Submitted:
25 September 2023
Posted:
27 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. The bioreactor for obtaining a Ca. Accumulibacter-enriched culture
2.2. Determination of the oxygen uptake rates
2.3. Determination of dynamics of substrate and phosphate in batch experiments
2.4. Molecular techniques
2.5. Analytical techniques
2.6. Statistical data processing
3. Results
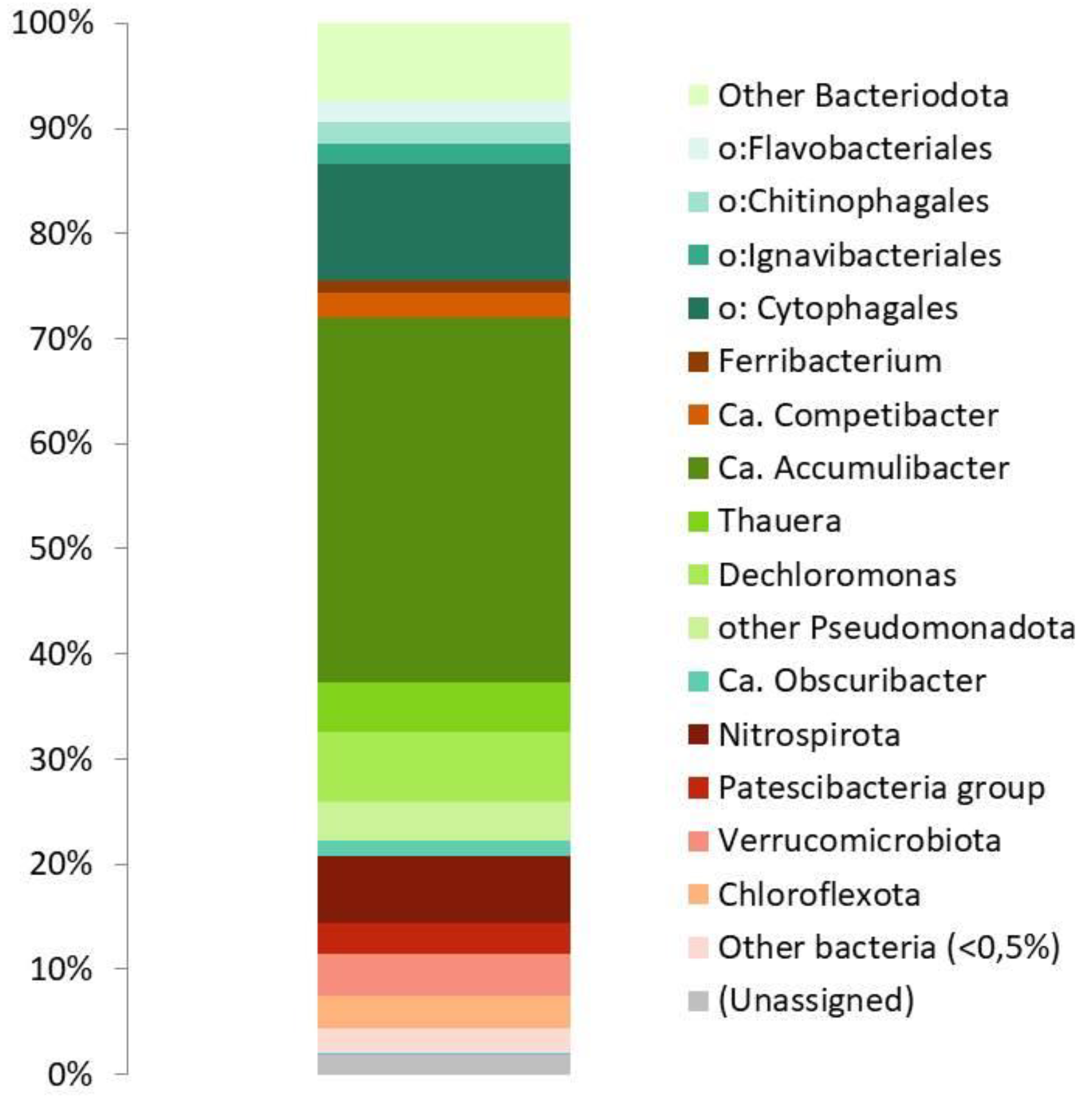
3.1. Bioreactor operation and microbial community composition
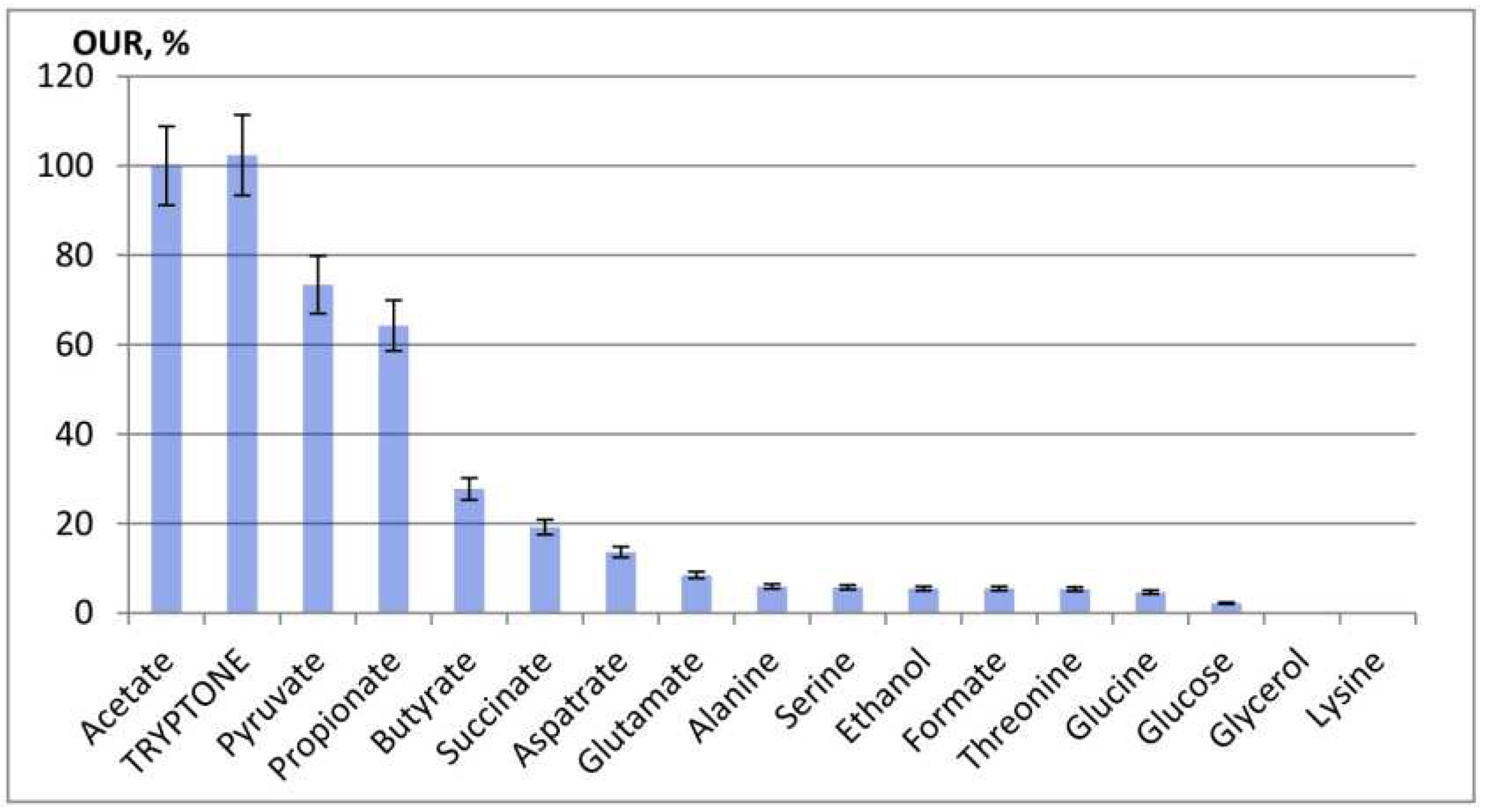
3.2. Oxygen consumption
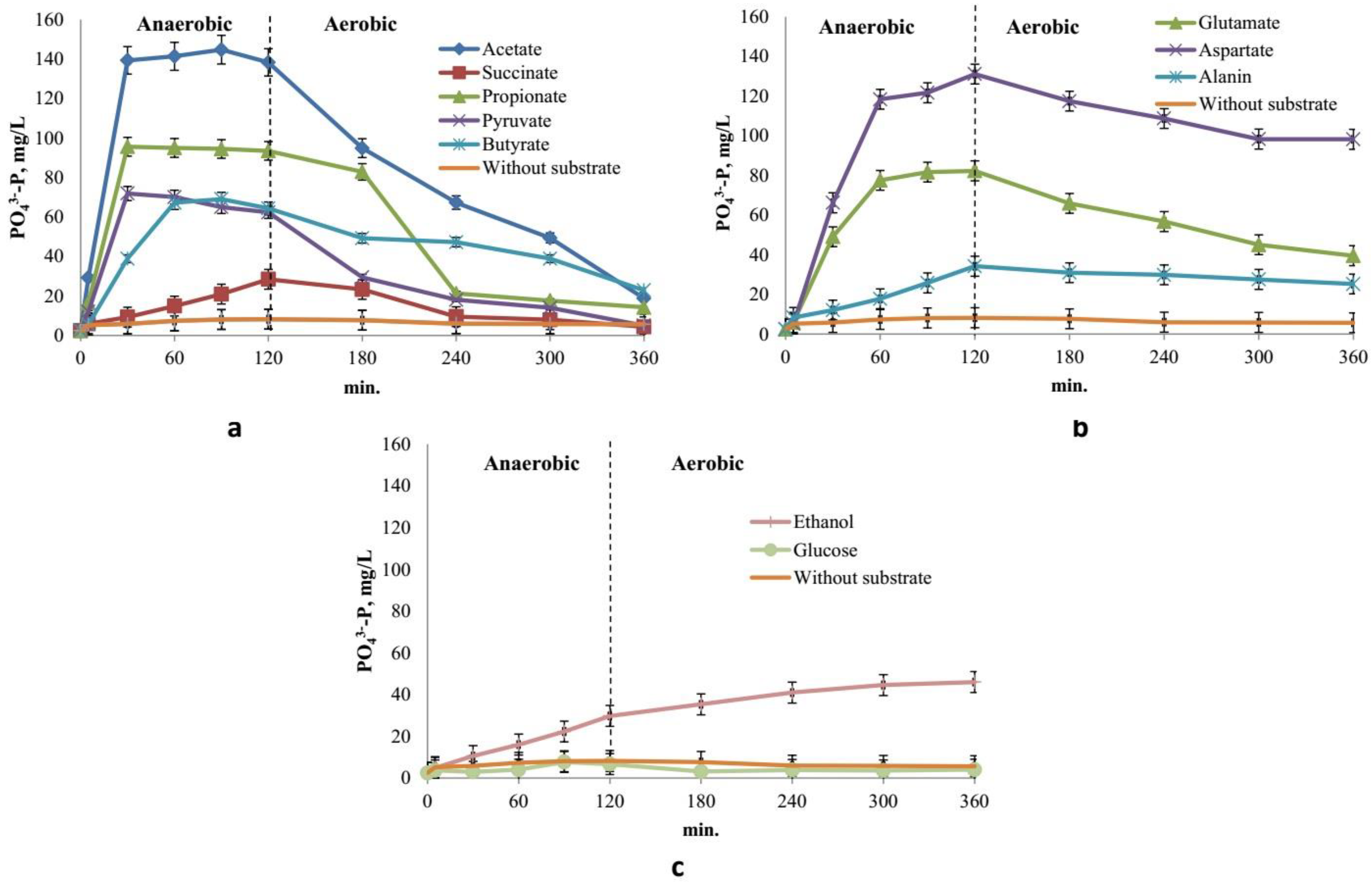
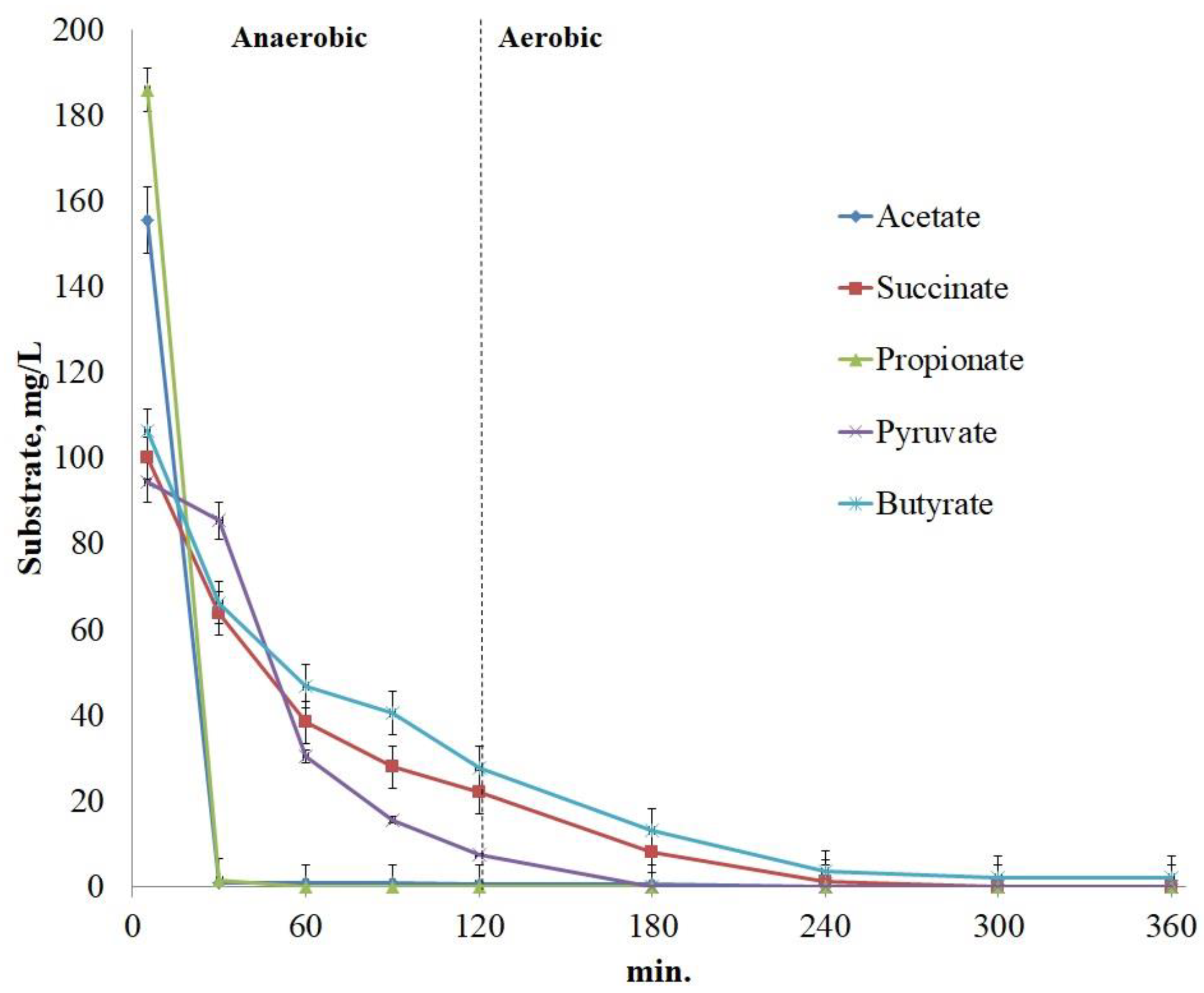
3.3. Cycle of phosphate release/uptake under anaerobic/aerobic conditions
3.4. Abilities of Ca. Accumulibacter transport systems
4. Discussion
4.1. Response of Ca. Accumulibacter to the substrates
4.1.1. Volatile fatty acids
4.1.2. Pyruvate
4.1.3. Succinate
4.1.4. Amino acids
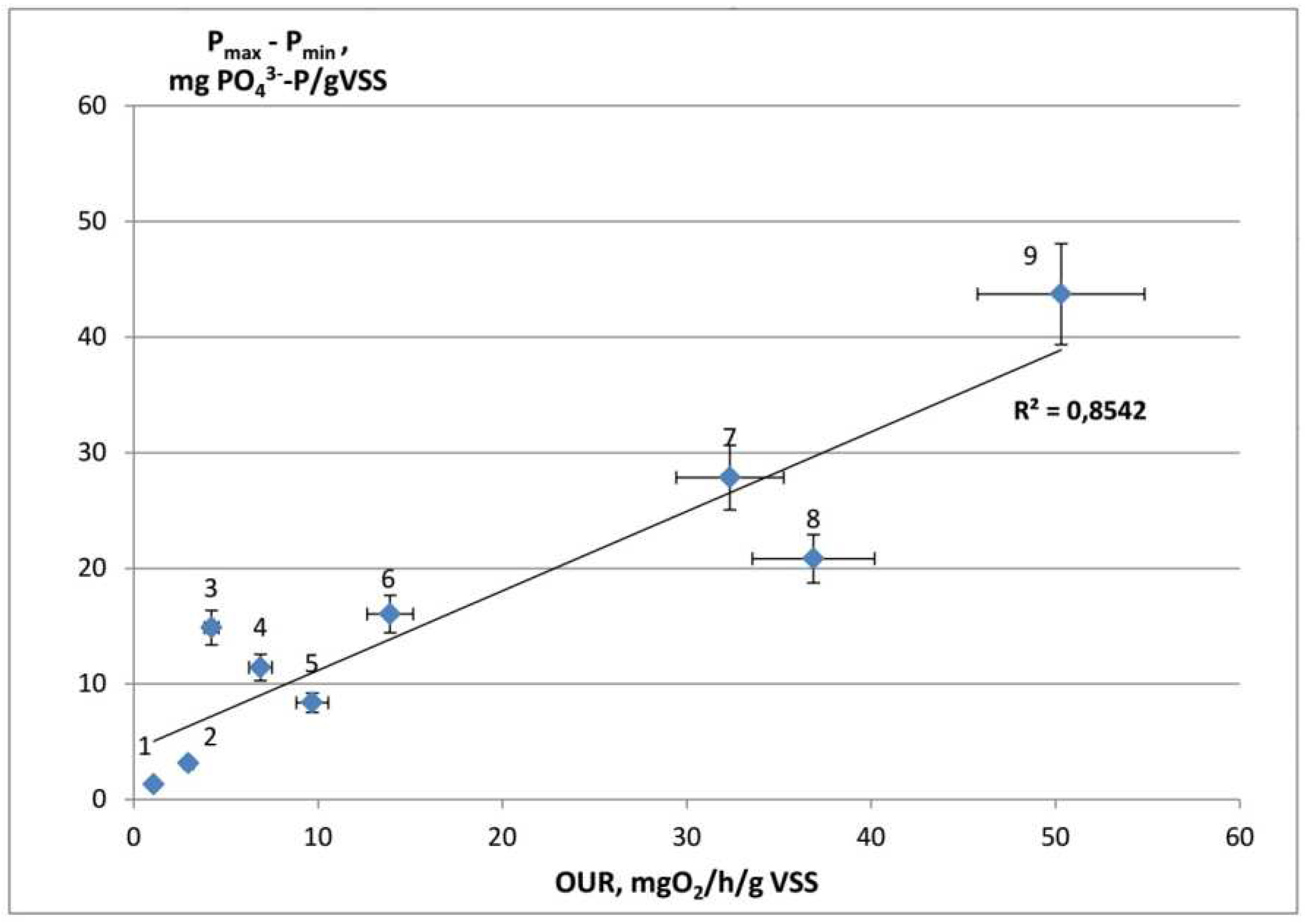
4.2. Correlation between OUR and phosphate uptake during the anaerobic stage
