1. Introduction
Lung cancer is the most prevalent and aggressive malignant neoplasm worldwide, causing substantial mortality, with higher incidence rates among men and ranking as the third most common cancer among women. It remains the leading cause of cancer-related death worldwide, a trend attributed primarily to population aging in developed countries and widespread tobacco consumption in less developed regions [
1]. Cancer cells can undergo uncontrolled proliferation and migrate to distant sites, leading to metastasis. Moreover, they can evade cell death mechanisms, making the development of effective treatments challenging.
Dehydroepiandrosterone (DHEA), the most abundant hormone in human plasma, has demonstrated a robust antiproliferative effect and the ability to induce cell death in various tumor cells. Previous research by our group has unveiled its potential to inhibit the proliferation of breast cancer cells [
2] and cervical cancer cells, including C33A, CaSki, and HeLa [
3]. DHEA also inhibits the migration of breast cancer cells such as MCF-7, MDA-MB-231, and Hs578T [
4], as well as cervical cancer cells like InBl and SiHa [
5]. Additionally, DHEA induces cell death in MCF-7 cells [
4]. Our studies have also demonstrated that DHEA inhibits events associated with the metastatic process in breast tumor cell lines [
6,
7]. Furthermore, various researchers have highlighted DHEA´s ability to induce autophagy in tumor cells. For example, Rovito and collaborators demonstrated that DHEA induces phosphorylation of the Bcl-2 protein, promoting its dissociation from Beclin-1 and resulting in autophagy induction in MCF-7 breast cancer cells [
8]. Another study revealed that DHEA induces autophagic cell death in human hepatoma cells (HepG2) through the NK-NRF2-p62 axis [
9].
However, the impact of DHEA on lung cancer cells remains a subject of debate. While some studies have suggested limited chemopreventive effects on lung tumor epithelial cell lines [
10], others have indicated potential inhibition of chemically induced lung cancer in animal models [
11,
12]. Given the conflicting evidence in the literature, this study seeks to elucidate the effects of DHEA on the proliferation, migration, autophagy, and viability of several lung cancer cell lines.
By addressing this knowledge gap, we aim to contribute to a better understanding of the potential role of DHEA in lung cancer, with potential implications for future therapeutic and preventive strategies.
3. Discussion
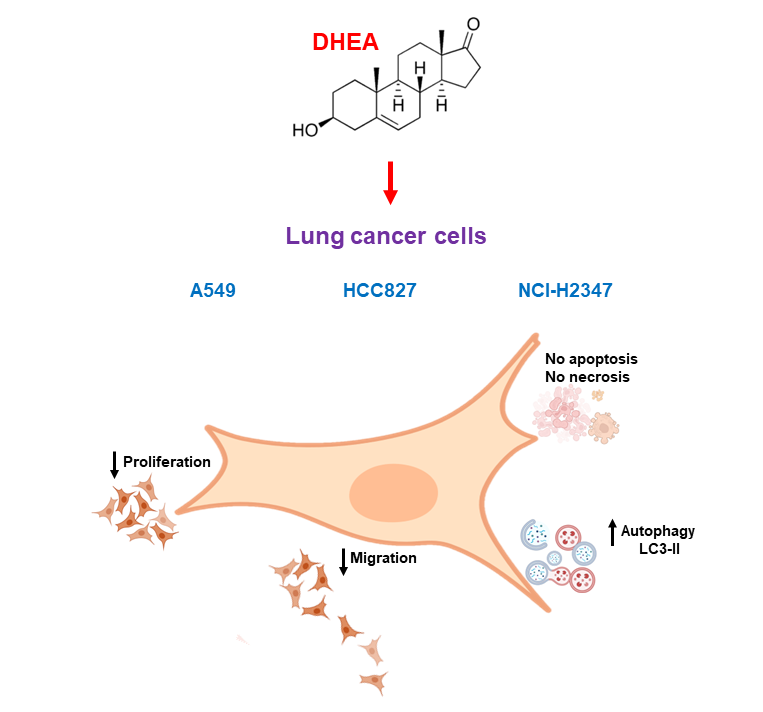
Lung cancer is known to have a high incidence and is responsible for many deaths worldwide. Conversely, research has demonstrated that DHEA exhibits protective effects against diverse cancer cells. However, limited information is available regarding its impact on lung tumor cells. Therefore, this study aims to investigate the effects of DHEA on the proliferation, migration, autophagy, and viability of various lung tumor cells.
