1. Introduction
1-1. Facial nerve and facial palsy
The facial nerve is a mixed nerve containing both motor and sensory functions. Motor nerves are responsible for movement of the muscles of the face and neck, and contain parasympathetic components that are responsible for the secretory actions of lacrimal and salivary glands. It also contains specialized sensory nerves in the anterior two-thirds of the tongue that detect taste and general sensory nerves that are responsible for deep perception of the auricle, posterior wall of the ear canal, ear lobe, and soft tissue of the face. Thus, the facial nerve consists of two efferent nerves and two afferent nerves and collectively serves four functions. The facial nerve can be classified into upper and lower segments based on its motor nucleus. The upper segment of the motor nucleus receives inputs from both sides of the brain – cross fibers and non-cross fibers from the pons – governing facial expressions of both sides of the face. In contrast, nerve fibers in the lower segment of the motor nucleus are exclusively innervated by the contralateral cerebral cortex. Thus, in cases where upper lesions occur on one side of the motor nucleus, because the upper face is controlled by both hemispheres, paralysis mainly manifests in the facial expression muscles at the bottom of the face around the lower region of the mouth. This paralysis does not affect the expression muscles of the forehead or eye circumference or impair taste, salivation, or tear secretion. In contrast, in the case of a lesion below the unilateral motor nucleus, paralysis affects both upper and lower facial muscles on the lesion side as a whole [
1,
2].
Although facial nerve palsy is not a life-threatening disease, it has a devastating effect on those afflicted, impacting their emotional state and social lives. Thus, effective treatment and positive prognosis are of paramount importance. When damage to the cell body of a neuron is sufficient to destroy it, the neuron can no longer survive. However, if the axon is only partially cut and the cell body is not damaged, the neuron can regenerate the axon. Moreover, under appropriate conditions, this cell may re-form synapses to cells with which it had previously synapsed, allowing for the possibility of full restoration of function. Changes in nerve fibers after nerve damage vary depending on the degree of damage. In the case of mild damage such as neuropraxia, nerve fibers go through the process of local demyelination and remyelination; but in case of the severe damage, axonal degeneration and regeneration occur [
3,
4].
1-2. Definition and types of autophagy
The term ‘autophagy’ is derived from the Greek words ‘auto’ meaning self and ‘phagy’ meaning to eat. There are three types of autophagy: (1) microautophagy, in which intracellular substances directly enter the lysosome through invagination of the lysosomal membrane; (2) macroautophagy, where an autophagosome composed of a double lipid membrane surrounds a substance and fuses with lysosomes; and (3) chaperone-mediated autophagy, which degrades proteins with specific target motifs through a process mediated by a chaperone complex and lysosomal-associated membrane protein type 2A. Where not otherwise specified, ‘autophagy’ generally refers to macroautophagy. Autophagy is further classified into aggrephagy, lipophagy, mitophagy, plexophagy, ribophagy and xenophagy, depending on the nature of the material that is loaded and digested. Autophagy plays an important role not only in the degradation of protein aggregates, but also in the removal of damaged intracellular organelles, including mitochondria, endoplasmic reticulum and peroxisomes; it also is involved in removing extracellular pathogens, such as bacteria, viruses, and parasites. In various organisms, including mice, whole-body or tissue-specific deletion of autophagy-related genes (Atg) causes serious disorders and death [
5,
6], supporting the hypothesis that autophagy is an important process in maintaining health.
