1. Introduction
In recent years, the increasing demand for alternative food shifted research toward new kinds of nutrients. One of the most important new scientific trends in food research is about cannabinoids that are naturally present in Cannabis Sativa L (CS), a dioecious plant widely diffused, belonging to the Cannabaceae family. In the past, it was widely used as a drug and a source of textile fiber [
1]. The genus Cannabis is divided into three classes: the first, named C. Sativa L. and commonly known as hemp, rich in fiber; the second one, C. indica Lam., is the drug-type, containing high levels of narcotic compound Δ9- tetrahydrocannabinol (Δ9-THC); the third, C.ruderalis Janish shows intermediate properties between the first two [
2]. While the drug type was widely investigated for a long time thanks to its pharmaceutical properties, scientific research is now focused on the fiber-type, both for food and pharmaceutical purposes [
2,
3]. Moreover, hemp seeds are widely used in food to extract high nutritional value oil, rich in polyunsaturated fatty acids [
3], but hemp inflorescences are also characterized by a wide range of chemical species, among which the most interesting are cannabinoids [
2] that can be used also as supplements in foods and not only for therapeutic purposes. Cannabinoids belong to the terpenophenolics class, being generated by the alkylation of an alkyl resorcinol and a monoterpene oligomeric unit. The main fraction of cannabinoids in fiber-type is composed of cannabidiolic acid (CBDA) and cannabigeloric acid (CBGA), with their decarboxylated types, respectively cannabidiol (CBD) and cannabigerol (CBG) [
2,
4].
CBD is a very important and well-known molecule because of its antioxidant, anti-inflammatory and antibiotic activity, anxiolytic and neuroprotective properties [
5,
6]. The extraction method chosen to extract CBD is based on the raw material source and the desired grade of selectivity. CBD-enriched extracts can be obtained employing ethanol, which is the preferred solvent thanks to its selectivity and the easy separation method necessary to obtain CBD extracts, but is characterized by poor yield [
2,
7]. Yields can be improved by raising the extraction time or temperature, although long exposition time to high temperatures could degrade thermolabile cannabinoids [
7]. Another technique to obtain cannabinoids is the Soxhlet extraction, in which solvent reflux guarantees the maximum phytochemicals extraction, even if it was found that it can compromise the integrity of the cannabinoids extracted [
7].
To obtain CBD-enriched extracts, a decarboxylation stage could be inserted before the extraction process, in order to promote the conversion of CBDA into CBD [
8,
9]. In recent years, CO
2 supercritical fluid extraction has been investigated as a green alternative to the classic solvent extraction [
10]. The yield of extraction of this method depends on the operative condition, pressure and temperature. In particular, yield increases with pressure, along with a decrease in the extraction time [
3]. In some cases, 5%v/v ethanol is added to CO
2 to improve the extraction of cannabinoids, while it has been demonstrated that using only CO
2 increases the CBD purity in extracts [
11].
The CBD oil from inflorescences and hemp seed oil properties have recently encouraged studies on food products based upon hemp oil and protein, in which the positive effects of hemp protein are accompanied by the antioxidant and anti-inflammatory properties of CBD-rich hemp oil [
3,
12].
In this paper, several methods for the extraction of CBD and its precursor CBDA from hemp inflorescences and stalks were investigated together with the oil extraction from protein flour. The extracts were analyzed with the HPLC method and the antioxidant power was tested. The protein flour, still rich in oil, was added with CBD oil and used for dough formulation designed for protein snacks because baked goods can be used as an excellent way to avoid exposure to direct heat and enjoy the many advantages of CBD. Finally, the rheological properties of several dough formulations, based on hemp protein and CBD-enriched oil, were also investigated.
2. Materials and Methods
2.1. Raw materials
Flowers and stalks were manually divided from the plant obtaining hemp inflorescences (HI) and hemp stalks (HS). The hemp Futura 75 was supplied by Le Querce S.r.l (Montalto Uffugo, CS, Italy) and certified with a content of Δ9-THC below 0.3% (w/w) while the protein flour (PF) was kindly provided by Eco Officina Agraria S.r.l (Arezzo, Italy). The PF is composed of fats 24%w/w, carbohydrates 9%w/w, proteins 57%w/w and moisture 10%w/w.
For the extraction process, only absolute ethanol (VWR Chemicals, France) as solvent was used. Proteic doughs were prepared using: PF, Hi-Maize® 260, which is a resistant starch (Ingredion, USA), Greek Yogurt (Fage, Luxembourg) and tap water. The CO2 (purity >99.99%) was supplied by SIAD Spa (Bergamo, Italy) for supercritical extraction.
2.2. Extraction methods
For antioxidant recovery, different methods of extraction were used: supercritical fluid extraction using CO2 as supercritical fluid (SFE), soxhlet (SE) and dynamic maceration (DME) using ethanol as solvent.
The extractions were performed on hemp inflorescences (HI), stalks (HS) and proteic flour (HPF) on the raw material after manual separation without further treatments and after a decarboxylation process (DHI, DHS, DHPF) at 140°C, for 30 min in a ventilated oven (FD 53, Binder, Germany), according to literature procedure [
8].
