Submitted:
25 September 2023
Posted:
27 September 2023
You are already at the latest version
Abstract
Keywords:
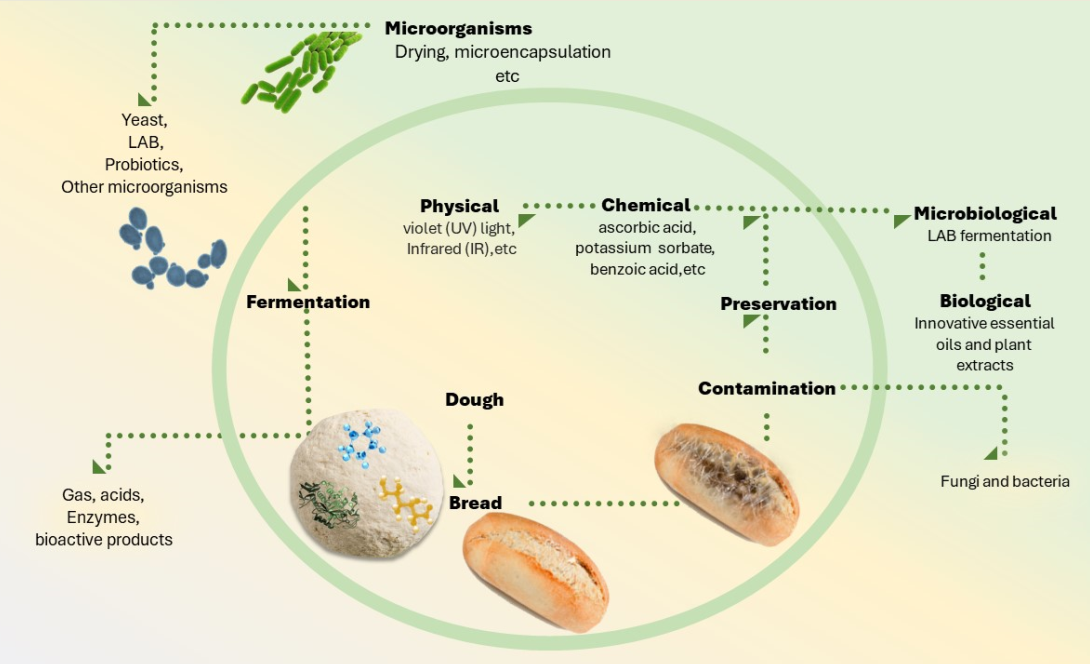
1. Introduction
2. Microorganisms and probiotics in baking
3. Drying and encapsulation processes of probiotics and microorganisms
3.1. Freeze drying
3.2. Spray drying
3.3. Fluidized bed drying
3.4. Vacuum drying
4. Preservation methods
4.1. Physical agents
4.2. Chemical agents
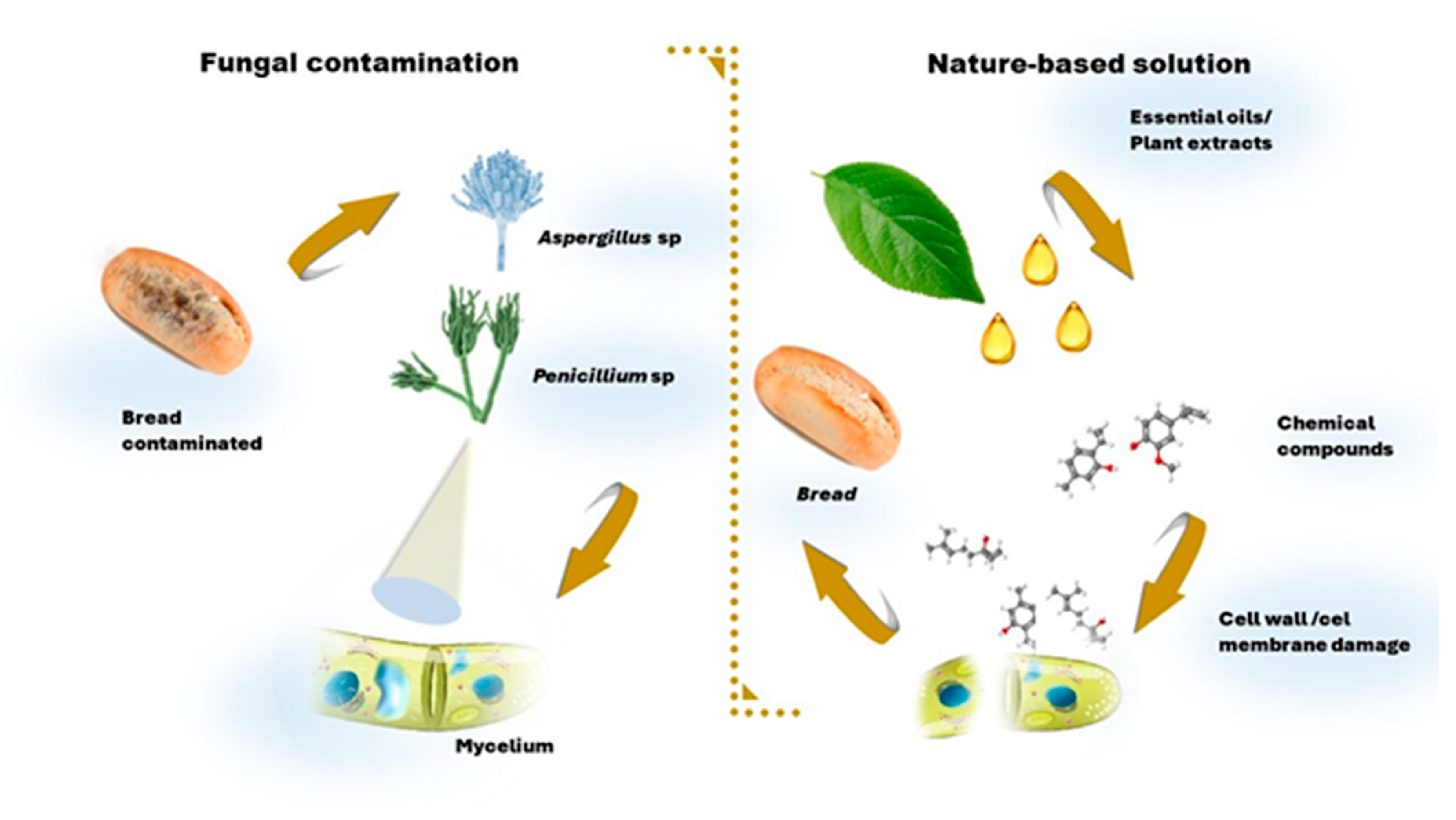
4.3. Natural and biological agents: essential oil, plant extracts, LAB, yeasts, and enzymes
4.4. Essential oils
4.5. Plant extracts
4.6. Lactic acid bacteria (LAB) and yeasts
4.7. Enzymes
5. Conclusion
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arranz-Otaegui, A.; Gonzalez Carretero, L.; Ramsey, M.N.; Fuller, D.Q.; Richter, T. Archaeobotanical Evidence Reveals the Origins of Bread 14,400 Years Ago in Northeastern Jordan. Proceedings of the National Academy of Sciences 2018, 115, 7925–7930. [CrossRef]
- Chiron, H. La fermentation du pain : histoire et modernité. Cahiers François Viète 1999, 81–96. [CrossRef]
- Dong, Y.; Karboune, S. A Review of Bread Qualities and Current Strategies for Bread Bioprotection: Flavor, Sensory, Rheological, and Textural Attributes. Comp Rev Food Sci Food Safe 2021, 20, 1937–1981. [CrossRef]
- Ribet, L.; Dessalles, R.; Lesens, C.; Brusselaers, N.; Durand-Dubief, M. Nutritional Benefits of Sourdoughs: A Systematic Review. Advances in Nutrition 2023, 14, 22–29. [CrossRef]
- Heredia-Sandoval, N.; Valencia-Tapia, M.; Calderón De La Barca, A.; Islas-Rubio, A. Microbial Proteases in Baked Goods: Modification of Gluten and Effects on Immunogenicity and Product Quality. Foods 2016, 5, 59. [CrossRef]
- Akamine, I.T.; Mansoldo, F.R.P.; Cardoso, V.S.; De Souza Dias, E.P.; Vermelho, A.B. Hydrolase Activities of Sourdough Microorganisms. Fermentation 2023, 9, 703. [CrossRef]
- Akamine, I.T.; Mansoldo, F.R.P.; Vermelho, A.B. Probiotics in the Sourdough Bread Fermentation: Current Status. Fermentation 2023, 9, 90. [CrossRef]
- Marco, I.D.; Silva, C.M.D.; Moraes, J.O.D.; Menezes, L.A.A.; Miotto, M.; Laurindo, J.B.; Lindner, J.D.D. A Systematic Review of Drying Methods and Their Impact on Technological Characteristics of Sourdough Type III. Biotechnology Research and Innovation 2022, 6, e2022003. [CrossRef]
- Reese, A.T.; Madden, A.A.; Joossens, M.; Lacaze, G.; Dunn, R.R. Influences of Ingredients and Bakers on the Bacteria and Fungi in Sourdough Starters and Bread. mSphere 2020, 5, e00950-19. [CrossRef]
- Rahman, M.; Islam, R.; Hasan, S.; Zzaman, W.; Rana, M.R.; Ahmed, S.; Roy, M.; Sayem, A.; Matin, A.; Raposo, A.; et al. A Comprehensive Review on Bio-Preservation of Bread: An Approach to Adopt Wholesome Strategies. Foods 2022, 11, 319. [CrossRef]
- Axel, C.; Brosnan, B.; Zannini, E.; Furey, A.; Coffey, A.; Arendt, E.K. Antifungal Sourdough Lactic Acid Bacteria as Biopreservation Tool in Quinoa and Rice Bread. International Journal of Food Microbiology 2016, 239, 86–94. [CrossRef]