1-3. Molecular mechanisms controlling autophagy
Since the first identification of autophagy-related genes in yeast by Ohsumi et al. in 1993, more than 35 Atg genes have been discovered [
7,
8]. In addition to being intricately regulated by the proteins it produces, the autophagy process is under negative regulation by mTOR (mammalian target of rapamycin) and positive regulation by AMPK (adenosine monophosphate-activated protein kinase). The process itself can be divided into four major steps: 1) initiation and vesicle nucleation, 2) vesicle elongation, 3) fusion and degradation, and 4) termination. The initiation step is regulated by the ULK1 protein, with the ULK1-containing complex, ULK1-Atg13-Atg101-FIP200, dissociating from the mTORC1 complex as a result of nutrient deficiency-dependent dephosphorylation of ULK1. This leads to increased activation of the beclin 1/Vps34 complex (beclin 1-Atg14L-Vps15-Vps34 complex), inducing the production of PI3P (phosphatidylinositol-3-phosphate) and initiating nucleation of vesicles with a double-membrane structure, facilitated by the DFCP1 and WIPI proteins that gather at the site. Vesicles subsequently elongate through the actions of proteins that constitute the two ubiquitin-like conjugation systems, ATG12-ATG5-ATG16 and LC3-phosphatidylethanolamine, resulting in the formation of an autophagosome. The resulting mature autophagosome then fuses with a lysosome to form an autolysosome, within which isolated intracellular substances or organelles are degraded by lysosomal hydrolytic enzymes. As a consequence, nutrients (e.g., amino acids) released through autolysosomal digestion increase the activation of mTOR protein, a major negative regulator of the mTORC1 complex, ultimately leading to termination of the autophagy process [
9,
10].
2. Induction of autophagy in various diseases
The autophagy process, involving the transport of intracellular substances surrounded by a double membrane structure to a lysosome and subsequent autolysomal degradation, acts together with the ubiquitin proteasome system to play an important role in maintaining intracellular protein quality control. Autophagy also plays an important role in the degradation of protein aggregates and the removal of damaged intracellular organelles and extracellular pathogens. Thus, not surprisingly, dysregulation of autophagy can act as a major cause of various diseases. In the absence of sufficient nutrients to generate adequate ATP, autophagy also breaks down proteins into amino acids, lipid droplets into fatty acids, and glycogen into glucose, thereby serving a regulatory function that maintains intracellular energy balance. Conversely, under energy-replete conditions, autophagic degradation of energy sources (proteins, fat particles, and sugar sources) is suppressed and their storage increases. However, disruptions in the regulation of autophagy result in an energy imbalance [