For SE, 15 ± 1 g of sample and 400 mL of ethanol were used. The samples were placed into a thimble filter and located in the middle of Soxhlet extractor. The extraction process was carried out at 78°C for 8 hours after which ethanol enriched in extract hemp extracts was collected [
13].
For DME, 1 g of sample was used and an amount of ethanol of 40 mL, using the solvent/sample ratio suggested by literature [
2]. Following the Brighenti procedure, the DME was carried out at room temperature for 15 min, under magnetic stirring. Afterwards, the ethanol was recovered and the exhaust sample was extracted again by the same procedure for a second time. The extract obtained was collected and the exhaust hemp sample was used for a third cycle by adding 20 mL of ethanol, obtaining a final extract quantity of 100 mL.
By Rotavapor system (Heidolph G3, Hei-VAP Value), the extracts were isolated and the yield was calculated by weighting. Extractions were done in triplicate and the maximum yield was computed according to the following equation:
Determination was done in triplicate.
For SFE, 25±1 g of grounded samples were used. SFEs were carried out in a laboratory-scale plant (Spe-ed SFE, Applied Separations, Allentown (PA), USA) at 40 °C and 360 bar. The SFE procedure is composed of a first phase of 30 minutes, in a static mode, followed by dynamic phases of 15 min. Between a dynamic phase and another one 15 minutes of static phase are set The total extraction time is 140 min. Extracts obtained were collected in a volumetric flask and weighed during extraction. The maximum yield was calculated according to Equation (1).
To evaluate the maximum oil yield using flour, a Soxhlet extraction was performed with 15 ± 1 g of HPF and 400 mL of n-hexane of analytical grade (Sigma-Aldrich Co., Milano, Italy). After extraction, the oil was isolated thanks to a Rotavapor at 50 °C (Heidolph G3, Hei-VAP Value) and the yield was evaluated by Equation (1).
2.3. Proteic dough preparation
The recipes used in this work for the protein snacks are reported in
Table 1. An addition of 1% w/w on the final dough weight of CBD oil extracted from inflorescences was added to enrich the formulation.
The mixing of the ingredients was carried out with Brabender Farinograh (Belotti Instrument, Italy), mixed with a speed of mixing of 63 rpm, at 24°C. The mixing time for all the samples was 20 minutes.
2.4. HPLC analysis
CBD and CBDA content in the extracts were evaluated with HPLC. Analyses were performed using a Smartline HPLC system (Knauer, Berlin, Germany), consisting of a degasser, a pump, and a UV detector 2600. Chromatographic separation was accomplished by 150 mm x 2 mm i.d., with precolumn, C18 Ascentis (Supelco, Darmstadt, Germany). The column temperature was set at 32°C. The mobile phase was composed of 0.1% v/v formic acid in acetonitrile (A) and 0.1% v/v formic acid in water (B), at a flow rate of 0.1 mL/min. According to Brighenti et al. (2017) [
2], the gradient elution during analysis varies as follows: 0-13 min 60% A; 13 - 17 min from 60 to 80% A, 17-22 min from 80% to 90% A, which was maintained for eight minutes and finally a 15 minutes post-running time was imposed. This method is validated for quality control of pharmaceutical products, according to ICH guidelines [
2].
Absorbance spectra were recorded every 1 s, between 200 and 450 nm, with a bandwidth of 8 nm. Chromatograms were acquired at 210, 220, 235 and 275 nm. The measurements were performed in triplicate. Both for CBD and CBDA a calibration curve was evaluated by performing HPLC analysis on different concentrations of CBD and CBDA standards (Sigma ALDRICH Co, 3050 Spruce Street St Louis MO 63103 USA). Before the injection into the HPLC system, the extracts were filtered by using a 0.45 mPTFE filter.
2.5. Antioxidant activity
The radical scavenging properties of the extracts towards the 1,1-diphenyl-2-picryl-hydrazil (DPPH) radical were investigated as described by Iacopetta et al. [
14], with some modifications. Briefly, 20 μL of each extract, properly dissolved in ethanol, were mixed with 180 μL of a 0.1 mM DPPH solution in methanol, in a 48-well plate to obtain seven different concentrations (from 100 to 5000 µg/mL. As negative control, 20 μL of ethanol in 180 μL of DPPH methanol solution were diluted. The mixture, was then shaked and incubated at room temperature, for 30 min, protected from the light. The scavenging activity was determined at 517 nm by the means of a microplate reader and DPPH radical scavenging was expressed as inhibition percentages (%IDPPH) of the tested extracts compared to the initial concentration of DPPH (control), according to:
where A0 is the control reaction absorbance and A is the absorbance the presence of samples at 517 nm. IC50 values determination was obtained from the IDPPH percentages, using GraphPad Prism 9 software (GraphPad Inc., San Diego, CA, USA). As positive control, Trolox was employed. All experiments were performed three times, each in triplicate. Data are expressed as mean values ± standard deviation (SD).
where A0 is the control reaction absorbance and A is the absorbance the presence of samples at 517 nm. IC50 values determination was obtained from the IDPPH percentages, using GraphPad Prism 9 software (GraphPad Inc., San Diego, CA, USA). As positive control, Trolox was employed. All experiments were performed three times, each in triplicate. Data are expressed as mean values ± standard deviation (SD).
