Submitted:
26 September 2023
Posted:
27 September 2023
You are already at the latest version
Abstract
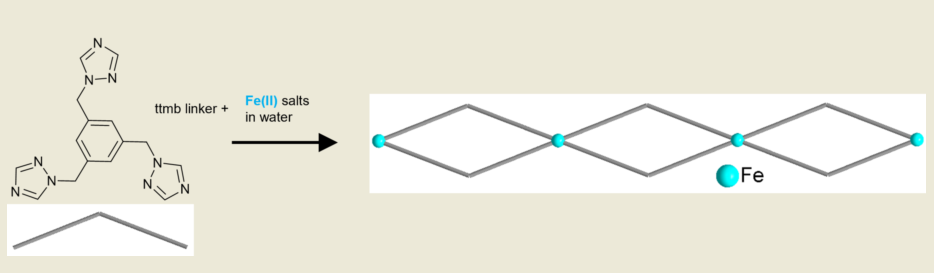
Keywords:
1. Introduction
2. Materials and Methods
2.1. Synthesis
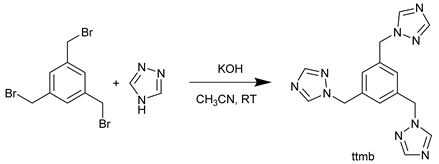
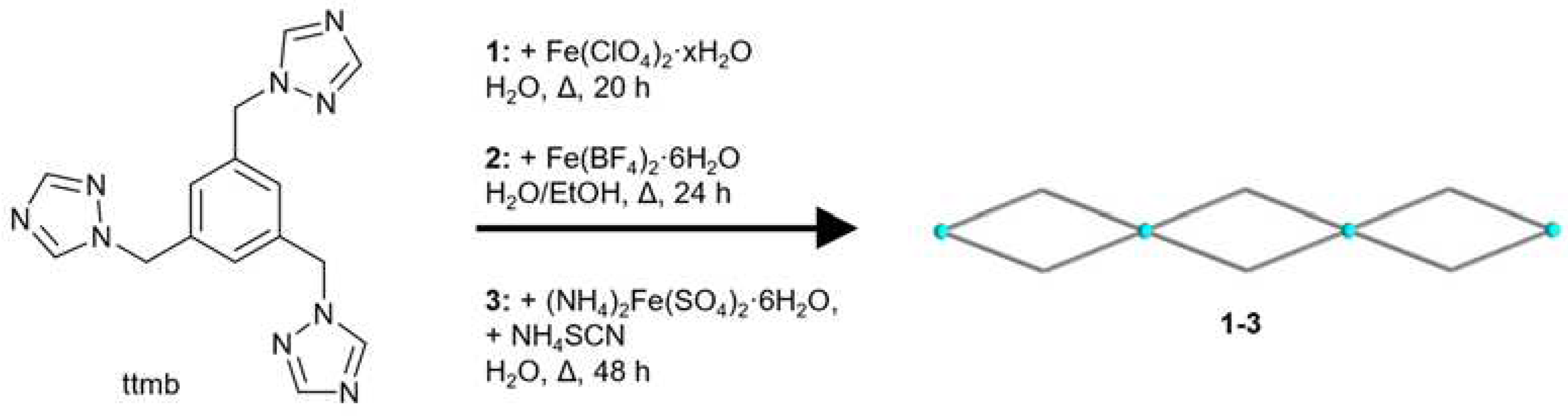
2.1.2. Synthesis of [Fe(H2O)2(ttmb)2](ClO4)2·4H2O (1)
2.1.3. Synthesis of [Fe(H2O)2(ttmb)2](BF4)2·4H2O (2)
2.1.4. Synthesis of [Fe(ttmb)2(NCS)2] (3)
3. Results and Discussion
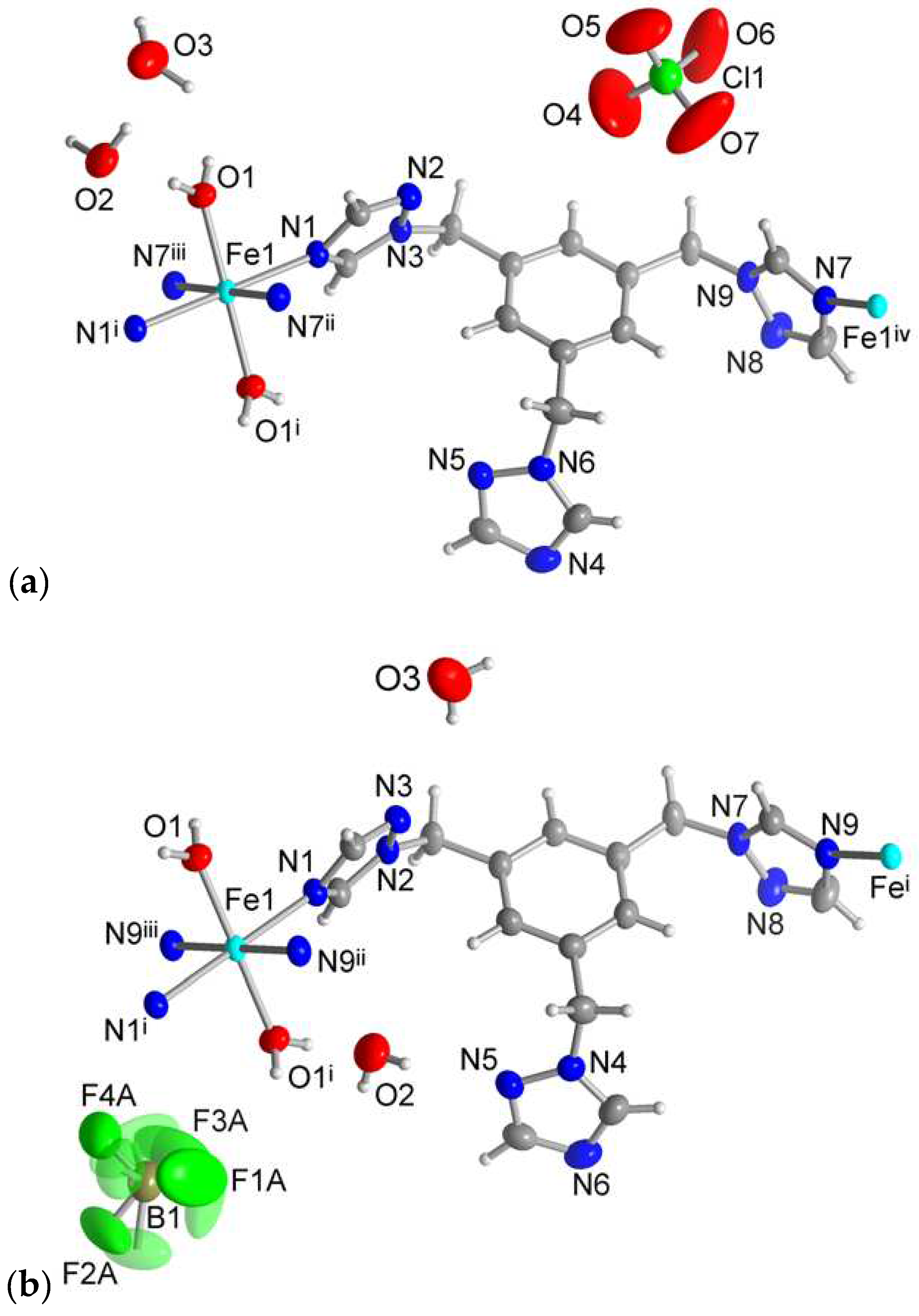
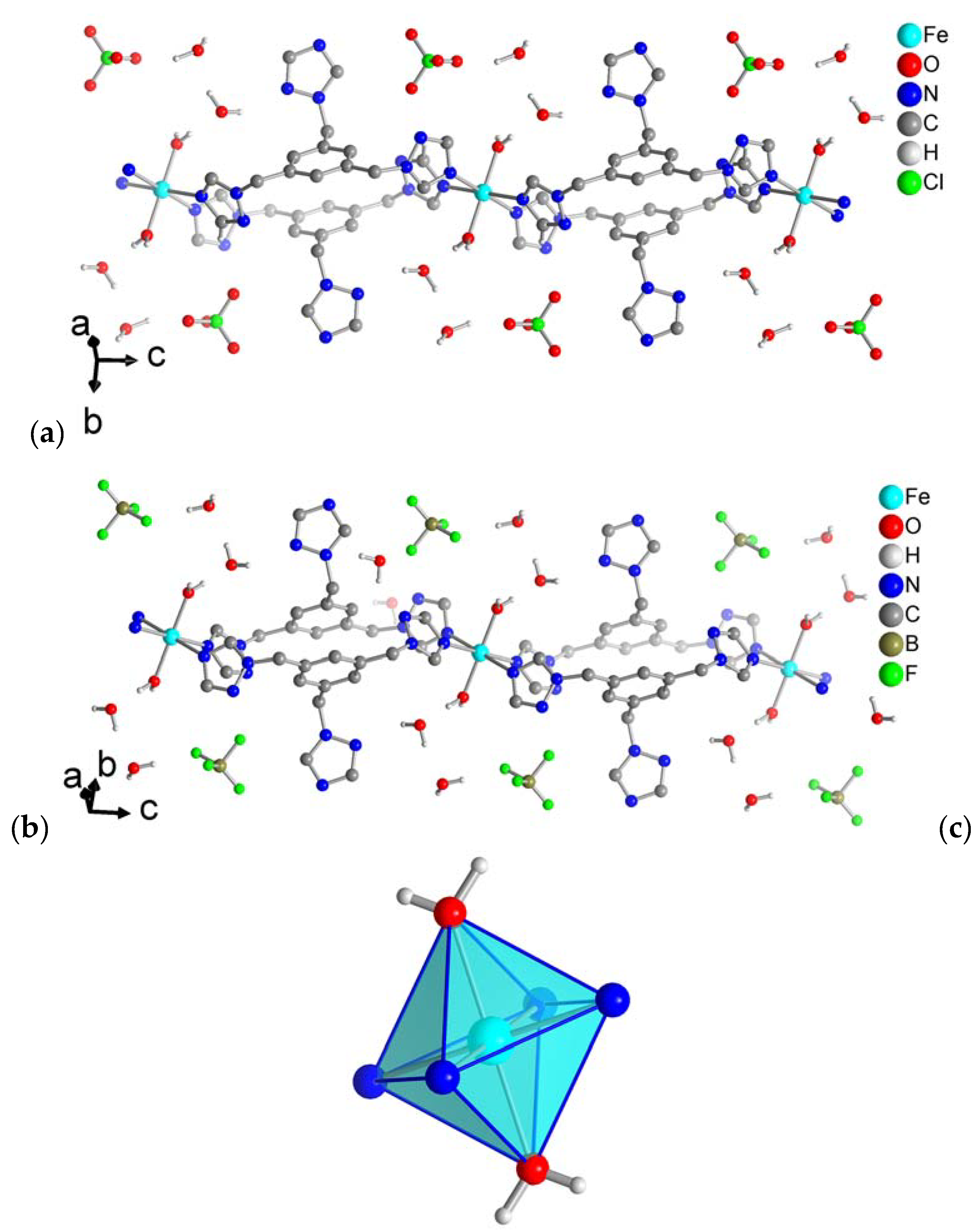
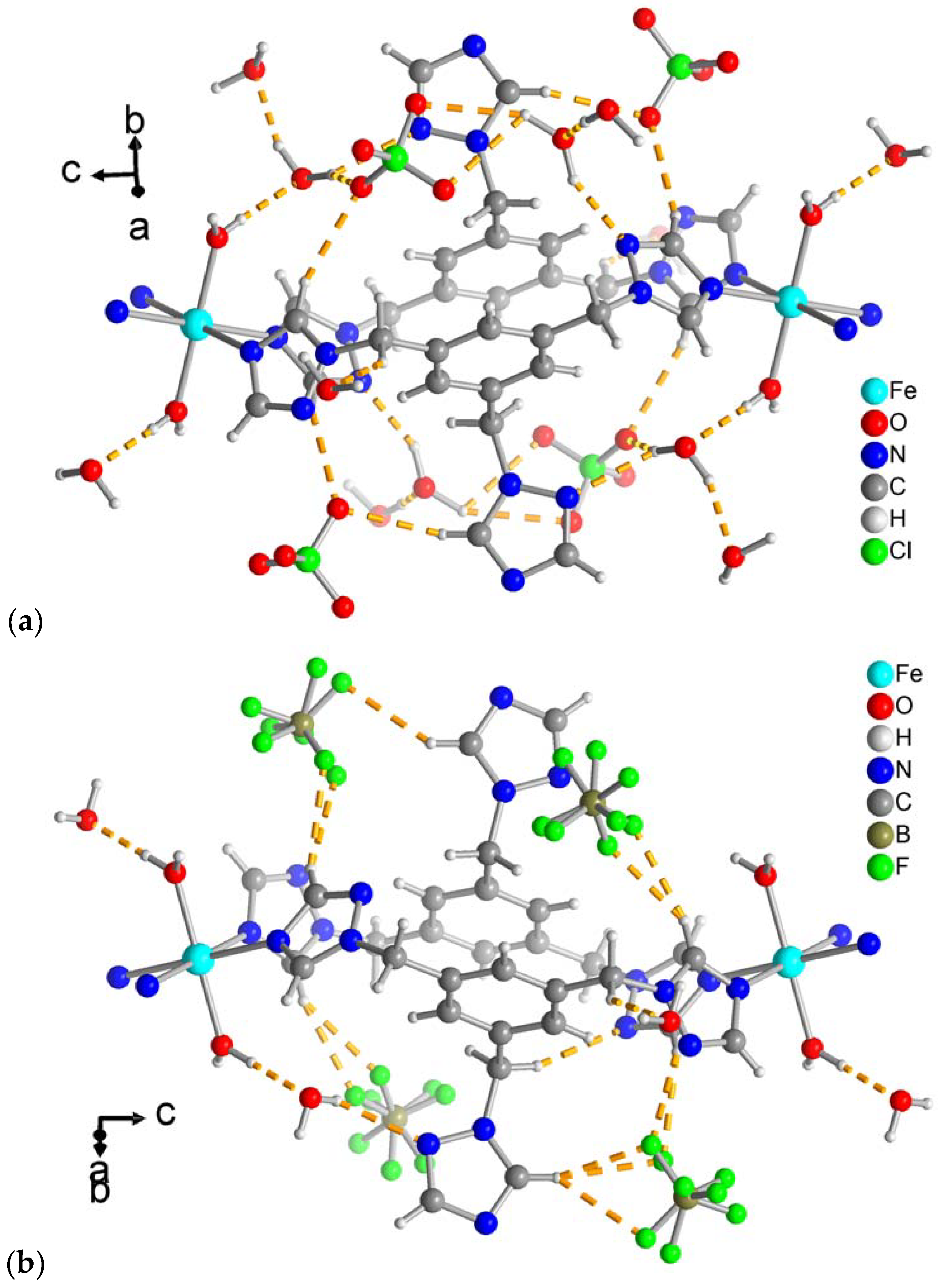
3.1. Crystal structure of [Fe(H2O)2(ttmb)2](X)2·4H2O (1: X = ClO4; 2: X = BF4)
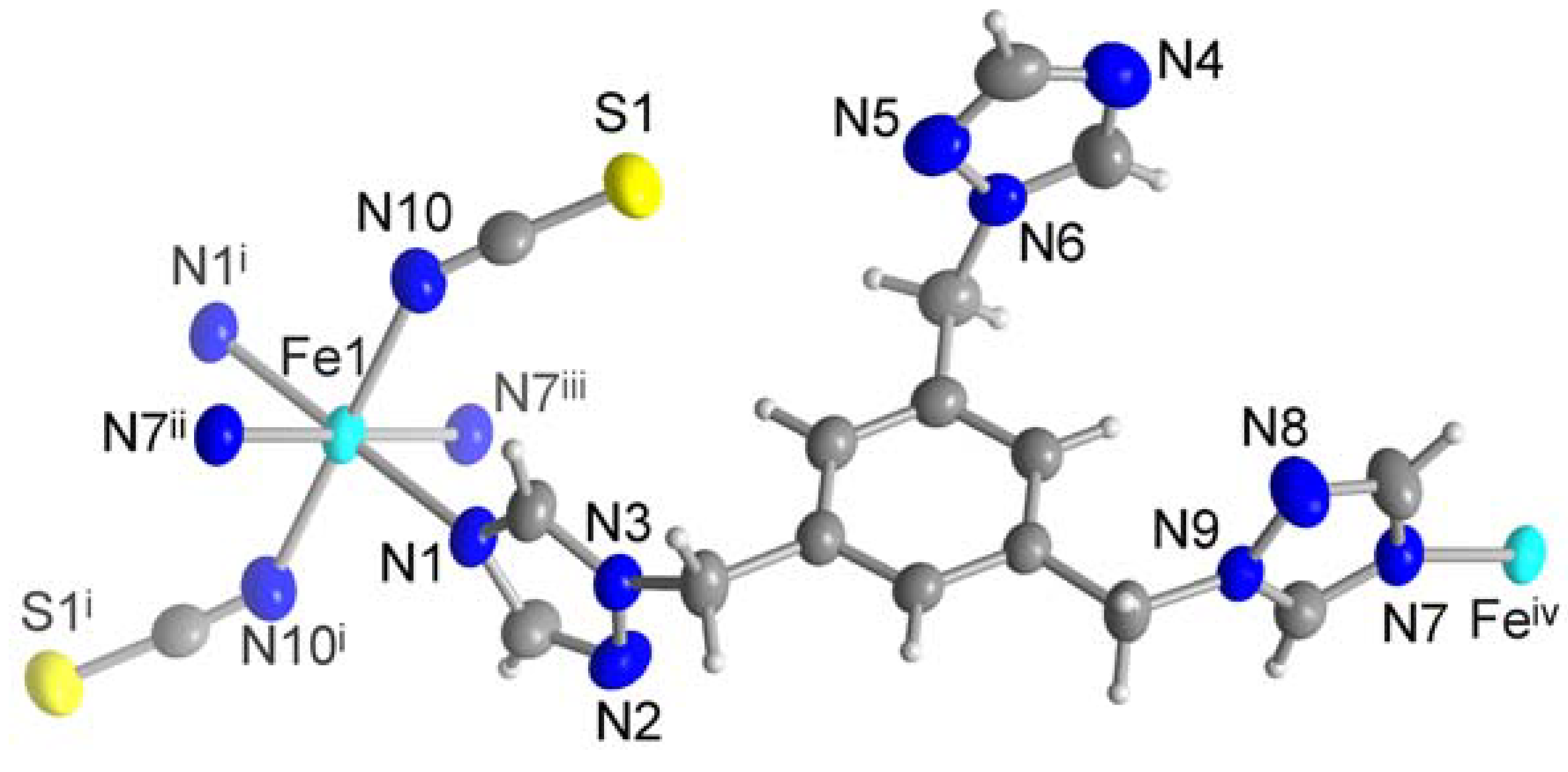
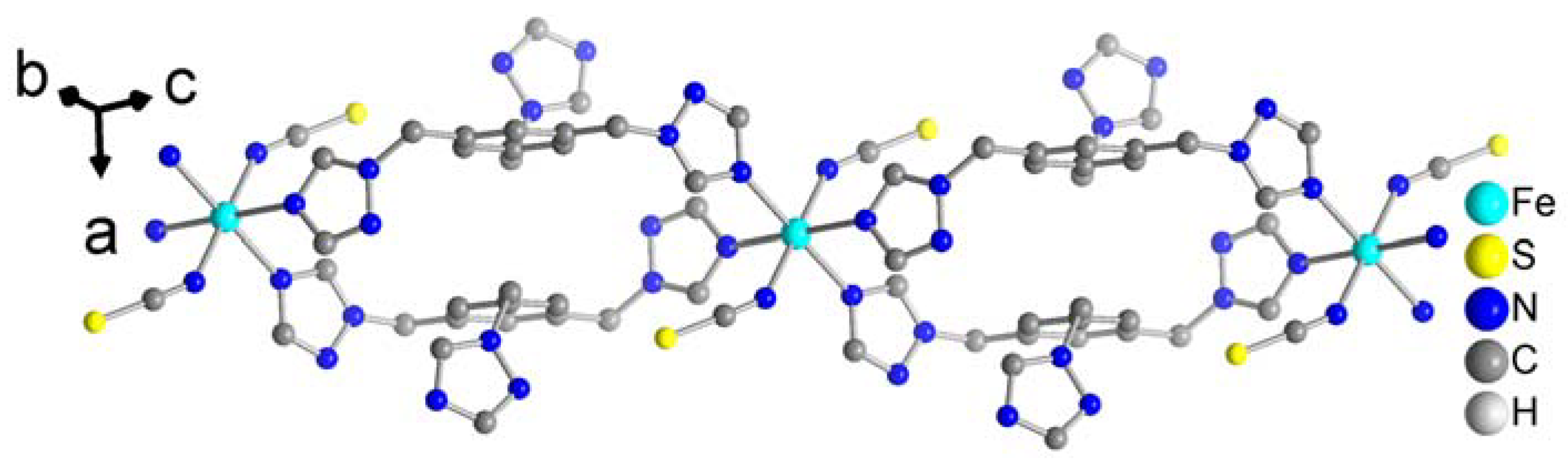
3.2. Crystal Structure of [Fe(ttmb)2(NCS)2] (3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination polymers. Design, analysis and application; Royal Society of Chemistry: Cambridge, UK, 2009; ISBN 978-0-85404-837-3. [Google Scholar]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O'Keeffe, M.; Suh, M.P.; Reedijk, J. Coordination polymers, metal–organic frameworks and the need for terminology guidelines. CrystEngComm 2012, 14, 3001. [Google Scholar] [CrossRef]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Paik Suh, M.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Matveevskaya, V.; Pavlov, D.; Yakunenkov, A.; Potapov, A. Coordination Polymers Based on Highly Emissive Ligands: Synthesis and Functional Properties. Materials 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.L.; Vittal, J.J. One-dimensional coordination polymers: complexity and diversity in structures, properties, and applications. Chem. Rev. 2011, 111, 688–764. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Robin, A.Y.; Fromm, K.M. Coordination polymer networks with O- and N-donors: What they are, why and how they are made. Coord. Chem. Rev. 2006, 250, 2127–2157. [Google Scholar] [CrossRef]