- Islam, F.; Saeed, F.; Imran, A.; Shehzadi, U.; Ali, R.; Nosheen, F.; Chauhan, A.; Asghar, A.; Ojukwu, M. Bio-Preservatives and Essential Oils as an Alternative to Chemical Preservatives in the Baking Industry: A Concurrent Review. J Food Sci Technol 2023. [CrossRef]
- Cao, H.; Wang, X.; Wang, X.; Guan, X.; Huang, K.; Zhang, Y. Effect of Storage Conditions on the Textural Properties and in Vitro Digestibility of Wheat Bread Containing Whole Quinoa Flour. Food Bioscience 2022, 49, 101921. [CrossRef]
- Jideani, V.A. Bread Storage and Preservation. In Encyclopedia of Food Security and Sustainability; Elsevier, 2019; pp. 593–604 ISBN 978-0-12-812688-2.
- Kline, L.; Sugihara, T.F. Microorganisms of the San Francisco Sour Dough Bread Process. Applied Microbiology 1971, 21, 459–465.
- Yang, H.; Liu, T.; Zhang, G.; He, G. Intraspecific Diversity and Fermentative Properties of Saccharomyces Cerevisiae from Chinese Traditional Sourdough. LWT 2020, 124, 109195. [CrossRef]
- Zhang, G.; Sun, Y.; Sadiq, F.A.; Sakandar, H.A.; He, G. Evaluation of the Effect of Saccharomyces Cerevisiae on Fermentation Characteristics and Volatile Compounds of Sourdough. Journal of Food Science and Technology 2018, 55. [CrossRef]
- Kaplan, S.L. Good Bread Is Back: A Contemporary History of French Bread, the Way It Is Made, and the People Who Make It; Duke University Press, 2006; ISBN 978-0-8223-8828-9.
- Koistinen, V.M.; Mattila, O.; Katina, K.; Poutanen, K.; Aura, A.M.; Hanhineva, K. Metabolic Profiling of Sourdough Fermented Wheat and Rye Bread. Scientific Reports 2018, 8. [CrossRef]
- Chavan, R.S.; Chavan, S.R. Sourdough Technology-A Traditional Way for Wholesome Foods: A Review. Comprehensive Reviews in Food Science and Food Safety 2011, 10, 169–182. [CrossRef]
- De Vuyst, L.; Comasio, A.; Kerrebroeck, S.V. Sourdough Production: Fermentation Strategies, Microbial Ecology, and Use of Non-Flour Ingredients. Critical Reviews in Food Science and Nutrition 2023, 63, 2447–2479. [CrossRef]
- Gänzle, M.G.; Zheng, J. Lifestyles of Sourdough Lactobacilli – Do They Matter for Microbial Ecology and Bread Quality? International Journal of Food Microbiology 2019, 302, 15–23. [CrossRef]
- Probiotics in Food Health and Nutritional Properties and Guidelines for Evaluation FAO FOOD AND NUTRITION PAPER.
- Jiang, S.; Cai, L.; Lv, L.; Li, L. Pediococcus Pentosaceus, a Future Additive or Probiotic Candidate. Microbial Cell Factories 2021, 20, 1–14. [CrossRef]
- Anisha, A.H.N.; Anandham, R.; Kwon, S.W.; Gandhi, P.I.; Gopal, N.O. Evaluation of Bacillus Spp. as Dough Starters for Adhirasam - A Traditional Rice Based Fermented Food of Southern India. Braz. J. Microbiol. 2015, 46, 1183–1191. [CrossRef]
- Rocha, J.M.; Malcata, F.X. On the Microbiological Profile of Traditional Portuguese Sourdough. Journal of Food Protection 1999, 62, 1416–1429. [CrossRef]
- Li, Z.; Zheng, M.; Zheng, J.; Gänzle, M.G. Bacillus Species in Food Fermentations: An Underappreciated Group of Organisms for Safe Use in Food Fermentations. Current Opinion in Food Science 2023, 50, 101007. [CrossRef]
- Akbari, S.; Rahmatzai, N.; Mominzai, M.A. Antimicrobial Activity of Lactic Acid Bacteria in Rope Producing Strains of Bacillus from Wheat Bread. Science and Education 2023, 4, 29–37.
