1. Introduction
Since the 1950s, veterinary antibiotics (VAs) have been extensively used to prevent and control livestock diseases. Antibiotic use for human and veterinary purposes increased globally by 65% between 2000 and 2015 [
1]. VA production and use in South Korea has steadily increased, with 938 tons sold in 2018 [
2]. VAs have been indiscriminately used in animal feed for the past five decades to increase and ensure profits with negligible environmental considerations [
3]. These VAs, usually residues remaining in animal manure, can contaminate soil and water bodies when the manure is used as fertilizer [
4,
5,
6,
7]. Plants growing in these contaminated soils absorb the VA residues, disturbing both enzymatic and non-enzymatic antioxidant systems, affecting the PSII pigment system, and ultimately lowering the plant's productivity [
3,
8,
9].
Even at low concentrations, irrigation water contaminated with VAs has been reported to cause adverse effects on plants and humans through bioconcentration [
10,
11]. Seo et al. [
12] identified eight VAs that pose the highest environmental risks in the Korean Republic. Among these, three antibiotics, amoxicillin (AMX, 0.1 mg L
-1), chlortetracycline (CTC, 0.3 mg L
-1), and oxytetracycline (OTC, 0.05 mg L
-1), have been found to be persistent in soil, with frequent detection in aquatic systems and high absorption by plants.
Recently, several studies have evaluated the effects of VAs on plant physiology and the soil environment [
6,
7,
13,
14]. However, all of these studies were conducted in hydroponic or greenhouse environments which did not reflect plant uptake and accumulation of antibiotics under open field conditions. Therefore, this study aimed to assess the absorption-translocation by rice plants of AMX, CTC, and OTC unintentionally introduced with irrigation water during cultivation and the impact of these VAs on plant growth and crop yield.
2. Materials and methods
2.1. Experimental Site
The experiments were conducted from June 2021 to November 2021 in the Jangsu-gun district, Jeollabuk-do Province, South Korea (35° 42' 46.26"N, 127° 31' 12.216"E) (
Figure 1).
2.2. Experimental Design, Agricultural Activity, and Sample Collection
The background levels of VAs in previous studies detected in the water ecosystem in South Korea were as follows: AMX: 0.1 mg L
-1, CTC: 0.3 mg L
-1, and OTC: 0.05 mg L
-1 [
12,
13,
14].
The different combinations for each treatment are detailed in
Table 1. In brief, three different doses of VAs were used in this study: background level, ten-fold background level, and fifty-fold background level. The experiments were performed in a randomized complete block design with three replicates. The three VAs used in the experiment were obtained from Sigma Aldrich (Seoul, Korea; 99%).
The VAs was dissolved in a watering can and uniformly watered into each plot. The plot size was 4 m in length and 2.5 m in width. Rice cultivation was carried out in all of the experimental plots under the same conditions as in the rest of the field. The
Japonica cultivar
‘Shindongjin’ was chosen for this experiment. Organic farmyard manure was not used to avoid unexpected antibiotic contamination. Spring plowing was conducted April 30. The experimental paddy plots received basal fertilization and were plowed May 25 and maintained under flooded conditions. Approximately four rice seedlings (35 days old) per hill were transplanted by hand on June 1. Additional fertilizers were applied June 20 (maximum tilling stage), July 25 (panicle formation stage), and August 10 (booting stage). Inorganic fertilizers (N–P
2O
5–K
2O = 90–45–57 kg ha
−1) were applied equally to all plots according to the guidelines of the Rural Development Administration (RDA) [
15]. The water level in the experimental plots was maintained at 5-10 cm until 25 days after transplantation. The experimental plots were then drained of all water for 3 days at the late tilling stage, and the water level was maintained thereafter at 2 to 3 cm until 10 days prior to harvest. The rice plants were harvested on October 15. Borders were constructed between plots to prevent the exchange of irrigation water and VAs. Each plot had an independent water inlet and outlet. Rice plants were kept healthy via prophylactic sprays of recommended insecticides and fungicides as necessary. The fields were kept weed-free by hand weeding conducted at 2-week intervals [
16].
About 500 g of soil and two hills of rice plant samples (including underground and upper ground parts) were collected at 15, 30, 45, 60, 75, 90, and 105 days after transplant (DAT). The samples were collected from five random sites per experimental plot. Soil samples were taken from a depth of 0-10 cm. All samples were lyophilized and stored at -80°C until further analysis. Brown rice samples were collected at harvesting and subsequently lyophilized and stored at -80°C after threshing and removing the outer hulls.