- Naik, A.D.; Dîrtu, M.M.; Railliet, A.P.; Marchand-Brynaert, J.; Garcia, Y. Coordination Polymers and Metal Organic Frameworks Derived from 1,2,4-Triazole Amino Acid Linkers. Polymers 2011, 3, 1750–1775. [Google Scholar] [CrossRef]
- Hoskins, B.F.; Robson, R. Infinite polymeric frameworks consisting of three dimensionally linked rod-like segments. J. Am. Chem. Soc. 1989, 111, 5962–5964. [Google Scholar] [CrossRef]
- Horike, S.; Shimomura, S.; Kitagawa, S. Soft porous crystals. Nat. Chem. 2009, 1, 695–704. [Google Scholar] [CrossRef]
- Kitagawa, S.; Noro, S.; Nakamura, T. Pore surface engineering of microporous coordination polymers. Chem. Commun. 2006, 701–707. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular chemistry: Concepts and perspectives : a personal account built upon the George Fisher Baker lectures in chemistry at Cornell University [and] Lezioni Lincee, Accademia nazionale dei Lincei, Roma; VCH: Weinheim, New York, 1995; ISBN 3527293124. [Google Scholar]
- Mu, Y.; Han, G.; Ji, S.; Hou, H.; Fan, Y. Coordination polymers based on a flexible bis(triazole) ligand and aromatic polycarboxylate anions: syntheses, topological structures and photoluminescent properties. CrystEngComm 2011, 13, 5943. [Google Scholar] [CrossRef]
- Du, M.; Bu, X.-H.; Huang, Z.; Chen, S.-T.; Guo, Y.-M.; Diaz, C.; Ribas, J. From metallacyclophanes to 1-D coordination polymers: role of anions in self-assembly processes of copper(II) and 2,5-bis(3-pyridyl)-1,3,4-oxadiazole. Inorg. Chem. 2003, 42, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Chand, D.K.; Biradha, K.; Fujita, M.; Sakamoto, S.; Yamaguchi, K. A molecular sphere of octahedral symmetry. Chem. Commun. 2002, 2486–2487. [Google Scholar] [CrossRef]