- Song, S.H. Analysis of Microflora Profile in Korean Traditional Nuruk. J. Microbiol. Biotechnol. 2013, 23, 40–46. [CrossRef]
- Shangpliang, H.N.J.; Tamang, J.P. Metagenomics and Metagenome-Assembled Genomes Mining of Health Benefits in Jalebi Batter, a Naturally Fermented Cereal-Based Food of India. Food Research International 2023, 172, 113130. [CrossRef]
- Menezes, L.A.A.; Sardaro, M.L.S.; Duarte, R.T.D.; Mazzon, R.R.; Neviani, E.; Gatti, M.; De Dea Lindner, J. Sourdough Bacterial Dynamics Revealed by Metagenomic Analysis in Brazil. Food Microbiology 2020, 85, 103302. [CrossRef]
- Arora, K.; Ameur, H.; Polo, A.; Di Cagno, R.; Rizzello, C.G.; Gobbetti, M. Thirty Years of Knowledge on Sourdough Fermentation: A Systematic Review. Trends in Food Science and Technology 2021, 108, 71–83. [CrossRef]
- Van Kerrebroeck, S.; Maes, D.; De Vuyst, L. Sourdoughs as a Function of Their Species Diversity and Process Conditions, a Meta-Analysis. Trends in Food Science and Technology 2017, 68, 152–159. [CrossRef]
- Damián, M.R.; Cortes-Perez, N.G.; Quintana, E.T.; Ortiz-Moreno, A.; Garfias Noguez, C.; Cruceño-Casarrubias, C.E.; Sánchez Pardo, M.E.; Bermúdez-Humarán, L.G. Functional Foods, Nutraceuticals and Probiotics: A Focus on Human Health. Microorganisms 2022, 10, 1065. [CrossRef]
- Minervini, F.; Celano, G.; Lattanzi, A.; Tedone, L.; de Mastro, G.; Gobbetti, M.; de Angelis, M. Lactic Acid Bacteria in Durum Wheat Flour Are Endophytic Components of the Plant during Its Entire Life Cycle. Applied and Environmental Microbiology 2015, 81, 6736–6748. [CrossRef]
- Struyf, N.; Vandewiele, H.; Herrera-Malaver, B.; Verspreet, J.; Verstrepen, K.J.; Courtin, C.M. Kluyveromyces Marxianus Yeast Enables the Production of Low FODMAP Whole Wheat Breads. Food Microbiology 2018, 76. [CrossRef]
- Wittwer, A.; Howell, K. Rising Stars in the Bakery: Novel Yeasts for Modern Bread. Microbiol. Aust. 2022, 43, 75–78. [CrossRef]
- Korcari, D.; Secchiero, R.; Laureati, M.; Marti, A.; Cardone, G.; Rabitti, N.S.; Ricci, G.; Fortina, M.G. Technological Properties, Shelf Life and Consumer Preference of Spelt-Based Sourdough Bread Using Novel, Selected Starter Cultures. LWT 2021, 151, 112097. [CrossRef]
- Warburton, A.; Silcock, P.; Eyres, G.T. Impact of Sourdough Culture on the Volatile Compounds in Wholemeal Sourdough Bread. Food Research International 2022, 161, 111885. [CrossRef]
- De Vuyst, L.; Harth, H.; Van Kerrebroeck, S.; Leroy, F. Yeast Diversity of Sourdoughs and Associated Metabolic Properties and Functionalities. International Journal of Food Microbiology 2016, 239, 26–34. [CrossRef]
- Palla, M.; Agnolucci, M.; Calzone, A.; Giovannetti, M.; Di Cagno, R.; Gobbetti, M.; Rizzello, C.G.; Pontonio, E. Exploitation of Autochthonous Tuscan Sourdough Yeasts as Potential Starters. International Journal of Food Microbiology 2019, 302, 59–68. [CrossRef]
- Corona, O.; Alfonzo, A.; Ventimiglia, G.; Nasca, A.; Francesca, N.; Martorana, A.; Moschetti, G.; Settanni, L. Industrial Application of Selected Lactic Acid Bacteria Isolated from Local Semolinas for Typical Sourdough Bread Production. Food Microbiology 2016, 59, 43–56. [CrossRef]
- Sidari, R.; Martorana, A.; Zappia, C.; Mincione, A.; Giuffrè, A.M. Persistence and Effect of a Multistrain Starter Culture on Antioxidant and Rheological Properties of Novel Wheat Sourdoughs and Bread. Foods 2020, 9, 1258. [CrossRef]
- Koj, K.; Pejcz, E. Rye Dietary Fiber Components upon the Influence of Fermentation Inoculated with Probiotic Microorganisms. Molecules 2023, 28, 1910. [CrossRef]
- Li, H.; Li, B.; Gao, L.; Ge, R.; Cui, X.; Zhou, J.; Li, Z. Gamma-Aminobutyric Acid (GABA) Promotes Characteristics of Levilactobacillus Sp. LB-2. LWT 2023, 184, 115014. [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-Parabiotics: The New Horizons in Microbial Biotherapy and Functional Foods. Microb Cell Fact 2020, 19, 168. [CrossRef]
- Limbad, M.; Gutierrez Maddox, N.; Hamid, N.; Kantono, K. Sensory and Physicochemical Characterization of Sourdough Bread Prepared with a Coconut Water Kefir Starter. Foods 2020, 9, 1165. [CrossRef]
- Kook, S.Y.S.Y.; Lee, Y.; Jeong, E.C.E.C.; Kim, S. Immunomodulatory Effects of Exopolysaccharides Produced by Bacillus Licheniformis and Leuconostoc Mesenteroides Isolated from Korean Kimchi. Journal of Functional Foods 2019, 54, 211–219. [CrossRef]
- De Angelis, M.; Bottacini, F.; Fosso, B.; Kelleher, P.; Calasso, M.; Di Cagno, R.; Ventura, M.; Picardi, E.; van Sinderen, D.; Gobbetti, M. Lactobacillus Rossiae, a Vitamin B12 Producer, Represents a Metabolically Versatile Species within the Genus Lactobacillus. PLoS One 2014, 9, e107232. [CrossRef]
- Hansen, A.; Schieberle, P. Generation of Aroma Compounds during Sourdough Fermentation: Applied and Fundamental Aspects. Trends in Food Science & Technology 2005, 16, 85–94. [CrossRef]
- Lau, S.W.; Chong, A.Q.; Chin, N.L.; Talib, R.A.; Basha, R.K. Sourdough Microbiome Comparison and Benefits. Microorganisms 2021, 9, 1355. [CrossRef]
- Nuobariene, L.; Hansen, Å.S.; Arneborg, N. Isolation and Identification of Phytase-Active Yeasts from Sourdoughs. LWT - Food Science and Technology 2012, 48, 190–196. [CrossRef]
- Nuobariene, L.; Arneborg, N.; Hansen, Å.S. Foodbalt 2014 Phytase Active Yeasts Isolated From Bakery Sourdoughs. Foodbalt 2014, 223–227.
- Baye, K.; Guyot, J.-P.; Icard-Vernière, C.; Rochette, I.; Mouquet-Rivier, C. Enzymatic Degradation of Phytate, Polyphenols and Dietary Fibers in Ethiopian Injera Flours: Effect on Iron Bioaccessibility. Food Chemistry 2015, 174, 60–67. [CrossRef]
- Iglesias-Puig, E.; Monedero, V.; Haros, M. Bread with Whole Quinoa Flour and Bifidobacterial Phytases Increases Dietary Mineral Intake and Bioavailability. LWT - Food Science and Technology 2015, 60, 71–77. [CrossRef]
- Karaman, K.; Sagdic, O.; Durak, M.Z. Use of Phytase Active Yeasts and Lactic Acid Bacteria Isolated from Sourdough in the Production of Whole Wheat Bread. LWT - Food Science and Technology 2018, 91. [CrossRef]
- Chen, J.; Pang, H.; Wang, L.; Ma, C.; Wu, G.; Liu, Y.; Guan, Y.; Zhang, M.; Qin, G.; Tan, Z. Bacteriocin-Producing Lactic Acid Bacteria Strains with Antimicrobial Activity Screened from Bamei Pig Feces. Foods 2022, 11, 709. [CrossRef]
- Reis, J.A.; Paula, A.T.; Casarotti, S.N.; Penna, A.L.B. Lactic Acid Bacteria Antimicrobial Compounds: Characteristics and Applications. Food Eng Rev 2012, 4, 124–140. [CrossRef]