We employed three human lung cancer cell lines for our experiments: NCI-H2347 cells, which are adenocarcinoma cells derived from non-small cell lung cancer; and HCC827 and A549 cells, derived from lung carcinoma. These cell lines were exposed to varying concentrations of DHEA (1 μM, 10 μM, and 100 μM) for 24 and 48 hours. Proliferation was assessed using crystal violet staining; cell viability and count via trypan blue staining; MTT reduction assay was employed to indirectly measure the viable cells in the culture; and migration was evaluated using a wound healing assay.
Because one μM DHEA had no effect on the before-mentioned processes, autophagy and cell death were assessed in cells treated with only 10 μM and 100 μM DHEA. Autophagy was analyzed using an autophagy detection kit, while annexin-V-FITC/IP staining and caspase-3/7 activity assays were employed to evaluate cell death.
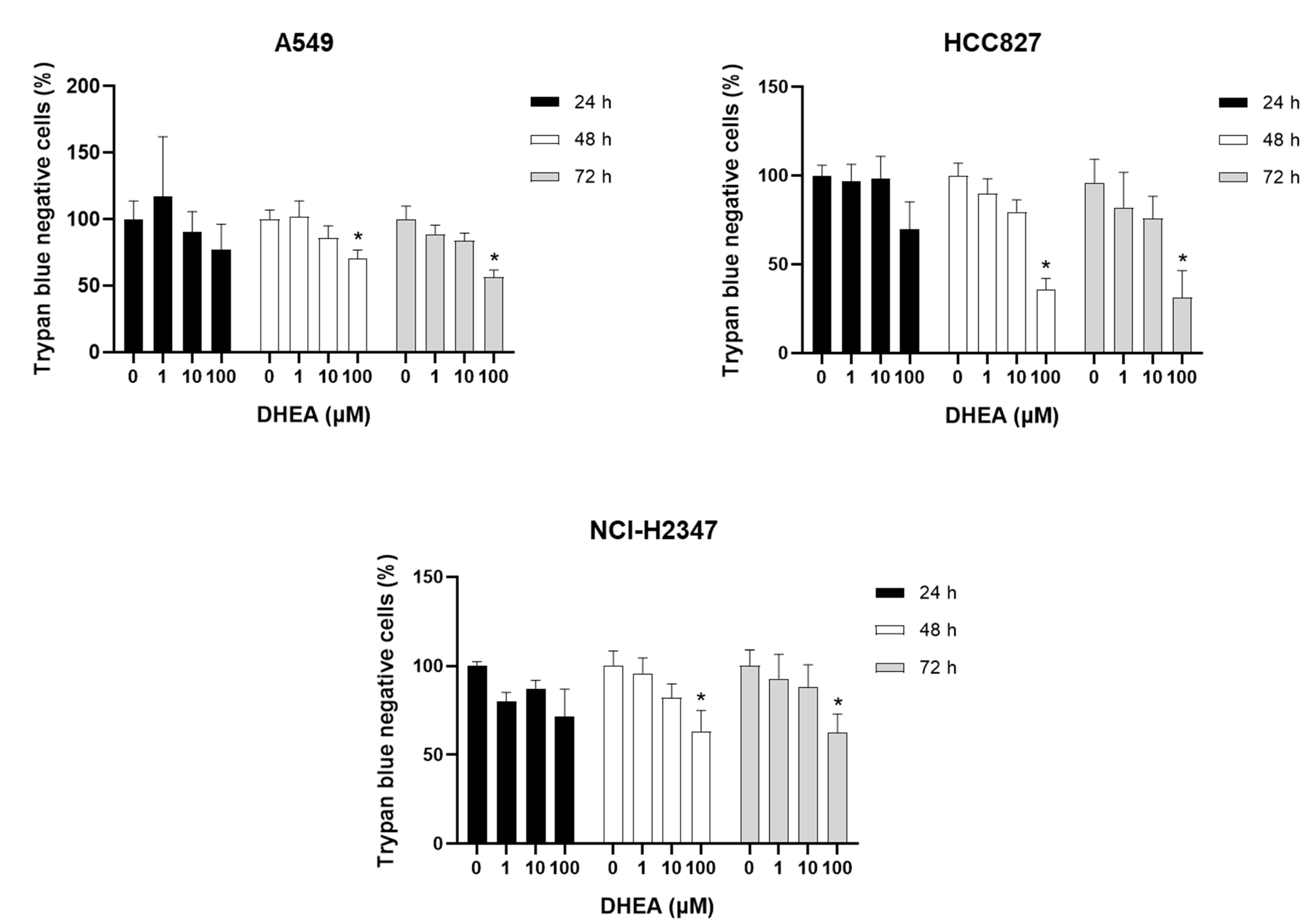
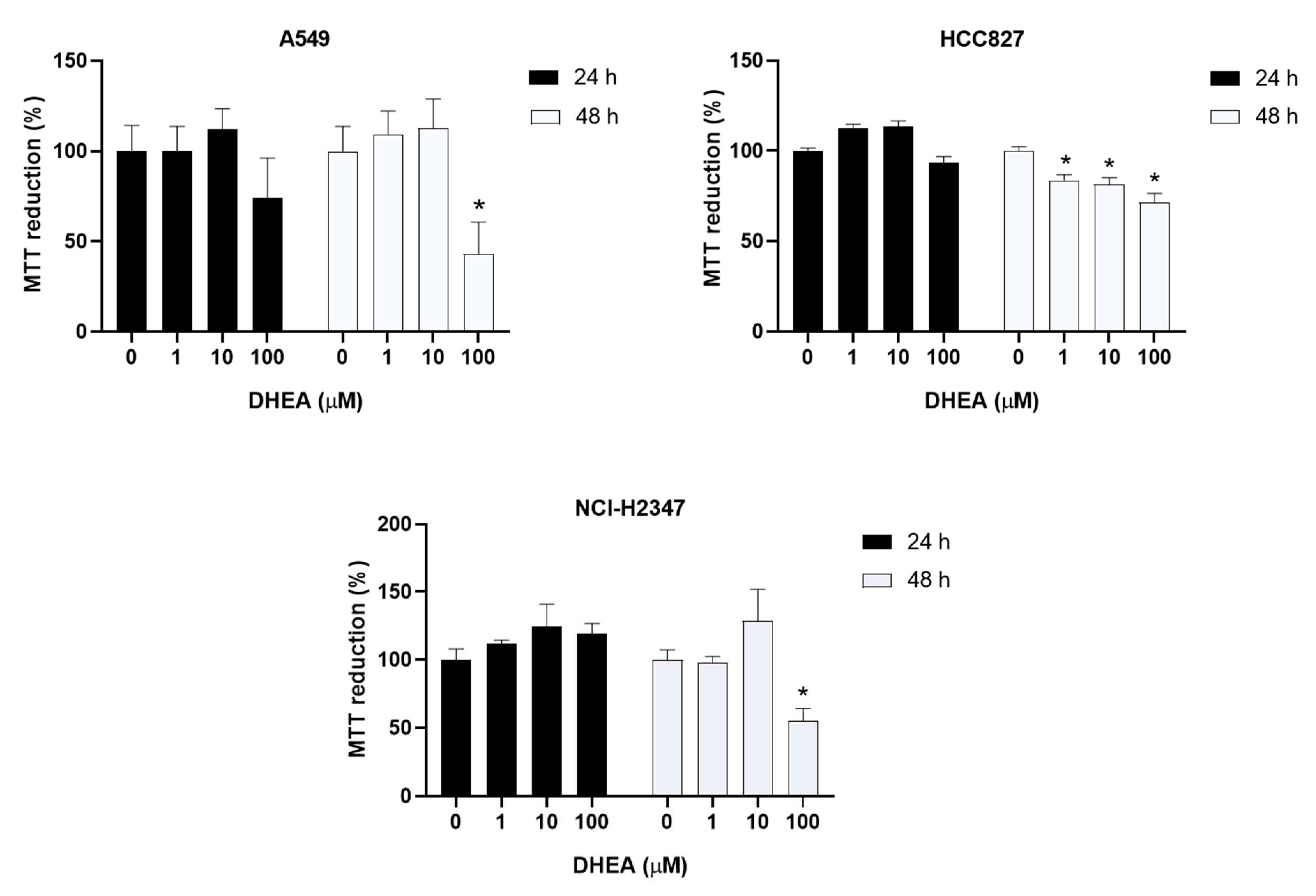
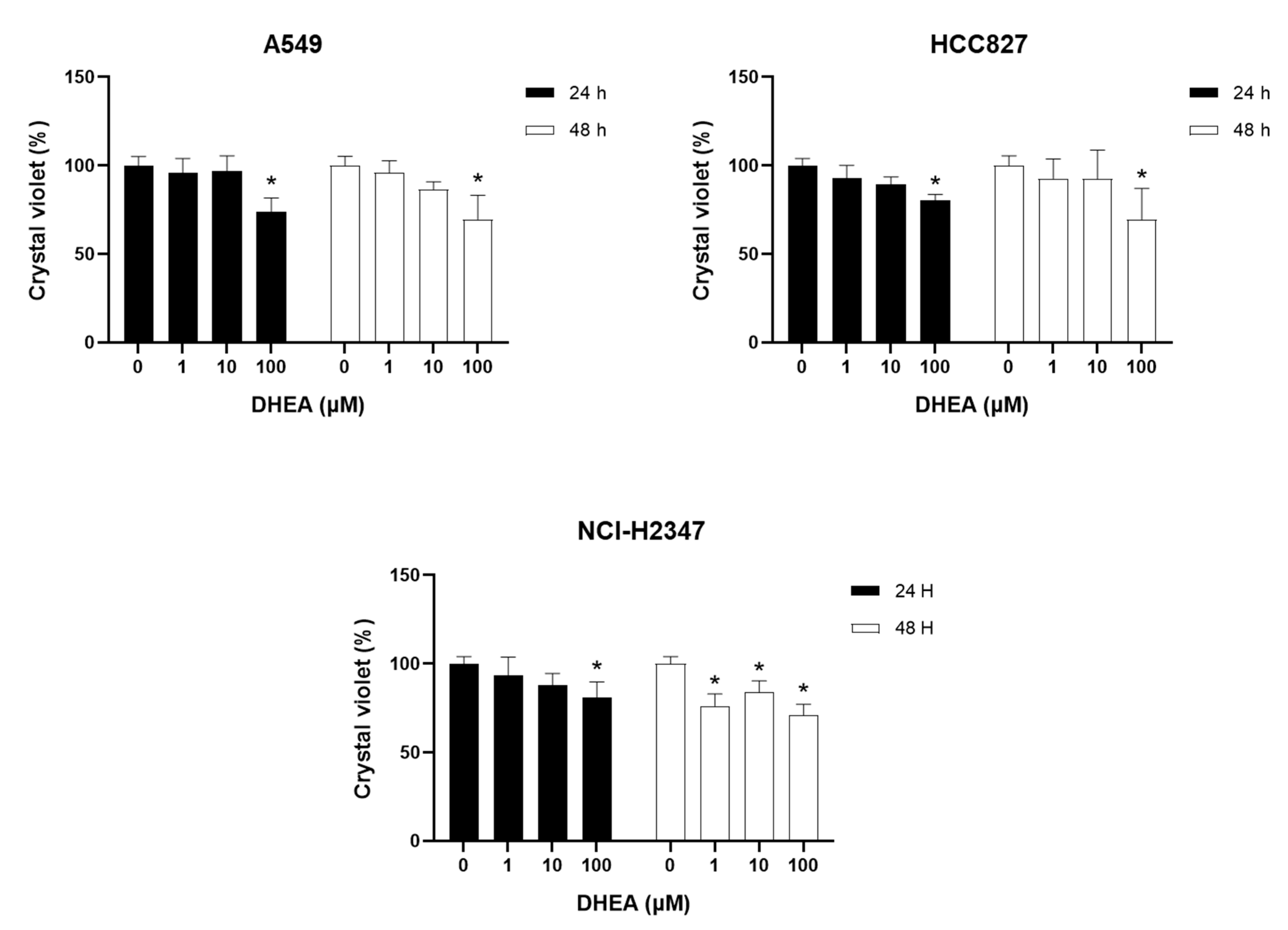
Our findings reveal that DHEA significantly decreased the growth rate, as measured by crystal violet staining, and inhibited MTT reduction in all lung tumor cells after 48 hours of treatment with 100 μM (
Figure 1 and
Figure 2). Since a decrease in the growth rate can be associated with the inhibition of cell proliferation or a cytotoxic effect, we opted to assess cell numbers using trypan blue staining after DHEA exposure. The results indicate that DHEA induces a notable reduction in cell numbers at 48 hours, which becomes even more pronounced at 72 hours (
Figure 3). Several studies support the antiproliferative effect of DHEA on tumor cells derived from various sources, including breast (MCF-7 and MDA-MB-231), colon (Caco-2 and HT29), melanoma (B16), myeloma (U-266, NOP-2, and IL-KM3), neuroblastoma, and cervix (C33A, CaSki, and HeLa) [13-17]. However, there have also been observations of DHEA stimulating proliferation in the same cell lines, as well as in others such as prostate (LNCaP) and melanoma (A375-SM) [
18,
19]. It has been demonstrated that DHEA inhibits the proliferation of MCF-7 cells at pharmacological concentrations. However, interestingly, it increases their proliferation at physiological concentrations [
2,
13], suggesting that DHEA can have a dual role depending on the concentration used. Furthermore, the effect of DHEA on proliferation may vary depending on the specific cell type, resulting in either a pronounced or a mild effect when the same concentrations are applied to different cell lines. Our study found that all used cells responded in a similar way to the antiproliferative effect induced by DHEA; however, A549 and NCI-H2347 cells were particularly more responsive in MTT reduction. We observed that these cells exhibit a higher proliferation rate compared to the other lung cancer cells used, suggesting a potential therapeutic strategy for tumors characterized by excessive and uncontrolled proliferation in the future.