- Friese, V.A.; Kurth, D.G. From coordination complexes to coordination polymers through self-assembly. Current Opinion in Colloid & Interface Science 2009, 14, 81–93. [Google Scholar] [CrossRef]
- Li, W.; Kim, Y.; Li, J.; Lee, M. Dynamic self-assembly of coordination polymers in aqueous solution. Soft Matter 2014, 10, 5231–5242. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-L.; Sun, W.-Y.; Zhu, H.-L.; Yu, K.-B.; Tang, W.-X. A two-dimensional network constructed via 72-membered heart-shaped macrocycles, a copper(II) complex with 1,3,5-tris(imidazol-1-ylmethyl)-2,4,6-trimethylbenzene and diethylenetriamine ligands. Inorg. Chim. Acta 1999, 295, 129–135. [Google Scholar] [CrossRef]
- Shi, X.; Tan, D.; Liu, Y.; Liang, G.; Zhang, X. Syntheses, structures and fluorescence properties of two novel polymers based on a flexible tripodal ligand 1,3,5-tris((1H-1,2,4-triazol -1-yl)methyl)benzene. J. Mol. Struct. 2014, 1074, 134–139. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Li, X.; Hou, H.; Fan, Y. Structure analysis and catalytic property of a microporous framework based on a flexible tripodal ligand with novel conformations. J. Mol. Struct. 2011, 996, 110–114. [Google Scholar] [CrossRef]
- Yin, X.-J.; Zhou, X.-H.; Gu, Z.-G.; Zuo, J.-L.; You, X.-Z. Syntheses and physical properties of three-dimensional coordination polymers with the flexible tripodal ligand 1,3,5-tris(1,2,4-triazol-1-ylmethyl)benzene. Inorg. Chem. Commun. 2009, 12, 548–551. [Google Scholar] [CrossRef]
- Shi, Z.; Pan, Z.; Zhang, C.; Zheng, H. Syntheses, structures, and properties of six cobalt(II) complexes based on a tripodal tris(4-(1H-1,2,4-triazol-1-yl)phenyl)amine ligand. Dalton Trans. 2015, 44, 16854–16864. [Google Scholar] [CrossRef]
- Li, B.Z.; Liu, X.G.; Wang, Z.H.; Li, B.L.; Zhang, Y. A novel two-dimensional network cadmium(II) coordination polymer containing one 1,4-bis(1,2,4-triazol-1-yl)butane and double dicyanamide bridges. Acta Crystallogr. C 2006, 62, m10–2. [Google Scholar] [CrossRef] [PubMed]