11,
12].
Ongoing studies have investigated potential positive or negative effects of autophagy on human diseases, but the underlying mechanisms have proven more complex than expected. Consequently, whether and how autophagy is involved in maintaining human health or the development of disease remains unclear. Activation of autophagy in cancer acts as a tumor suppressor, whereas inactivation of autophagy allows survivals of cancer cells under nutrient-poor conditions. In muscular disorders, autophagy may increase as a compensatory response to defects in lysosome function, whereas inactivation of autophagy can lead to the accumulation of autophagosomes, potentially impairing cell function. In neurodegeneration, autophagy activation contributes to the timely removal of protein aggregates before they become toxic, whereas autophagy inactivation might trigger cell death in neurons burdened with aggregated proteins. In the case of pathogen infection, autophagy activation serves as a cellular defense against invasion of bacteria and viruses; conversely, inactivation of autophagy allows pathogens to establish a replicative niche [
11,
12,
13,
14].
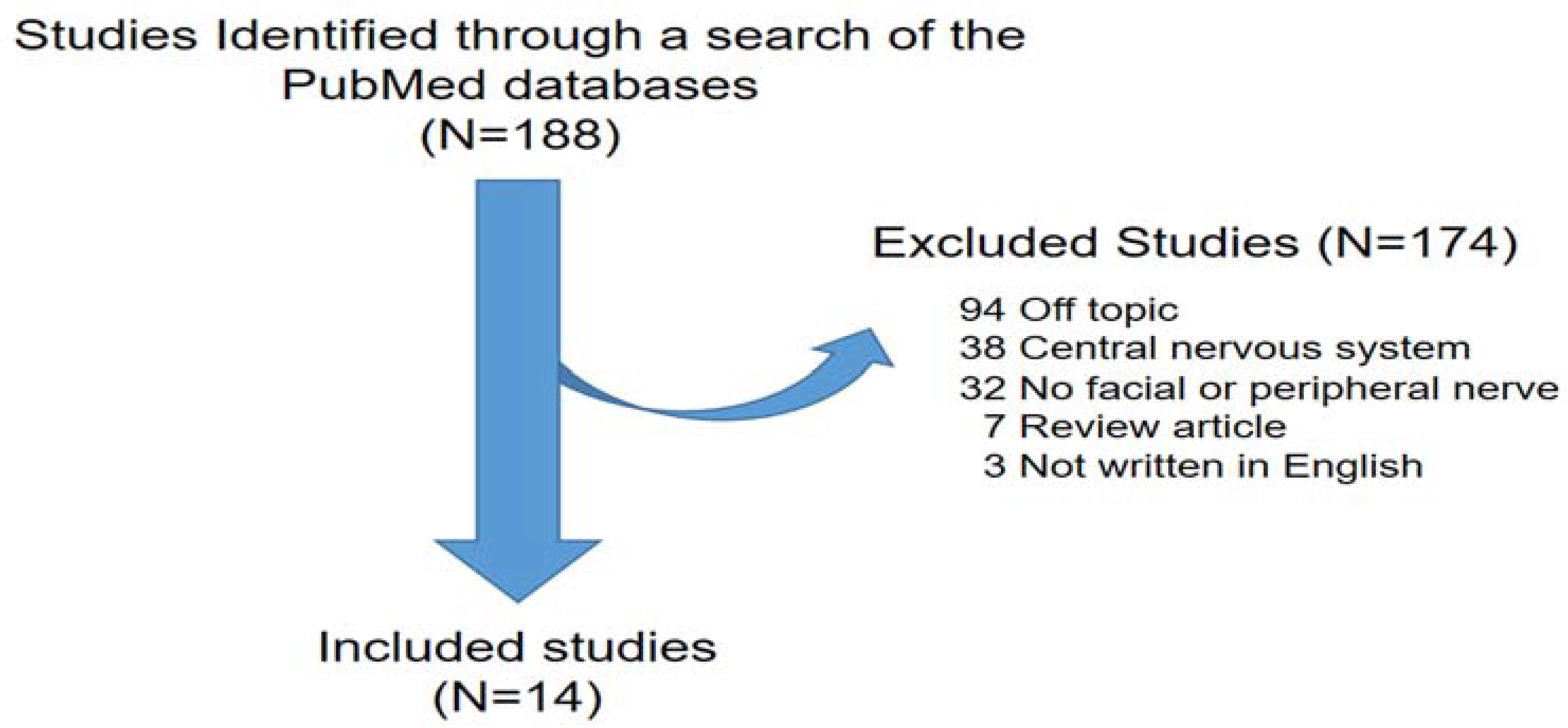
While the involvement of autophagy has been studied in various diseases, its induction and contribution to nerve degeneration and regeneration after facial nerve injury (FNI) has not yet been established. To bridge this knowledge gap and shed light on the clearance of damaged nerve debris and innate immune induced by facial nerve damage, we conducted a review of the relevant literature. To this end, we analyzed and summarized the results of previous studies on the involvement of autophagy in nerve regeneration. Our search encompassed literature databases, focusing on studies published in English. Studies were included if they 1) were prospective or retrospective investigations of autophagy and facial or peripheral nerves; 2) considered facial nerve degeneration and regeneration; and 3) included human patients and/or animal studies on autophagy and facial or peripheral nerves. From 1993 to 2023, we identified 188 studies based on search terms from PubMed electronic database. No autophagy, no facial or peripheral nerve, review article, off topic, not written in English were excluded. A total of 14 peripheral nerve studies that met these criteria, including 11 involving sciatic nerves, 2 involving facial nerves, and 1 involving the inferior alveolar nerve, were included in this review (
Figure 1).