(A0 is the absorbance of the control reaction and A is the absorbance in presence of samples, at 730 nm).
IC50 values were derived from the %IABTS using GraphPad Prism 9 software (GraphPad Inc., San Diego, CA, USA). As well in this case, Trolox was used as positive control. The experiments were performed three times, each in triplicate. Data are expressed as mean values ± standard deviation (SD).
2.6. Rheological characterization
The doughs were characterized by rheological measurements performing tests in dynamic mode. Frequency sweep tests were carried out at 20°C, 40°C and 60°C in the linearity region, in the range of 0.1-10 Hz, preliminary evaluated by stress sweep test and time sweep test. Furthermore, temperature ramp tests in heating mode were performed from 20°C to 100°C, at 1°C/min, at 1 Hz, in linearity. All the rheological tests were carried out using a rotational rheometer (HAAKE MARS III, Thermo Fisher Scientific, Braunschweig, Germany) equipped with a parallel plate geometry (diameter= 20 mm, gap = 2 mm ±0.1 mm), and a Peltier system to control the temperature. All measurements were conducted twice. To avoid water evaporation phenomena during tests, silicone oil of 120 cSt (VWR Chemicals, Briare, France) was used.
The frequency sweep tests were interpreted using the weak gel model [
15]:
where G* is the complex modulus,
is the frequency, A is a measure of gel strength, and z is a measure of gel structuration.
2.7. Baking trials and characterization
The formulations studied by rheological technique were baked in a ventilated oven (Unox XF013, Stefania, Italy) for 30 min at 180°C and then for 15 min at 140°C.
The snack bars were characterized post-baking by color measurements performed by colorimeter (Croma Meter CR-400, Konica Minolta, Japan), in the CieLab space. The colors were defined by color coordinates in CieLab Space: L*, the axis of lightness, a*, the axis of red/green transition, and b*, the axis of yellow/blue transition. Moreover, by the tristimulus values X, Y and Z, the browning index (BI) was evaluated as reported in Eq. 5 [
16].
where x=X/(X+Y+Z). Preliminary calibration was done before the measurements.
CBD content was also quantified in the protein snacks after baking by extraction peformed by maceration in ethanol for 12 h (1 g sample in 50 mL ethanol) and evaluated following the HPLC methods reported in the procedure described in 2.4 section.
3. Results and discussion
3.1. Extraction and characterization of extracts
All the extractions from the different parts of hemp were optimized to obtain the yield maximum. Therefore, several extractions and different techniques were performed and compared. Solvent selection for the soxhlet and dynamic extraction was evaluated according to the literature to obtain an oil rich in CBD and CBDA from flour, flowers and stalks and a green technique, the SFE-CO
2, was performed to extract oil from all the samples and compare the quantity of oil and the quality among the different methods [
3].
All solvent extraction processes were conducted using ethanol, which is recognized in the literature as the optimal solvent for the extraction of polar molecules such as cannabinoids [
2]. The data obtained by the extractions are shown in
Table 2, and it is possible to see that by using dynamic maceration no appreciable amount of extract was obtained from the stalks. In contrast, extraction from inflorescences leads to appreciable amounts of substances as also reported by other authors [
11]. Moreover, the values obtained with Soxhlet are higher than DME for the inflorescences compared to the SE. Looking at the SFE extraction, it is possible to observe that the quantity is intermediate between DME and SE.
As concern stalks, it is possible to obtain an extract only by SE, probably because of the higher temperature used compared to the dynamic extraction. The results obtained confirm the critical role played by temperature as also reported by Brighenti et al. [
2], who analysed different extraction processes.
The oil extraction from flour, which is obtained from hemp seed, was performed by three different methods. The SFE method was used because it is a good green technology to use to obtain safe and ready-to-use oil from plants, while SE and DME were performed with ethanol, which is a polar solvent, and also the best choice to obtain the maximum yield of extraction of polar species, then in cannabinoids. From the results, it is possible to understand and quantify the oil yield and also if it is possible to extract with it a certain quantity of polar substances in this way and without any co-solvent, as reported by [
2]. The obtained data show that 75% w/w of the amount of the total fats is recoverable from the PF thanks to SFE, confirming the good affinity of CO
2 with the lipophilic substances. The result obtained is interesting compared to the maximum amount of oil that can be extracted by Soxhlet methodology. In fact, a maximum oil yield of 88% is obtained using hexane as solvent, similarly to the extraction trend found by [
3].
All the samples obtained were analyzed by HPLC to identify the non-psychoactive species extracted from the hemp plant.