- Woschko, D.; Millan, S.; Ceyran, M.-A.; Oestreich, R.; Janiak, C. Synthesis of a Chiral 3,6T22-Zn-MOF with a T-Shaped Bifunctional Pyrazole-Isophthalate Ligand Following the Principles of the Supramolecular Building Layer Approach. Molecules 2022, 27, 5374. [Google Scholar] [CrossRef]
- Aromí, G.; Barrios, L.A.; Roubeau, O.; Gamez, P. Triazoles and tetrazoles: Prime ligands to generate remarkable coordination materials. Coordination Chemistry Reviews 2011, 255, 485–546. [Google Scholar] [CrossRef]
- Haasnoot, J.G. Mononuclear, oligonuclear and polynuclear metal coordination compounds with 1,2,4-triazole derivates as ligands. Coord. Chem. Rev. 2000, 200-202, 131–185. [Google Scholar] [CrossRef]
- Garcia, Y.; Adarsh, N.N.; Naik, A.D. Crystal engineering of Fe(II) spin crossover coordination polymers derived from triazole or tetrazole ligands. Chimia (Aarau) 2013, 67, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Yi, L.; Cheng, P.; Liao, D.-Z.; Yan, S.-P. Synthesis and characterization of a 3D coordination polymer based on trinuclear triangular CuII as secondary building units. Inorg. Chem. 2006, 45, 5799–5803. [Google Scholar] [CrossRef]
- Ouellette, W.; Liu, H.; O'Connor, C.J.; Zubieta, J. Solid-state coordination chemistry of copper(II) tetrazolates: anion control of frameworks constructed from trinuclear copper(II) building blocks. Inorg. Chem. 2009, 48, 4655–4657. [Google Scholar] [CrossRef]
- Ouellette, W.; Hudson, B.S.; Zubieta, J. Hydrothermal and structural chemistry of the zinc(II)- and cadmium(II)-1,2,4-triazolate systems. Inorg. Chem. 2007, 46, 4887–4904. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Chen, X.-M. Crystal engineering of binary metal imidazolate and triazolate frameworks. Chem. Commun. 2006, 1689–16990. [Google Scholar] [CrossRef]
