1. Introduction
Leishmaniases are neglected infectious diseases caused by protozoa of the genus Leishmania, whose clinical manifestations may depend on the parasite species and the host's immune profile, among other factors [
1]. Cutaneous Leishmaniasis (CL) in Brazil is caused by species belonging to the Sub-Genus
Viannia of Leishmania, while,
L.
infantum is the principal agent of Visceral Leishmaniasis in Europe and Brazil [
2,
3]. Leishmania infections are of great clinical relevance to humans and veterinarians. However, there is dearth of treatment options [
4]. The need to find new therapeutic and clinical approaches to treat leishmaniasis has prompted an intense search for new compounds to improve the treatment of the disease [
5].
Molecules composed of urea substituents have shown encouraging results in controlling tumor cells. These compounds inhibit protein translation due to the phosphorylation of the translation initiation factor 2 (eIF2) subunit alpha (eIF2α) in eukaryotes [
6]. eIF2α is essential for forming the ternary translation initiation complex between eIF2.GTP.tRNAiMet, enabling protein synthesis in eukaryotic cells [
7]. Phosphorylation of eIF2α leads to a global attenuation of cellular protein synthesis. Different kinases can mediate this phosphorylation [
8]. HRI (heme-regulated inhibitor) can lead to phosphorylation of the alpha subunit resulting from heme complex deprivation in mammals [
9]. PERK (protein kinase R-like kinase) can be activated due to the presence of endoplasmic reticulum stress and by the presence of pathogens such as viruses and
Leishmania, phosphorylating eIF2alpha [
10], GCN2 (general control nonderepressible 2) can also phosphorylate this factor in response to amino acid deprivation [
11] and, finally, PKR (protein kinase R), which is capable of inducing the phosphorylation of eIF2α after its activation due to binding to the double-stranded RNA, particularly in response to viral infection. The evaluation of the urea substituent library by the subsequent structure-activity relationship identified N-aryl-N-cyclohexyl urea as a specific analog and potent HRI activator inhibiting the proliferation of all cancer cells tested in including estrogen receptor-positive MCF7 breast cancer and BRAF-mutated melanoma cancer cell lines,[
12].
It has already been described that the phosphorylation of eIF2α is essential for the differentiation of
Leishmania, mediated by the activation of PERK [
13]. Our group has been studying the effects of N'N-Diarylureas on the viability of
Leishmania parasites, since these drugs have been defined as activators of eIF2α-kinases, this could result in an attenuation of the parasite's translational process, as well as altering its differentiation mechanisms. When tested on trypanosomatids of the genus
Trypanosoma, urea substituents could inhibit proliferation by decreasing the rate of infection and the number of parasites. Among the library of compounds tested, I-17 was the most promising molecule in reducing,
T.
brucei and,
T.
cruzi growth with a high therapeutic index [
14]. I-17 also displayed significant activity against
Listeria monocytogenesis by inhibiting pathogen trafficking [
15].
2. Materials and Methods
I-17 Synthesis and biological evaluation of I-17 was originally described in [
16], I-17 was purified to >98% and dried as a white powder. The compound was solubilized in DMSO as 20 mM stock solution.
2.1. Cell lines and culture conditions
The RAW 264.7 macrophages (ATCC: TIB-71) were maintained in DMEM culture medium (GIBCO) plus 10% (v/v) inactivated fetal bovine serum (GIBCO) and 100U/mL penicillin and 100 mg/mL streptomycin (Invitrogen). The cells were cultured in 100 mm plates and grown in an incubator in an atmosphere of 10% CO2 at 37°C. Cells were removed from the plates with 0.2% trypsin plus 0.5 mM EDTA.
THP1 cells were differentiated into macrophage-like cells using PMA 20 ng/ml. Subsequently, cells were exposed to I-17 at different concentrations (2 µM -80 µM) or DMSO alone. Wells containing medium-only and untreated cells were used as controls.