After air-drying soil samples in the shade at room temperature for a week, they were ground by pestle and sieved into 2-mm particles. About 5 g of sample was poured into a glass funnel (1,000 mL volume) joined to a vacuum filter flask (pore filter size: 11 μm). Then, 50 mL acetone (Sigma Aldrich; 98%) was poured over the soil sample. The VAs in liquid solution were extracted and poured into a 200-mL round-bottom flask and solvent was removed on a rotary evaporator. Subsequently, 0.2 g disodium ethylene diamine tetraacetate (Na2EDTA, ≥98.5%; Sigma Aldrich) was added to the residue and diluted with 200 mL of Milli-Q water (Sigma Aldrich; 99.99%). Subsequently, the mixture was treated with an ultrasonic sonicator (Ultrasonic cleaner; HES-SON Ultrasonic Co., Seoul, Korea). The liquid, after passage through a 0.45-μm pore size syringe filter (Advantec Toyo Kaisha Ltd., Tokyo, Japan) for purification, was poured into Falcon tubes. Finally, 100 µL of the liquid mixture was withdrawn and stored in 100-µL amber glass bottles for further analysis.
Plant samples (including roots, stems, and brown rice) were thawed and dried in an oven (Jung Do Science Co., Jeollabuk, Korea) at 60°C. The dried plant tissues were ground into powder. About 2 g of each sample was dispersed twice with 40 mL acetone (Sigma Aldrich; 98%) by vortex mixing for 60 s. The mixture was then treated with an ultrasonic sonicator for 15 min, followed by centrifugation (Benchtop Centrifuge VS-4000N; Global Science Industry, Gangwon, Korea) under air-cooling at 12,000 rpm for 5 min. The combined extracts were pipetted into a 200 mL round-bottom flask and the solvent was removed by rotary-evaporation. About 0.2 g of ≥98.5% Na2EDTA was added, and the mixture was diluted with 200 mL Milli-Q water (Sigma Aldrich; 99.99%). The solution was briefly ultrasonicated and then poured through syringe filters (size: 0.4 μm) into falcon tubes. Finally, 100 µL of the liquid mixture was withdrawn and stored in 100 µL amber glass bottles for further analysis.
2.3. Liquid Chromatography-mass Spectrometry (LC-MS) Analysis
The concentration of VAs in both soil and plants was determined using an LC-MS system with an HPLC system (1200 HPLC; Agilent Technologies, Santa Clara, CA, USA), attached to a detector (1260 Infinity, variable wavelength detector; Agilent), a pump (G1312B binary pump; Agilent), and a column compartment (1200 series thermostatted column compartment G1316B; Agilent) for separation. The mobile phase gradient consisted of phase A comprising 0.1% formic acid in distilled water, and phase B comprising 0.1% formic acid (Sigma Aldrich; 98%) in acetonitrile on a Kinetex column (2.6 µm C18. 50 x 2.1 mm; Phenomenex, Torrance, CA, USA) with a flow rate of 0.5 mL min
-1. The gradient program was configured as follows: 0 min, A: B = 95: 5; 1 minute, A: B = 95: 5, 12 minutes, A: B = 0: 100, 14 minutes, A: B = 95: 5, and 20 minutes, A: B = 95:5. Mass spectrometric analysis was performed in the positive electron ionization mode, with the mobile phase flow into the chamber set at 11 mL min
-1 and the source temperature kept at 300°C. The detector signal for amino acids was recorded from 0-21 min, and the ions were scanned from 190 to 400 m/z. The concentration of VAs in different plant organs and soil was obtained from LC-MS analysis and then used to calculate the bio-concentration factor (BCF) of the individual antibiotics according to Zayed et al. [
17].
2.4. Chlorophyll Content and Grain Yield
Approximately 0.25 g of fresh leaves were weighed and placed in a test tube containing 25 mL of 80% acetone (Sigma Aldrich; 98%). The tubes were left in a dark room for 48 hours at room temperature.
The optical density of the chlorophyll was measured at 645 nm and 663 nm with a UV spectrophotometer (OPTIZEN 2120UV; Mecasys Co., Ltd., Daejeon, Korea). Chlorophyll content in plants was calculated using the formulas of Arnon [
18].
The chlorophyll content was expressed as mg g-1 of fresh leaves.
The rice yield was studied based on the standards of the RDA [
19]. Rice was collected in the center of each experimental plot in an area of 3.3 m
2. Approximately 80 panicles in each plot were cut and stored separately in a laboratory. The harvested rice was dried at room temperature for 2-3 days and then threshed. The seeds were re-dried in an oven at 40°C until they reached a moisture content of about 14%. Grain yield was determined by weighing the harvested rice (tons ha
-1).
