1. Introduction
Obstructive Sleep Apnea (OSA) is a respiratory condition identified by the partial or complete obstruction of the upper air passages during sleep [
1]. In the pediatric population, most studies report an average prevalence of 4% [
2,
3,
4]. Sleep-disordered breathing (SDB) is more common in children aged 2 to 8 years and during adolescence. The first peak of incidence is primarily associated with adenoid and tonsil hypertrophy, while the second peak is predominantly linked to obesity [
5]. The prevalence reaches 6% in patients aged 2 to 8 years, 4.7% between 8 and 11 years, and 4.3% between 16 and 19 years [
6].
The complications of SDB in the pediatric context can be categorized into major groups, namely neurobehavioral and cognitive complications [
7], growth retardation [
8], and metabolic and cardiovascular issues [
9,
10]. Individual differences have been noted in response to hypoxia, hypercapnia, and changes in airway pressure during SDB [
11]. Furthermore, alterations in proinflammatory cytokine patterns have also been observed in patients with OSAS [
12,
13]. These inflammatory changes might have implications in the pathogenesis of the conditions and the development of its numerous complications [
14]. As the number of children with a heightened risk of OSAS grows, a significant portion of these individuals will need ongoing medical transition monitoring into their young adulthood [
15].
In adult ages, OSA is strongly correlated with various ocular diseases commonly encountered in ophthalmic practice. Some of these ocular consequences are reversible, but if not adequately treated, they can also permanently threaten the patient's vision [
16,
17]. Recently, it has been suggested that children with OSAS might exhibit ocular disorders. Ocular comorbidities could begin in childhood with OSAS, persist, and worsen in subsequent ages if not appropriately manage [
18].
This literature review aims to examine the association between OSAS and eye health in children. Studies exploring how OSAS might negatively impact children's eye health in various aspects will be searched for and analyzed, highlighting limitations and gaps in knowledge that may necessitate further research to solidify the current evidence.
2. Materials and Methods
A search was conducted using three databases: PUBMED/MEDLINE, SCOPUS, and WebOfScience (Access date June 25, 2023), employing specific keywords to identify relevant studies for the review. Keywords included factors such as age (children, infants, adolescents), ocular diseases (eye, cornea, retina, optic nerve), and sleep disorders (disordered sleep breathing, obstructive sleep apnea, polysomnography). These choices aimed to explore the relationship between children's eye health and sleep disorders, tailored to the requirements of each search engine.