2.2. Parasites
Leishmania (Leishmania) amazonensis, strain WHOM/BR/75/Josefa, was maintained in Schneider Insect Medium (Sigma) supplemented with 10% SFB (Gibco). The promastigote forms in the stationary phase (day 5 of the culture) were used for the infections. L. (L.) infantum strain MHOM/TN/80/IPT1 and MHOM/IT/08/31U, as well as two clinical isolates, were also used in this study. L. infantum promastigotes were cultivated at 26 °C in Evans’ Modified Tobie’s Medium (EMTM)
2.3. Viability assays and IC50 determination
Cell viability tests were performed as follows: 2x105 RAW 264.7 macrophages were plated one day before and exposed to different concentrations of compounds I-17 for 48 hours, and the MTT (Cell Titer Prolif Assay) or the MTS tests (Cell Titer 96H Aqueous Non-Radioactive Cell Proliferation Assay, Promega) were used to test cell viability. For the determination of the 50% inhibition concentration (IC50), the calculation of surviving cells (%) = (AT-AB) / (AC-AB) x 100 was used, where AC is the absorbance of the untreated sample, AT is the absorbance of the treated samples, and AB is the absorbance of the blank. To determine the viability of the parasites, promastigotes were exposed to different concentrations of the compounds for 48 hours.
2.4. Infection index
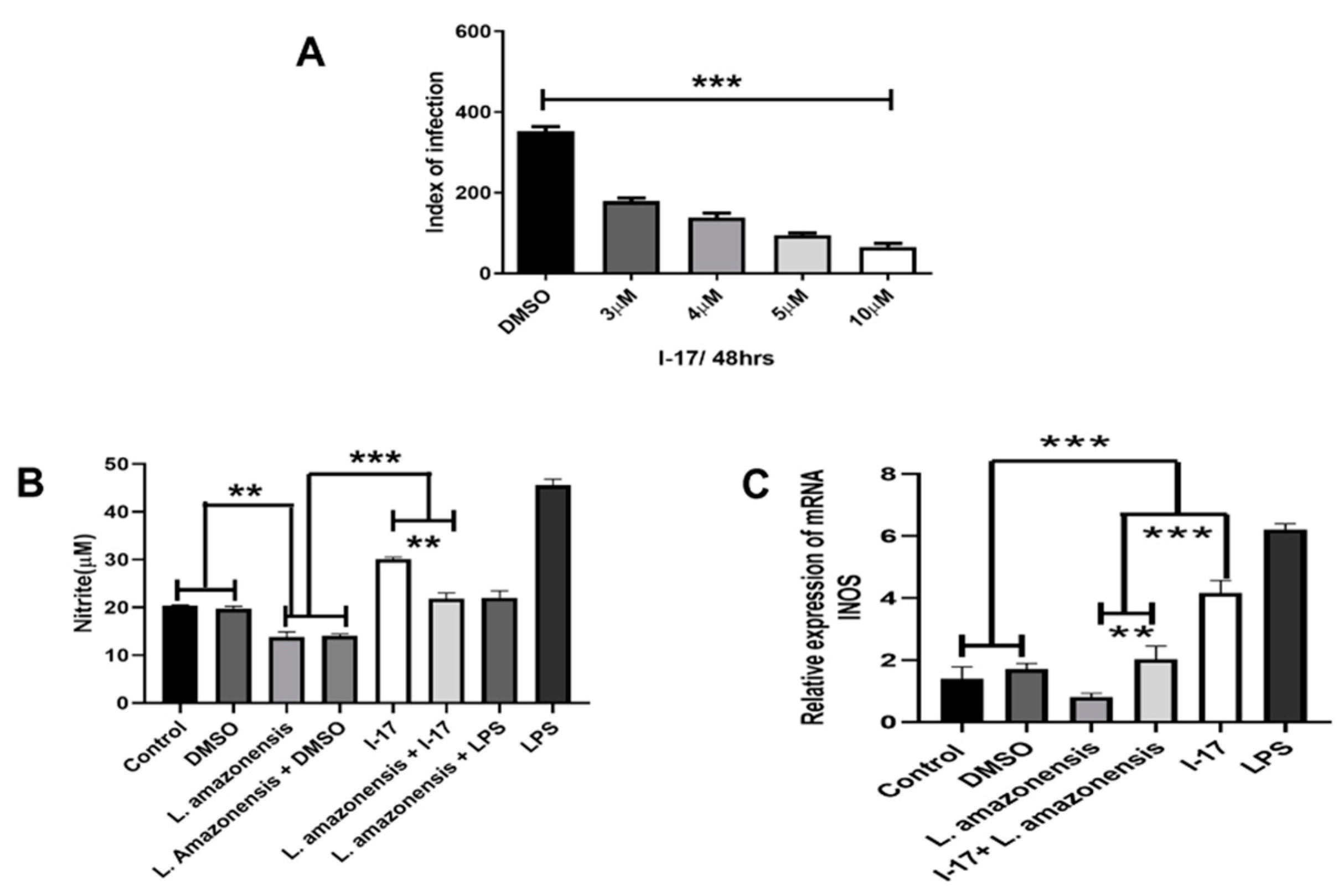
For the determination of the Infection Index, RAW 264.7 cells were plated at a concentration of 5x104 in a 24-well plate the previous day and infected with, L. amazonensis (10:1) for 24 hours and then exposed to various concentrations of I-17 for 48 hours. The infection index was calculated as follows: the percentage of infected macrophages multiplied by the number of amastigotes per macrophage.