Before the extract analysis, the standard solutions of CBDA e CBD were analyzed and the calibration curves were generated to identify and quantify the cannabinoids. According to the literature [
2], CBDA and CBD are distinguishable as they have different UV spectra. In particular, the CBDA spectrum shows three different absorption maxima, the stronger being at 220-223 nm, the second one at 266-270 nm, and the weaker at 305 nm. On the other hand, the CBD spectrum is characterized by two absorption maxima, the first around 210-215 nm, and the second at 270 nm.
Table 3 reports the results obtained from HPLC characterization in terms of CBDA and CBD components from HI and PF.
HPLC analysis shows that for all the investigated samples the decarboxylation process increases the CBD quantity and decreases the CBDA concentration. In addition, the results show that dynamic maceration is the optimal extraction technique that can maximize the CBD yield as reported also by Brighenti (2017) [
2]. Soxhlet extraction, despite being an exhaustive extraction, probably causes the degradation of cannabinoids because of the high temperature necessary and the long extraction times involved, so the obtained values of CBD and CBDA were lower than those obtained by the other techniques [
17]. As expected, the apolarity of the supercritical fluid used generally lowers the amount of cannabinoids extracted compared to ethanol methods. Then, even if the hemp oil yield from PF is high, it is not as rich in cannabinoids as oil from HI. The obtained values are in line with the literature data [
17,
18].
Finally, analysis of the stalks reveals the presence of CBD in higher concentrations than CBDA already in their native state. Decarboxylation for HI and PF increases the CBD amount, making them an interesting waste product to use for CBD recovery from the hemp plant [
19]. This result is in agreement with what is reported in the literature, where the use of stalks for the recovery of CBD-rich oil for biofuels is shown [
20,
21].
3.2. Antioxidant activity results
To date, different studies demonstrated that many compounds extracted from natural matrices possess an interesting antioxidant activity [
22]. It is known that oxidative stress, characterized by a higher presence of Reactive Oxygen Species (ROS), can induce dramatic effects by damaging important intracellular structures, amongst them DNA, proteins and lipids [
23]. These effects can be prevented by employing compounds with antioxidant properties, which can scavenge ROS and other radicals. Since the investigation of the antioxidant activity is influenced by several variables, several proper assays are required for assessing the antioxidant effects of an extract [
24]. In this study, two different in vitro assays, namely ABTS and DPPH, were employed to evaluate the ability in free-radical scavenging of the extracts (for further details, see experimental section). As a positive control, a water-soluble analogue of vitamin E (Trolox) was adopted. The calculated IC50 values, expressed in µM, are reported in
Table 4. First, the best ability in scavenging the ABTS radical was exhibited by DHI and HI, showing IC50 values of 38.1±1.1 and 85.3±2.4 µg/mL, respectively, which are about two- and five-fold higher than that of Trolox (15.9±1.3 µg/mL). The result, related to the extract obtained with DM methodology, is in agreement with the extracted CBD values, evaluated by HPLC investigation, which are the highest among the samples investigated with antioxidant assay measurements. A similar activity was detected for DHS (IC50 = 87.2±2.2 µg/mL), whereas a lower one was found for HS (IC50 = 125.1±3.3 µg/mL). In both cases, Soxhlet extraction from stalks leads to lower extracted CBD quantities and, therefore, lower antioxidant capacity of the extracts. Finally, HPF and DHPF, extracted by the SFE process, were not able to scavenge the ABTS radical (IC50 > 2000 µg/mL). According to HPLC analysis, all decarboxylated samples, which resulted in a higher amount of CBD, show higher antioxidant activity than the untreated samples.
A different behaviour was recorded for the ability to scavenge the DPPH radical. Indeed, the best scavenging ability was found for DHS (IC50 = 348.5±2.4 µg/mL), followed by DHI and HS, whose values were very similar (IC50 of 535.7±4.7 and 550.1±4.5 µg/mL, respectively). Again, HFP and DHFP were unable to scavenge the DPPH radical (IC50 > 5000 µg/mL). In conclusion, the extracts demonstrated better activity in inhibiting the radical ABTS compared to the DPPH, in agreement with Dawidowicz (2021) [
25].
3.3. Rheology
In light of the good yield of hemp oil from PF and the high quantity of antioxidants, a possible way to introduce bioactive compounds in foods is to enrich the hemp oil from seeds with the extract from the inflorescences, to increase the antioxidant effect of foods. Then, doughs were prepared with the PF to have formulations suitable for the preparation of protein bars (25% protein, dry basis) and resistant starch (Hi-Maize) to improve the dough structure [
26] and 5%w/w on fat basis of oil obtained from HI by DME was added to enrich in CBD the bar. All the samples were characterized as reported in the materials and methods section.