Articles in languages other than English were excluded to avoid potential language barriers. Bibliographic reviews, isolated clinical cases, case series, and letters were not considered as they might lack the depth required for the present analysis. Studies involving adult participants (aged 18 and above) were excluded to maintain focus exclusively on the pediatric population. Additionally, duplicate studies—those published multiple times or found in various data sources—were excluded to prevent duplications and ensure data integrity.
3. Results
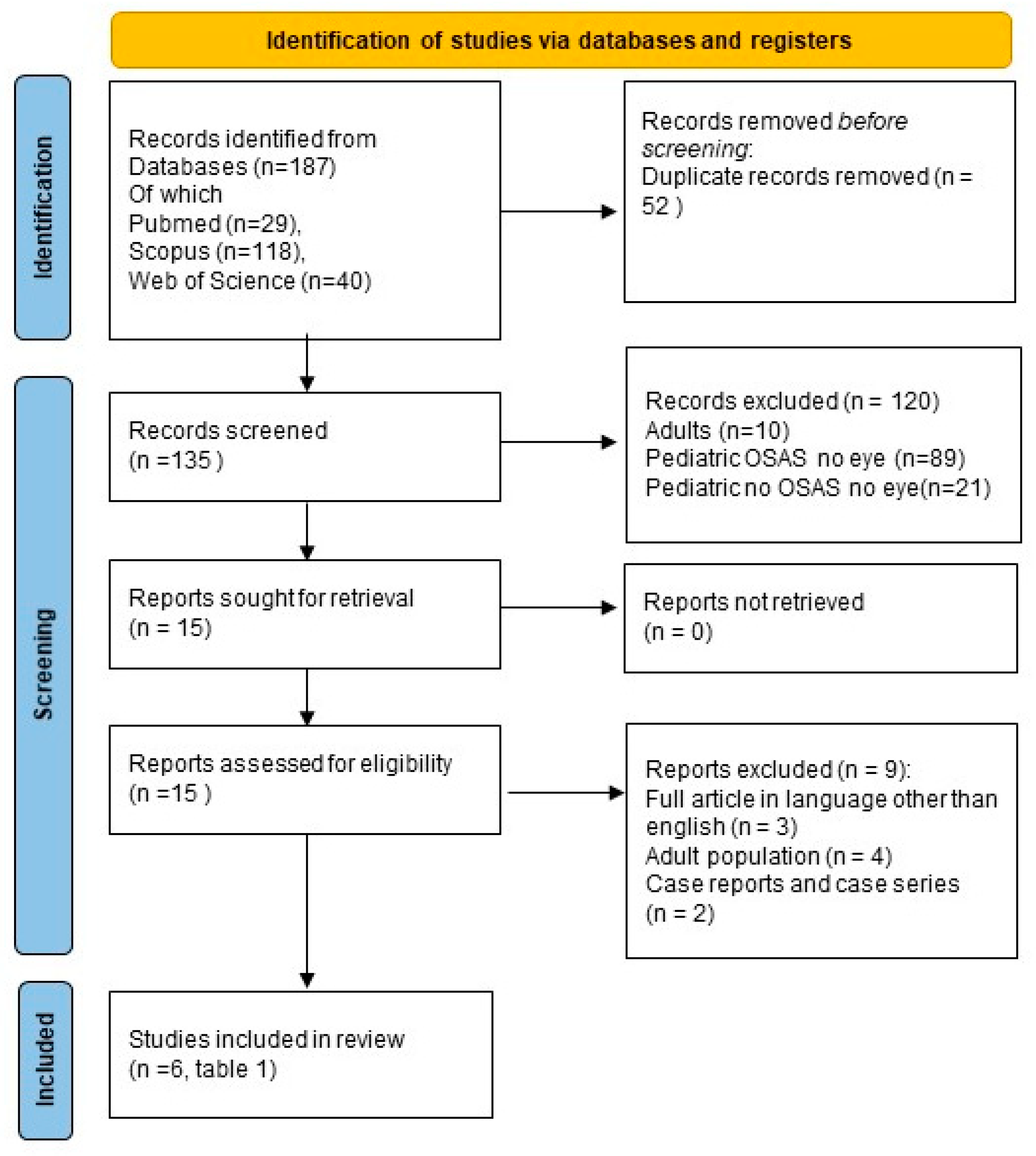
The initial search identified a total of 187 articles, comprising 29 from PubMed, 118 from Scopus, and 40 from Web of Science. Duplicate articles were subsequently excluded, removing 52 articles, leaving 29 from PubMed, 92 from Scopus, and 14 from Web of Science for further consideration (
Figure 1, PRISMA). Concerning article relevance, 120 articles were excluded. Among these, 10 were associated with the adult population, while 110 pertained to the pediatric population. The latter group of articles was further categorized into three subgroups: articles excluded because they solely addressed OSAS without any mention of ocular pathology (89 articles identified), articles related to ocular pathology but not OSAS (no articles identified), and articles not associated with either OSAS or ocular pathology (21 articles identified). Subsequently, three articles with full text in languages other than English were excluded. Additionally, two articles were excluded due to their classification as case reports or case series, and four articles were excluded because they pertained to adulthood. Ultimately, the search yielded 6 relevant articles on the topic (
Table 1), all related to the pediatric population and available in English.
The identified studies investigating retinal characteristics are three, with the first one published by Cinici et al. [
19]. Compared to healthy controls, this study examined optic nerve thickness in children with OSAS and adenotonsillar hypertrophy. A total of 88 patients were enrolled in the study, but no significant correlation was found between the presence of OSAS and RNFL thickness (ranging from -0.031 to +0.016 in the right and left eyes, p>0.05). However, it was highlighted that the age at examination might be a risk factor for developing eye issues in patients with OSAS (r=+0.107, p<0.05). The primary constraint of this study was the lack of polysomnography (PSG), necessitating the utilization of the OSA-18 survey as a substitute.