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wentzel, M.C.; Loewenthal, R.E., Ekama, G.A.; Marais, G.V.R. Enhanced polyphosphate organism cultures in activated sludge systems – Part 1: Enhanced culture development. Water S.A. 1988, 14, 81–92.
- Schuler, A.J.; Jenkins, D. Enhanced biological phosphorus removal from wastewater by biomass with different phosphorus contents, part1: Experimental Results and Comparison with Metabolic Models. Water Environ. Res. 2003, 75, 485–498. [CrossRef]
- Welles, L.; Abbas, B.; Sorokin, D.Y.; Lopez-Vazquez C.M.; Hooijmans C.M, van Loosdrecht M.C.M.; Brdjanovic D. Metabolic response of 'Candidatus Accumulibacter phosphatis' clade II to changes in the influent P/C ratio. Front. Microbiol. 2017, 7, 2121. https://doi.org/10.3389/fmicb.2016.02121. [CrossRef]
- Oehmen, A.; Lemos, P.C.; Carvalho, G.; Yuan, Z.; Keller, J.; Blackall, L.L.; Reis, M.A.M. Advances in enhanced biological phosphorus removal: from micro to macro scale. Water Res. 2007, 41, 2271–2300. [CrossRef]
- Dorofeev, A.G.; Nikolaev, Y.A.; Mardanov, A.V.; Pimenov, N.V. Role of phosphate-accumulating bacteria in biological phosphorus removal from wastewater. Appl. Biochem. Microbiol. 2020, 56, 3-18. https://doi.org/10.1134/S0003683820010056. [CrossRef]
- Zhao, W.; Bi, X.; Peng, Y.; Bai, M. Research advances of the phosphorus-accumulating organisms of Candidatus Accumulibacter, Dechloromonas and Tetrasphaera: Metabolic mechanisms, applications and influencing factors. Chemosphere 2022, 307 Part 1, 135675. https://doi.org/10.1016/j.chemosphere.2022.135675. [CrossRef]
- Paez-Watson, T.; van Loosdrecht, M.C.M.; Wahl, S.A. Predicting the impact of temperature on metabolic fluxes using resource allocation modelling: Application to polyphosphate accumulating organisms. Water Res. 2023, 228, 119365. https://doi.org/10.1016/j.watres.2022.119365. [CrossRef]
- Dorofeev, A.G.; Nikolaev, Y.A.; Mardanov, A.V.; Pimenov, N.V. Cyclic metabolism as a mode of microbial existence. Microbiology 2019, 88, 402-415. https://doi.org/10.1134/S0026261719040052. [CrossRef]
