1. Introduction
Over 250 000 men every year will be diagnosed with prostate cancer, and of these, 30 000 men will die of their disease. Radical prostatectomy remains an integral part of the treatment algorithm, particularly in men with T2-3 tumors with a Gleason score. In many clinical contexts, laparoscopy is gradually replacing conventional surgical procedures. Laparoscopy is associated with diminished mortality and less overall and severe morbidity; as well, laparoscopy has curtailed the incidence of surgical site infections, urinary tract infections, bleeding, sepsis, and septic shock [
1]. Unfortunately, laparoscopic procedures require peritoneal carbon dioxide insufflation. Carbon dioxide (CO
2) insufflation also result in various adverse events such as the potential to increase cerebral blood flow, intracranial pressure (ICP), and intraocular pressure. The increase in intra-abdominal pressure increases venous resistance, thereby decreasing blood return to the heart. However, in a hypervolemic state, the increase in mean systemic pressure overrides the resistance, resulting in increased blood return to the heart . Pneumoperitoneum also increases cerebral blood flow due to an increase in partial pressure CO
2 and an increase in catecholamine release independent of partial pressure CO
2 . A minimum of 10–15 min is required for PaCO2 elevation after pneumoperitoneum in laparoscopic procedures. In the late stage of increased ICP, the diffusion of CO2 from the peritoneum results in a reflex arterial vasodilation in the CNS, thus further increasing ICP. Compression of the lower lobes of the lungs creates a ventilation/perfusion mismatch, further increasing PaCO2[
2].
All this results in an acute rise in ICP that may be harmful to patients with head injury or unrecognized intracranial mass lesions. Raised ICP decreases cerebral perfusion pressure, and sudden changes are more deleterious The gas negatively affects not only the viscera, but also it affects the central nervous system because the resultant pneumoperitoneum leads to increased intracranial pressure (ICP) [
3]. Surgeons rarely use computer tomography and magnetic resonance imaging to assess intraoperative ICP. The “gold standard” method for monitoring intracranial pressure (ICP) includes using invasive, intraparenchymal, or intraventricular devices that are often only available in specialist neurocritical care units [
4]. Intracranial pressure (ICP) monitoring and external CSF drainage are fundamental to the management of neurosurgical patients in the critical care setting, and current methods have evolved considerably since the early days of neurosurgery. Invasive measurement is the most accurate tool to determine ICP, but it is associated with numerous complications, such as brain damage, bleeding, and infection [
5].
The optic nerve is an outward form of the diencephalon during embryogenesis. It is wrapped by the nerve sheath, which is derived from three layers of meninges and protrudes towardthe orbit. Thus, the cerebrospinal fluidmoves freely between the intracranial and intraorbital subarachnoid spaces. The intraorbital subarachnoid space surrounds the optic nerve, and it is subject to the same pressure changes as the intracranial subarachnoid space [
6]. Raised intracranial pressure (ICP) may cause secondary brain ischemia that adds insult to pathologies like traumatic brain injury, stroke and intracranial hemorrhages. It can cause complications like visual impairment, reversible or permanent neurological problems, seizures, stroke and even death.[
7,
8]
The optic nerve is responsive to ICP. The optic nerve can be thought of as an outpouching of intact brain tissue; the intraorbital component is fully encapsulated by dura, arachnoid, and pia matter, enabling the optic nerve sheath to transmit cerebral spinal fluid and fluctuate in size based on intracranial pressure. The meningeal cover of the optic nerve is the continuation of the dural and subarachnoid space. The bulbous portion of the optic nerve, approximately 3 mm posterior to the globe, appears to be the most distensible and sensitive to ICP on the basis of in-vivo models of elevated ICP and artificial creation of elevated ICP in cadaveric models.
Ocular sonography is safely used for ophthalmic evaluation since more than thirty years [
9], and has a fast learning curve:novice sonologists need only 25 scans to obtain adequate results [
10], with limited variability in measurement of ONSD, as median intra-observer and inter-observer variations were shown to be respectively less than 0.2 and 0.3 mm [
11]
Several studies have shown that measurement of ONSD is a quick and non-invasive method of detecting increased ICP and can be used as a strong and accurate predictor of increased ICP. It is also known as a method of accurately and quickly monitoring changes in ICP. Despite the popularity of ultrasound measurement of the ONSD, this technique was used mainly for the emergency situation or critical care [
12].
Ultrasonographic measurement of the optic nerve sheath diameter (ONSD) [
13,
14] is a favourable noninvasive procedure to assess the ICP of patients who undergo urologic surgery. It has become a popular approach for detecting elevated ICP. The procedure is based on the fact that hemodynamic disturbances during laparoscopic surgery lead to increased ICP. Any increase in cerebrospinal fluid pressure increases the diameter of the optic nerve sheath, and the optic nerve sheath directly communicates with the subarachnoid space. Normal ICP values correlate with ONSD values (range of 4.8‒6 mm) [
15,
16]. Furthermore, ultrasound examination is a simple, reproducible, inexpensive, and widely available modality [
17]. Thus, ultrasonographic measurement of the optic nerve sheath diameter is an ideal means to noninvasively assess ICP.