2.5. Statistical Analysis
All calculations, including standard deviation analysis, were completed in Excel (Microsoft, Redmond, WA, USA). Data were analyzed by analyzing variance (ANOVA) and Tukey's test of significance at p < 0.05 to determine the differences between VA treatments. Most statistical analyses and graphical illustrations were performed using GraphPad Prism 20.0 (GraphPad, La Jolla, CA, USA). Duncan's Multiple Range Test at 95% confidence intervals separated mean differences in rice yield using MSTAT-C software (Michigan State University, East Lansing, MI, USA).
3. Results and Discussion
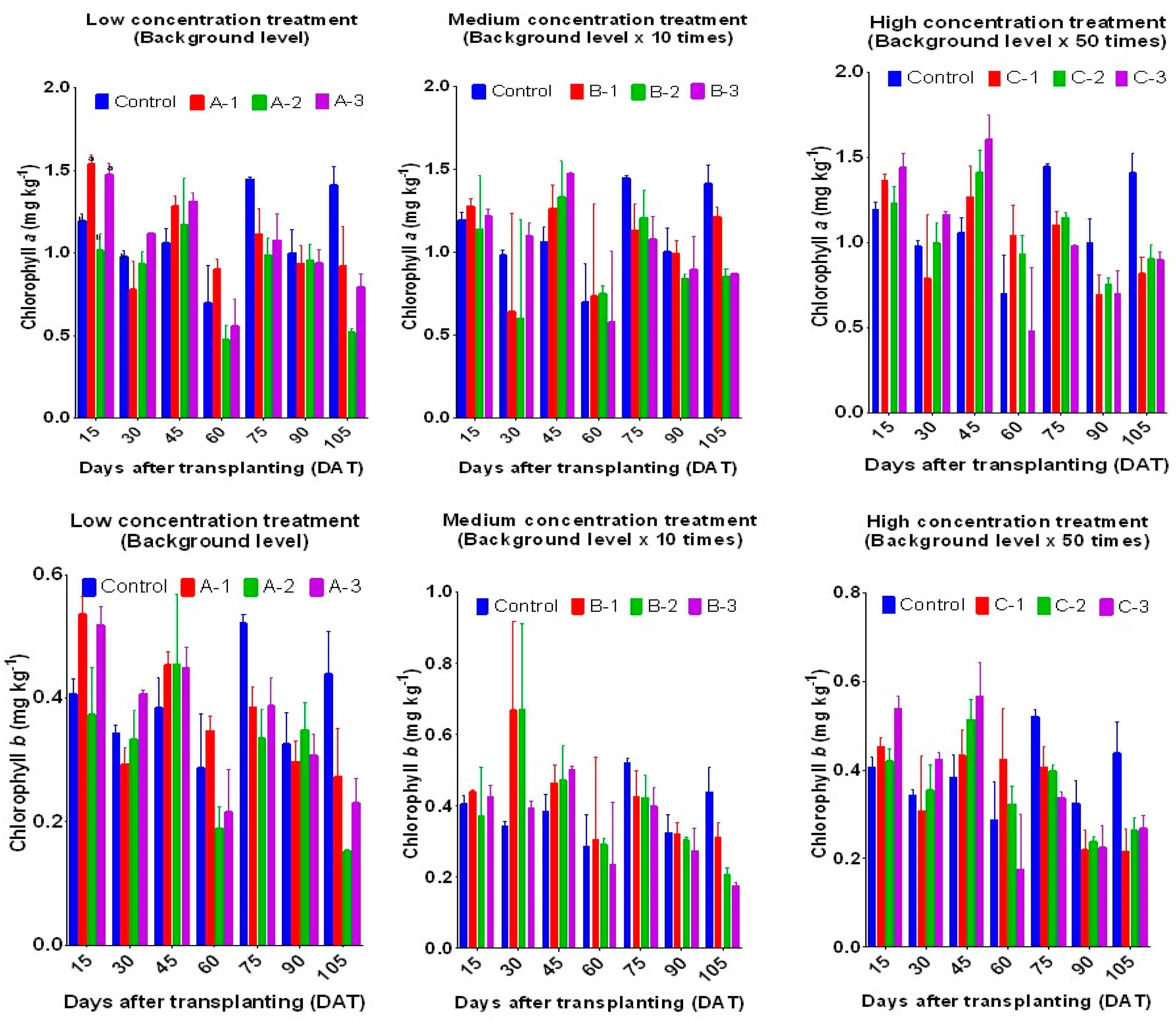
3.1. Effects of Veterinary Antibiotics in Paddy Irrigation Water on Plant Chlorophyll Content
The fluctuation in chlorophyll content indicates pollutant-induced plant stress [
20]. Total chlorophyll (total Chl), chlorophyll
a (Chl
a), and chlorophyll
b (Chl
b) were significantly affected by treatment with VAs, as shown in
Figure 2. During the rice plants' early growth stage, the chlorophyll content was higher in the VA-treated groups compared to the control (P<0.05). During the late growth stages, total Chl content decreased by 10-20% and 35-45% in the ten-fold and fifty-fold background level groups, respectively. These results indicate that exposure to VAs can severely affect the photosynthetic pathway and biomass accumulation.