It has been observed that specific biological processes, such as autophagy and apoptosis, share interactions among their components and can be induced by similar stimuli, indicating a complex crosstalk between them [
20]. Identical death signals can trigger apoptosis and necrosis; autophagy and necrosis can be activated in parallel or sequentially with shared or opposing targets [
20]. Given the intricate interconnection of these processes, we also assessed apoptosis and necrosis in lung tumor cells exposed to DHEA. Both annexin-V-FITC/IP as caspases-3/7 activation assays showed no induction of apoptosis and necrosis (see supplementary Figures).
Metastasis is the leading cause of cancer-related mortality, and cell migration is crucial in this process [
21]. Therefore, we evaluated the effect of DHEA on the migration of lung cancer cells. For this purpose, we used the wound healing assay, which is a simple and cost-effective method allowing the study of directional cell migration
in vitro [
22]. Our results demonstrated that lung tumor cells treated with DHEA for 48 hours exhibited a significant decrease in wound closure (Figures 4, 5, and 6), suggesting that DHEA can affect the migration capacity of cells, preventing them from invading other organs and thus inhibiting metastasis. In a previous study, we showed that DHEA inhibited the migration of breast cancer cells MDA-MB-231, reversing the mesenchymal phenotype by increasing the expression of an epithelial marker (E-cadherin) and decreasing the expression of mesenchymal proteins such as N-cadherin, vimentin, and Snail [
7], which was mediated through the PI3K/Akt pathway [
22]. Although these studies were conducted in breast cancer cells, similar mechanisms are likely involved in the effects of DHEA on lung cancer cells.
Autophagy is a catabolic process that tumor cells undergo in response to stress stimuli. It involves the recycling of both organelles and proteins to maintain cellular homeostasis. However, excessive autophagy can result in programmed cell death, referred to as autophagic cell death [
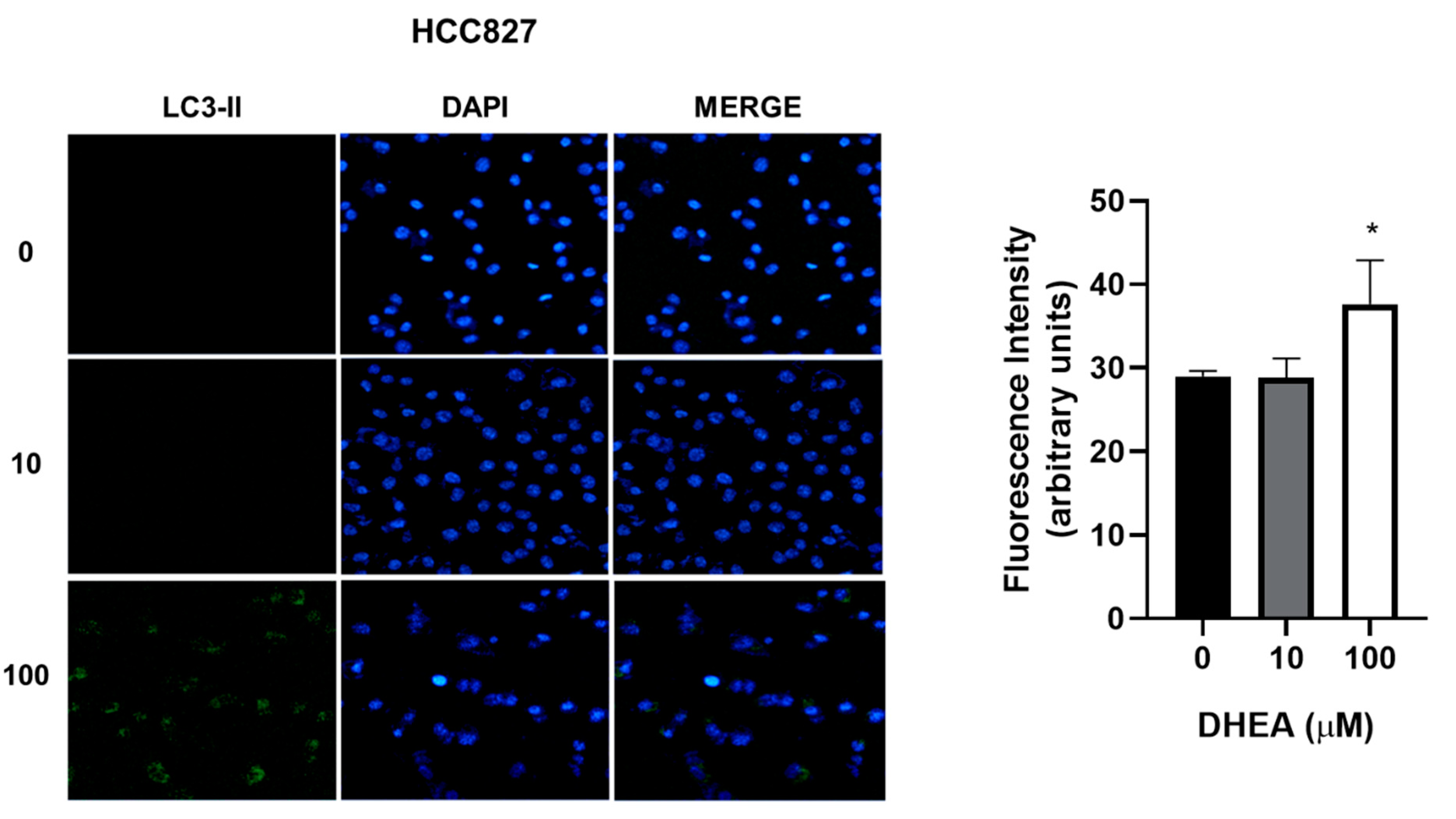
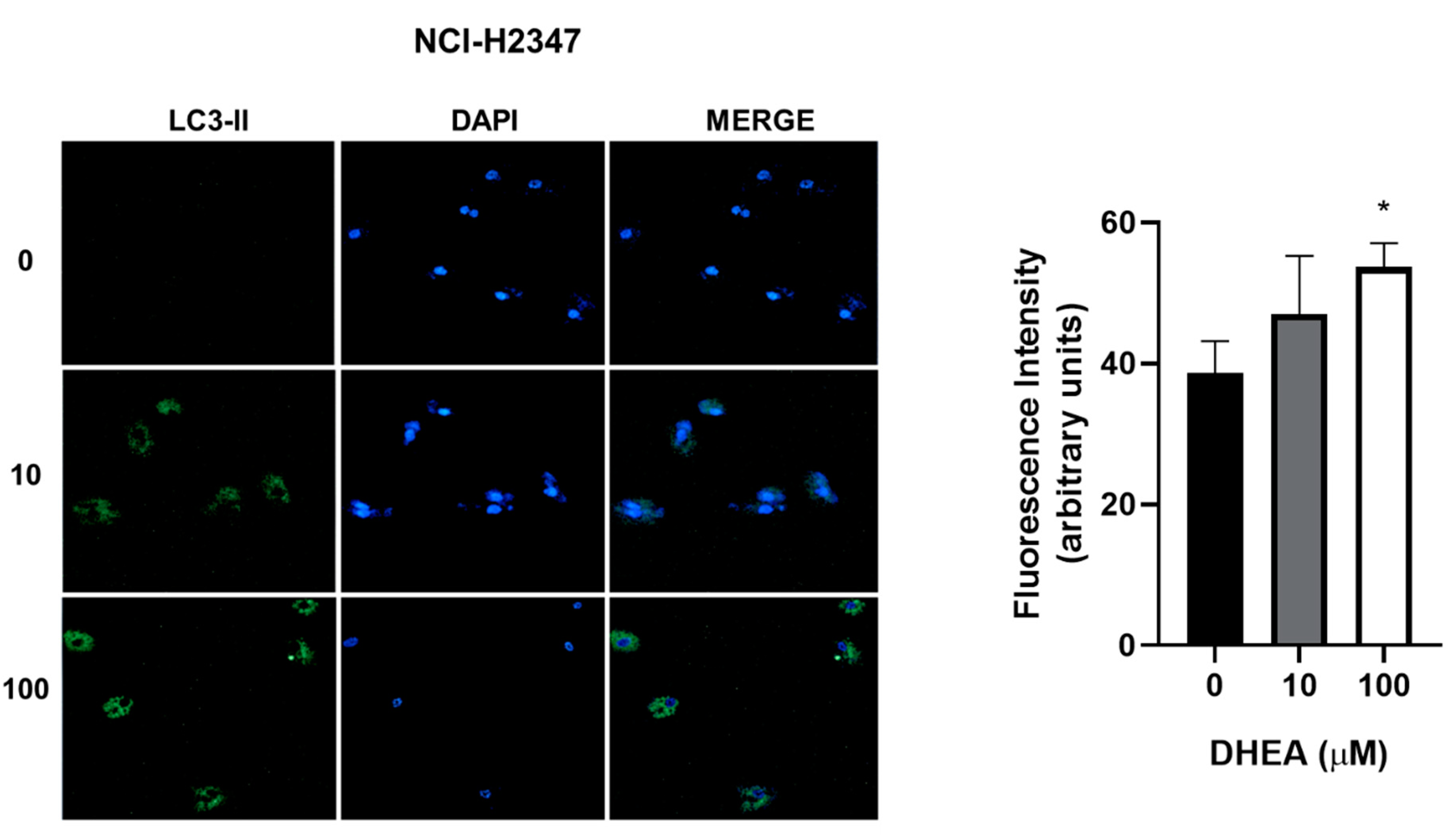
23]. Therefore, we investigated the impact of DHEA on autophagy in lung tumor cells. The results obtained in this work demonstrated that DHEA at 100 μM induced autophagy in all three lung tumor cell lines (
Figure 7,
Figure 8 and