During urological laparoscopic surgery, the patient is placed in the Trendelenburg position, which has a major effect on intra- and postoperative cardiovascular and respiratory function. We noticed, that a lot of patiets after operation in Tredelenburg position have got conjunctival chemosis and edema, postoperative delirium, cognitive dysfunction and delayed awakening.
Thus: our goal was to determine whether the Trendelenburg position affects ONSD. Additionally, we investigated whether changes in ONSD were associated with postoperative nausea or vomiting, and delirium, and prolonged awakening time.
2. Materials and Methods
This study is a single center prospective observational study. The Bioethics Committee of the Jagiellonian University in Cracow, Poland approved this study on 23/02/2018 (No. 1072.6120.30.2018). The study was registered at ClinicalTrials.gov (NCT03485612) All study participants provided written consent to participate voluntarily. We excluded persons under 18 years of age and who had undergone prior ophthalmological procedures. Exclusion criteria were hemodynamic instability; history of eye surgery; current eyeball disease (e.g., cataract, glaucoma, retinal detachment); increased intracranial pressure (e.g., brain tumor, spinal cord lesion); and current medications known to influence emergence from anesthesia (e.g., benzodiazepines, opioids).
From April 2018 to August 2018, participants underwent urological laparoscopic surgery with general anaesthesia in the Trendelenburg position. Standard monitoring during anaesthesia included arterial oxygen saturation, ECG, and noninvasive arterial blood pressure. Premedication was not used. The standard anaesthesia consisted of propofol (1.5-2 mg / kg body weight iv) and fentanyl (1-2 μg / kg body weight iv) for induction. Cisatracurium (0.2 mg / kg body weight iv) was administered prior to intubation. Neuromuscular blockade monitoring was performed with an NMT device. Blouses of cisatracurium was adjusted to maintain two counts of train of four (TOF) and stopped at least 30 min before the end of surgery. Anaesthesia was maintained with sevoflurane (MAC 1-1.5) and fentanyl boluses (0.05‒1 μg / kg body weight iv). Mechanical ventilation was delivered at the tidal volume of 8 ml / kg body weight. The respiratory rate was tied to the end-expiratory CO2 (etCO2) concentration with a view to maintaining etCO2 in the 35-45 mm Hg range. The first measurement of ONSD was obtained before patients were placed in the Trendelenburg position. Then the Trendelenburg position was instituted by tilting the operating table to ca. 45 degrees. Patients remained in that position during the operation. After surgery, the patients were placed in a horizontal position, and the second measurement of ONSD was obtained. During the procedure, the pressure of the pneumoperitoneum was 10-15 mm Hg. After surgery, residual neuromuscular blockade was reversed with Neostigmine (2 mg i.v.)