2.5. GRIESS Test
To measure the production of Nitric Oxide (NO), RAW 264.7 cells were plated at a concentration of 2x105 and subsequently infected for 24 hours with, L. amazonensis (10:1) and treated with the compound at a concentration of 5 µM. The GRIESS test was carried out as described by the manufacturer (G4410 Sigma-Aldrich)
2.6. RT-PCR Assays
Real-time PCR reactions were performed using the StepOne Real-Time PCR System (Applied Biosystems). The reactions were carried out in triplicate, using the GoTaq qPCR Master Mix kit (Promega), with the concentration of the primers described in
Table 1, 7, 5 μL of SYBR green PCR master mix, 1 μL of cDNA and nuclease-free water (Promega), in a final volume of 15 μL. The analysis was carried out using StepOne version 2. 0 software (Applied Biosystems) using the ∆∆CT method. Primers utilized GAPDH Forward 5’- TGCACCACCACCTGCTTAGC- 3´ and GAPDH Reverse 5’ GGCATGGACTGTGGTCATGAG- 3’Mu-iNOS-F: 5′-CAGCTGGGCTGTACAAACCTT- 3′ and Mu-NOS2-R: 5′-CATTGGAAGTGAAGCGTTTCG-3′.
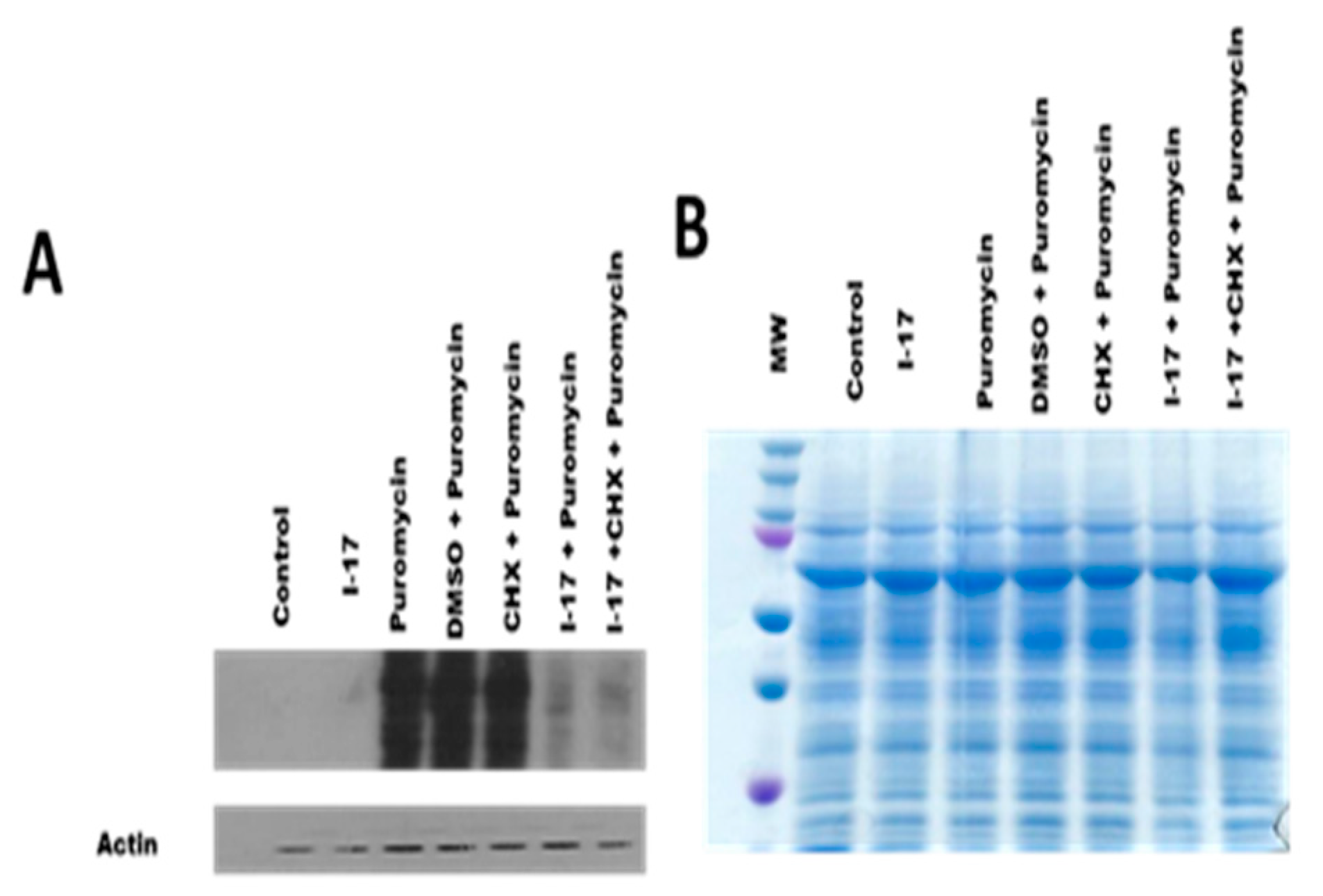
2.7. Puromycin incorporation assay
We tested puromycin incorporation in Leishmania extracts to test whether I-17 would inhibit translation initiation. L. amazonensis promastigotes were pretreated with 5 μM of I-17 compound for 2 hours or 10 μM of cycloheximide and then treated with 10 μM of puromycin (P4512- Sigma-Aldrich) for 2 hours. Western Blot was performed to determine puromycin incorporation in the treated cells. The blots were incubated separately with primary antibodies Anti-Puromycin (MERCK MABE343) β-actin (Sigma-Aldrich A2543).