Figure 9). Similar findings have been reported in other types of tumor cells. In the human hepatoma cell line HepG2, DHEA induces autophagic cell death, associated with generating reactive oxygen species (ROS) [
9]. Panada and colleagues synthesized N-alkynyl-17-aminosteroids and N-alkynyl-20-aminosteroids (based on dehydroepiandrosterone and pregnenolone, respectively) and demonstrated mitochondrial membrane potential alterations and increased autophagy in C6 rat glioma cells [
24]. These findings regarding DHEA's effect on autophagy in tumor cells are significant. DHEA could potentially serve as a promising therapeutic agent for targeting tumor cells through autophagy, especially in cases where apoptosis resistance is observed [
9]. In this work, only the autophagic process was evaluated. Therefore, it will be imperative to determine if DHEA can induce autophagic death in the lung cancer cells used.
The current treatment of cancer patients with chemotherapy agents lacks specificity, as it affects both tumor and normal cells with a high proliferative rate, leading to damage in healthy cells and consequent side effects [
25]. However, DHEA has demonstrated a beneficial impact on other normal cells. For instance, in endothelial cells derived from human umbilical cords, DHEA has shown a protective effect against TNF-α and oxLDL-induced inflammatory responses [
26,
27], indicating that DHEA could have a specific result against tumor cells. However, determining its effect on other normal cells, including lung normal cells, will be very important.
In general, all used lung tumor cells have similar responses to DHEA, despite it has been reported that DHEA has pleiotropic effects, which can vary depending on the cell type. The differences in the analyzed cell lines may be attributed to the differential protein expression. A study investigating the involvement of phosphatidylethanolamine-binding protein 4 (PEBP4) in the growth, proliferation, apoptosis, and invasion of non-small cell lung cancer (NSCLC) found that PEBP4 enhanced HCC827 cell proliferation and invasion ability while inhibiting apoptosis [
28]. HCC827 cells may express this protein, which could confer resistance to the effects of DHEA. Other morphological characteristics of cells, such as epithelial or mesenchymal phenotypes and specific biomarkers, can also influence the cellular response to drugs. The overactivation of the PI3K/Akt pathway has been observed in various human cancers, particularly in cells exhibiting a mesenchymal or highly aggressive phenotype [
29]. Our research group demonstrated that DHEA affects mesenchymal phenotype through the PI3K pathway [
22]. Therefore, a similar mechanism might be in the cell lines exhibiting a more mesenchymal phenotype and greater activation of the PI3K pathway. Therefore, DHEA could exert its effects on lung tumor cells through this pathway.