- Marques, R.; Santos, J.; Nguyen, H.; Carvalho, G.; Noronha, J.P.; Nielsen, P.H.; Reis, M.A. M.; Oehmen, A. Metabolism and ecological niche of Tetrasphaera and Ca. Accumulibacter in enhanced biological phosphorus removal. Water Res. 2017, 122, 159–171. https://doi.org/10.1016/j.watres.2017.04.072. [CrossRef]
- Brand, V.R.; Crosby, L.D.; Criddle, C.S. Niche differentiation among three closely related Competibacteraceae clades at a fullscale activated sludge wastewater treatment plant and putative linkages to process performance. Appl. Environ. Microbiol. 2019, 85, e02301-18. https://doi.org/10.1128/AEM.02301-18. [CrossRef]
- Kong, Y.; Nielsen, J.L.; Nielsen, P.H. Microautoradiographic study of Rhodocyclus-related polyphosphate-accumulating bacteria in full-scale enhanced biological phosphorus removal plants. Appl. Environ. Microbiol. 2004, 70, 5383-5390. [CrossRef]
- Levantesi, C.; Serafim, L.S.; Crocetti, G.R.; Lemos, P.C.; Rossetti, S.; Blackall, L.L.; Reis, M.A.M.; Tandoi, V. Analysis of the microbial community structure and function of a laboratory scale enhanced biological phosphorus removal reactor. Environ. Microbiol. 2002, 4, 559–569. [CrossRef]