2.8. Statistical analysis
Two-way ANOVA analyzed data for independent samples followed by Bonferroni's Multiple Comparison Test (with no designated control group), using GraphPad Prism 6 software (San Diego, CA, USA). Data were presented as the mean values ± standard error of three independent experiments' mean (SEM). Comparisons between means were statistically significant when p < 0.05.
3. Results
Viability tests were carried out on RAW 264.7 and THP1 macrophages to determine cell tolerance to the I-17 compound, seeking a broader window to use the compound safely to resolve the infection without leading to macrophage death. Stationary promastigote cultures of,
L.
amazonensis and,
L.
infantum were also submitted to the MTT test upon a wide range of concentrations of I-17. The EC50 values were consistent with those of other trypanosomatids, such as,
T.
cruzi and,
T.
brucei [
14].
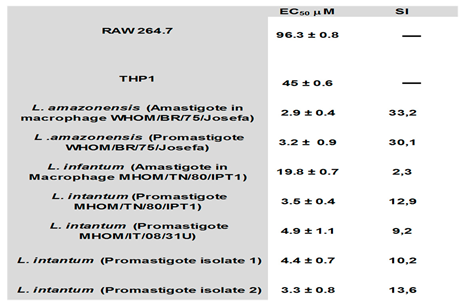
The Ideal concentration for the reduction of amastigote load in infected macrophages was determined by calculating the EC50 from the results obtained through the infection index carried out with both
Leishmania species. The selectivity index (SI) was calculated from the ratio obtained between the EC50 values of host cells and parasites,
Table 1. The results obtained in clinical isolates of,
L.
infantum were similar to those obtained with the reference strains, data not shown. I-17 was effective in both promastigotes and intracellular amastigotes,
Table 1.