- Wang, H.-P.; Wang, H.-L.; Li. B.-L. Synthesis, Structure, Lumiescence and Thermal Stability Properties of a New (3,4)-Connected 2D Zn Coordination Polymer. J. Struct. Chem. 2020, 10, 1835–1840. [Google Scholar] [CrossRef]
- Li, H.; Liu, G.; Liu, T.T.; Zhang, H.Y.; Yue, F.; Wang, J.D. Syntheses of triazole-bridged cadmium coordination polymer with luminescence properties. Russ J Coord Chem 2011, 37, 8–11. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Luan, J.; Wang, X.-L.; Zhang, J.-W.; Liu, G.-C.; Tian, A.-X. Construction and properties of cobalt(ii )/copper(ii ) coordination polymers based on N-donor ligands and polycarboxylates mixed ligands. RSC Adv 2014, 4, 62430–62445. [Google Scholar] [CrossRef]
- Schweifer, J.; Weinberger, P.; Mereiter, K.; Boca, M.; Reichl, C.; Wiesinger, G.; Hilscher, G.; van Koningsbruggen, P. J.; Koojiman, H.; Grunert, M.; Linert, W. catena-[µ-Tris(1,2-bis(tetrazol-1-yl)ethane-N4,N4´)iron(II)] bis(tetrafluoroborate): synthesis, structure, spectroscopic and magnetic characterization of a chain-type coordination polymer spin-crossover compound. Inorg. Chim. Acta 2002, 339, 297–306. [Google Scholar] [CrossRef]
- Absmeier, A.; Bartel, M.; Carbonera, C.; Jameson, G.N.L.; Werner, F.; Reissner, M.; Caneschi, A.; Létard, J.-F.; Linert, W. Mutual Influence of Spacer Length and Noncoordinating Anions on Thermal and Light-Induced Spin-Crossover Properties of Iron(II)–α,ω-Bis(tetrazol-1-yl)alkane Coordination Polymers. Eur J Inorg Chem 2007, 2007, 3047–3054. [Google Scholar] [CrossRef]
- Li, D.-P.; Zhou, X.-H.; Liang, X.-Q.; Li, C.-H.; Chen, C.; Liu, J.; You, X.-Z. Novel Structural Diversity of Triazolate-Based Coordination Polymers Generated Solvothermally with Anions. Crystal Growth & Design 2010, 10, 2136–2145. [Google Scholar] [CrossRef]