- Pijuan, M.; Baeza, J.A.; Casas, C.; Lafuente J. Response of an EBPR population developed in an SBR with propionate to different carbon sources. Water Sci. Technol. 2004, 50, 131–138. [CrossRef]
- Begum, S.A.; Batista, J.R. Impact of butyrate on microbial selection in enhanced biological phosphorus removal systems. Environ. Technol. 2014, 35, 2961–2972. [CrossRef]
- Wang, L.; Liu, J.; Oehmen, A.; Le, C.; Geng, Y.; Zhou Y. Butyrate can support PAOs but not GAOs in tropical climates. Water Res. 2021, 193, 116884. https://doi.org/10.1016/j.watres.2021.116884. [CrossRef]
- Tian. Y.; Chen, H.; Chen, L.; Deng, X.; Hu, Z.; Wang, C.; Wei, C.; Qiu, G.; Wuertz, S. Glycine adversely affects enhanced biological phosphorus removal. Water Res. 2022, 209, 117894. https://doi.org/10.1016/j.watres.2021.117894. [CrossRef]
- Nguyen, H.T.; Kristiansen, R.; Vestergaard, M.; Wimmer, R.; Nielsen, P.H. Intracellular accumulation of glycine in polyphosphate-accumulating organisms in activated sludge, a novel storage mechanism under dynamic anaerobic-aerobic conditions. Appl. Environ. Microbiol. 2015, 81, 4809–4818. [CrossRef]