- Siragusa, S.; De Angelis, M.; Di Cagno, R.; Rizzello, C.G.; Coda, R.; Gobbetti, M. Synthesis of Gamma-Aminobutyric Acid by Lactic Acid Bacteria Isolated from a Variety of Italian Cheeses. Appl Environ Microbiol 2007, 73, 7283–7290. [CrossRef]
- Diana, M.; Rafecas, M.; Quílez, J. Free Amino Acids, Acrylamide and Biogenic Amines in Gamma-Aminobutyric Acid Enriched Sourdough and Commercial Breads. Journal of Cereal Science 2014, 60, 639–644. [CrossRef]
- Venturi, M.; Galli, V.; Pini, N.; Guerrini, S.; Granchi, L. Use of Selected Lactobacilli to Increase γ-Aminobutyric Acid (GABA) Content in Sourdough Bread Enriched with Amaranth Flour. Foods 2019, 8, 218. [CrossRef]
- Da Ros, A.; Polo, A.; Rizzello, C.G.; Acin-Albiac, M.; Montemurro, M.; Di Cagno, R.; Gobbetti, M. Feeding with Sustainably Sourdough Bread Has the Potential to Promote the Healthy Microbiota Metabolism at the Colon Level. Microbiology Spectrum 2021, 9. [CrossRef]
- Polak, T.; Mejaš, R.; Jamnik, P.; Kralj Cigić, I.; Poklar Ulrih, N.; Cigić, B. Accumulation and Transformation of Biogenic Amines and Gamma-Aminobutyric Acid (GABA) in Chickpea Sourdough. Foods 2021, 10, 2840. [CrossRef]
- Kiepś, J.; Dembczyński, R. Current Trends in the Production of Probiotic Formulations. Foods 2022, 11, 2330. [CrossRef]
- Arepally, D.; Reddy, R.S.; Goswami, T.K.; Coorey, R. A Review on Probiotic Microencapsulation and Recent Advances of Their Application in Bakery Products. Food Bioprocess Technol 2022, 15, 1677–1699. [CrossRef]
- Rajam, R.; Subramanian, P. Encapsulation of Probiotics: Past, Present and Future. Beni-Suef University Journal of Basic and Applied Sciences 2022, 11. [CrossRef]
- Misra, S.; Pandey, P.; Panigrahi, C.; Mishra, H.N. A Comparative Approach on the Spray and Freeze Drying of Probiotic and Gamma-Aminobutyric Acid as a Single Entity: Characterization and Evaluation of Stability in Simulated Gastrointestinal Conditions. Food Chemistry Advances 2023, 3, 100385. [CrossRef]
- Liu, H.; Cui, S.W.; Chen, M.; Li, Y.; Liang, R.; Xu, F.; Zhong, F. Protective Approaches and Mechanisms of Microencapsulation to the Survival of Probiotic Bacteria during Processing, Storage and Gastrointestinal Digestion: A Review. Critical Reviews in Food Science and Nutrition 2019, 59, 2863–2878. [CrossRef]
- Chen, J.; Wang, Q.; Liu, C.-M.; Gong, J. Issues Deserve Attention in Encapsulating Probiotics: Critical Review of Existing Literature. Critical Reviews in Food Science and Nutrition 2017, 57, 1228–1238. [CrossRef]
- Li, B.; Tian, F.; Liu, X.; Zhao, J.; Zhang, H.; Chen, W. Effects of Cryoprotectants on Viability of Lactobacillus Reuteri CICC6226. Appl Microbiol Biotechnol 2011, 92, 609–616. [CrossRef]
- Sun, H.; Zhang, M.; Liu, Y.; Wang, Y.; Chen, Y.; Guan, W.; Li, X.; Wang, Y. Improved Viability of Lactobacillus Plantarum Embedded in Whey Protein Concentrate/Pullulan/Trehalose Hydrogel during Freeze Drying. Carbohydrate Polymers 2021, 260, 117843. [CrossRef]
- Bagad, M.; Pande, R.; Dubey, V.; Ghosh, A.R. Survivability of Freeze-Dried Probiotic Pediococcus Pentosaceus Strains GS4, GS17 and Lactobacillus Gasseri (ATCC 19992) during Storage with Commonly Used Pharmaceutical Excipients within a Period of 120 Days. Asian Pacific Journal of Tropical Biomedicine 2017, 7, 921–929. [CrossRef]
- Romyasamit, C.; Saengsuwan, P.; Boonserm, P.; Thamjarongwong, B.; Singkhamanan, K. Optimization of Cryoprotectants for Freeze-Dried Potential Probiotic Enterococcus Faecalis and Evaluation of Its Storage Stability. Drying Technology 2022, 40, 2283–2292. [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A Review of Microencapsulation Methods for Food Antioxidants: Principles, Advantages, Drawbacks and Applications. Food Chemistry 2019, 272, 494–506. [CrossRef]
- Rezvankhah, A.; Emam-Djomeh, Z.; Askari, G. Encapsulation and Delivery of Bioactive Compounds Using Spray and Freeze-Drying Techniques: A Review. Drying Technology 2020, 38, 235–258. [CrossRef]
- Singh, S.; Gupta, R.; Chawla, S.; Gauba, P.; Singh, M.; Tiwari, R.K.; Upadhyay, S.; Sharma, S.; Chanda, S.; Gaur, S. Natural Sources and Encapsulating Materials for Probiotics Delivery Systems: Recent Applications and Challenges in Functional Food Development. Front. Nutr. 2022, 9, 971784. [CrossRef]
- Rajam, R.; Karthik, P.; Parthasarathi, S.; Joseph, G.S.; Anandharamakrishnan, C. Effect of Whey Protein - Alginate Wall Systems on Survival of Microencapsulated Lactobacillus Plantarum in Simulated Gastrointestinal Conditions. Journal of Functional Foods 2012, 4, 891–898. [CrossRef]
- Poozesh, S.; Akafuah, N.K.; Campbell, H.R.; Bashiri, F.; Saito, K. Experimental and Mathematical Tools to Predict Droplet Size and Velocity Distribution for a Two-Fluid Nozzle. Fluids 2020, 5, 231. [CrossRef]
- Wang, N.; Fu, N.; Chen, X.D. The Extent and Mechanism of the Effect of Protectant Material in the Production of Active Lactic Acid Bacteria Powder Using Spray Drying: A Review. Current Opinion in Food Science 2022, 44, 100807. [CrossRef]
- Bhagwat, A.; Bhushette, P.; Annapure, U.S. Spray Drying Studies of Probiotic Enterococcus Strains Encapsulated with Whey Protein and Maltodextrin. Beni-Suef Univ J Basic Appl Sci 2020, 9, 33. [CrossRef]
- Minj, S.; Anand, S. Development of a Spray-Dried Conjugated Whey Protein Hydrolysate Powder with Entrapped Probiotics. Journal of Dairy Science 2022, 105, 2038–2048. [CrossRef]
- Arslan, S.; Erbas, M.; Tontul, I.; Topuz, A. Microencapsulation of Probiotic Saccharomyces Cerevisiae Var. Boulardii with Different Wall Materials by Spray Drying. LWT - Food Science and Technology 2015, 63, 685–690. [CrossRef]
- Padhmavathi, V.; Shruthy, R.; Preetha, R. Chitosan Coated Skim Milk-Alginate Microspheres for Better Survival of Probiotics during Gastrointestinal Transit. J Food Sci Technol 2023, 60, 889–895. [CrossRef]
- Haron, N.S.; Zakaria, J.H.; Mohideen Batcha, M.F. Recent Advances in Fluidized Bed Drying. IOP Conf. Ser.: Mater. Sci. Eng. 2017, 243, 012038. [CrossRef]
- Koh, W.Y.; Lim, X.X.; Tan, T.-C.; Kobun, R.; Rasti, B. Encapsulated Probiotics: Potential Techniques and Coating Materials for Non-Dairy Food Applications. Applied Sciences 2022, 12, 10005. [CrossRef]
- Sánchez-Portilla, Z.; Melgoza-Contreras, L.M.; Reynoso-Camacho, R.; Pérez-Carreón, J.I.; Gutiérrez-Nava, A. Incorporation of Bifidobacterium Sp. into Powder Products through a Fluidized Bed Process for Enteric Targeted Release. Journal of Dairy Science 2020, 103, 11129–11137. [CrossRef]