A decrease in chlorophyll content was reported to cause a reduced rate of photosynthesis, leading to a reduction in crop yield (Liu, Lv, Xu et al. 2018). Our results corroborate previous studies that have evaluated the effects of VAs on plants (Riaz et al. 2017; Tasho et al. 2020). In an earlier report by Liu et al. [
21], the addition of antibiotics had a discernible impact on the levels of total Chl, Chl
a, Chl
b, and the Chl
a/Chl
b ratio in
Phragmites australis. Antibiotics at concentrations greater than 100 μg L
-1 were found to disrupt the normal chlorophyll content of
Phragmites australis, while antibiotic concentrations between 0.1 and 1 μg L
-1 were found to enhance chlorophyll content. In addition, the Chl
a/Chl
b ratio in plants increased with higher concentrations of antibiotics [
21]. VAs at low concentrations promote the synthesis of nucleic acids and intracellular proteins, while at high concentrations, VAs interfere with photosynthetic machinery, including the pigment system and respiratory proteins [
22].
3.2. Soil Residues of Veterinary Antibiotics Introduced into Irrigation Water
AMX residues were not detected in soil when the rice paddy field was exposed to the background level and ten-fold background level; however, at the fifty-fold background level, AMX residues were detected with concentrations ranging from 2.74 ± 0.90 to 6.07 ± 1.88 μg kg
-1 (
Table 6). The residual concentration of AMX in soil was not found to be affected by treatment frequency. CTC and OTC residues were found in the soil at all monitoring intervals and all three treatment levels, with levels ranging from 0.34 ± 0.11 to 1.47 ± 0.09 μg kg
-1 and 0.28 ± 0.09 to 1.14 ± 0.09 μg kg
-1, respectively (
Table 2). Moreover, the residual concentrations of CTC and OTC were not affected by the number of treatments.
According to Braschi et al. [
23], hydrolysis is one of the main degradation mechanisms for VAs, including the tetracycline class (e.g., CTC and OCT) and the β-lactam class (e.g., AMX). In soil, half-life values of AMX ranged from 0.43 to 0.57 days, while those for CTC were 34 days [
24]. OTC is found in soil with a half-life of 30 – 40 days [
25,
26]. VAs in the soil-water system are highly dependent on antibiotic half-life, which was evident from the results depicting the absence of AMX at low and medium concentrations. The prolonged persistence of AMX in the soil at fifty-fold background level may be due to the inhibition of soil microbial activity, which results in reduced degradation. This is a concentration-dependent phenomenon of VAs that has also been reported in previous studies [
27]. These residual concentrations of VAs in soil have been reported to inhibit microbial growth in soil, affect soil microbial community composition and potentially change the ecological functions of the soil [
28,
29]. Therefore, the current contamination of VAs in the water source is a critical concern for agro-ecosystems.
3.3. Absorption-Translocation and Bioconcentration of VAs into Rice Plants
Our report found that AMX was evident in stems, roots, and grains (
Table 3). AMX was not detected in rice roots, stems, and brown rice at ten-fold background level. However, it was detected in stems after a six split-dose treatment. At fifty-fold background level, AMX was absorbed and translocated into roots and stems regardless of treatment frequency, except in stems with two split-dose and four split-dose treatments. The detected concentration ranged from 19.2±0.9 to 79.3±0.9 μg kg
-1. Likewise, AMX was not detected in stems and roots at ten-fold background level, although trace concentrations were detected in stems and roots prior to the harvest stage. Additionally, AMX was also not detected in brown rice treated at ten-fold background level.