4. Methods and Materials
4.1. Materials
DMEM high glucose, RPMI-1640 medium, and other cell culture reagents were acquired from GIBCO/BRL (Grand Island, NY, USA). All plastic materials for cell culture were obtained from Sarstedt (Numbrecht, DE). Newborn calf serum (NBCS) was purchased from Biowest (Nuaillé, France). Trans-Dehydroepiandrosterone (DHEA) and trypan blue solution were acquired from Sigma Aldrich (St. Louis, MO, USA). Crystal violet solution for staining was acquired for Hycel. The annexin-V-FITC reagent was obtained from BioLegend (San Diego, CA, USA); PI solution and annexin-V binding buffer were purchased from BD Pharmingen (San Jose, CA, USA). The caspase-Glo 3/7 assay was from Promega (Madison, WI, USA). The autophagy assay kit was obtained from Abcam (Cambridge, UK).
4.2. Cell lines and culture
NCI-H2347 (adenocarcinoma derived from human non-small cell lung cancer), A549 and HCC827 cells (adenocarcinomas, both isolated from the human lung) were used as models of lung tumor cells. NCI-H2347 and A549 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) high glucose, and HCC827 cells were cultured in the RPMI-1640 medium. Both media were supplemented with 10% NBCS and 1% antibiotic-antimycotic solution. Cells were exposed to different pharmacological concentrations of DHEA (1, 10, and 100 μM) for 24 and 48 hours. The cells were incubated at 37°C with 5% CO2 and 95% relative humidity.
4.3. Cell proliferation and viability
Proliferation, cell number, and viability were assessed using crystal violet, trypan blue staining, and MTT reduction, respectively, following previously established protocols [
3]. Cells were seeded in 96-well plates at a density of 3 × 10
3 cells per well and incubated for 24 and 48 hours with DHEA (1, 10, and 100 μM) previously dissolved in 99% ethanol.
For crystal violet staining, cells were fixed with 100 μL of 1.1% glutaraldehyde prepared in HEPES buffer (150 mM NaCl, 10.9 mM HEPES, 4.4 mM KCl, 12.2 mM glucose, pH 7.4) for 15 minutes at room temperature. Cells were washed with water, air-dried, and stained with 100 μL of 0.1% crystal violet solution (crystal violet in 200 mM formic acid buffer, pH 6) for 20 minutes. Excess crystal violet was removed by washing the cells several times with water. The crystal violet incorporated into the cells was solubilized with 100 μL of a 10% acetic acid solution, and the optical density at 590 nm was determined.
For trypan blue staining, at the end of exposure, cells were detached with trypsin and mixed with trypan blue solution 1:1 and observed under a microscope. The number of stained and no stained cells was counted.
For MTT reduction, after treatment with DHEA, cells were incubated with 20 μL per well of MTT (5 mg/mL) for four hours at 37°C. The medium was removed, and the formazan salt formed was solubilized with acid isopropanol (0.04 N HCl). Optical density was measured at 570 nm. Optical density was measured using a microplate spectrophotometer (Benchmark Plus, BioRad, Hercules, CA, USA).
4.4. Apoptotic and necrotic death determination
Apoptotic and necrotic cell death were examined by flow cytometry using a dual staining with annexin-V-FITC and propidium iodine according to manufacturer’s instructions as previously described [
30]. Cells (10 × 10
3 per well) were seeded in 6-well plates and exposed to 10 and 100 µM DHEA for 24 hours. After, cells were incubated with 100 µL annexin buffer containing PI staining solution and FITC annexin-V staining solution, at room temperature in the dark for 15 minutes. Subsequently, the samples were immediately analyzed using a FACSaria flow cytometer (Becton-Dickinson, CA, USA). Data were processed using FlowJo 8.7 software (Stanford University).
4.5. Caspase-3/7 activity assay
The activity of caspase-3/7 was determined using the Caspase-Glo 3/7 Assay. A549, HCC872, and NCI-H2347 cells were placed in 96-well plates at a density of 3 ×103 cells/well in 100 µL medium, and 24 hours after attachment, the cells were treated with 10 and 100 µM of DHEA or untreated for 12 hours. At the end of the experiment, the caspase-Glo3/7 reagent was added to cells and the plate was incubated at room temperature for 2 hours. The luminescence values were measured in a multiwell plate reader (Biotek Synergy HT).
4.6. Migration assay
Cell migration was evaluated using the scratch motility assay, also known as the wound healing assay [
31]. The cells were seeded in 12-well plates and allowed to grow to 100% confluence. Subsequently, cell monolayers were scratched using a 200 μL pipette tip to create a wound. To eliminate floating cells, the plates were washed twice with a HEPES buffer containing Ca
+2 and Mg
+2 (0.1 g MgCl
2, 0.1 g CaCl
2). Then, cells were incubated for 24 and 48 hours with a culture medium containing 2% NCBS and either without (control) or with 1, 10, and 100 μM DHEA. Migrating cells were captured in images at 0, 24, and 48 hours. The percentage of open wound area (the portion of the wound not occupied by cells) was calculated using Image J software (version 1.50i).
4.7. Autophagy determination
For each experiment, 3 × 103 cells were cultured on 12-well plates. When the wells reached 50% confluence, cells were exposed to 10 and 100 μM of DHEA for 48 hours. After the treatment, autophagy was assessed using a detection kit. Cells were washed twice with 1X assay buffer, and then 100 μL of the microscopy dual detection reagent (2 μL of green detection reagent + 1 μL of nuclear stain in 1 mL of 1X assay buffer) was added to each sample. The samples were protected from light and incubated for 30 minutes at 37°C. Following incubation, cells were washed with 100 μL of 1X assay buffer, fixed with 4% formaldehyde for 20 minutes, and washed three times with 1X assay buffer. Finally, cells were observed under an LSM 700 Zeiss confocal microscope, and the images were processed using Zen Software (Zeiss, Germany).
4.8. Statistical analysis
A minimum of three independent experiments were performed for each condition. The results are presented as the mean ± standard deviation (SD). Multiple comparisons were conducted using one-way analysis of variance (ANOVA) followed by Tukey's pairwise comparison using GraphPad Prism (version 5.01). For apoptosis, results were analyzed by one-way ANOVA followed by Dunnett´s multiple comparison test. Values of p<0.05 were considered statistically significant.
Author Contributions
Methodology, investigation, formal analysis, data curation, validation, software, C.M.-M., Z.C.-V., I.P.-A., and A.V.-M.; writing-review and editing, C.M.-M., Z.C.-V.; resources, C.M.-M.; conceptualization, resources, writing-original draft preparation, supervision, project administration, R.L.-M.; funding acquisition, C.M.-M, and R.L.-M. All authors have read and agreed to the published version of the manuscript.