4. Conclusions
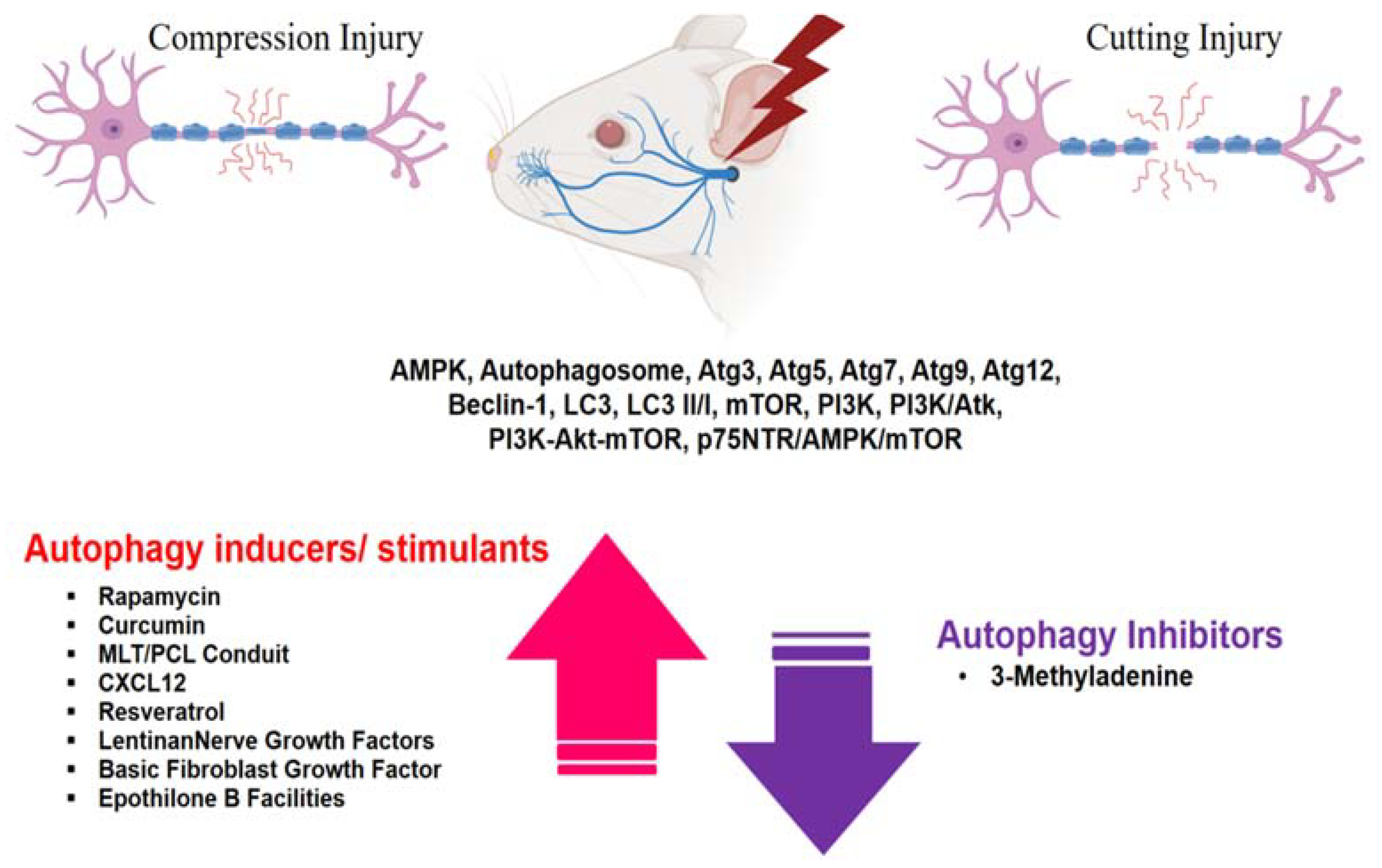
Although research on the facial nerve remains limited, studies of autophagy conducted on peripheral nerves, including the sciatic nerve, have yielded noteworthy findings. These investigations have revealed the expression of a number of autophagy-related substances in compression and transection nerve injury models, including AMPK, Atg3, Atg5, Atg7, Atg9, Atg12, beclin-1, LC3, LC3 II/I, mTOR, PI3K, PI3K/Atk, PI3K-Akt-mTOR and p75NTR/AMPK/mTOR, as well as changes in autophagosome dynamics. In the case of PNI, administration of autophagy inducers or inhibitors increased or decreased autophagy-related substances, suggesting that autophagy is closely related to peripheral nerve degeneration and regeneration. It is imperative that future studies delve in other unexplored autophagy-related factors post nerve damage and further explore the intricate interplay between autophagy, inflammation- and immunity-related factors, and nerve regeneration-related factors.