CTC absorption into stems, roots, and brown rice is exhibited in
Table 4. CTC was identified in rice roots and stems in all treatment groups, regardless of treatment concentration, frequency, or crop growth stage. However, at the background level, CTC was not detected in brown rice in the groups that received two split-dose or four split-dose treatments. The highest detected concentration of CTC in brown rice was 25.1±0.4 μg kg
-1 when treated with VAs at fifty-fold background level with a six split-dose treatment. OTC absorption was detected in stems, roots, and brown rice (
Table 4). OTC displayed a substantially comparable absorption transition pattern as CTC. Overall, this experiment demonstrated that VA accumulation was directly proportional to VA treatment concentration, which is consistent with other research conducted under hydroponic conditions [
30,
31]. More frequent application of VAs into the water had shown better uptake and translocation into plant parts as compared with a less regular supply. Under less regular supply, the water-soluble VAs were efficiently degraded at outdoor temperatures and light [
32] or runoff with groundwater [
33,
34]. VAs belonging to the same antibiotic class had similar transposition-absorption patterns owing to similar solubility, affinity, and absorption mechanisms [
35]. Earlier studies suggested that VAs accumulate in roots in higher concentrations [
36]. The octanol/water division of antibiotics is closely related to the translocation of antibiotic uptake into plants (log K
ow). CTC and OTC, non-ionized compounds with log K
ow values less than 0, were mobile into both xylem and phloem. The concentrations of CTC and OTC detected in stems were 4 and 10 times higher than those in brown rice (
Table 4 and
Table 5).
AMX (0 < log K
ow <3) did not translocate into brown rice at the ten-fold background level. AMX was detected in brown rice when treated with fifty-fold background level (
Table 3). BCF values were calculated to evaluate the rate of uptake of antibiotics from the soil by plants. The mean BCF values for brown rice, roots, and stems of rice plants grown in the experimental plots are presented in
Table 6. The uptake and translocation of VAs by rice plants varied significantly. Interestingly, CTC and OTC were more easily translocated in rice plants than AMX. AMX, a member of the β-lactam class, accumulated at the lowest concentration compared to CTC and OTC across all treatments. However, the BCF values in this study were extremely low. The low BCF values for antibiotics suggest that rice plants have a low ability to absorb and retain antibiotics in their tissues. Even only at trace levels, the accumulation of VAs in brown rice may be harmful to human health through ingestion. The findings indicate that these widely used veterinary antibiotics might hamper crop production, leave residues in the soil, and pose risks to human health via bioaccumulation if introduced into the agro-ecosystem unintentionally.
Table 6.
Bioconcentration factor (BCF) of different organs of rice plants grown in the experimental plots.
Table 6.
Bioconcentration factor (BCF) of different organs of rice plants grown in the experimental plots.