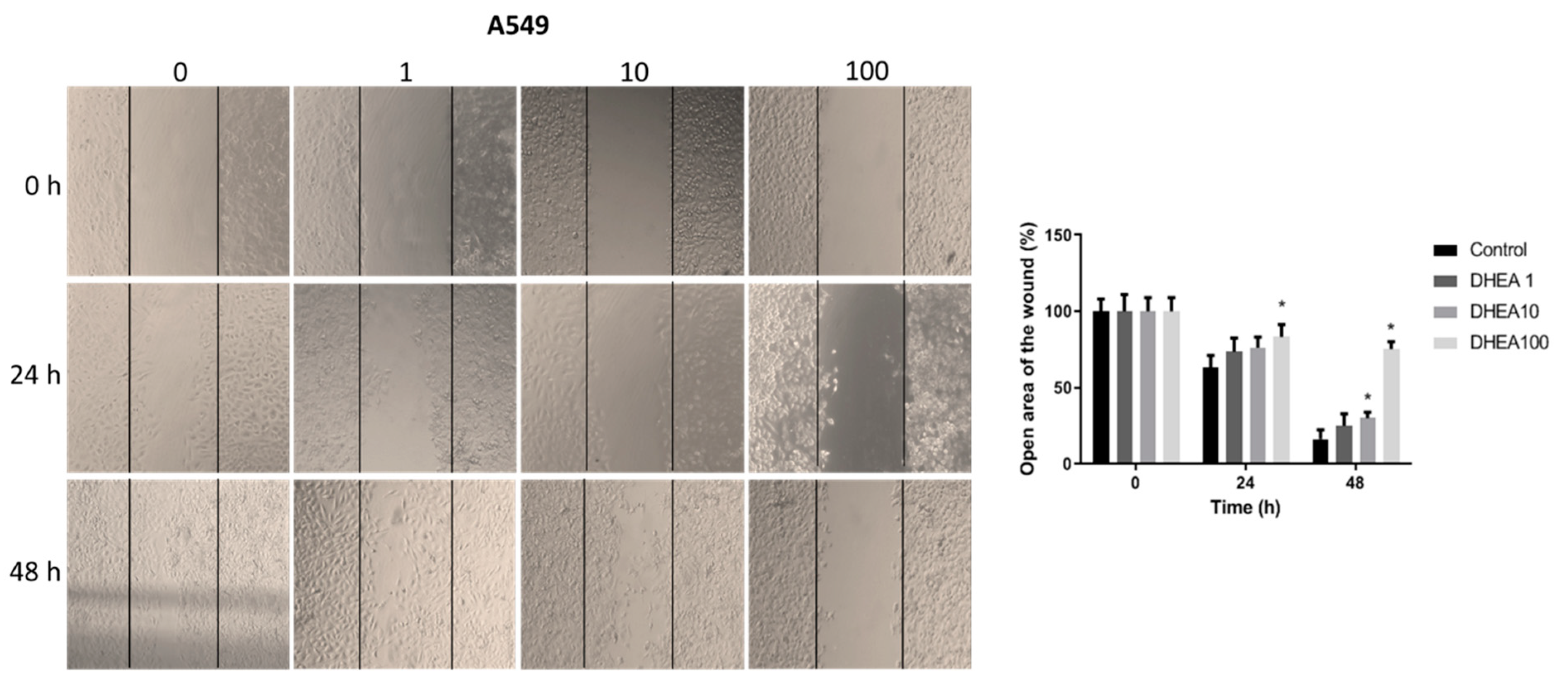
Figure 1.
Effect of DHEA on trypan blue staining. Cells (3 × 103/well) were exposed to 0, 1, 10, and 100 μM DHEA for 24 and 48 hours. Viability and cell number were measured by trypan blue staining. Results were expressed as percentages (mean ± standard deviation) of three independent experiments. * p<0.05 compared with control.
Figure 1.
Effect of DHEA on trypan blue staining. Cells (3 × 103/well) were exposed to 0, 1, 10, and 100 μM DHEA for 24 and 48 hours. Viability and cell number were measured by trypan blue staining. Results were expressed as percentages (mean ± standard deviation) of three independent experiments. * p<0.05 compared with control.
Figure 2.
Effect of DHEA on MTT reduction. Cells (3 × 103/well) were exposed to 0, 1, 10, and 100 μM DHEA for 24 and 48 hours, and MTT reduction was measured. Results were expressed as percentages (mean ± standard deviation) of three independent experiments. * p<0.05 compared with control.
Figure 2.
Effect of DHEA on MTT reduction. Cells (3 × 103/well) were exposed to 0, 1, 10, and 100 μM DHEA for 24 and 48 hours, and MTT reduction was measured. Results were expressed as percentages (mean ± standard deviation) of three independent experiments. * p<0.05 compared with control.
Figure 3.
Effect of DHEA on crystal violet staining. Cells (3 × 103/well) were exposed to 0, 1, 10, and 100 μM DHEA for 24 and 48 hours. Cell number was measured by crystal violet staining. Results were expressed as percentages (mean ± standard deviation) of three independent experiments. * p<0.05 compared with control.
Figure 3.
Effect of DHEA on crystal violet staining. Cells (3 × 103/well) were exposed to 0, 1, 10, and 100 μM DHEA for 24 and 48 hours. Cell number was measured by crystal violet staining. Results were expressed as percentages (mean ± standard deviation) of three independent experiments. * p<0.05 compared with control.
Figure 4.
Effect of DHEA on A549 cell migration. Cells (100 × 103/well) were cultured in 12-well plates and, when reached confluence, were exposed to 0, 1, 10, and 100 μM DHEA for 24 and 48 hours, and migration was evaluated by wound healing assay. Images of cells at 10X magnification were taken at 0, 24, and 48 hours after treatment with an inverted microscope (Olympus CKX41). Analysis of the open area of the wound was performed with the ImageJ 1.50i software (Wayne Rasband, National Institutes of Health, USA). Results were expressed as mean ± standard deviation of three independent experiments. * p<0.05 compared with control.
Figure 4.
Effect of DHEA on A549 cell migration. Cells (100 × 103/well) were cultured in 12-well plates and, when reached confluence, were exposed to 0, 1, 10, and 100 μM DHEA for 24 and 48 hours, and migration was evaluated by wound healing assay. Images of cells at 10X magnification were taken at 0, 24, and 48 hours after treatment with an inverted microscope (Olympus CKX41). Analysis of the open area of the wound was performed with the ImageJ 1.50i software (Wayne Rasband, National Institutes of Health, USA). Results were expressed as mean ± standard deviation of three independent experiments. * p<0.05 compared with control.