- Schottel, B.L.; Chifotides, H.T.; Shatruk, M.; Chouai, A.; Pérez, L.M.; Bacsa, J.; Dunbar, K.R. Anion-pi interactions as controlling elements in self-assembly reactions of Ag(I) complexes with pi-acidic aromatic rings. J. Am. Chem. Soc. 2006, 128, 5895–5912. [Google Scholar] [CrossRef] [PubMed]
- Busch, D.H. Structural Definition of Chemical Templates and the Prediction of New and Unusual Materials. Am. Chem. Soc. 1992, 12, 389–395. [Google Scholar] [CrossRef]
- Anderson, S.; Anderson, H.L.; Sanders, J.K.M. Expanding roles for templates in synthesis. Acc. Chem. Res. 1993, 26, 469–475. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, L.-Y.; Liu, X.-G.; Wang, J.; Li, B.-L.; Li, H.-Y. Structural versatility of seven copper(ii) coordination polymers constructed with the long flexible ligand 1,4-bis(1,2,4-triazol-1-yl)butane. CrystEngComm 2011, 13, 6090. [Google Scholar] [CrossRef]
- Tanaka, D.; Kitagawa, S. Template Effects in Porous Coordination Polymers. Chem. Mater. 2008, 20, 922–931. [Google Scholar] [CrossRef]
- Halper, S.R.; Do, L.; Stork, J.R.; Cohen, S.M. Topological Control in Heterometallic Metal-Organic Frameworks by Anion Templating and Metalloligand Design. J. Am. Chem. Soc. 2006, 128, 15255–15268. [Google Scholar] [CrossRef] [PubMed]
- Beer, P.D.; Gale, P.A. Anion Recognition and Sensing: The State of the Art and Future Perspectives. Angew. Chem. Int. Ed. 2001, 40, 486–516. [Google Scholar] [CrossRef]
- Huang, X.-C.; Zhang, J.-P.; Chen, X.-M. A new route to supramolecular isomers via molecular templating: nanosized molecular polygons of copper(I) 2-methylimidazolates. J. Am. Chem. Soc. 2004, 126, 13218–13219. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-C.; Zhang, J.-P.; Lin, Y.-Y.; Chen, X.-M. Triple-stranded helices and zigzag chains of copper(I) 2-ethylimidazolate: solvent polarity-induced supramolecular isomerism. Chem. Commun. 2005, 2232–2234. [Google Scholar] [CrossRef]