Simsek et al. [
20] examined the optic nerve in 76 patients to assess differences between OSAS patients and controls and identify discrepancies before and after adenotonsillectomy (A&T) in OSAS patients. OSAS patients had higher intraocular pressure (IOP) compared to controls (p<0.05), but there were no significant differences in optic nerve density. After surgery, the superior optic nerve thickness significantly increased in OSAS patients (p<0.05), proportionally to the severity of the condition. The thickness of the inferior optic nerve also exhibited an increment in OSAS patients, though it did not demonstrate any significant correlation with the severity of the condition. The study's main limitation was the lack of PSG utilization for OSAS diagnosis. The result suggests that surgery can improve optic nerve thickness in OSAS patients and that higher IOP may increase the risk of eye issues in moderate to severe OSAS patients.
The study by Bayraktar and Şimşek [
21] evaluated choroidal alterations in children with OSAS. The study assessed eye health in pediatric patients with OSAS, focusing on choroidal thickness. 151 patients were involved, with 109 having OSAS and 42 controls. Choroid was evaluated using optical coherence tomography (OCT), and the results indicated a significant nasal choroidal thinning in OSAS patients compared to controls (p<0.05). However, the study had limitations such as the absence of PSG and post-A&T analysis and lack of information on disease duration. The result suggests that OSAS may harm children's eye health, particularly on the choroid.
Ye et al. [
22] examined the effect of adenoidectomy on children with OSAS, using optical coherence tomography angiography (OCT-A) to assess retinal perfusion. 62 children with OSAS were included, and a significant improvement in vascular density in the parafoveal area (p < 0.01) and reduction in the foveal avascular zone were observed after the intervention (t = 4.50, p < 0.05) and an increase in the deep capillary plexus (t = - 4.43, p < 0.05). The authors concluded that OCT-A can be considered a valuable method to evaluate the effects of adenoidectomy in pediatric OSAS cases.
Ye et al. [
23] assessed retinal vasculature in OSAS patients compared to healthy controls, utilizing OCT-A to evaluate the choroid. 132 patients were involved, 66 cases and 66 controls, and the results indicated significantly lower (p<0.05) values of various vascular indices in the macular superficial/deep capillary plexus (SCP/DCP) and Foveal Avascular Zone in deep capillary plexus flow area zone (FAZ in DCP) in OSAS patients compared to controls (p<0.05). The study had limitations, such as the absence of PSG and non-measurement of arterial pressure, but it still suggests that OSAS may harm retinal vasculature.
Finally, the last identified article is by Bonacci et al. [
18]. Conducted in Italy on 72 patients, the study evaluated the effect of OSAS on eye health. Patients were divided into two groups, with and without OSAS, and were investigated with overnight respiratory polygraphy. The assessment was performed using various tools, and the results in OSAS patients were found to be significantly different compared to controls in terms of corneal thickness (0.9 ± 0.5 vs. 0.6 ± 0.3, respectively; p = 0.02) and average retinal nerve fiber layer thickness (102.8 ± 10.5 µm vs. 98.1 ± 12.3 µm, respectively; p = 0.012). The study suggests that OSAS may harm corneal thickness and retinal health [
18].
In summary (
Table 1), three studies investigating retinal characteristics [
18,
19,
20], three examining choroid in OSAS patients [
21,
22,
23], and two on cornea [
18,
20] have been identified. The results indicate that OSAS may harm eye health, particularly in the cornea and optic nerve. However, some studies had limitations, including the absence of PSG for OSAS diagnosis [
19,
20,
21]. Home respiratory polygraphy was used in one study [
18].
Table 1 presents two prospective observational studies that evaluated ocular parameters before and after otorhinolaryngological interventions. Surgical intervention and A&T improved conditions in OSAS patients [
20,
22]. In the first study, post-A&T intervention reduced IOP and RNFL thickness [
20]. In the second study, improvements in retinal vascularization were observed after adenoidectomy, with increased vascularization parameters [
22].
4. Discussion
Out of the six identified studies, three are related to the correlation between pediatric OSAS and choroidal alterations [
21,
22,
23], and another three investigate the correlation with retinal and optic nerve changes [
18,
19,
20]; two of the latter also considers corneal alterations [
18,
20]. Two studies observed ocular changes after otorhinolaryngological intervention [
20,
22]. However, in both studies [
20,
22], PSG was not utilized/stated as a diagnostic method for OSA. These studies revealed that otorhinolaryngological interventions (such as A&T and surgery for adenoid and tonsil hypertrophy) positively impacted ocular parameters in patients with OSA, suggesting potential connections between upper airway issues and ocular changes. Five studies employed control groups to aid in comparing outcomes in patients with OSA to those without, thereby mitigating or controlling disruptive variables [
18,
19,
20,
21,
23]. Only one study employed instrumental diagnosis (overnight respiratory polygraphy) of OSAS [
18].