| Treatment |
AMX |
CTC |
OTC |
| Root |
Stem |
Brown rice |
Root |
Stem |
Brown rice |
Root |
Stem |
Brown rice |
| A-1 |
0.000 |
0.000 |
0.000 |
0.181 |
0.200 |
0.000 |
0.148 |
0.000 |
0.000 |
| A-2 |
0.000 |
0.000 |
0.000 |
0.050 |
0.057 |
0.000 |
0.055 |
0.055 |
0.001 |
| A-3 |
0.000 |
0.000 |
0.000 |
0.046 |
0.052 |
0.007 |
0.050 |
0.049 |
0.002 |
| B-1 |
0.000 |
0.000 |
0.000 |
0.043 |
0.049 |
0.010 |
0.046 |
0.045 |
0.002 |
| B-2 |
0.000 |
0.000 |
0.000 |
0.043 |
0.053 |
0.010 |
0.046 |
0.044 |
0.002 |
| B-3 |
0.000 |
0.000 |
0.000 |
0.048 |
0.063 |
0.012 |
0.053 |
0.052 |
0.003 |
| C-1 |
0.008 |
0.005 |
0.000 |
0.047 |
0.067 |
0.013 |
0.051 |
0.049 |
0.003 |
| C-2 |
0.024 |
0.018 |
0.005 |
0.043 |
0.063 |
0.014 |
0.046 |
0.044 |
0.003 |
| C-3 |
0.023 |
0.014 |
0.018 |
0.026 |
0.075 |
0.018 |
0.052 |
0.045 |
0.004 |
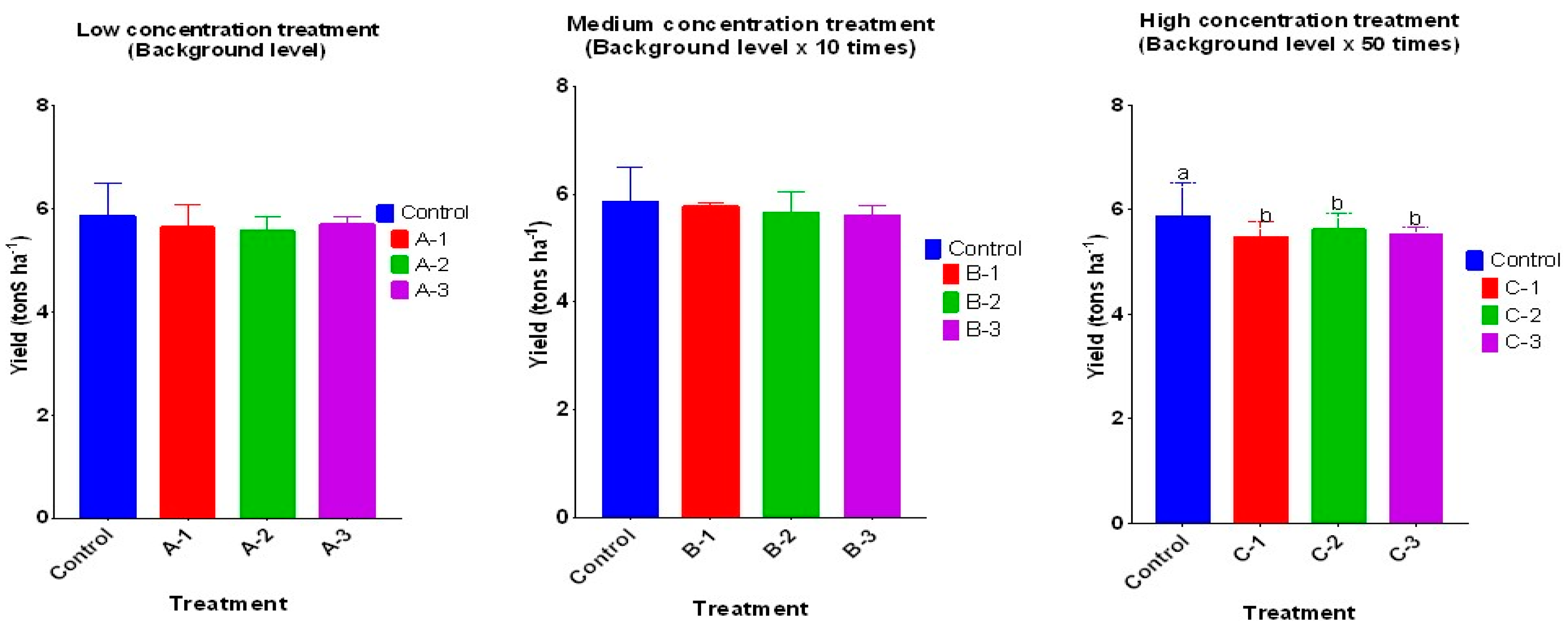
3.4. The Effects of VAs in Paddy Irrigation Water on Rice Yield
When irrigation water containing unintentionally introduced VAs was applied, rice grain yields were found to slightly decrease compared to the control (P<0.05) (
Figure 3). As shown in section 3.1, total Chl content in the VA treatments decreased by 35-45% compared to the control. A reduction in chlorophyll content might be responsible for reduced yields of rice grain. Since the structure and physicochemical properties of VAs and exchangeable Ca
2+ are compatible, antibiotic-Ca
2+ complexes can be formed, resulting in reduced Ca
2+ uptake in plants [
37].
4. Conclusion
The consistent increase in usage of VAs in agriculture and their unintentional disposal in soil have raised a huge concern for the safety of plant and human health. The current findings contribute to a better understanding of the absorption-translocation and phytotoxicity of VAs in rice plants under field conditions. Absorption-translocation of VAs into plants was directly proportional to the concentration and frequency of VAs supplemented with paddy irrigation water. In addition, significant variations in the uptake capacities of roots, stems, and grains of rice plants indicated tissue-specific absorption. The bioaccumulation of VAs in rice plants significantly varied depending on the particular class of antibiotics. Furthermore, soil residues of VAs were evident in the experimental plots, with the highest concentrations being CTC, followed by OTC and AMX. In the future, it will be interesting to decipher the relationship between antibiotic dilution and hormetic effects in plants. Overall, plant uptake of these VAs could be a risk to food safety.
Author Contributions
Visualization, resources, supervision, project administration, funding acquisition, and writing—review by J.Y. Cho. Resources, investigation, formal analysis, and data curation by H.S. Jeon. Resources, supervision, and methodology by I.H. Seo. Conceptualization, methodology, investigation, formal analysis, software, data curation, writing—original draft preparation, writing—review and editing by V.H. Duong. All authors have read and agree to the published version of the manuscript.
Funding
This paper was supported by research funds of Jeonbuk National University in 20022.