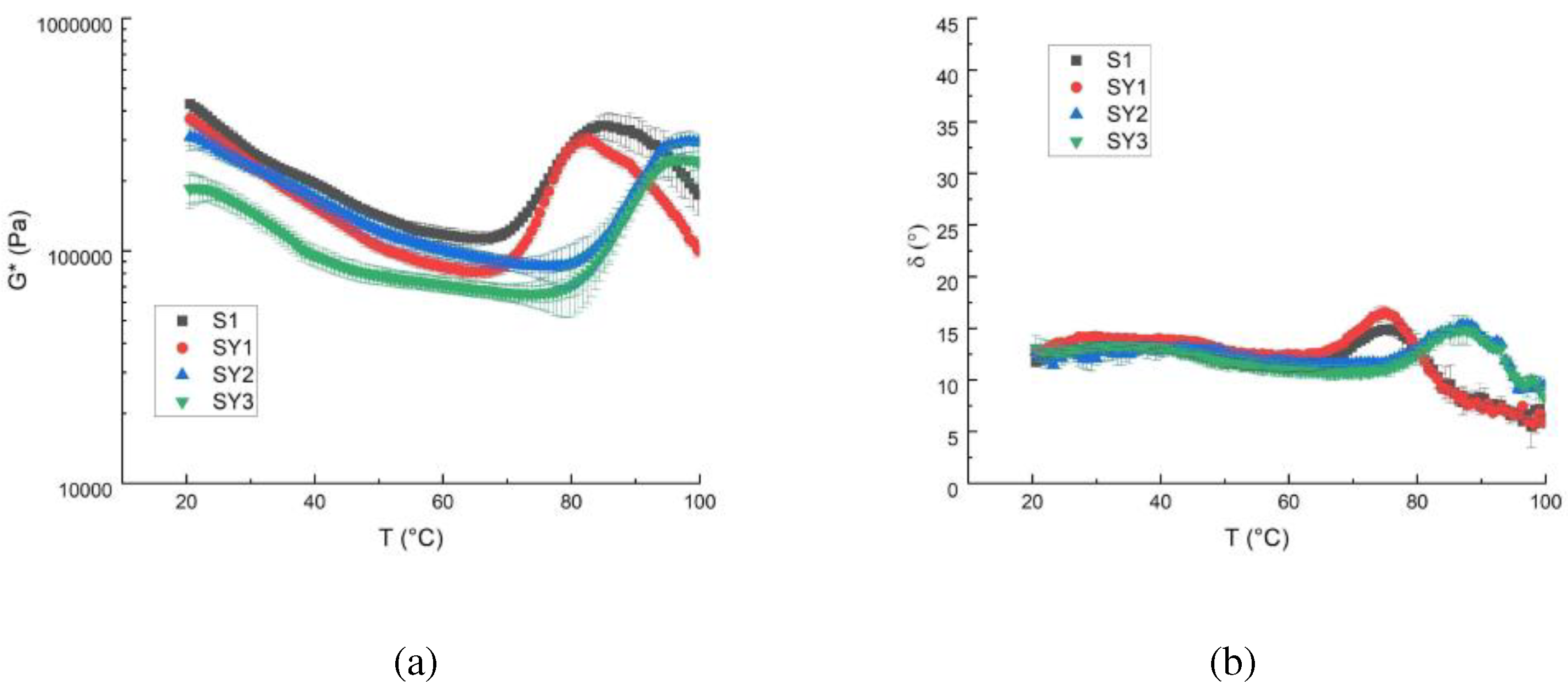
For all samples, the temperature ramp test shows the complex modulus evolution that decreases with temperature increase. The typical trend of the complex modulus, for starch-based systems, shows a first G* decay, corresponding to an increase in molecular mobility caused by the increase in temperature, followed by a more or less sharp increase of G*. This last increase is due to the gelatinisation phenomena that modify the material microstructure [
3]. The possible accentuated growth of the complex module depends on the nature and origin of the flour as well as on the quantity of water, etc. The doughs obtained with or without yogurt show an increase of G* after 60°C. S1 and SY1 show similar behaviour and similar gelatinization temperature, in agreement with other literature formulations [
27], while samples richer in Greek yogurt show a shift of the G* curve toward higher values of gelatinization temperature.
The yogurt proteins have probably a competitive effect against resistant starch in the gelatinization process.
Moreover, the angle phase (
Figure 1b) values suggest a solid-like behaviour for all samples in the temperature range investigated, with values ranging between 5° and 25°.