In adults, OSAS increases the risk of glaucoma, ischemic optic neuropathy [
24,
25], and floppy eyelid syndrome [
17,
26]. "Self-reported snoring" is associated with reduced retinal thickness and vascular density [
27]. Patients with OSAS exhibit reduced RNFL thickness, Ganglion Cell Complex (GCC) thickness, and perifoveal vascular density, accompanied by retinal and optic nerve ischemic injury [
28,
29]. OSAS may correlate with non-arteritic anterior ischemic optic neuropathy (NAION) through endothelial damage, hypoxia, and reduced perfusion [
17,
30]. It is linked to dry eye syndrome [
17], keratoconus [
16,
17], and central serous chorioretinopathy. OSAS heightens the risk of Retinal Vein Occlusion (RVO) and diabetic retinopathy [
17,
31], causing damage to retinal vessels [
17,
31].
In pediatric studies of OSAS, ocular changes have been observed, including optic nerve thickness, choroidal layer, retinal vascularization, and cornea. OSAS in children can impact eye health, with increased retinal vascular density after treatment [
22] and reduced choroidal thickness in patients with adenotonsillar hypertrophy [
21]. Despite the absence of severe cases, OSAS could have a negative impact, necessitating regular monitoring [
23]. An immediate correlation between OSAS and optic nerve thickness in children is not evident and contradictory results have been reported. OSAS raises intraocular pressure and reduces optic nerve thickness, but treatment improves the optic nerve [
20]. In pediatric patients with OSAS, the cornea exhibits anomalies and optic nerve thickness increases, possibly due to intermittent hypoxia [
18].
In summary, studies in adults suggest that OSAS is clearly associated with ocular risks such as glaucoma, ischemic optic neuropathy, floppy eyelid syndrome, and various retinal and corneal alterations [
16,
17,
25,
28,
29,
30,
31,
32,
33]. In pediatric patients with OSAS, studies show similar ocular changes, including optic nerve thickness, choroidal layer, retinal vascularization, and cornea, with evidence of improvements in retinal vascularization after treatment [
18,
19,
20,
21,
22,
23]. However, OSAS seems not to exhibit an immediate correlation with optic nerve thickness in children, although age might still play a role [
19]. Overall, both in adults and children, OSAS can contribute to various ocular disorders, but there might be differences in specific risks and observed alterations between the two populations.
The research conducted so far appears to have some methodological shortcomings that could impact the results' validity and reliability. Firstly, to our knowledge, the research is based on only 6 studies [
18,
19,
20,
21,
23], and it's possible that the sample size might not be sufficiently large to obtain representative and reliable results. The absence of PSG usage in the diagnosis could raise doubts about the accuracy of the diagnosis itself and may affect the proper identification of patients with OSAS [
19,
20,
21,
22,
23]. Lastly, if the patients with OSAS have other medical conditions that could influence ocular parameters, these factors should be considered in the analysis [
18].
5. Conclusions
The results of the studies indicate a potential negative impact of OSAS on children's eye health. These studies have examined various aspects, including optic nerve thickness, choroidal layer, and retinal and corneal vascularization alterations. Overall, OSAS could adversely affect ocular health through multiple mechanisms. Therefore, regular monitoring by an ophthalmologist for children affected by OSAS is paramount. However, it's important to note that the research conducted so far has some methodological shortcomings that could impact the results' validity and reliability. Therefore, while the studies emphasize the significance of considering OSAS as a potential influencing factor in pediatric ocular pathologies, further investigations are necessary using more accurate diagnostic methods and a larger sample size to obtain more robust and generalizable results.