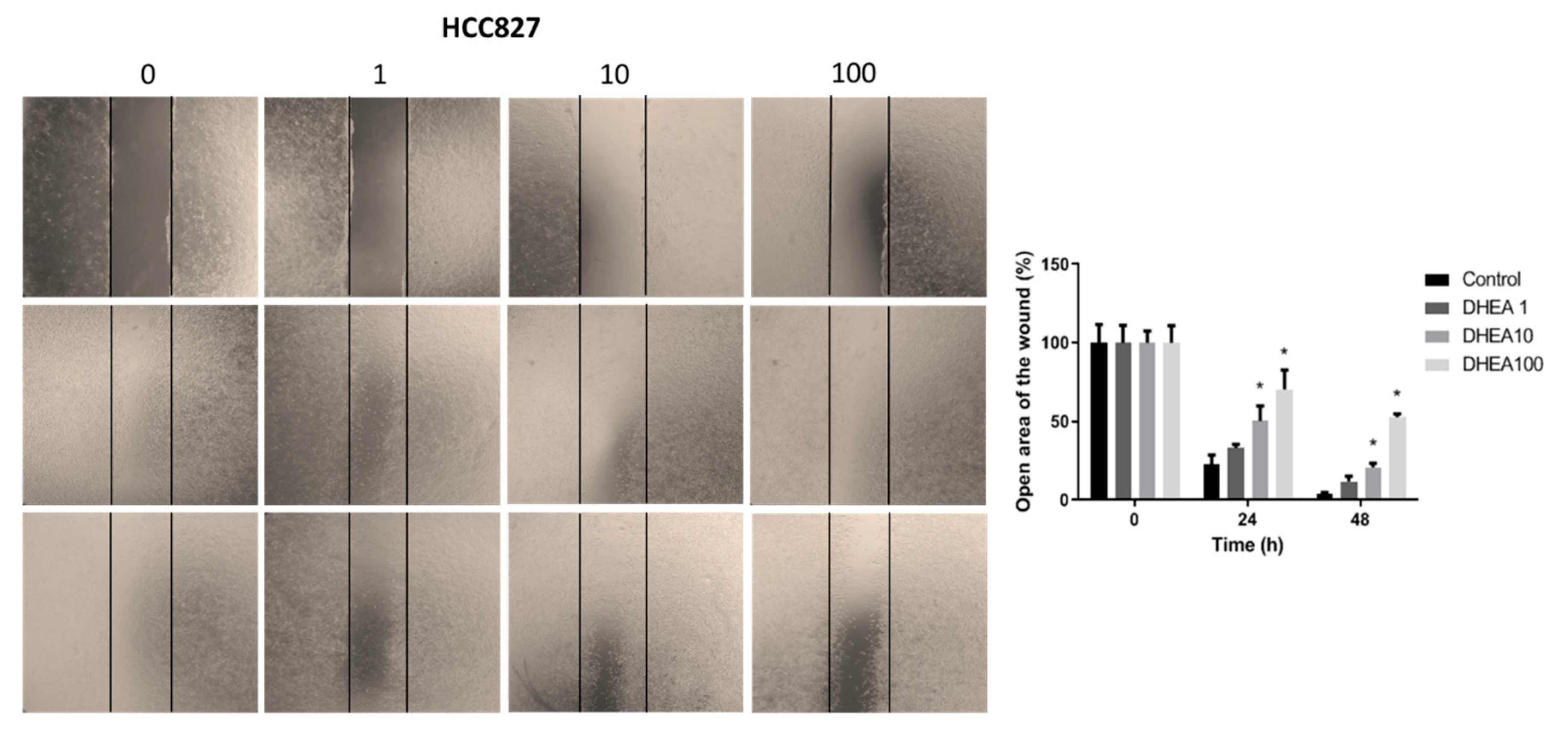
Figure 5.
Effect of DHEA on HCC827 cell migration. Cells (100 × 103/well) were cultured in 12-well plates and, when reached confluence, were exposed to 0, 1, 10, and 100 μM DHEA for 24 and 48 hours, and migration was evaluated by wound healing assay. Images of cells at 10X magnification were taken at 0, 24, and 48 hours after treatment with an inverted microscope (Olympus CKX41). Analysis of the open area of the wound was performed with the ImageJ 1.50i software (Wayne Rasband, National Institutes of Health, USA). Results were expressed as mean ± standard deviation of three independent experiments. * p<0.05 compared with control.
Figure 5.
Effect of DHEA on HCC827 cell migration. Cells (100 × 103/well) were cultured in 12-well plates and, when reached confluence, were exposed to 0, 1, 10, and 100 μM DHEA for 24 and 48 hours, and migration was evaluated by wound healing assay. Images of cells at 10X magnification were taken at 0, 24, and 48 hours after treatment with an inverted microscope (Olympus CKX41). Analysis of the open area of the wound was performed with the ImageJ 1.50i software (Wayne Rasband, National Institutes of Health, USA). Results were expressed as mean ± standard deviation of three independent experiments. * p<0.05 compared with control.
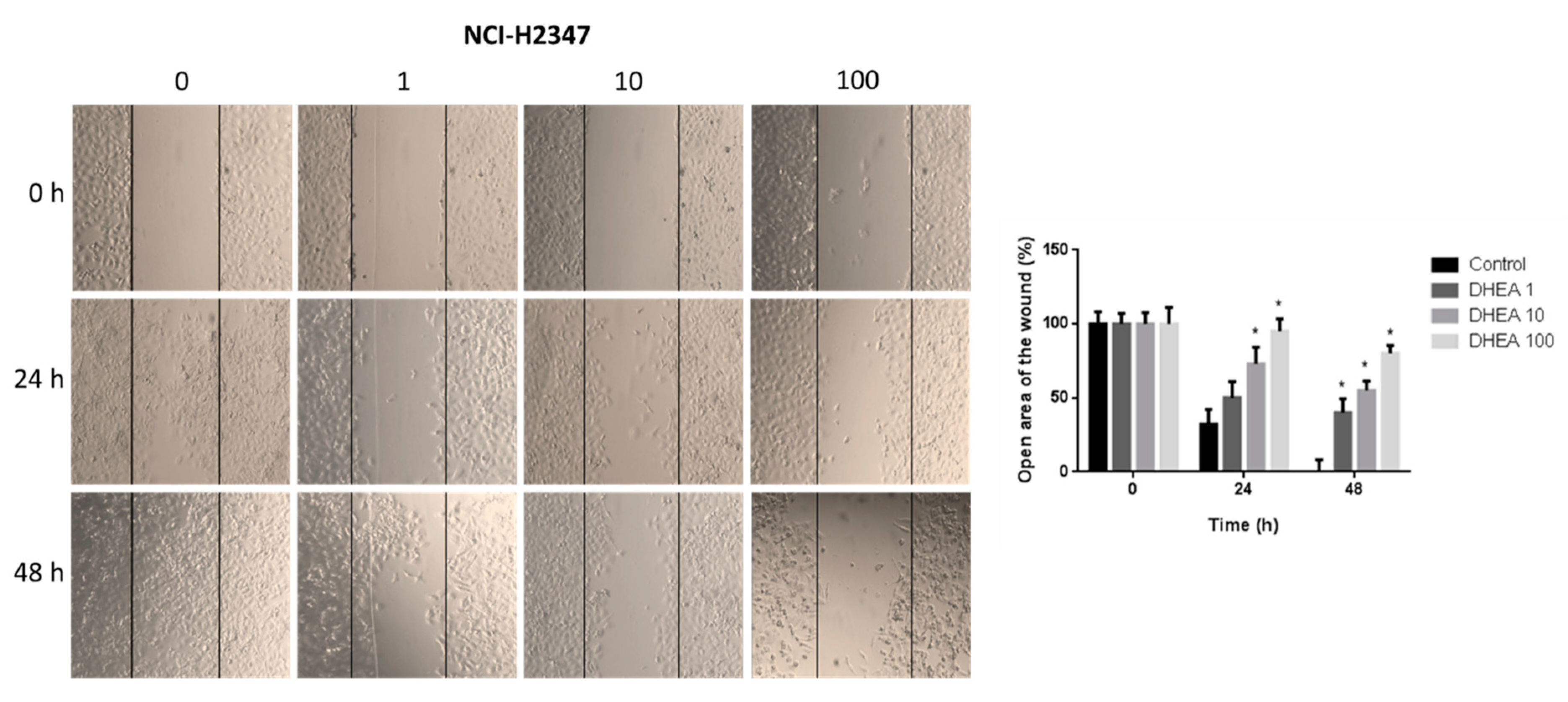
Figure 6.