The difference in the tolerance levels of the macrophages to the parasites made it feasible to use the compound in further experiments.
We decided to test whether I-17 effectively attenuated protein translation in Leishmania promastigotes. To pursue this goal, we tested puromycin incorporation in protein extracts pre-treated with puromycin.
Figure 1 shows the reduction of puromycin incorporation in I-17-treated promastigotes, thus suggesting that the I-17 compound attenuates the translation in these parasites.
We aimed to investigate a possible immunomodulatory effect of I-17 on infected macrophages. The production of Nitric Oxide (NO) is a critical factor for controlling Leishmania infection and depends on Nitric Oxide Synthase (NOS2) induction.
Figure 2A shows the dose-effect response in the infection index. We selected the dose of 5μM to investigate the expression of NOS2,
Figure 2B, and NO production. Our data show that I-17 treatment leads to the expression of NOS2 and NO production.
4. Discussion
Cutaneous and visceral leishmaniasis are prominent neglected diseases worldwide, affecting hundreds of thousands of people. The development of vaccines is still in process, while prevention requires a consistent and expensive governmental measure. In many countries, leishmaniasis treatment relies on applying drugs such as antimonial intramuscular injections as the first-line therapy. Liposomal amphotericin B is used in visceral leishmaniasis and mucosal leishmaniasis. However, the administration of amphotericin B demands the medical attention. Antimonial injections may lead to undesirable side effects, and long-term administration frequently results in patients' low adherence to the treatment, and oral treatment still relies on miltefosine, [
17]. The large number of
Leishmania species related to human infections and the impact of viral coinfections challenge the perception of the actual effectiveness of the ongoing therapeutics in leishmaniasis treatment.
As a proof of principle, we decided to use the compound I-17 in
Leishmania parasites to test the hypothesis that inhibitors of translation initiation will reduce promastigote replication and infection index. Previously, we have screened 25 analogs of 1,3-diarylureas and 1-((1,4-trans)-4-aryloxy cyclohexyl-3-arylureas (cHAUs) against
Trypanosoma cruzi [
16] and the compound I-17 inhibited epimastigotes and intracellular amastigotes forms. This class of compounds inhibits protein translation by activating eIF2alpha-kinases and further blockage of translation initiation. The screening of di-substituted ureas in
L.
amazonensis and
L.
infantum corroborated that I-17 was the most effective compound in reducing promastigote and amastigote growth. The EC50 in promastigotes of
both Leishmania species ranged from 3,0 to 5.0 μM. At the same time, the reduction of the infection index showed significant differences.
L. infantum (MHOM/TN/80/IPT1) amastigotes exhibited more resistance, with an SI of 2,2, while the infection index of
L.
amazonensis infection showed an EC50 of 3,0 with an SI of 33,2. Further studies are necessary to clarify whether and why
L.
infantum amastigotes are more resistant to I-17.
The production of NO by infected macrophages is associated with
controlling Leishmania infection and is one mark of the M1 macrophages. Cells infected with
L.
amazonensis classically show a reduction in NO since the subversion of the pathway is important for the successful establishment of infection by the parasite [
18,
19]. Our data showed that NOS2 expression and NO production are augmented in I-17-treated either infected and non-infected macrophages, suggesting a metabolic modulatory effect of I-17, which is justifiable because the activation of HRI phosphorylates eIF2α, favoring the generation of nitric oxide in cells [
20], a fact that corroborates previous work developed with these compounds that can activate HRI to obtain translation inhibition [
21]
Our previous work showed that I-17 treatment leads to the activation of an HRI-like kinase in
T.
cruzi and the phosphorylation of eIF2α [
22]. Our data through testing puromycin incorporation revealed that I-17 blocked mRNA translation, most likely due to inhibiting translation initiation. Work is underway to describe the eIF2alpha kinase activated in
Leishmania by I-17.
5. Conclusions
In conclusion, the data obtained with I-17 supports the notion that I-17 activates an eIF2alpha kinase in parasites and can induce NO production in host cells, developing a hostile milieu for the growth of intracellular amastigotes. The high SI justifies further in vivo studies to test I-17 and may pave the way for developing more effective drugs against Leishmania based on di-substitute ureas and the inhibition of protein translation.