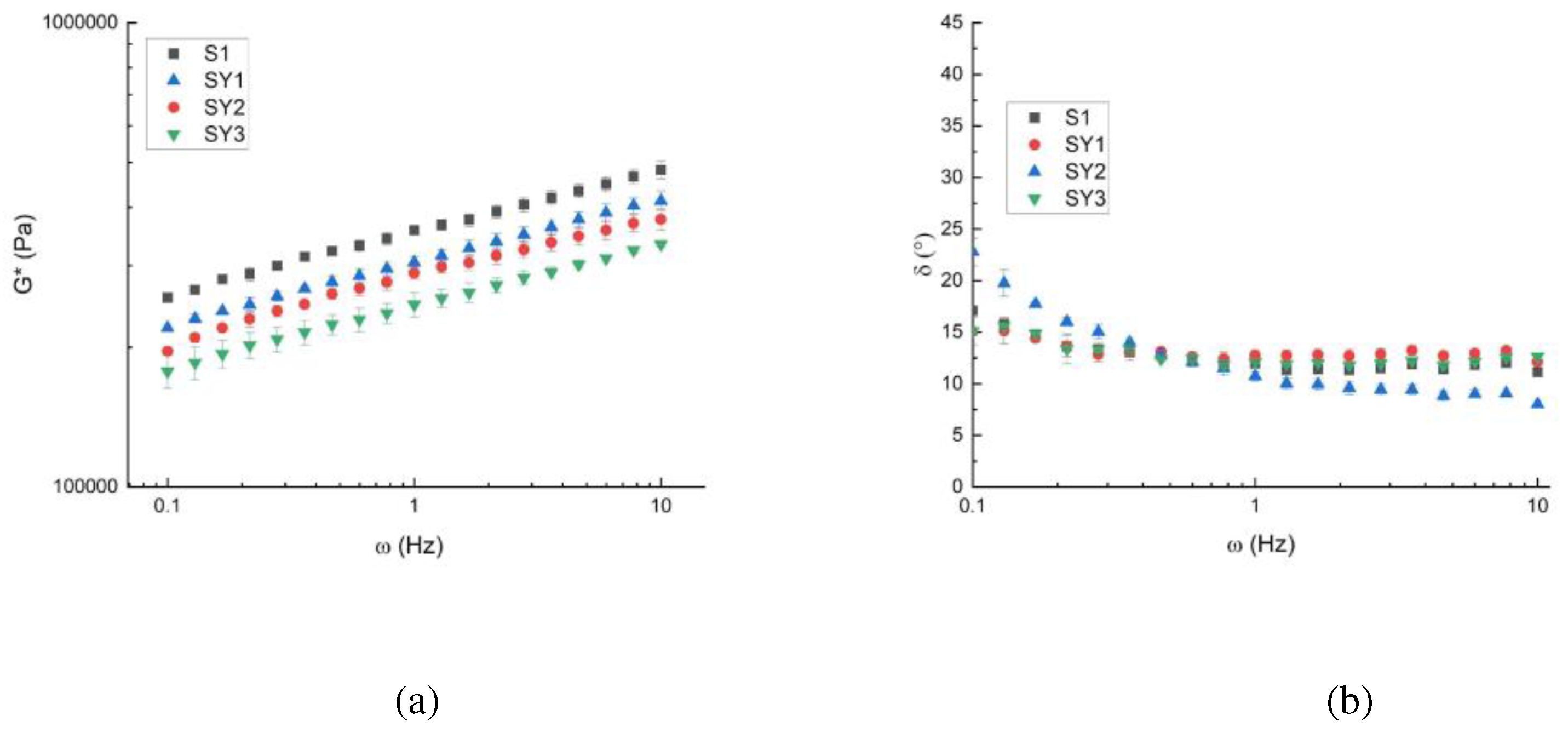
Figure 2 shows the G* and the angle phase values at different frequencies and 20° C. It is possible to see that the systems are solid-like because the δ lower than 45°. The consistency of the dough decreases (G* lowers) increasing the Greek yogurt quantity even if the structure doesn’t change too much as shown by the δ trend (
Figure 2b).
The same trend was observed for frequency sweep tests performed at 40°C and 60°C and all data were interpreted by the weak-gel model [
15]. The weak-gel parameters are reported in
Table 5.
From the data reported in
Table 5, it can be observed that for each temperature, the A parameter decreases from S1 to SY3, indicating that the addition of Greek yogurt has the effect of weakening the sample without changing the structure, as shown by
z values. On the other hand, for each sample the strength of the structure decreases with the increase in temperature and, also in this case, the z parameter is almost constant with the temperature.
3.3. Application
The formulations studied in the rheological analysis were used for the preparation of snack bars. In
Figure 3, the picture before (a) and after (b) baking of the different studied doughs were reported.
In particular, observing
Figure 3 (a), the yogurt increase in the dough gradually leads to a lighter color of the snack bar, becoming more evident in the SY3 formulation. After baking (
Figure 3 (b)), the difference in the coloring of the four formulations is markedly less visible, with similar values of color parameters. L*, a* and b* are reported in
Table 6. In fact, samples show similar values but the browning index reveals an effect in the samples prepared with yogurt. Specifically speaking, the BI parameter increases with yogurt increase suggesting for samples after cooking a more pronounced browning increasing the yogurt quantity. This effect, in agreement with literature results [
28], is related to Maillard reactions that occur in cooking, due to the content of sugars. In particular, although Greek yogurt contains lactose in low amounts [
29], the latter is involved in Maillard reactions and promotes the browning of the samples [
28].
From HPLC analysis the presence of CBD in the cooked dought was verified and quantified. In particular, a concentration of 0.36±0.01 mg
CBD/g
snack was found. The value obtained is in line with the CBD concentration extracted from the protein flour (as reported in
Table 1). this result suggests that incorporating antioxidants into the formulation can preserve their function and increase the added value of the food.