Author Contributions
Conceptualization, M.Z. and E.B.; methodology, M.Z.; validation, G.P. and E.P.; investigation, M.Z. and L.N.; resources, M.Z and E.P..; data curation, M.Z.; writing—original draft preparation, M.Z anf E.B..; writing—review and editing, E.B. and L.N.; visualization, G.P. and E.P.; supervision, G.P. and E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sateia, M.J. International classification of sleep disorders-third edition: highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.J.; Chae, K.Y. Obstructive sleep apnea syndrome in children: Epidemiology, pathophysiology, diagnosis and sequelae. Korean J Pediatr 2010, 53, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Favaro, S.; Hilliard, T.; Henderson, J. Obstructive sleep apnoea in children. Paediatrics and Child Health 2009, 19, 271–275. [Google Scholar] [CrossRef]
- Marcus, C.L.; Brooks, L.J.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Schechter, M.S.; Sheldon, S.H.; Spruyt, K.; Ward, S.D.; et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012, 130, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Giuca, M.R.; Carli, E.; Lardani, L.; Pasini, M.; Miceli, M.; Fambrini, E. Pediatric Obstructive Sleep Apnea Syndrome: Emerging Evidence and Treatment Approach. ScientificWorldJournal 2021, 2021, 5591251. [Google Scholar] [CrossRef] [PubMed]
- Inoshita, A.; Kasai, T.; Matsuoka, R.; Sata, N.; Shiroshita, N.; Kawana, F.; Kato, M.; Ikeda, K. Age-stratified sex differences in polysomnographic findings and pharyngeal morphology among children with obstructive sleep apnea. J Thorac Dis 2018, 10, 6702–6710. [Google Scholar] [CrossRef]
- Zaffanello, M.; Ferrante, G.; Zoccante, L.; Ciceri, M.L.; Nosetti, L.; Tenero, L.; Piazza, M.; Piacentini, G. Predictive Power of Oxygen Desaturation Index (ODI) and Apnea-Hypopnea Index (AHI) in Detecting Long-Term Neurocognitive and Psychosocial Outcomes of Sleep-Disordered Breathing in Children: A Questionnaire-Based Study. J Clin Med 2023, 12. [Google Scholar] [CrossRef]
- Zaffanello, M.; Piacentini, G.; La Grutta, S. Beyond the growth delay in children with sleep-related breathing disorders: a systematic review. Panminerva Med 2020, 62, 164–175. [Google Scholar] [CrossRef]
- Tagetti, A.; Bonafini, S.; Zaffanello, M.; Benetti, M.V.; Vedove, F.D.; Gasperi, E.; Cavarzere, P.; Gaudino, R.; Piacentini, G.; Minuz, P.; et al. Sleep-disordered breathing is associated with blood pressure and carotid arterial stiffness in obese children. J Hypertens 2016. [Google Scholar] [CrossRef]
- Zaffanello, M.; Piacentini, G.; Pietrobelli, A.; Fava, C.; Lippi, G.; Maffeis, C.; Gasperi, E.; Nosetti, L.; Bonafini, S.; Tagetti, A.; et al. Ambulatory clinical parameters and sleep respiratory events in a group of obese children unselected for respiratory problems. World J Pediatr 2017, 13, 577–583. [Google Scholar] [CrossRef]
- Brockbank, J.C. Update on pathophysiology and treatment of childhood obstructive sleep apnea syndrome. Paediatr Respir Rev 2017, 24, 21–23. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Gozal, D. Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef]
- Maniaci, A.; Iannella, G.; Cocuzza, S.; Vicini, C.; Magliulo, G.; Ferlito, S.; Cammaroto, G.; Meccariello, G.; De Vito, A.; Nicolai, A.; et al. Oxidative Stress and Inflammation Biomarker Expression in Obstructive Sleep Apnea Patients. J Clin Med 2021, 10. [Google Scholar] [CrossRef]
- Huang, Y.S.; Guilleminault, C.; Hwang, F.M.; Cheng, C.; Lin, C.H.; Li, H.Y.; Lee, L.A. Inflammatory cytokines in pediatric obstructive sleep apnea. Medicine (Baltimore) 2016, 95, e4944. [Google Scholar] [CrossRef]
- Zaffanello, M.; Franchini, M.; Piacentini, G. Pediatric Sleep-Disordered Breathing and Long-Term Complications: Clinical and Health Implications. In J Clin Med; Switzerland, 2022; Volume 11.