Effect of DHEA on NCI-H2347 cell migration. Cells (100 × 103/well) were cultured in 12-well plates and, when reached confluence, were exposed to 0, 1, 10, and 100 μM DHEA for 24 and 48 hours, and migration was evaluated by wound healing assay. Images of cells at 10X magnification were taken at 0, 24, and 48 hours after treatment with an inverted microscope (Olympus CKX41). Analysis of the open area of the wound was performed with the ImageJ 1.50i (Wayne Rasband, National Institutes of Health, USA). Results were expressed as mean ± standard deviation of three independent experiments. * p<0.05 compared with control.
Figure 6.
Effect of DHEA on NCI-H2347 cell migration. Cells (100 × 103/well) were cultured in 12-well plates and, when reached confluence, were exposed to 0, 1, 10, and 100 μM DHEA for 24 and 48 hours, and migration was evaluated by wound healing assay. Images of cells at 10X magnification were taken at 0, 24, and 48 hours after treatment with an inverted microscope (Olympus CKX41). Analysis of the open area of the wound was performed with the ImageJ 1.50i (Wayne Rasband, National Institutes of Health, USA). Results were expressed as mean ± standard deviation of three independent experiments. * p<0.05 compared with control.
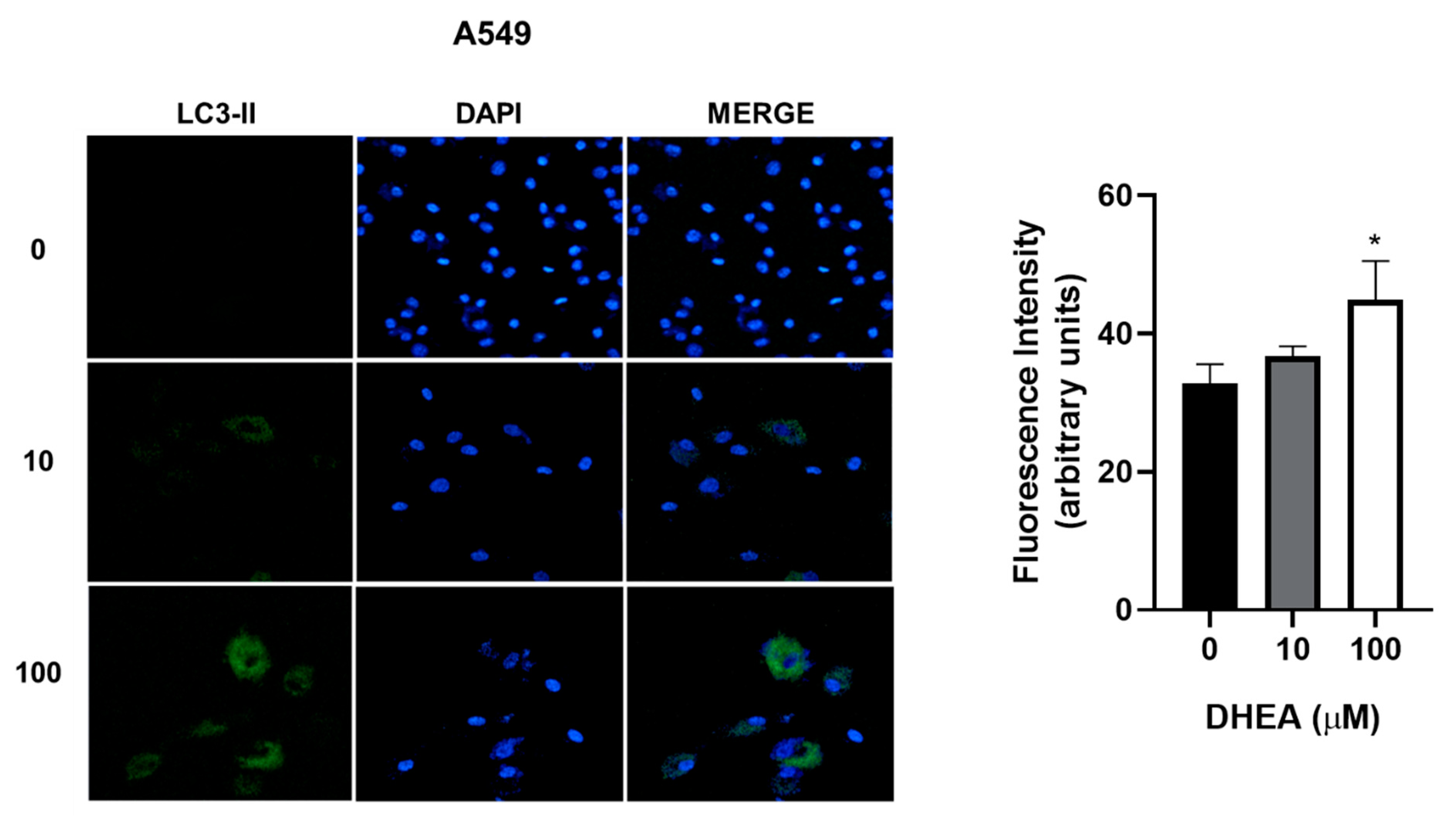
Figure 7.
Effect of DHEA on autophagy of A549 cells. Cells (3 × 103/well) were cultured in 12-well plates and exposed to 0, 10, and 100 μM DHEA for 48 hours. Images of cells at a 20X magnification were taken with a confocal microscope (Zeiss, LSM 700) of a representative assay. Images were analyzed using the ZEN 2012 (blue edition) software. Results were expressed as mean ± standard deviation of three independent experiments. * p<0.05 compared with control.
Figure 7.
Effect of DHEA on autophagy of A549 cells. Cells (3 × 103/well) were cultured in 12-well plates and exposed to 0, 10, and 100 μM DHEA for 48 hours. Images of cells at a 20X magnification were taken with a confocal microscope (Zeiss, LSM 700) of a representative assay. Images were analyzed using the ZEN 2012 (blue edition) software. Results were expressed as mean ± standard deviation of three independent experiments. * p<0.05 compared with control.
Figure 8.
Effect of DHEA on autophagy of HCC827 cells. Cells (3 × 103/well) were cultured in 12-well plates and exposed to 0, 10, and 100 μM DHEA for 48 hours. Images of cells at a 20X magnification were taken with a confocal microscope (Zeiss, LSM 700) of a representative assay. Images were analyzed using the ZEN 2012 (blue edition) software. Results were expressed as mean ± standard deviation of three independent experiments. * p<0.05 compared with control.
Figure 8.
Effect of DHEA on autophagy of HCC827 cells. Cells (3 × 103/well) were cultured in 12-well plates and exposed to 0, 10, and 100 μM DHEA for 48 hours. Images of cells at a 20X magnification were taken with a confocal microscope (Zeiss, LSM 700) of a representative assay. Images were analyzed using the ZEN 2012 (blue edition) software. Results were expressed as mean ± standard deviation of three independent experiments. * p<0.05 compared with control.
Figure 9.
Effect of DHEA on autophagy of NCI-H2347 cells. Cells (3 × 103/well) were cultured in 12-well plates and exposed to 0, 10, and 100 μM DHEA for 48 hours. Images of cells at a 20X magnification were taken with a confocal microscope (Zeiss, LSM 700) of a representative assay. Images were analyzed using the ZEN 2012 (blue edition) software. Results were expressed as mean ± standard deviation of three independent experiments. * p<0.05 compared with control.
Figure 9.
Effect of DHEA on autophagy of NCI-H2347 cells. Cells (3 × 103/well) were cultured in 12-well plates and exposed to 0, 10, and 100 μM DHEA for 48 hours. Images of cells at a 20X magnification were taken with a confocal microscope (Zeiss, LSM 700) of a representative assay. Images were analyzed using the ZEN 2012 (blue edition) software. Results were expressed as mean ± standard deviation of three independent experiments. * p<0.05 compared with control.