Author Contributions
Conceptualization, S.G.Y.; Data curation, Y.J.K., D.K.Y., S.Y.J., S.S.K., J.L., and J.H.Y.; Formal analysis, J.L., J.H.Y., and J.M.L.; Funding acquisition: S.S.K. and S.G.Y.; Methodology, D.C.P., Y.J.K., D.K.Y., and J.M.L.; validation, Project administration, D.C.P. and S.G.Y.; Visualization, D.K., D.C.P., and S.S.K.; Writing - original draft, D.K.Y., Y.J.K., D.C.P., and S.G.Y.; Writing - review & editing, D.C.P. and S.G.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgement
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF 2018R1A6A1A03025124)(NRF 2019R1A2C1086807)(NRF 2022R1A2C1091779). This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HV22C0233). The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, K.H. The Role of Autophagy in the Pathogenesis and Treatment of Metabolic Diseases. Molecular and Cellular Biology Newsletter. 2004, 1–9. [Google Scholar] [CrossRef]
- Dobie, R.A. Test of facial nerve function. In Otolaryngology Head and Neck Surgery, St. Louis Mosby, 3rd ed.; Cummings, C.W., Fredrickson, J.M., Harker, L.A., et al., Eds.; 1998; pp. 2757–2766. [Google Scholar]
- Byun, J.Y. Facial paralysis disorders. Anatomy and evaluation of facial nerve. In Korean Society of Otorhinolaryngology−Head and Neck Surgery, 3rd ed.; Seoul. KoonJa, 2018; pp. 913–932. [Google Scholar]
- Hu, Y. Axon injury induced endoplasmic reticulum stress and neurodegeneration. Neural Regen Res. 2016, 11, 1557–1559. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, M.S. Autophagy–a key player in cellular and body metabolism. Nat Rev Endocrinol. 2014, 10, 322–37. [Google Scholar] [CrossRef]
- Ichimura, Y.; Komatsu, M. Pathophysiological role of autophagy: lesson from autophagy-deficient mouse models. Exp Anim. 2011, 60, 329–345. [Google Scholar] [CrossRef]
- Tsukada, M.; Ohsumi, Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993, 333, 169–174. [Google Scholar] [CrossRef]
- Mizushima, N.; Yoshimori, T.; Ohsumi, Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 2011, 27, 107–132. [Google Scholar] [CrossRef]
- Russell, R.C.; Yuan, H.X.; Guan, K.L. Autophagy regulation by nutrient signaling. Cell Res. 2013, 24, 42–57. [Google Scholar] [CrossRef]
- Nixon, R.A. The role of autophagy in neurodegenerative disease. Nature medicine. 2013, 19, 983–997. [Google Scholar] [CrossRef]
- Shntani, T.; Klionsky, D.J. Autophagy in health and disease; A double-edged sword. Science. 2004, 306, 990–995. [Google Scholar] [CrossRef]
- Cuervo, A.M. Autophagy; in sickness and in health. Trends in Cell Biol. 2004, 14, 70–77. [Google Scholar] [CrossRef]
- Mehrpour, M.; Esclatine, A.; Beau, I.; Codogno, P. Autophagy in health and disease. 1. Regulation and significance of autophagy: an overview. Am J Physiol Cell Physiol. 2010, 298, C776–85. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.M.; Ryter, S.W.; Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 2013, 368, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, J.A.; Carty, L.; Iruarrizaga-Lejarreta, M.; Palomo-Irigoyen, M.; Varela-Rey, M.; Jessen, K.R. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J Cell Biol. 2015, 210, 153–68. [Google Scholar] [CrossRef] [PubMed]
- Romeo-Guitart, D.; Leiva-Rodriguez, T.; Forés, J.; Casas, C. Improved Motor Nerve Regeneration by SIRT1/Hif1a-Mediated Autophagy. Cells. 2019, 30, 1354. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Chen, L.; Zhang, H.X.; Li, S.F.; Li, C.X. Autophagy Promotes Peripheral Nerve Regeneration and Motor Recovery Following Sciatic Nerve Crush Injury in Rats. J Mol Neurosci. 2016, 58, 416–23. [Google Scholar] [CrossRef] [PubMed]