- Vilar, R. Anion-templated synthesis. Angew. Chem. Int. Ed Engl. 2003, 42, 1460–1477. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Y.; Liang, N.; Li, B.; Zhang, Y. Two nickel coordination polymers with flexible ligand 1,3,5-tri(1,2,4-triazol-1-ylmethyl)-2,4,6-trimethylbenzene. Inorg. Chem. Commun. 2009, 12, 1161–1163. [Google Scholar] [CrossRef]
- Gimeno, N.; Vilar, R. Anions as templates in coordination and supramolecular chemistry. Coordination Chemistry Reviews 2006, 250, 3161–3189. [Google Scholar] [CrossRef]
- Kim, H.-J.; Zin, W.-C.; Lee, M. Anion-directed self-assembly of coordination polymer into tunable secondary structure. J. Am. Chem. Soc. 2004, 126, 7009–7014. [Google Scholar] [CrossRef]
- Vilar, R. Anion-templated synthesis. Angew. Chem. Int. Ed Engl. 2003, 42, 1460–1477. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Y.; Liang, N.; Li, B.; Zhang, Y. Two nickel coordination polymers with flexible ligand 1,3,5-tri(1,2,4-triazol-1-ylmethyl)-2,4,6-trimethylbenzene. Inorganic Chemistry Communications 2009, 12, 1161–1163. [Google Scholar] [CrossRef]
- Lankshear, M.D.; Beer, P.D. Strategic anion templation. Coordination Chemistry Reviews 2006, 250, 3142–3160. [Google Scholar] [CrossRef]
- Shi, Q.; Cao, R.; Sun, D.-F.; Hong, M.-C.; Liang, Y.-C. Solvothermal syntheses and crystal structures of two metal coordination polymers with double-chain structures. Polyhedron 2001, 20, 3287–3293. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; Wood, P.A. Mercury 4.0: from visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Agilent; Agilent Technologies Ltd, Yarnton, Oxfordshire, England, 2014.
- APEX2, data collection program for the CCD area-detector system, Version 2.1-0, Bruker Analytical X-ray Systems, Madison (WI), USA,1997–2014.
- SAINT, data reduction and frame integration program for the CCD area-detector system, Bruker Analytical X-ray Systems, Madison(WI), USA, 1997–2014.