- Oyserman, B.O.; Noguera, D.R.; del Rio, T.G.; Tringe. S.G.; McMahon, K.D. Metatranscriptomic insights on gene expression and regulatory controls in Candidatus Accumulibacter phosphatis. ISME J. 2016, 10, 810–822. [CrossRef]
- Qiu, G.; Liu, X.; Saw, N.; Law, Y.; Zuniga-Montanez, R.; Thi, S.S.; Nguyen, T.Q.N.; Nielsen, P.H.; Williams, R.B.H.; Wuertz, S. Metabolic traits of Candidatus Accumulibacter clade IIF strain SCELSE-1 using amino acids as carbon sources for enhanced biological phosphorus removal. Environ. Sci. Technol. 2020, 54, 2448–2458. https://doi.org/10.1021/acs.est.9b02901. [CrossRef]
- Ziliani, A.; Bovio-Winkler, P.; Cabezas, F.; Etchebehere, C.; Garcia, H.A.; López-Vázquez, C.M.; Brdjanovic, D.; van Loosdrecht, M.C.M.; Rubio-Rincón, F.J. Putative metabolism of Ca. Accumulibacter via the utilization of glucose. Water Res. 2023, 229, 119446. https://doi.org/10.1016/j.watres.2022.119446. [CrossRef]
- McMahon, K.D.; He, S.; Oehmen, A. The microbiology of phosphorus removal. In Microbial Ecology of Activated Sludge; Seviour, R., Nielsen, P.H., Eds.; IWA, London, 2010; pp. 281–319.