Institutional Review Board Statement
The study did not require ethical approval.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Stefan, M.G.; Loghin, F. 2020. Antibiotics in the environment: causes and consequences. Med. Pharm. Rep. 2020, 93, 231–240. [Google Scholar] [PubMed]
- Kim, J.P.; Jin, D.R.; Lee, W.; Chae, M.; Park, J. Occurrence and removal of veterinary antibiotics in livestock wastewater treatment plants, South Korea. Processes 2020, 8, 720–732. [Google Scholar] [CrossRef]
- Tasho, R.P.; Cho, J.Y. Veterinary antibiotics in animal waste, its distribution in soil and uptake by plants: A review. Sci. Total Environ. 2016, 563, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; Gonçalves, C.A.; de Brito, J.C.M.; Souza, A.M.; da Silva Cruz, F.V.; Bicalho, E.M.; Figueredo, C.C.; Garcia, Q.S. Ciprofloxacin induces oxidative stress in duckweed (Lemna minor L.): Implications for energy metabolism and antibiotic-uptake ability. J. Hazard. Mater. 2017, 328, 140–149. [Google Scholar] [CrossRef]
- Migliore, L.; Cozzolino, S.; Fiori, M. Phytotoxicity to and uptake of enrofloxacin in crop plants. Chemosphere 2003, 52, 1233–1244. [Google Scholar] [CrossRef]
- Pan, M.; Wong, C.K.; Chu, L.M. Distribution of antibiotics in wastewater-irrigated soils and their accumulation in vegetable crops in the Pearl River Delta, southern China. J. Agric. Food Chem. 2014, 62, 11062–11069. [Google Scholar] [CrossRef]
- Jalloul, G.; Keniar, I.; Tehrani, A.; Boyadjian, C. Antibiotics contaminated irrigation water: An Ooerview on its impact on edible crops and visible light active titania as potential photocatalysts for irrigation water treatment. Front. Environ. Sci. 2021, 9, 642–671. [Google Scholar] [CrossRef]
- Liu, X.; Lv, Y.; Xu, K.; Xiao, X.; Xi, B.; Lu, S. Response of ginger growth to a tetracycline-contaminated environment and residues of antibiotic and antibiotic resistance genes. Chemosphere 2018, 201, 137–143. [Google Scholar] [CrossRef]
- Di Marco, G.; Gismondi, A.; Canuti, L.; Scimeca, M.; Volpe, A.; Canini, A. Tetracycline accumulates in I beris sempervirens L. through apoplastic transport inducing oxidative stress and growth inhibition. Plant Biol. 2014, 16, 792–800. [Google Scholar] [CrossRef]
- Minden, V.; Deloy, A.; Volkert, A.M.; Leonhardt, S.D.; Pufal, G. Antibiotics impact plant traits, even at small concentrations. AoB Plants 2017, 9, 1–19. [Google Scholar] [CrossRef]
- Iwu, C.D.; Korsten, L.; Okoh, A.I. The incidence of antibiotic resistance within and beyond the agricultural ecosystem: A concern for public health. Microbiol. Open 2020, 9, 1035–1063. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.H.; Choi, J.K.; Kim, S.K.; Min, H.K.; Jung, Y.S. Prioritizing environmental risks of veterinary antibiotics based on the use and the potential to reach environment. Kor. J. Soil Sci. Fertil. 2007, 40, 43–50. [Google Scholar]
- Kim, H.R.; Park, S.B.; Kim, S.C. Monitoring of antibiotics in the soil and sediment near at the animal feeding operation and wastewater treatment plant. Kor. J. Soil Sci. Fertil. 2017, 50, 285–292. [Google Scholar] [CrossRef]
- Chung, S.S.; Zheng, J.; Burket, S.; Brooks, B. Select antibiotics in leachate from closed and active landfills exceed thresholds for antibiotic resistance development. Environ. Intl. 2018, 115, 89–96. [Google Scholar] [CrossRef]
- RDA (Rural Development Administration). Fertilization standard of crop plants. 2017, Jeonju, South Korea.
- Cho, J.Y.; Son, J.G.; Song, C.H.; Hwang, S.A.; Lee, Y.M.; Jeongm, S.Y.; Chung, B.Y. Integrated nutrient management for environmental-friendly rice production in salt-affected rice paddy fields of Saemangeum reclaimed land of South Korea. Paddy Water Environ 2008, 6, 263–273. [Google Scholar] [CrossRef]
- Zayed, A.; Gowthaman, S.; Terry, N. Phytoaccumulation of trace elements by wetland plants: I. Duckweed. J. Environ. Qual. 1998, 27, 715–721. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant physiol. 1949, 24, 24–31. [Google Scholar] [CrossRef]
- RDA (Rural Development Administration). Standard investigation methods for agriculture experiment. 1995, Suwon, South Korea.