- Stummer, S.; Toegel, S.; Rabenreither, M.-C.; Unger, F.M.; Wirth, M.; Viernstein, H.; Salar-Behzadi, S. Fluidized-Bed Drying as a Feasible Method for Dehydration of Enterococcus Faecium M74. Journal of Food Engineering 2012, 111, 156–165. [CrossRef]
- Wirunpan, M.; Savedboworn, W.; Wanchaitanawong, P. Survival and Shelf Life of Lactobacillus Lactis 1464 in Shrimp Feed Pellet after Fluidized Bed Drying. Agriculture and Natural Resources 2016, 50, 1–7. [CrossRef]
- Vorländer, K.; Bahlmann, L.; Kwade, A.; Finke, J.H.; Kampen, I. Effect of Process Parameters, Protectants and Carrier Materials on the Survival of Yeast Cells during Fluidized Bed Granulation for Tableting. Pharmaceutics 2023, 15, 884. [CrossRef]
- Wu, C.-H.; Liu, Y.-C.; Ou, S.-F.; Chen, S.-T.; Kuo, J.-M.; Hsueh, Y.-H. Improving Acid Resistance and Characteristics of Microencapsulated Lactobacillus Brevis RK03 Using Top Fluid Bed Drying Technology. Process Biochemistry 2021, 110, 1–8. [CrossRef]
- Haldar, L.; Gandhi, D.N. Development of Vacuum-dried Probiotic Milk Powder with Bacillus Coagulans. Int J of Dairy Tech 2020, 73, 283–291. [CrossRef]
- Ermis, E. A Review of Drying Methods for Improving the Quality of Probiotic Powders and Characterization. Drying Technology 2022, 40, 2199–2216. [CrossRef]
- Misra, S.; Pandey, P.; Dalbhagat, C.G.; Mishra, H.N. Emerging Technologies and Coating Materials for Improved Probiotication in Food Products: A Review. Food Bioprocess Technol 2022, 15, 998–1039. [CrossRef]
- Broeckx, G.; Vandenheuvel, D.; Claes, I.J.J.; Lebeer, S.; Kiekens, F. Drying Techniques of Probiotic Bacteria as an Important Step towards the Development of Novel Pharmabiotics. International Journal of Pharmaceutics 2016, 505, 303–318. [CrossRef]
- Foerst, P.; Kulozik, U.; Schmitt, M.; Bauer, S.; Santivarangkna, C. Storage Stability of Vacuum-Dried Probiotic Bacterium Lactobacillus Paracasei F19. Food and Bioproducts Processing 2012, 90, 295–300. [CrossRef]
- Santivarangkna, C.; Kulozik, U.; Foerst, P. Effect of Carbohydrates on the Survival of Lactobacillus Helveticus during Vacuum Drying. Lett Appl Microbiol 2006, 42, 271–276. [CrossRef]
- Conrad, P.B.; Miller, D.P.; Cielenski, P.R.; De Pablo, J.J. Stabilization and Preservation of Lactobacillus Acidophilus in Saccharide Matrices. Cryobiology 2000, 41, 17–24. [CrossRef]
- Ambros, S.; Foerst, P.; Kulozik, U. Temperature-Controlled Microwave-Vacuum Drying of Lactic Acid Bacteria: Impact of Drying Conditions on Process and Product Characteristics. Journal of Food Engineering 2018, 224, 80–87. [CrossRef]
- EL Houssni, I.; Khedid, K.; Zahidi, A.; Hassikou, R. The Inhibitory Effects of Lactic Acid Bacteria Isolated from Sourdough on the Mycotoxigenic Fungi Growth and Mycotoxins from Wheat Bread. Biocatalysis and Agricultural Biotechnology 2023, 50, 102702. [CrossRef]
- Debonne, E.; Van Bockstaele, F.; Van Driessche, M.; De Leyn, I.; Eeckhout, M.; Devlieghere, F. Impact of Par-Baking and Packaging on the Microbial Quality of Par-Baked Wheat and Sourdough Bread. Food Control 2018, 91. [CrossRef]
- Moe, N.C.; Basbasan, A.J.; Winotapun, C.; Hararak, B.; Wanmolee, W.; Suwanamornlert, P.; Leelaphiwat, P.; Boonruang, K.; Chinsirikul, W.; Chonhenchob, V. Application of Lignin Nanoparticles in Polybutylene Succinate Based Antifungal Packaging for Extending the Shelf Life of Bread. Food Packaging and Shelf Life 2023, 39, 101127. [CrossRef]
- Basbasan, A.J.; Hararak, B.; Winotapun, C.; Wanmolee, W.; Leelaphiwat, P.; Boonruang, K.; Chinsirikul, W.; Chonhenchob, V. Emerging Challenges on Viability and Commercialization of Lignin in Biobased Polymers for Food Packaging: A Review. Food Packaging and Shelf Life 2022, 34, 100969. [CrossRef]
- Esakkimuthu, E.S.; DeVallance, D.; Pylypchuk, I.; Moreno, A.; Sipponen, M.H. Multifunctional Lignin-Poly (Lactic Acid) Biocomposites for Packaging Applications. Front. Bioeng. Biotechnol. 2022, 10, 1025076. [CrossRef]
- Axel, C.; Zannini, E.; Arendt, E.K. Mold Spoilage of Bread and Its Biopreservation: A Review of Current Strategies for Bread Shelf Life Extension. Critical Reviews in Food Science and Nutrition 2017, 57. [CrossRef]
- Saranraj, P.; Geetha, M. Microbial Spoilage of Bakery Products and Its Control by Preservatives. International Journal of Pharmaceutical & biological archives 2012, 3, 38–48.
- Debonne, E.; Giannotti, G.; Verbeke, C.; Eeckhout, M.; Devlieghere, F. Growth/No-Growth Models of Propionic and Sorbic Acid for Bread and Cake Molds. Food Control 2023, 152, 109872. [CrossRef]
- Quattrini, M.; Liang, N.; Fortina, M.G.; Xiang, S.; Curtis, J.M.; Gänzle, M. Exploiting Synergies of Sourdough and Antifungal Organic Acids to Delay Fungal Spoilage of Bread. International Journal of Food Microbiology 2019, 302, 8–14. [CrossRef]
- Jackson-Davis, A.; White, S.; Kassama, L.S.; Coleman, S.; Shaw, A.; Mendonca, A.; Cooper, B.; Thomas-Popo, E.; Gordon, K.; London, L. A Review of Regulatory Standards and Advances in Essential Oils as Antimicrobials in Foods. Journal of Food Protection 2023, 86, 100025. [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.; Vazquez-Olivo, G.; Heredia, J. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [CrossRef]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential Oils: A Promising Eco-Friendly Food Preservative. Food Chemistry 2020, 330, 127268. [CrossRef]
- Konfo, T.R.C.; Djouhou, F.M.C.; Koudoro, Y.A.; Dahouenon-Ahoussi, E.; Avlessi, F.; Sohounhloue, C.K.D.; Simal-Gandara, J. Essential Oils as Natural Antioxidants for the Control of Food Preservation. Food Chemistry Advances 2023, 2, 100312. [CrossRef]
- -ur-Rehman, S.; Hussain, S.; Nawaz, H.; Mushtaq Ah, M.; Anjum Murt, M.; Jaffar Riz, A. Inhibitory Effect of Citrus Peel Essential Oils on the Microbial Growth of Bread. Pakistan J. of Nutrition 2007, 6, 558–561. [CrossRef]
- Mukurumbira, A.R.; Shellie, R.A.; Keast, R.; Palombo, E.A.; Muir, B.W.; Jadhav, S.R. The Antimicrobial Efficacy of Native Australian Essential Oils in Liquid and Vapour Phase against Foodborne Pathogens and Spoilage Microorganisms. Food Control 2023, 151, 109774. [CrossRef]
- Cheng, C.; Zou, Y.; Peng, J. Oregano Essential Oil Attenuates RAW264.7 Cells from Lipopolysaccharide-Induced Inflammatory Response through Regulating NADPH Oxidase Activation-Driven Oxidative Stress. Molecules 2018, 23, 1857. [CrossRef]
- Debonne, E.; Van Bockstaele, F.; Samapundo, S.; Eeckhout, M.; Devlieghere, F. The Use of Essential Oils as Natural Antifungal Preservatives in Bread Products. Journal of Essential Oil Research 2018, 30, 309–318. [CrossRef]