- Smolders, G.J.; van der Meij, J.; van Loosdrecht, M.C.M.; Heijnen, J.J. Stoichiometric model of the aerobic metabolism of the biological phosphorus removal process. Biotechnol. Bioeng. 1994, 44, 837–848. [CrossRef]
- Welles, L.; Lopez-Vazquez, C.M.; Hooijmans, C.M.; van Loosdrecht, M. C. M.; Brdjanovic, D. Impact of salinity on the aerobic metabolism of phosphate-accumulating organisms. Appl. Microbiol. Biotechnol. 2015, 99, 3659–3672. [CrossRef]
- Pelevina, A.V.; Berestovskaya, Y.Y.; Grachev, V.A.; Dorofeeva, I.K.; Sorokin, V.V.; Dorofeev, A.G.; Kallistova, A.Yu.; Nikolaev, Yu.A.; Kotlyarov, R.Yu.; Beletskii, A.V.; Ravin, N.V.; Pimenov, N.V.; Mardanov, A.V. A Microbial Consortium Removing Phosphates under Conditions of Cyclic Aerobic-Anaerobic Cultivation. Microbiology 2021, 90, 66–77. https://doi.org/10.1134/S0026261721010082. [CrossRef]
- Pelevina, A.V.; Grouzdev, E.V.; Berestovskaya, Yu.Yu.; Dorofeev, A.G.; Nikolaev, Yu.A.; Kallistova, A.Yu.; Beletsky, A.V.; Ravin, N.V.; Pimenov, N.V.; Mardanov, A.V. New insight into the granule formation in the reactor for enhanced biological phosphorus removal. Front. Microbiol. 2023, in press.
- Brdjanovic, D.; van Loosdrecht, M.C.M.; Hooijmans, C.M.; Alaerts, G.J.; Heijnen, J.J. Temperature effects on physiology of biological phosphorus removal. J. Environ. Eng. 1997, 123, 144–153. [CrossRef]
- Schuler, A.J. Jenkins, D. Enhanced biological phosphorus removal from wastewater by biomass with different phosphorus contents, part II: anaerobic adenosine triphosphate utilization and acetate uptake rates. Water Environ. Res. 2003, 75, 512–522. [CrossRef]
- Bassin, J.P.; Winkler, M.-K.H.; Kleerebezem, R.; Dezotti, M.; van Loosdrecht, M.C.M. Improved phosphate removal by selective sludge discharge in aerobic granular sludge reactors. Biotechnol. Bioeng. 2012, 109, 1919–1928. [CrossRef]
- Acevedo. B.; Murgui, M.; Borr´as, L.; Barat, R. New insights in the metabolic behaviour of PAO under negligible poly-P reserves. Chem. Eng. J. 2017, 311, 82–90. [CrossRef]
- Smolders, G.J.F.; van der Meij, J.; van Loosdrecht, M.C.M.; Heijnen, J.J. Model of the Anaerobic Metabolism of the Biological Phosphorus Removal Process: Stoichiometry and pH Influence. Biotechnol. Bioeng. 1994, 43, 461-470. [CrossRef]
- Liu, W.-T.; Mino, T.; Matsuo, T.; Nakamura, K. Biological phosphorus removal processes - Effect of pH on anaerobic substrate metabolism. Water Sci. Technol. 1996, 34, 25-32. [CrossRef]
- Ren, T.; Chi, Y.; Wang, Y.; Shi, X.; Jin, X.; Jin, P. Diversified metabolism makes novel Thauera strain highly competitive in low carbon wastewater treatment. Water Res. 2021, 206, 117742. https://doi.org/10.1016/j.watres.2021.117742. [CrossRef]
- Oehmen, A.; Zeng, R.J.; Yuan, Z.; Keller, J. Anaerobic metabolism of propionate by polyphosphate-accumulating organisms in enhanced biological phosphorus removal systems. Biotechnol. Bioeng. 2005, 1, 43-53. [CrossRef]
- L´opez-V´azquez, C.M.; Hooijmans, C.M.; Brdjanovic, D.; Gijzen, H.J.; van Loosdrecht, M. C.M. A practical method for quantification of phosphorus- and glycogen accumulating organism populations in activated sludge systems. Water Environ. Res. 2007, 79, 2487–2498. [CrossRef]
- 35. Diaz, R.; Mackey, B.; Chadalavada, S.; Kainthola, J.; Heck. P.; Goel, R. Enhanced Bio-P removal: Past, present, and future – A comprehensive review. Chemosphere 2022, 309, 136518. https://doi.org/10.1016/j.chemosphere.2022.136518. [CrossRef]
- Ribas, D.; Soares-Silva, I.; Vieira, D.; Sousa-Silva, M.; Sá-Pessoa, J.; Azevedo-Silva, J.; Viegas, S.C.; Arraiano, C.M.; Diallinas, G.; Paiva, S.; Soares, P.; Casal, M. The acetate uptake transporter family motif “NPAPLGL (M/S)” is essential for substrate uptake. Fungal Genet. Biol. 2019, 122, 1-10. [CrossRef]
- Gimenez, R.; Nuñez, M.F.; Badia, J.; Aguilar, J.; Baldoma, L. The gene yjcG, cotranscribed with the gene acs, encodes an acetate permease in Escherichia coli. J. Bacteriol. 2003, 185, 6448-6455. [CrossRef]
- Jolkver, E.; Emer, D.; Ballan, S.; Krämer, R.; Eikmanns, B.J.; Marin, K. Identification and characterization of a bacterial transport system for the uptake of pyruvate, propionate, and acetate in Corynebacterium glutamicum. J. Bacteriol. 2009, 191, 940-948.
