J.K.; de Souto Barreto, P.; Fougere, B.; Rolland, Y.; Vellas, B.; Cesari, M. Chronic inflammation and sarcopenia: A regenerative cell therapy perspective. Exp Gerontol 2018, 103, 115–123. [Google Scholar] [CrossRef]
- Salem, R.; Gilbertsen, M.; Butt, Z.; Memon, K.; Vouche, M.; Hickey, R.; Baker, T.; Abecassis, M.M.; Atassi, R.; Riaz, A.; et al. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin Gastroenterol Hepatol 2013, 11, 1358–1365 e1351. [Google Scholar] [CrossRef]
- Salem, R.; Gordon, A.C.; Mouli, S.; Hickey, R.; Kallini, J.; Gabr, A.; Mulcahy, M.F.; Baker, T.; Abecassis, M.; Miller, F.H.; et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology 2016, 151, 1155–1163 e1152. [Google Scholar] [CrossRef] [PubMed]
- Martelletti, C.; Ricotti, A.; Gesualdo, M.; Carucci, P.; Gaia, S.; Rolle, E.; Burlone, M.E.; Okolicsanyi, S.; Mattalia, A.; Pirisi, M.; et al. Radioembolization vs sorafenib in locally advanced hepatocellular carcinoma with portal vein tumor thrombosis: A propensity score and Bayesian analysis. J Dig Dis 2021, 22, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Vilgrain, V.; Pereira, H.; Assenat, E.; Guiu, B.; Ilonca, A.D.; Pageaux, G.P.; Sibert, A.; Bouattour, M.; Lebtahi, R.; Allaham, W.; et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol 2017, 18, 1624–1636. [Google Scholar] [CrossRef] [PubMed]
- Chow, P.K.H.; Gandhi, M.; Tan, S.B.; Khin, M.W.; Khasbazar, A.; Ong, J.; Choo, S.P.; Cheow, P.C.; Chotipanich, C.; Lim, K.; et al. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients with Hepatocellular Carcinoma. J Clin Oncol 2018, 36, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Garin, E.; Tzelikas, L.; Guiu, B.; Chalaye, J.; Edeline, J.; De Baere, T.; Tacher, V.; Robert, C.; Assenat, E.; Terroir-Cassou-Mounat, M.; et al. Major impact of personalized dosimetry using 90Y loaded glass microspheres SIRT in HCC: Final overall survival analysis of a multicenter randomized phase II study (DOSISPHERE-01). Journal of Clinical Oncology 2020, 38, 516–516. [Google Scholar] [CrossRef]
- Spreafico, C.; Sposito, C.; Vaiani, M.; Cascella, T.; Bhoori, S.; Morosi, C.; Lanocita, R.; Romito, R.; Chiesa, C.; Maccauro, M.; et al. Development of a prognostic score to predict response to Yttrium-90 radioembolization for hepatocellular carcinoma with portal vein invasion. J Hepatol 2018, 68, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.Y.; Lee, J.H.; Sinn, D.H.; Hur, M.H.; Hong, J.H.; Park, M.K.; Cho, H.J.; Choi, N.R.; Lee, Y.B.; et al. Long-Term Outcomes of Transarterial Radioembolization for Large Single Hepatocellular Carcinoma: A Comparison to Resection. J Nucl Med 2022, 63, 1215–1222. [Google Scholar] [CrossRef]
- Lewandowski, R.J.; Gabr, A.; Abouchaleh, N.; Ali, R.; Al Asadi, A.; Mora, R.A.; Kulik, L.; Ganger, D.; Desai, K.; Thornburg, B.; et al. Radiation Segmentectomy: Potential Curative Therapy for Early Hepatocellular Carcinoma. Radiology 2018, 287, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Guiu, B.; Garin, E.; Allimant, C.; Edeline, J.; Salem, R. TARE in Hepatocellular Carcinoma: From the Right to the Left of BCLC. Cardiovasc Intervent Radiol 2022, 45, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).