- Da Silva, B.D.; Bernardes, P.C.; Pinheiro, P.F.; Fantuzzi, E.; Roberto, C.D. Chemical Composition, Extraction Sources and Action Mechanisms of Essential Oils: Natural Preservative and Limitations of Use in Meat Products. Meat Science 2021, 176, 108463. [CrossRef]
- Ju, J.; Xu, X.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Inhibitory Effects of Cinnamon and Clove Essential Oils on Mold Growth on Baked Foods. Food Chemistry 2018, 240, 850–855. [CrossRef]
- Nielsen, P.V.; Rios, R. Inhibition of Fungal Growth on Bread by Volatile Components from Spices and Herbs, and the Possible Application in Active Packaging, with Special Emphasis on Mustard Essential Oil. International Journal of Food Microbiology 2000, 60, 219–229. [CrossRef]
- Guynot, M.E.; Ramos, A.J.; Seto, L.; Purroy, P.; Sanchis, V.; Marin, S. Antifungal Activity of Volatile Compounds Generated by Essential Oils against Fungi Commonly Causing Deterioration of Bakery Products. J Appl Microbiol 2003, 94, 893–899. [CrossRef]
- Passone, M.A.; Girardi, N.S.; Ferrand, C.A.; Etcheverry, M. Invitro Evaluation of Five Essential Oils as Botanical Fungitoxicants for the Protection of Stored Peanuts from Aspergillus Flavus and A. Parasiticus Contamination. International Biodeterioration & Biodegradation 2012, 70, 82–88. [CrossRef]
- Xing, Y.; Xu, Q.; Li, X.; Che, Z.; Yun, J. ANTIFUNGAL ACTIVITIES OF CLOVE OIL AGAINST RHIZOPUS NIGRICANS, ASPERGILLUS FLAVUS AND PENICILLIUM CITRINUM IN VITRO AND IN WOUNDED FRUIT TEST: ANTIFUNGAL ACTIVITIES OF CLOVE OIL. Journal of Food Safety 2012, 32, 84–93. [CrossRef]
- Otoni, C.G.; Pontes, S.F.O.; Medeiros, E.A.A.; Soares, N.D.F.F. Edible Films from Methylcellulose and Nanoemulsions of Clove Bud ( Syzygium Aromaticum ) and Oregano ( Origanum Vulgare ) Essential Oils as Shelf Life Extenders for Sliced Bread. J. Agric. Food Chem. 2014, 62, 5214–5219. [CrossRef]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove Essential Oil (Syzygium Aromaticum L. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health. Molecules 2021, 26, 6387. [CrossRef]
- Aljabeili, H.S.; Barakat, H.; Abdel-Rahman, H.A. Chemical Composition, Antibacterial and Antioxidant Activities of Thyme Essential Oil (<I>Thymus Vulgaris</I>). FNS 2018, 09, 433–446. [CrossRef]
- Kumar, A.; Shukla, R.; Singh, P.; Prasad, C.S.; Dubey, N.K. Assessment of Thymus Vulgaris L. Essential Oil as a Safe Botanical Preservative against Post Harvest Fungal Infestation of Food Commodities. Innovative Food Science & Emerging Technologies 2008, 9, 575–580. [CrossRef]
- Askarne, L.; Talibi, I.; Boubaker, H.; Boudyach, E.H.; Msanda, F.; Saadi, B.; Serghini, M.A.; Ait Ben Aoumar, A. In Vitro and in Vivo Antifungal Activity of Several Moroccan Plants against Penicillium Italicum, the Causal Agent of Citrus Blue Mold. Crop Protection 2012, 40, 53–58. [CrossRef]
- Mani López, E.; Valle Vargas, G.P.; Palou, E.; López Malo, A. Penicillium Expansum Inhibition on Bread by Lemongrass Essential Oil in Vapor Phase. Journal of Food Protection 2018, 81, 467–471. [CrossRef]
- Teodoro, R.A.R.; De Barros Fernandes, R.V.; Botrel, D.A.; Borges, S.V.; De Souza, A.U. Characterization of Microencapsulated Rosemary Essential Oil and Its Antimicrobial Effect on Fresh Dough. Food Bioprocess Technol 2014. [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus Officinalis, L.): A Review. Medicines 2018, 5, 98. [CrossRef]
- Prakash, B.; Singh, P.; Kedia, A.; Dubey, N.K. Assessment of Some Essential Oils as Food Preservatives Based on Antifungal, Antiaflatoxin, Antioxidant Activities and in Vivo Efficacy in Food System. Food Research International 2012, 49, 201–208. [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Antifungal Activity of Lemon (Citrus Lemon L.), Mandarin (Citrus Reticulata L.), Grapefruit (Citrus Paradisi L.) and Orange (Citrus Sinensis L.) Essential Oils. Food Control 2008, 19, 1130–1138. [CrossRef]
- Singh, G.; Maurya, S.; deLampasona, M.P.; Catalan, C.A.N. A Comparison of Chemical, Antioxidant and Antimicrobial Studies of Cinnamon Leaf and Bark Volatile Oils, Oleoresins and Their Constituents. Food and Chemical Toxicology 2007, 45, 1650–1661. [CrossRef]
- Friedlein, U.; Dorn-In, S.; Schwaiger, K. Antimicrobial Effects of Plant Extracts against Clostridium Perfringens with Respect to Food-Relevant Influencing Factors. Journal of Food Protection 2021, 84, 1809–1818. [CrossRef]
- Singh, A.A.; Naaz, Z.T.; Rakaseta, E.; Perera, M.; Singh, V.; Cheung, W.; Mani, F.; Nath, S. Antimicrobial Activity of Selected Plant Extracts against Common Food Borne Pathogenic Bacteria. Food and Humanity 2023, 1, 64–70. [CrossRef]
- Obulesu, M. Effect of Plant Extracts against Alzheimer’s Disease. In Plant Extracts in Neurodegenerative Diseases; Elsevier, 2022; pp. 1–15 ISBN 978-0-323-95762-5.
- Jiménez, M.C.; Prieto, K.; Lasso, P.; Gutiérrez, M.; Rodriguez-Pardo, V.; Fiorentino, S.; Barreto, A. Plant Extract from Caesalpinia Spinosa Inhibits Cancer-Associated Fibroblast-like Cells Generation and Function in a Tumor Microenvironment Model. Heliyon 2023, 9, e14148. [CrossRef]
- Kola, V.; Carvalho, I.S. Plant Extracts as Additives in Biodegradable Films and Coatings in Active Food Packaging. Food Bioscience 2023, 54, 102860. [CrossRef]
- Czubaszek, A.; Czaja, A.; Sokół-Łętowska, A.; Kolniak-Ostek, J.; Kucharska, A.Z. Quality of Bread Enriched with Microencapsulated Anthocyanin Extracts during in Vitro Simulated Digestion. Journal of Cereal Science 2023, 113, 103724. [CrossRef]
- Maibam, B.D.; Chakraborty, S.; Nickhil, C.; Deka, S.C. Effect of Euryale Ferox Seed Shell Extract Addition on the in Vitro Starch Digestibility and Predicted Glycemic Index of Wheat-Based Bread. International Journal of Biological Macromolecules 2023, 226, 1066–1078. [CrossRef]
- Balasubramaniam, V.G.; Ramakrishnan, S.R.; Antony, U. Opportunities and Challenges of Plant Extracts in Food Industry. In Plant Extracts: Applications in the Food Industry; Elsevier, 2022; pp. 295–315 ISBN 978-0-12-822475-5.
- Negi, P.S. Plant Extracts for the Control of Bacterial Growth: Efficacy, Stability and Safety Issues for Food Application. International Journal of Food Microbiology 2012, 156, 7–17. [CrossRef]
- Bhalla, R.; Bhalla, N.; Norton, L. Bread Preservation Efficacy and Antifungal Activity of Six Wood and Leaf Essential Oils. Editors’ Remarks.