Author Contributions
The work was conceptualized by U.G.L., L.G., and B.H.A. J.M.M., K.D.T., J.V.S., and A.D. were in charge of methodology and investigation; U.G.L. and L.C. carried out formal analysis; resources by M.C., B.H.A., B.H.A. and U.G.L.; data curation by J.V.S.; U.G.L. and J.V.L.S wrote the original draft preparation and L.C. and B.H.A. reviewed and edited the manuscript; U.G.L. and L.G. supervised the experiments. All authors have read and agreed to the published version of the manuscript.
Funding
Synthesis of I-17 was supported by NIH/NCI grant #1RO1CA152312 to B.H. Aktas. This work was partially supported by the Department of Biomolecular Sciences of the University of Urbino and by Fano Ateneo to L. Gallucci and Fundação Carlos Chagas Filho de Apoio a Pesquisa do Estado do Rio de Janeiro (FAPERJ) to U. Gazos Lopes.
Data Availability Statement
All the research data are available to the corresponding author upon request.
Acknowledgments
The authors are in debt to Renato Silva for the technical assistance.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Hong, A.; Zampieri, R.A.; Shaw, J.J.; Floeter-Winter, L.M.; Laranjeira-Silva, M.F. One Health Approach to Leishmaniases: Understanding the Disease Dynamics through Diagnostic Tools. Pathogens. 2020, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Pinart, M.; Rueda, J.R.; Romero, G.A.; Pinzón-Flórez, C.E.; Osorio-Arango, K.; Silveira Maia-Elkhoury, A.N.; Reveiz, L.; Elias, V.M.; Tweed, J.A. Interventions for American cutaneous and mucocutaneous leishmaniasis. Cochrane Database Syst Rev. 2020, 8, CD004834. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Conceição, C.; Pereira, A.; Rocha, R.; Ortuño, M.; Muñoz, C.; Jumakanova, Z.; Pérez-Cutillas, P.; Özbel, Y.; Töz, S.; et al. The estimated distribution of autochthonous leishmaniasis by Leishmania infantum in Europe in 2005-2020. PLoS Negl Trop Dis. 2023, 17, e0011497. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Miró, G.; Baneth, G.; Bourdeau, P.; Breitschwerdt, E.; Capelli, G.; Cardoso, L.; Day, M.J.; Dobler, G.; Ferrer, L.; et al. Canine Leishmaniasis Control in the Context of One Health. Emerg Infect Dis. 2019, 25, 1–4. [Google Scholar] [CrossRef]
- Abdellahi, L.; Iraji, F.; Mahmoudabadi, A.; Hejazi, S.H. Vaccination in Leishmaniasis: A Review Article. Iran Biomed. J. 2022, 26, 1–35. [Google Scholar] [CrossRef]
- Aktas, B.H.; Qiao, Y.; Ozdelen, E.; Schubert, R.; Sevinc, S.; Harbinski, F.; Grubissich, L.; Singer, S.; Halperin, J.A. Small-Molecule targeting of translation initiation for cancer therapy. Oncotarget. 2013, 4, 1606–1617. [Google Scholar] [CrossRef]
- Komar, A.A.; Merrick, W.C. A Retrospective on eIF2A-and Not the Alpha Subunit of eIF2. Int J Mol Sci. 2020, 21, 2054. [Google Scholar] [CrossRef]
- Chesnokova, E.; Bal, N.; Kolosov, P. Kinases of eIF2a Switch Translation of mRNA Subset during Neuronal Plasticity. Int J Mol Sci. 2017, 18, 2213. [Google Scholar] [CrossRef]
- Burwick, N.; Aktas, B.H. The eIF2-alpha kinase HRI: A potential target beyond the red blood cell. Expert Opin Ther Targets. 2017, 21, 1171–1177. [Google Scholar] [CrossRef]
- Dias-Teixeira, K.L.; Calegari-Silva, T.C.; Medina, J.M.; Vivarini, Á.C.; Cavalcanti, Á.; Teteo, N.; Santana, A.K.M.; Real, F.; Gomes, C.M.; Pereira, R.M.S.; et al. Emerging Role for the PERK/eIF2α/ATF4 in Human Cutaneous Leishmaniasis. Sci Rep. 2017, 7, 17074. [Google Scholar] [CrossRef]