- Barr J.J.; Dutilh, B.E.; Skennerton, C.T.; Fukushima, T.; Hastie M.L.; Gorman, J.J.; Tyson, G.W.; Bond, P.L. Metagenomic and metaproteomic analyses of Accumulibacter phosphatis-enriched floccular and granular biofilm. Environ. Microbiol. 2016, 18, 273-287. [CrossRef]
- Liu, X.-W.; Wang, H.-H.; Chen, J.-Y.; Li, X.-T.; Chen, G.-Q. Biosynthesis of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) by recombinant Escherichia coli harboring propionyl-CoA synthase gene (prpE) or propionate permease gene (prpP). Biochem. Eng. J. 2009, 43, 72-77. [CrossRef]
- Arun, V.; Mino, T.; Matsuo, T. Metabolism of carboxylic acids located in and around the glycolytic pathway and the TCA cycle in the biological phosphorus removal process. In Proceedings of the Fourteenth Biennial Conference of the International Association on Water Pollution Research and Control, Brighton, U.K., 18–21 July, 1988. https://doi.org/10.1016/B978-1-4832-8439-2.50038-9. [CrossRef]
- Satoh, H.; Ramey, W.D.; Koch F.A.; Oldham W.K.; Mino, T.; Matsuo T. Anaerobic substrate uptake by the enhanced biological phosphorus removal activated sludge treating real sewage. Water Sci. Technol. 1996, 34, 9-16. [CrossRef]
- Skory, C.D.; Hector, R.E.; Gorsich, S.W.; Rich J.O. Analysis of a functional lactate permease in the fungus Rhizopus. Enzyme Microb. Technol. 2010, 46, 43-50. [CrossRef]
- Song, H.; Lee, S.Y. Production of succinic acid by bacterial fermentation. Enzyme Microbial Technol. 2006, 39, 352–361. [CrossRef]
- Wainaina, S.; Lukitawesa; Awasthi, K.M.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437-458. https://doi.org/10.1080/21655979.2019.1673937. [CrossRef]
- Putri, D.N.; Sahlan, M.; Montastruc, L.; Meyer, M.; Negny, S.; Hermansyah, H. Progress of fermentation methods for bio-succinic acid production using agro-industrial waste by Actinobacillus succinogenes. Energy Rep. 2020, 6, 234–239. https://doi.org/10.1016/j.egyr.2019.08.050. [CrossRef]
- 47. Hosie, A. H. F.; Poole, P. S. Bacterial ABC transporters of amino acids. Res. microbiol. 2001, 152, 259-270. [CrossRef]
- Wang, S.; Yan, Z.; Wang, P.; Zheng, X.; Fan, J. Comparative metagenomics reveals the microbial diversity and metabolic potentials in the sediments and surrounding seawaters of Qinhuangdao mariculture area. PloS one 2020, 15, e0234128. https://doi.org/10.1371/journal.pone.0234128. [CrossRef]
- Oehmen, A.; Yuan, Z.; Blackall, L.L.; Keller, J. Comparison of acetate and propionate uptake by polyphosphate accumulating organisms and glycogen accumulating organisms. Biotechnol. Bioeng. 2005, 91, 162–168. [CrossRef]
| Substrate | OUR, mg O2/(g TSS h) |
Anaerobic phase | Aerobic phase | ||
|---|---|---|---|---|---|
| Рmax, mg РО43--P/L |
P-release/ substrate- uptake, P-mol/S-mol |
Average P release rate in the first 30 min, mg РО43--P/(L h) |
Рmax - Рmin, mg РО43--P/L |
||
| Acetate | 32,2 | 144,7 | 1,4 | 273,6 | 125,9 |
| Pyruvate | 23,6 | 65,0 | 1,7 | 138,8 | 60 |
| Propionate | 20,7 | 94,5 | 1,6 | 186,2 | 80,2 |
| Butyrate | 8,9 | 69,1 | 2,1 | 72,8 | 46,2 |
| Succinate 1 | 6,2 | 28,5 | 1,3 | 13,7 | 24,1 |
| D,L-Aspartate | 4,4 | 131,1 | 2,0 2 | 127,3 | 32,9 |
| L- Glutamate | 2,7 | 82,3 | 2,1 2 | 93,1 | 42,8 |
| D,L-Alanine | 1,9 | 34,2 | 0,3 2 | 19,2 | 9 |
| D-Glucose | 1,1 | 7,7 | - | - | 3,7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