- Guidi, L.; Lo, P.E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174–190. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.H.; Liu, C.X.; Wang, Z.; Dong, J.; Zhu, G.F.; Huang, X. Potential effect and accumulation of veterinary antibiotics in Phragmites australis under hydroponic conditions. Ecol. Eng. 2013, 53, 138–143. [Google Scholar] [CrossRef]
- Migliore, L.; Brambilla, G.; Cozzolino, S.; Gaudio, L. Effect on plants of sulphadimethoxine used in intensive farming (Panicum miliaceum, Pisum sativum and Zea mays). Agric. Ecosyst. Environ. 1995, 52, 103–110. [Google Scholar] [CrossRef]
- Braschi, I.; Blasioli, S.; Fellet, C.; Lorenzini, R.; Garelli, A.; Pori, M.; Giacomini, D. Persistence and degradation of new β-lactam antibiotics in the soil and water environment. Chemosphere 2013, 93, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Halling-Sørensen, B.; Jacobsen, A.; Jensen, J.; SengeløV, G.; Vaclavik, E.; Ingerslev, F. Dissipation and effects of chlortetracycline and tylosin in two agricultural soils: A field-scale study in southern Denmark. Environ. Toxicol. Chem. 2005, 24, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Ling-Ling, L.; Huang, L.D.; Chung, R.S.; Ka-Hang, F.; Zhang, Y.S. Sorption and dissipation of tetracyclines in soils and compost. Pedosphere 2010, 20, 807–816. [Google Scholar]
- Wang, Q.; Yates, S.R. Laboratory study of oxytetracycline degradation kinetics in animal manure and soil. J. Agric. Food Chem. 2008, 56, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Chu, L. Phytotoxicity of veterinary antibiotics to seed germination and root elongation of crops. Ecotoxicol. Environ. Safety 2016, 126, 228–237. [Google Scholar] [CrossRef]
- Kotzerke, A.; Sharma, S.; Schauss, K.; Heuer, H.; Thiele-Bruhn, S.; Smalla, K.; Wilke, B.M.; Schloter, M. Alterations in soil microbial activity and N-transformation processes due to sulfadiazine loads in pig-manure. Environ. Pollut. 2008, 153, 315–322. [Google Scholar] [CrossRef]
- Keen, P.L.; Patrick, D.M. Tracking change: a look at the ecological footprint of antibiotics and antimicrobial resistance. Antibiotics 2013, 2, 191–205. [Google Scholar] [CrossRef]
- Boonsaner, M.; Hawker, D. Accumulation of oxytetracycline and norfloxacin from saline soil by soybeans. Sci. Total Environ. 2010, 408, 1731–1737. [Google Scholar] [CrossRef]
- Michelini, L.; Meggio, F.; Rocca, N.L.; Ferro, S.; Ghisi, R. Accumulation and effects of sulfadimethoxine in Salix fragilis L. plants: a preliminary study to phytoremediation purposes. Int. J. Phytoremediation 2012, 14, 388–402. [Google Scholar] [CrossRef]
- Martínez-Hernández, V.; Meffe, R.; López, S.H.; de Bustamante, I. The role of sorption and biodegradation in the removal of acetaminophen, carbamazepine, caffeine, naproxen and sulfamethoxazole during soil contact: a kinetics study. Sci. Total Environ. 2016, 559, 232–241. [Google Scholar] [CrossRef]
- Carlson, J.C.; Mabury, S.A. Dissipation kinetics and mobility of chlortetracycline, tylosin, and monensin in an agricultural soil in Northumberland County, Ontario, Canada. Environ. Toxicol. Chem. 2006, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Chu, L. Transfer of antibiotics from wastewater or animal manure to soil and edible crops. Environ. Pollut. 2017, 231, 829–836. [Google Scholar] [CrossRef]
- Park, Y.J.; Son, J.G. Phytotoxicity and Accumulation of antibiotics in water lettuce (Pistia stratiotes) and parrot feather (Myriophyllum aquaticum) plants under hydroponic culture conditions. Appl. Sci. 2022, 12, 630–641. [Google Scholar] [CrossRef]
- Azanu, D.; Mortey, C.; Darko, G.; Weisser, J.J.; Styrishave, B.; Abaidoo, R.C. Uptake of antibiotics from irrigation water by plants. Chemosphere 2016, 157, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Batchelder, A. Chlortetracycline and oxytetracycline effects on plant growth and development in soil systems. J. Environ. Qual. 1982, 11, 675–678. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).