- Ko, P.Y.; Yang, C.C.; Kuo, Y.L.; Su, F.C.; Jou, I.M. Schwann- Cell Autophagy, Functional Recovery, and Scar Reduction After Peripheral Nerve Repair. J Mol Neurosci. 2018, 64, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Inada, T.; Sato, H.; Hayashi, Y.; Hitomi, S.; Ando, M.; Shinoda, M. Rapamycin Accelerates Axon Regeneration Through Schwann Cell-mediated Autophagy Following Inferior Alveolar Nerve Transection in Rats. Neuroscience. 2021, 468, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, X.; Li, Q. Curcumin accelerates the repair of sciatic nerve injury in rats through reducing Schwann cells apoptosis and promoting myelinization. 2017, 92, 1103–1110. [Google Scholar] [CrossRef]
- Shintani, K.; Uemura, T.; Takamatsu, K. Protective effect of biodegradable nerve conduit against peripheral nerve adhesion after neurolysis. J Neurosurg. 2017, 20, 1–10. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chen, M.H.; Liao, S.Y. Multichanneled nerve guidance conduit with spatial gradients of neurotrophic factors and oriented nanotopography for repairing the peripheral nervous system. ACS Appl Mater Interfaces. 2017, 9, 37623–37636. [Google Scholar] [CrossRef]
- Salehi, M.; Naseri-Nosar, M.; Ebrahimi-Barough, S. Polyurethane/gelatin nanofibrils neural guidance conduit containing platelet-rich plasma and melatonin for transplantation of schwann cells. Cell Mol Neurobiol. 2018, 38, 703–713. [Google Scholar] [CrossRef]
- Qian, Y.; Han, Q.; Zhao, X.; Song, J.; Cheng, Y.; Fang, Z.; Qian, Y. 3D melatonin nerve scaffold reduces oxidative stress and inflammation and increases autophagy in peripheral nerve regeneration. J Pineal Res. 2018, 65, e12516. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Tang, T.; Zhu, J.; Tang, Y.; Sun, H.; Li, S. CXCL12 has therapeutic value in facial nerve injury and promotes Schwann cells autophagy and migration via PI3K-AKT-mTOR signal pathway. International journal of biological macromolecules 2019, 124, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ren, J.; Liu, Y.; Huang, D.; Lu, L. Resveratrol regulates the recovery of rat sciatic nerve crush injury by promoting the autophagy of Schwann cells. Life Sci. 2020, 256, 117959. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, M. Functional interactions of neurotrophins and neurotrophin receptors. Annu Rev Neurosci. 1995, 18, 223–53. [Google Scholar] [CrossRef]
- Zampieri, N.; Chao, M. Structural biology. The p75 NGF receptor exposed. Science. 2004, 304, 833–4. [Google Scholar] [CrossRef]
- Jiang, T.; Yu, J.T.; Zhu, X.C.; Wang, H.F.; Tan, M.S.; Cao, L. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol. 2014, 171, 3146–57. [Google Scholar] [CrossRef]
- Matsui, Y.; Takagi, H.; Qu, X.; Abdellatif, M.; Sakoda, H.; Asano, T. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007, 100, 914–22. [Google Scholar] [CrossRef]
- Li, R.; Li, D.; Wu, C.; Ye, L.; Wu, Y. , Yuan, Y.; Xiao, J. Nerve growth factor activates autophagy in Schwann cells to enhance myelin debris clearance and to expedite nerve regeneration. Theranostics. 2020, 10, 1649–1677. [Google Scholar] [CrossRef]
- Xiao, H.; Wei, C.; Liu, H.; Li, Z.; Zheng, C.; Luo, J. Lentinan alleviates sciatic nerve injury by promoting autophagy to remove myelin fragments. Phytother Res. 2023, 10. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, M.; Zhang, Z.; Sun, Y.; Wang, J.; Liu, Y. ADSCs Combined with Melatonin Promote Peripheral Nerve Regeneration through Autophagy. Int J Endocrinol. 2022, 5861553. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhang, H.; Xu, M.; Li, L.; Wu, M.; Zhang, S.; Ni, L. Delivery of Basic Fibroblast Growth Factor Through an In Situ Forming Smart Hydrogel Activates Autophagy in Schwann Cells and Improves Facial Nerves Generation via the PAK-1 Signaling Pathway. Front Pharmacol. 2022, 13, 778680. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, S.; Gao, J.; Hu, Y.; Chen, S.; Luo, X.; Zhang, H.; Luo, Z.; Huang, J. Epothilone B Facilitates Peripheral Nerve Regeneration by Promoting Autophagy and Migration in Schwann Cells. Frontiers in Cellular Neuroscience. 2020, 14, 143. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).