5. Conclusions
Cannabis Sativa L. plant is a very interesting potential source of functional molecules, in particular CBD and its acid precursor, CBDA. CBD is known as a non-psychoactive molecule, with antioxidant and anti-inflammatory power, widely used in the pharmaceutical field for medicinal purposes. Recently, in the food industry, the use of Cannabis Sativa L. was investigated, in particular for the production of healthy foods.
In this work, the possibility of recovery of CBDA and CBD from hemp plant wastes, inflorescences and stalks was studied. In particular, three different extraction methodologies were performed: dynamic maceration (DM), Soxhlet extraction (SE) and Supercritical fluid extraction with CO2. The extracts were analyzed by HPLC to identify and quantify CBD and CBDA species. The samples were studied also after the decarboxylation process, to obtain a higher CBD yield. Among all extraction processes investigated, DM results in the most efficient method, able to maximise CBD yield. In particular, decarboxylated samples and the inflorescences show a high CBD content. Extracts obtained with higher amounts of CBD confirm a good antioxidant power, with higher sensitivity to the ABTS method than to the DPPH method.
Finally, doughs were prepared with hemp flour adding CBD-enriched oil from HDI and they were characterized by rheological measurements in dynamic conditions. The rheological properties show a solid-like, with good mechanical strength and gelatinization temperatures around 80°C related to the presence of the resistant starch. The presence of Greek yogurt in the formulations reduces the consistency of the samples and shifts the gelatinization point to higher temperatures. The hemp-based snacks were also baked to obtain a finished food product and analyze the CBD quantity. A good quantity of CBD is present in the final food confirming the ability of the ingredients to preserve during heating at high temperatures the antioxidant properties.
This research highlights the possibility of recovering non-psychoactive antioxidant molecules from hemp, inflorescences and stalks, for use in the food industry in the production of high-value-added foods.
Author Contributions
Conceptualization, Noemi Baldino; methodology, Olga Mileti.; software, Mario F. O. Paleologo; validation, Olga Mileti and Francesca Romana Lupi.; formal analysis, Mario F. O. Paleologo; investigation, Mario F. O. Paleologo and Maria Marra; resources, Noemi Baldino; data curation, Noemi Baldino, Francesca R. Lupi and Domenico Iacopetta; writing—original draft preparation, Noemi Baldino and Olga Mileti; supervision, Domenico Gabriele. All authors have read and agreed to the published version of the manuscript
Funding
This research received no external funding
Data Availability Statement
Statement: The data used to support the findings of this study can be made available by the corresponding author upon request.
Acknowledgements
The authors are grateful to Regione Calabria (Italy), project “PSR Misura 16.2 dal titolo “DIVERSIFICAZIONE DI PRODOTTI DERIVANTI DA INFIORESCENZA E SEMI DI CANAPA” (CUP J82C22000640005)”, for funding an additional position and for the hemp inflorescences and raw materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Drinić, Z.; Vladic, J.; Koren, A.; Zeremski, T.; Stojanov, N.; Tomić, M.; Vidović, S. Application of Conventional and High-Pressure Extraction Techniques for the Isolation of Bioactive Compounds from the Aerial Part of Hemp (Cannabis Sativa L.) Assortment Helena. Ind Crops Prod 2021, 171, 113908. [Google Scholar] [CrossRef]
- Brighenti, V.; Pellati, F.; Steinbach, M.; Maran, D.; Benvenuti, S. Development of a New Extraction Technique and HPLC Method for the Analysis of Non-Psychoactive Cannabinoids in Fibre-Type Cannabis Sativa L. (Hemp). J Pharm Biomed Anal 2017, 143, 228–236. [Google Scholar] [CrossRef]
- Baldino, N.; Carnevale, I.; Mileti, O.; Aiello, D.; Lupi, F.R.; Napoli, A.; Gabriele, D. Hemp Seed Oil Extraction and Stable Emulsion Formulation with Hemp Protein Isolates. Applied Sciences (Switzerland) 2022, 12. [Google Scholar] [CrossRef]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis Sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer. Biomed Res Int 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, v. 9. 2019 v.9 no.1. [Google Scholar] [CrossRef]
- Baron, E.P. Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science. Headache: The Journal of Head and Face Pain 2018, 58, 1139–1186. [Google Scholar] [CrossRef]
- Nahar, L.; Uddin, S.J.; Alam, Md.A.; Sarker, S.D. Extraction of Naturally Occurring Cannabinoids: An Update. Phytochemical Analysis 2021, 32, 228–241. [Google Scholar] [CrossRef]