- Bao, Z.; Fan, M.; Hannachi, K.; Li, T.; Zhao, J.; Li, Y.; Qian, H.; Wang, L. Antifungal Activity of Star Anise Extract against Penicillium Roqueforti and Aspergillus Niger for Bread Shelf Life. Food Research International 2023, 172, 113225. [CrossRef]
- Torgbo, S.; Sukatta, U.; Kamonpatana, P.; Sukyai, P. Ohmic Heating Extraction and Characterization of Rambutan (Nephelium Lappaceum L.) Peel Extract with Enhanced Antioxidant and Antifungal Activity as a Bioactive and Functional Ingredient in White Bread Preparation. Food Chemistry 2022, 382, 132332. [CrossRef]
- Melini, V.; Melini, F. Strategies to Extend Bread and GF Bread Shelf-Life: From Sourdough to Antimicrobial Active Packaging and Nanotechnology. Fermentation 2018, 4, 9. [CrossRef]
- Dal Bello, F.; Clarke, C.I.; Ryan, L.A.M.; Ulmer, H.; Schober, T.J.; Ström, K.; Sjögren, J.; Van Sinderen, D.; Schnürer, J.; Arendt, E.K. Improvement of the Quality and Shelf Life of Wheat Bread by Fermentation with the Antifungal Strain Lactobacillus Plantarum FST 1.7. Journal of Cereal Science 2007, 45, 309–318. [CrossRef]
- Gerez, C.L.; Dallagnol, A.; Ponsone, L.; Chulze, S.; Font De Valdez, G. Ochratoxin A Production by Aspergillus Niger: Effect of Water Activity and a Biopreserver Formulated with Lactobacillus Plantarum CRL 778. Food Control 2014, 45, 115–119. [CrossRef]
- Ryan, L.A.M.; Zannini, E.; Dal Bello, F.; Pawlowska, A.; Koehler, P.; Arendt, E.K. Lactobacillus Amylovorus DSM 19280 as a Novel Food-Grade Antifungal Agent for Bakery Products. International Journal of Food Microbiology 2011, 146, 276–283. [CrossRef]
- Ryan, L.A.M.; Dal Bello, F.; Arendt, E.K. The Use of Sourdough Fermented by Antifungal LAB to Reduce the Amount of Calcium Propionate in Bread. International Journal of Food Microbiology 2008, 125, 274–278. [CrossRef]
- Rizzello, C.G.; Cassone, A.; Coda, R.; Gobbetti, M. Antifungal Activity of Sourdough Fermented Wheat Germ Used as an Ingredient for Bread Making. Food Chemistry 2011, 127, 952–959. [CrossRef]
- Garofalo, C.; Zannini, E.; Aquilanti, L.; Silvestri, G.; Fierro, O.; Picariello, G.; Clementi, F. Selection of Sourdough Lactobacilli with Antifungal Activity for Use as Biopreservatives in Bakery Products. J. Agric. Food Chem. 2012, 60, 7719–7728. [CrossRef]
- Cizeikiene, D.; Juodeikiene, G.; Paskevicius, A.; Bartkiene, E. Antimicrobial Activity of Lactic Acid Bacteria against Pathogenic and Spoilage Microorganism Isolated from Food and Their Control in Wheat Bread. Food Control 2013, 31, 539–545. [CrossRef]
- Leyva Salas, M.; Thierry, A.; Lemaître, M.; Garric, G.; Harel-Oger, M.; Chatel, M.; Lê, S.; Mounier, J.; Valence, F.; Coton, E. Antifungal Activity of Lactic Acid Bacteria Combinations in Dairy Mimicking Models and Their Potential as Bioprotective Cultures in Pilot Scale Applications. Front. Microbiol. 2018, 9, 1787. [CrossRef]
- Ouiddir, M.; Bettache, G.; Leyva Salas, M.; Pawtowski, A.; Donot, C.; Brahimi, S.; Mabrouk, K.; Coton, E.; Mounier, J. Selection of Algerian Lactic Acid Bacteria for Use as Antifungal Bioprotective Cultures and Application in Dairy and Bakery Products. Food Microbiology 2019, 82, 160–170. [CrossRef]
- Iosca, G.; Turetta, M.; De Vero, L.; Bang-Berthelsen, C.H.; Gullo, M.; Pulvirenti, A. Valorization of Wheat Bread Waste and Cheese Whey through Cultivation of Lactic Acid Bacteria for Bio-Preservation of Bakery Products. LWT 2023, 176, 114524. [CrossRef]
- Rizzello, C.G.; Lavecchia, A.; Gramaglia, V.; Gobbetti, M. Long-Term Fungal Inhibition by Pisum Sativum Flour Hydrolysate during Storage of Wheat Flour Bread. Appl Environ Microbiol 2015, 81, 4195–4206. [CrossRef]
- Schmidt, M.; Lynch, K.M.; Zannini, E.; Arendt, E.K. Fundamental Study on the Improvement of the Antifungal Activity of Lactobacillus Reuteri R29 through Increased Production of Phenyllactic Acid and Reuterin. Food Control 2018, 88, 139–148. [CrossRef]
- Leyva Salas, M.; Mounier, J.; Maillard, M.-B.; Valence, F.; Coton, E.; Thierry, A. Identification and Quantification of Natural Compounds Produced by Antifungal Bioprotective Cultures in Dairy Products. Food Chemistry 2019, 301, 125260. [CrossRef]
- Ribes, S.; Fuentes, A.; Talens, P.; Barat, J.M. Prevention of Fungal Spoilage in Food Products Using Natural Compounds: A Review. Critical Reviews in Food Science and Nutrition 2018, 58, 2002–2016. [CrossRef]
- Abbasi, A.; Sabahi, S.; Bazzaz, S.; Tajani, A.G.; Lahouty, M.; Aslani, R.; Hosseini, H. An Edible Coating Utilizing Malva Sylvestris Seed Polysaccharide Mucilage and Postbiotic from Saccharomyces Cerevisiae Var. Boulardii for the Preservation of Lamb Meat. International Journal of Biological Macromolecules 2023, 246, 125660. [CrossRef]
- Jin, J.; Nguyen, T.T.H.; Humayun, S.; Park, S.; Oh, H.; Lim, S.; Mok, I.-K.; Li, Y.; Pal, K.; Kim, D. Characteristics of Sourdough Bread Fermented with Pediococcus Pentosaceus and Saccharomyces Cerevisiae and Its Bio-Preservative Effect against Aspergillus Flavus. Food Chemistry 2021, 345, 128787. [CrossRef]
- Ramli, A.N.M.; Hong, P.K.; Abdul Manas, N.H.; Wan Azelee, N.I. An Overview of Enzyme Technology Used in Food Industry. In Value-Addition in Food Products and Processing Through Enzyme Technology; Elsevier, 2022; pp. 333–345 ISBN 978-0-323-89929-1.