- Sheldrick, G.M. SADABS: Area-Detector Absorption Correction, University of Göttingen, Göttingen, Germany, 1996.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2 : a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT - integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT - integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Brandenburg, K. , Diamond (Version 4.5), Crystal and Molecular Structure Visualization, Crystal Impact – K. Brandenburg & H. Putz Gbr, Bonn, Germany, 2009–2022.
- Shang, Q.; Zeng, T.; Gao, K.; Liu, N.; Cheng, Q.; Liao, G.; Pan, Z.; Zhou, H. A novel nitrogen heterocyclic ligand-based MOF: synthesis, characterization and photocatalytic properties. New J. Chem. 2019, 43, 16595–16603. [Google Scholar] [CrossRef]
- Garcia, Y.; Bravic, G.; Gieck, C.; Chasseau, D.; Tremel, W.; Gütlich, P. Crystal structure, magnetic properties, and 57Fe Mössbauer spectroscopy of the two-dimensional coordination polymers M(1,2-bis(1,2,4-triazol-4-yl)ethane)2(NCS)2 (MII = Fe, Co). Inorg. Chem. 2005, 44, 9723–9730. [Google Scholar] [CrossRef]
- Lavrenova, L.G.; Yudina, N.G.; Ikorskii, V.N.; Varnek, V.A.; Oglezneva, I.M.; Larionov, S.V. Spin-crossover and thermochromism in complexes of iron(II) iodide and thiocyanate with 4-amino-1,2,4-triazole. Polyhedron 1995, 14, 1333–1337. [Google Scholar] [CrossRef]
- Hesse, M.; Meier, H.; Zeeh, B.; Bienz, S.; Bigler, L.; Fox, T. Spektroskopische Methoden in der Organischen Chemie; 8 Auflage; Georg Thieme Verlag: Stuttgart, Germany, 2012; ISBN 9783135761084. [Google Scholar]
- Jordan, D.N.; Straßburg, P.G.; Woschko, D.; Carrella, L.M.; Cuignet, L.P.; Eickmeier, K.; Dronskowski, R.; Garcia, Y.; Rentschler, E.; Janiak, C. Interpenetration Phenomena via Anion Template Effects in Fe(II) and Co(II) Coordination Networks with a Bis-(1,2,4-triazole) Ligand. Polymers 2023, 15, 3286. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