- Ryu, B.R.; Islam, M.J.; Kalam Azad, M.O.; Go, E.J.; Rahman, M.H.; Rana, M.S.; Lim, Y.S.; Lim, J.D. Conversion Characteristics of Some Major Cannabinoids from Hemp (Cannabis Sativa l.) Raw Materials by New Rapid Simultaneous Analysis Method. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.-H.; Avula, B.; Radwan, M.; Wanas, A.; Antwerp, J.; Parcher, J.; Elsohly, M.; Khan, I. Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry. Cannabis Cannabinoid Res 2016, 1, 262–271. [Google Scholar] [CrossRef] [PubMed]
- De Vita, D.; Madia, V.N.; Tudino, V.; Saccoliti, F.; De Leo, A.; Messore, A.; Roscilli, P.; Botto, A.; Pindinello, I.; Santilli, G.; et al. Comparison of Different Methods for the Extraction of Cannabinoids from Cannabis. Nat Prod Res 2020, 34, 2952–2958. [Google Scholar] [CrossRef]
- Moreno, T.; Montanes, F.; Tallon, S.J.; Fenton, T.; King, J.W. Extraction of Cannabinoids from Hemp (Cannabis Sativa L.) Using High Pressure Solvents: An Overview of Different Processing Options. J Supercrit Fluids 2020, 161, 104850. [Google Scholar] [CrossRef]
- Korus, J.; Witczak, M.; Ziobro, R.; Juszczak, L. Hemp (Cannabis Sativa Subsp. Sativa) Flour and Protein Preparation as Natural Nutrients and Structure Forming Agents in Starch Based Gluten-Free Bread. LWT 2017, 84, 143–150. [Google Scholar] [CrossRef]
- Lewis-Bakker, M.M.; Yang, Y.; Vyawahare, R.; Kotra, L.P. Extractions of Medical Cannabis Cultivars and the Role of Decarboxylation in Optimal Receptor Responses. Cannabis Cannabinoid Res 2019, 4, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Fazio, A.; La Torre, C.; Barbarossa, A.; Ceramella, J.; Francomano, F.; Saturnino, C.; El-Kashef, H.; Alcaro, S.; Sinicropi, M.S. Annona Cherimola Mill. Leaf Extracts Affect Melanoma Cells Growth and Progression. Foods 2022, 11. [Google Scholar] [CrossRef]
- Gabriele, D.; de Cindio, B.; D’Antona, P. A Weak Gel Model for Foods. Rheol Acta 2001, 40, 120–127. [Google Scholar] [CrossRef]
- Gonzales, A.P.; Burin, L.; Buera, M. del P. Color Changes during Storage of Honeys in Relation to Their Composition and Initial Color. Food Research International 1999, 32, 185–191. [Google Scholar] [CrossRef]
- Christodoulou, M.C.; Christou, A.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Evaluation of Different Extraction Procedures for the Quantification of Seven Cannabinoids in Cannabis-Based Edibles by the Use of LC-MS. Journal of Food Composition and Analysis 2023, 115, 104915. [Google Scholar] [CrossRef]
- Citti, C.; Braghiroli, D.; Vandelli, M.A.; Cannazza, G. Pharmaceutical and Biomedical Analysis of Cannabinoids: A Critical Review. J Pharm Biomed Anal 2018, 147, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Szalata, M.; Dreger, M.; Zielińska, A.; Banach, J.; Szalata, M.; Wielgus, K. Simple Extraction of Cannabinoids from Female Inflorescences of Hemp (Cannabis Sativa L.). Molecules 2022, 27. [Google Scholar] [CrossRef]
- Marrot, L.; Candelier, K.; Valette, J.; Lanvin, C.; Horvat, B.; Legan, L.; DeVallance, D.B. Valorization of Hemp Stalk Waste Through Thermochemical Conversion for Energy and Electrical Applications. Waste Biomass Valorization 2022, 13, 2267–2285. [Google Scholar] [CrossRef]
- Duan, Y.; Tao, Z.; Zhu, A.; Len, C.; Wang, Y.; Yang, W. Enhanced Valorization of Hemp Stalk via Chemo-Catalytic and Hydrothermal Conversions. Fuel 2022, 327, 125059. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H. Bin Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int J Mol Sci 2017, 18. [Google Scholar]
- Milkovic, L.; Gasparovic, A.C.; Cindric, M.; Mouthuy, P.A.; Zarkovic, N. Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for Testing Antioxidant Activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Olszowy-Tomczyk, M.; Typek, R. CBG, CBD, Δ9-THC, CBN, CBGA, CBDA and Δ9-THCA as Antioxidant Agents and Their Intervention Abilities in Antioxidant Action. Fitoterapia 2021, 152, 104915. [Google Scholar] [CrossRef]
- Baldino, N.; Carnevale, I.; Laitano, F.; Lupi, F.; Curcio, S.; Gabriele, D. Formulation of Bread Model Doughs with Resistant Starch, Vegetable Proteins and Transglutaminase. European Food Research and Technology 2020, 246. [Google Scholar] [CrossRef]
- Istrate, A.M.; Dabija, A.; Codină, G.G.; Rusu, L. INFLUENCE OF HEMP FLOUR ON DOUGH RHEOLOGY AND BREAD QUALITY; 2021; Vol. 22;
- Iuga, M.; Boestean, O.; Ghendov-Mosanu, A.; Mironeasa, S. Impact of Dairy Ingredients on Wheat Flour Dough Rheology and Bread Properties. Foods 2020, 9. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Effects of Hydrocolloids and Processing Conditions on Acid Whey Production with Reference to Greek Yogurt. Trends Food Sci Technol 2016, 56, 61–76. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).