- Motta, J.F.G.; Freitas, B.C.B.D.; Almeida, A.F.D.; Martins, G.A.D.S.; Borges, S.V. Use of Enzymes in the Food Industry: A Review. Food Sci. Technol 2023, 43, e106222. [CrossRef]
- Dahiya, S.; Bajaj, B.K.; Kumar, A.; Tiwari, S.K.; Singh, B. A Review on Biotechnological Potential of Multifarious Enzymes in Bread Making. Process Biochemistry 2020, 99, 290–306. [CrossRef]
- Freitas, D.C.; Zambelli, R.A.; Ramos, M.V.; Oliveira, J.P.B.; Souza, P.F.N.; Santos, G.B.M.; Nagano, C.S.; Bezerra, L.P.; Silva, A.F.B.; Oliveira, J.S.; et al. Latex Peptidases Produce Peptides Capable of Delaying Fungal Growth in Bread. Food Chemistry 2022, 373. [CrossRef]
- Omedi, J.O.; Huang, J.; Huang, W.; Zheng, J.; Zeng, Y.; Zhang, B.; Zhou, L.; Zhao, F.; Li, N.; Gao, T. Suitability of Pitaya Fruit Fermented by Sourdough LAB Strains for Bread Making: Its Impact on Dough Physicochemical, Rheo-Fermentation Properties and Antioxidant, Antifungal and Quality Performance of Bread. Heliyon 2021, 7, e08290. [CrossRef]
| Strain | Cryoprotectant (w/v) | Survival rate after freeze-drying | References |
|---|---|---|---|
| Limosilactobacillus reuteri (formerly Lactobacillus reuteri) (CICC6226) | Sucrose (15%), skimmed milk (10%) | 95.98% ± 6.69 | [70] |
| L. plantarum (LP105) | Trehalose (5%), whey protein concentrate (10%), pullulan (4%) | 94.36% ± 1.06 | [71] |
| Pediococcus pentosaceus (GS4) | Skimmed milk powder (13%) | 81.76% ± 4.05 | [72] |
| Enterococcus faecalis (PK1202) | Skimmed milk powder (8%) | 96 % | [73] |
| Advantage | Disadvantages | References |
|---|---|---|
| Minimum damage to the product | Need of additional cryoprotectants | [74] |
| It provides a large surface area for the encapsulation | Lengthy drying time [24 – 36 h] | [75] |
| The most widely used method for sensitivity materials | Complex equipment and difficult to change the process | [76] |
| Porous structured powder due to sublimation of water | High capital and maintenance cost | [66] |
| Strain | Inlet/outlet air temperature | Protectant | Survival rate | References |
|---|---|---|---|---|
| Enterococcus rivorum (S22C) | 140 ºC± 2/60 ºC± 2 | Maltodextrin (12%), glucose (4%), whey protein (4%) | 92% | [80] |
| Lactobacillus acidophilus (ATCC4356) | 200 ºC/90 ºC | Whey protein (5%), maltodextrin (10%) | 84.87%± 0.02 | [81] |
| Saccharomyces cerevisiae var. boulardii | 125 ºC/61 ºC | maltodextrin (40.39%) | 92.84% | [82] |
| Lacticaseibacillusrhamnosus (formerly Lactobacillus rhamnosus) GG | 98 ºC±4/65±3 ºC | Lactose (10%), trehalose (10%) | 80% – 100% | [83] |
| Strain | Protectant | Temperature | Pressure | Time | Survival rate | Reference |
|---|---|---|---|---|---|---|
| Lacticaseibacillus paracasei (formely Lactobacillus paracasei) F19 | Trehalose 25% (w/w) | 15 ºC | 15 mbar | 22h | 70% | [95] |
| Lactobacillus helveticus | Sorbitol (1% w/w) | 43 ºC | 100 mbar | 12h | 18% | [96] |
| L. acidophilus | Trehalose (20% w/w) | Room temperature | 0.11 mbar | 96h | 37.9% | [97] |
| Advantages | Disadvantages | References |
|---|---|---|
| Reduced drying temperatures | long processing time | [94] |
| Higher drying rate | the dried product might have shrinkage | [92] |
| Reduced oxygen concentration | Denser structure | [98] |
| Essential Oils | Major compounds | Targeted Molds | Action | Reference |
|---|---|---|---|---|
| Clove (Syzygium aromaticum L.) | Eugenol, Acetyleugenol, Caryophyllene, Gallic Acid, Kaempferol, Quercetin, Tannins | Aspergillus flavus, A. niger, Aspergillus parasiticus, Eurotium amstelodami, Eurotium herbariorum, Eurotium repens, Eurotium rubrum, Penicillium corylophilum, Penicillium commune, P. roqueforti, Penicillium citrinum, Endomyces fibuliger, Rhizopus nigricans, Penicillium sp. | Reduced yeast and mold growth | [118,119,120,121,122,123] |
| Thyme (Thymus vugaris L.) | Thymol, Carvacrol, Linalool, P-Cymene, Camphene, Myrcene, Caryophyllene, Rosmarinic Acid | A. flavus, A. niger, Aspergillus terreus, Alternaria alternata, E. amstelodami, E. herbariorum, E. repens, Eurotium rubrum, Fusarium oxysporum, P. corylophilum, Penicillium italicum, Penicillium paneum | Bread shelf-life | [106,115,119,124,125,126] |
| Lemongrass (Cymbopogan citratus) | Citral, Geraniol, Limonen, Neral and Nerol, Myrcene and Citronellal | A. flavus, A. niger, E. amstelodami, E. Herbariorum, E. repens, E. rubrum, P. corylophilum, Penicillium expansum | Mold growth inhibited | [119,127] |
| Rosemary (Rosemary officinalis) | carnosic acid, carnosol, rosmarinic acid and hesperidin |
Penicillium sp. Aspergillus sp. |
Fungal generation reduced | [115,128,129] |
| Oregano (Origanum vulgare L.) | Carvacrol, Thymol, Rosmarinic Acid, P-Cymene, Terpinene, Linalool, Naringin, β-Caryophyllene | A. flavus, A. niger, Aspergillus fumigatus, Aspergillus ochraceus, A. parasiticus, A. terreus, Eurotium fibuliger, P. commune, P. roqueforti | Mold growth inhibited | [118] |
| Marjoram (Origanum majorana L.) | Terpinen-4-ol, α-Terpinene, γ-Terpinene, Linalool, Sabinene, P-Cymene, Myrcene, Thymol |
A. flavus Penicillium chrysogenum, Rhizopus spp. |
Protection of seeds during incubation Shelf life | [130] |
| Mandarin (Citrus reticulate L.) | Limonene, Myrcene, Linalool, γ-Terpinene, Nobiletin, Hesperidin, Rutin, Ascorbic Acid | A. flavus, A. niger, P. chrysogenum, Penicillium verrucosum | Mold growth inhibited | [131] |
| Cinnamon (Cinnamomum jersenianum Hand.-Mazz) | Cinnamaldehyde, Eugenol, Cinnamyl Acetate, Coumarin, Proanthocyanidins | A. flavus, A. niger, A. ochraceus, A. terreus, E. fibuliger, E. amstelodami, E. Herbariorum, E. repens, E. rubrum, P. corylophilum, P. citrinum; P. commune, Penicillium viridicatum, P. roqueforti | Reduction of the targeted mold growth | [118,119,132] |
| Antifungal lactic acid bacteria | Organism | Reference |
|---|---|---|
| L. plantarum FST 1.7 | Fusarium culmorum and Fusarium graminearum | [146] |
| L. plantarum CRL 778, L. reuteri CRL 1100, L. brevis CRL 772 and CRL 796 | Aspergillus, Fusarium, and Penicillium species | [147] |
| Lactobacillus amylovorus DSM 19280 | A. niger FST4.21, P. expansum FST 4.22, and P. roqueforti FST 4.11, F. culmorum FST 4.05 | [148] |
| L. plantarum | A. niger FST4.21, F. culmorum TMW 4.0754, p. expansum LTH S46 | [149] |
| L. plantarum LB1 F. rossiae LB5 | P. roqueforti DPPMAF1 | [150] |
| F. rossiae LD108 Companilactobacillus paralimentarius PB12 (formerly Lactobacillus paralimentarius) | Aspergillus japonicus, E. repens and Penicillium roseopurpureum | [151] |
| Latilactobacillus sakei (formerly Lactobacillus sakei) KTU05-6, Pediococcus acidilactici KTU05-7, P. pentosaceus KTU05-8, P. pentosaceus KTU05-9 and, P. pentosaceus KTU05-10 | Molds | [152] |
| L. amylovorus DSM19280 | Molds | [104] |
| L. plantarum L244 with Schleiferilactobacillus harbinensis L172 (formerly Lactobacillus harbinensis) | P. commune, Mucor racemosus and Rhodotorula mucilaginosa | [153] |
| L. plantarum CH1, Lc. paracasei B20 and Leuconostoc mesenteroides L1 | M. racemosus UBOCC-A-109155, P. commune UBOCC-A-116003, Yarrowia lipolytica UBOCC-A-216006, Aspergillus tubingensis AN, A. flavus T5 and Paecilomyces formosus AT | [154] |
| L. plantarum UMCC 2996, F. rossiae UMCC 3002, P. pentosaceus UMCC 3010 | A. flavus ITEM 7828, P. paneum ITEM 1381, A. niger ITEM 7090 | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