- Pedrotti, E.; Demasi, C.L.; Fasolo, A.; Bonacci, E.; Brighenti, T.; Gennaro, N.; Ferrari, M.; Marchini, G. Obstructive Sleep Apnea Assessed by Overnight Polysomnography in Patients With Keratoconus. Cornea 2018, 37, 470–473. [Google Scholar] [CrossRef]
- Liu, P.K.; Chiu, T.Y.; Wang, N.K.; Levi, S.R.; Tsai, M.J. Ocular Complications of Obstructive Sleep Apnea. J Clin Med 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Bonacci, E.; Fasolo, A.; Zaffanello, M.; Merz, T.; Brocoli, G.; Pietrobelli, A.; Clemente, M.; De Gregorio, A.; Longo, R.; Bosello, F.; et al. Early corneal and optic nerve changes in a paediatric population affected by obstructive sleep apnea syndrome. Int Ophthalmol 2022, 42, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Cinici, E.; Tatar, A. Thickness alterations of retinal nerve fiber layer in children with sleep-disordered breathing due to adenotonsillar hypertrophy. Int J Pediatr Otorhinolaryngol 2015, 79, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Simsek, A.; Bayraktar, C.; Dogan, S.; Uckardes, F.; Reyhan, A.H.; Sarikaya, Y.; Karatas, M.; Capkin, M. Retinal Nerve Fiber Layer Thickness Alteration in Apneic Children. Optom Vis Sci 2016, 93, 63–69. [Google Scholar] [CrossRef]
- Bayraktar, C.; Şimşek, A. Evaluation of choroidal thickness measurements in pediatric obstructive sleep apnea syndrome patients. Turk J Pediatr 2017, 59, 62–67. [Google Scholar] [CrossRef]
- Ye, H.; Zheng, C.; Lan, X.; Zhao, L.; Qiao, T.; Li, X.; Zhang, Y. Evaluation of retinal vasculature before and after treatment of children with obstructive sleep apnea-hypopnea syndrome by optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol 2019, 257, 543–548. [Google Scholar] [CrossRef]
- Ye, H.; Jin, C.; Li, X.; Zhao, L.; Li, Y.; Qiao, T. OCT-Angiography Comparison between Obstructive Sleep Apnea Children and Normal Subjects in China. Curr Eye Res 2021, 46, 355–360. [Google Scholar] [CrossRef]
- Wong, B.; Fraser, C.L. Obstructive Sleep Apnea in Neuro-Ophthalmology. J Neuroophthalmol 2019, 39, 370–379. [Google Scholar] [CrossRef]
- Farahvash, A.; Micieli, J.A. Neuro-Ophthalmological Manifestations of Obstructive Sleep Apnea: Current Perspectives. Eye Brain 2020, 12, 61–71. [Google Scholar] [CrossRef]
- Huon, L.K.; Liu, S.Y.; Camacho, M.; Guilleminault, C. The association between ophthalmologic diseases and obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath 2016, 20, 1145–1154. [Google Scholar] [CrossRef]
- Xiao, Y.; Shi, K.; Li, C.; Yang, K.; Zhu, X.; Su, B.; Ju, Y.; Lu, F.; Qu, J.; Li, M.; et al. Association of self-reported snoring with decreased retinal thickness and vessel density. Front Physiol 2022, 13, 917808. [Google Scholar] [CrossRef]
- Sun, C.L.; Zhou, L.X.; Dang, Y.; Huo, Y.P.; Shi, L.; Chang, Y.J. Decreased retinal nerve fiber layer thickness in patients with obstructive sleep apnea syndrome: A meta-analysis. Medicine (Baltimore) 2016, 95, e4499. [Google Scholar] [CrossRef]
- Zhao, X.J.; Yang, C.C.; Zhang, J.C.; Zheng, H.; Liu, P.P.; Li, Q. Obstructive Sleep Apnea and Retinal Nerve Fiber Layer Thickness: A Meta-analysis. J Glaucoma 2016, 25, e413–418. [Google Scholar] [CrossRef]
- Ahn, J.; Gorin, M.B. The Associations of Obstructive Sleep Apnea and Eye Disorders: Potential Insights into Pathogenesis and Treatment. Current Sleep Medicine Reports 2021, 7, 65–79. [Google Scholar] [CrossRef]
- Al Saeed, A.A.; AlShabib, N.S.; Al Taisan, A.A.; Kreary, Y.A. Association of Retinal Vascular Manifestation and Obstructive Sleep Apnea (OSA): A Narrative Review. Clin Ophthalmol 2021, 15, 3315–3320. [Google Scholar] [CrossRef]
- Fraser, C.L. Update on obstructive sleep apnea for neuro-ophthalmology. Curr Opin Neurol 2019, 32, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; He, X.T.T.; Kang, S.; Haqq, A.M.; MacLean, J.E. Preserved Sleep for the Same Level of Respiratory Disturbance in Children with Prader-Willi Syndrome. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).