- Stonyte, V.; Mastrangelopoulou, M.; Timmer, R.; Lindbergsengen, L.; Vietri, M.; Campsteijn, C.; Grallert, B. The GCN2/eIF2αK stress kinase regulates PP1 to ensure mitotic fidelity. EMBO Rep. 2023, 24, e56100. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ozel, D.; Qiao, Y.; Harbinski, F.; Chen, L.; Denoyelle, S.; He, X.; Zvereva, N.; Supko, J.G.; Chorev, M.; et al. Chemical genetics identify eIF2α kinase heme-regulated inhibitor as an anticancer target. Nat Chem Biol. 2011, 7, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, S.; Laverdière, M.; Chou, M.N.; Boilard, N.; Chow, C.; Papadopoulou, B. Translational control through eIF2alpha phosphorylation during the Leishmania differentiation process. PLoS ONE. 2012, 7, e35085. [Google Scholar] [CrossRef]
- Machado, F.C.; Franco, C.H.; Dos Santos Neto, J.V.; Dias-Teixeira, K.L.; Moraes, C.B.; Lopes, U.G.; Aktas, B.H.; Schenkman, S. Identification of di-substituted ureas that prevent growth of trypanosomes through inhibition of translation initiation. Sci Rep. 2018, 8, 4857. [Google Scholar] [CrossRef]
- Bahnan, W.; Boucher, J.; Gayle, P.; Shrestha, N.; Rosen, M.; Aktas, B.; Adkins, B.; Ager, A.; Khan, W.N.; and Schesser, K. 2017. The eIF2α kinase Heme Regulated Inhibitor (HRI) protects the host from infection by regulating intracellular pathogen trafficking. Infection and Immunity 2018, 86, e00707-17. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Takrouri, K.; Hee-Hwang, S.; Rana, S.; Halperin, H.; Natarajan, A.; Morisseau, M.; Hammock, B., Chorev; Aktas, B.H. Explorations of Substituted Urea Functionality for Discovery of New Activators of the Heme Regulated Inhibitor Kinase. Journal of Medicinal Chemistry 2013, 56, 9457–9470. [Google Scholar] [CrossRef]
- eBioMedicine. Leishmania: An urgent need for new treatments. EBioMedicine. 2023, 87, 104440. [Google Scholar] [CrossRef]
- Goodman, C.A.; Hornberger, T.A. Measuring protein synthesis with SUnSET: A valid alternative to traditional techniques? Exerc Sport Sci Rev. 2013, 41, 107–115. [Google Scholar] [CrossRef]
- Bardallo, R.G.; Panisello-Roselló, A.; Sanchez-Nuno, S.; Alva, N.; Roselló-Catafau, J.; Carbonell, T. Nrf2 and oxidative stress in liver ischemia/reperfusion injury. FEBS J. 2022, 289, 5463–5479. [Google Scholar] [CrossRef] [PubMed]
- Balestieri, F.M.; Queiroz, A.R.; Scavone, C.; Costa, V.M.; Barral-Netto, M.; Abrahamsohn Ide, A. Leishmania (L.) amazonensis-induced inhibition of nitric oxide synthesis in host macrophages. Microbes Infect. 2002, 4, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Calegari-Silva, T.C.; Vivarini, Á.C.; Miqueline, M.; Dos Santos, G.R.; Teixeira, K.L.; Saliba, A.M.; Nunes de Carvalho, S.; de Carvalho, L.; Lopes, U.G. The human parasite Leishmania amazonensis downregulates iNOS expression via NF-κB p50/p50 homodimer: Role of the PI3K/Akt pathway. Open Biol. 2015, 5, 150118. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Heim, R.A.; Wu, S. Nitric oxide: A regulator of eukaryotic initiation factor 2 kinases. Free Radic Biol Med. 2011, 50, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Du, R.; Reis Monteiro Dos Santos, G.R.; Yefidoff-Freedman, R.; Bohm, A.; Halperin, J.; Chorev, M.; Aktas, B.H. New activators of eIF2α Kinase Heme-Regulated Inhibitor (HRI) with improved biophysical properties. Eur J Med Chem. 2020, 187, 111973. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).