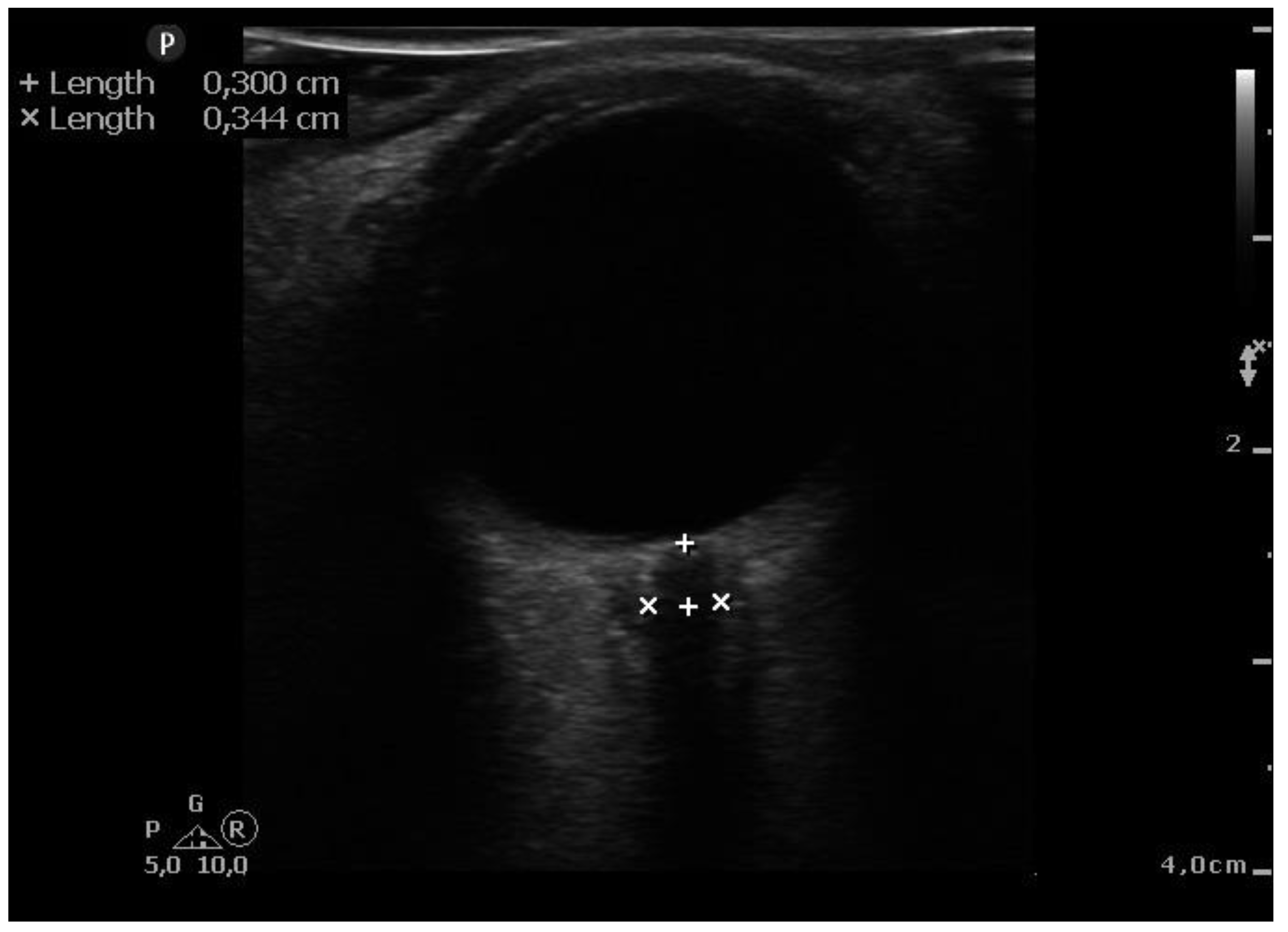
When general anesthesia induced and hemodynamically stable conditions were reached, ultra-sonographic measurements were taken. It was before introducing pneumoperitoneum (10-15 mmHg of insufflation pressure) and in the steep Trendelenburg position (45° incline) After applying water soluble lubricant, the probe was placed on the closed upper eyelid with minimal pressure in a direction parallel to the eyelid. Second measurements were taken after surgery end and immediately after position change to a supine position. ONSD ultrasound was performed using a 7.5 MHz line head L12-4 (PHILIPS SPARCK 22100 Bothell Everett Highway Bothell, WA, 98021 USA). (
Figure 1.) Two sonographers (T.S. and E. K.), who had performed > 50 scans measured ONSD, took three measurements of the same patient before and after the procedure, and the average of these measurements was taken as the final result. The ONSD measurement was standardized by measuring at a depth of 3 mm outside the posterior wall of the eyeball. Because other investigators [
16,
18] showed that ONSD > 5.0 mm correlated with increased intracranial pressure, and the cut-off point of 6.0 mm increases the sensitivity and specificity of the test, we used 6.0 mm as the reference value.
Attending anesthesiologist, checked the patient’s consciousness and evaluated if the patient met the pre-determined extubation criteria of (1) sufficient reflexes to protect the airway; (2) adequate gas exchange (respiration rate 10–30 breaths per minute and tidal volume ≥ 6 mL/kg); (3) TOF ratio ≥ 0.9; and (4) Observer Assessment of Alertness/Sedation Scale ≥ 2. Patients were extubated in the operating room and transferred to a post-anesthesia care unit for at least 3 hours. In the postoperative period, we monitored the incidence of nausea, delirium, and the time to patient awakening.
Statistical analysis
The calculation of the sample size was made using statistical power analysis based on the difference between the average ONSD values in the first 10 patients, for which the statistical power of 80% was considered. The test returned a minimum sample size of 69 patients. The statistical analysis was performed with Statistica 10.0 software. The Wilcoxon and Mann-Whitney U tests were used to determine the statistical significance of the ONSD differences and variables between groups. The significance level was set at 0.05.
3. Results
Seventy-three patients initially qualified for the study. Four patients had poor image quality, and no data were obtained; thus, they were excluded from the study, leaving 69 ASA I and ASA II patients. Sixty-one patients (88%) were men and 8 (12%) were women (
Table 1). The urological procedures included 45 prostatectomies, 11 resections of kidney tumours, 4 cystectomies, 4 lymphadenectomies, 3 Anderson-Hynes plasticities, and 2 others. The mean age of the patients was 63 ± 9 years. The average BMI value was 27.7 kg / cm
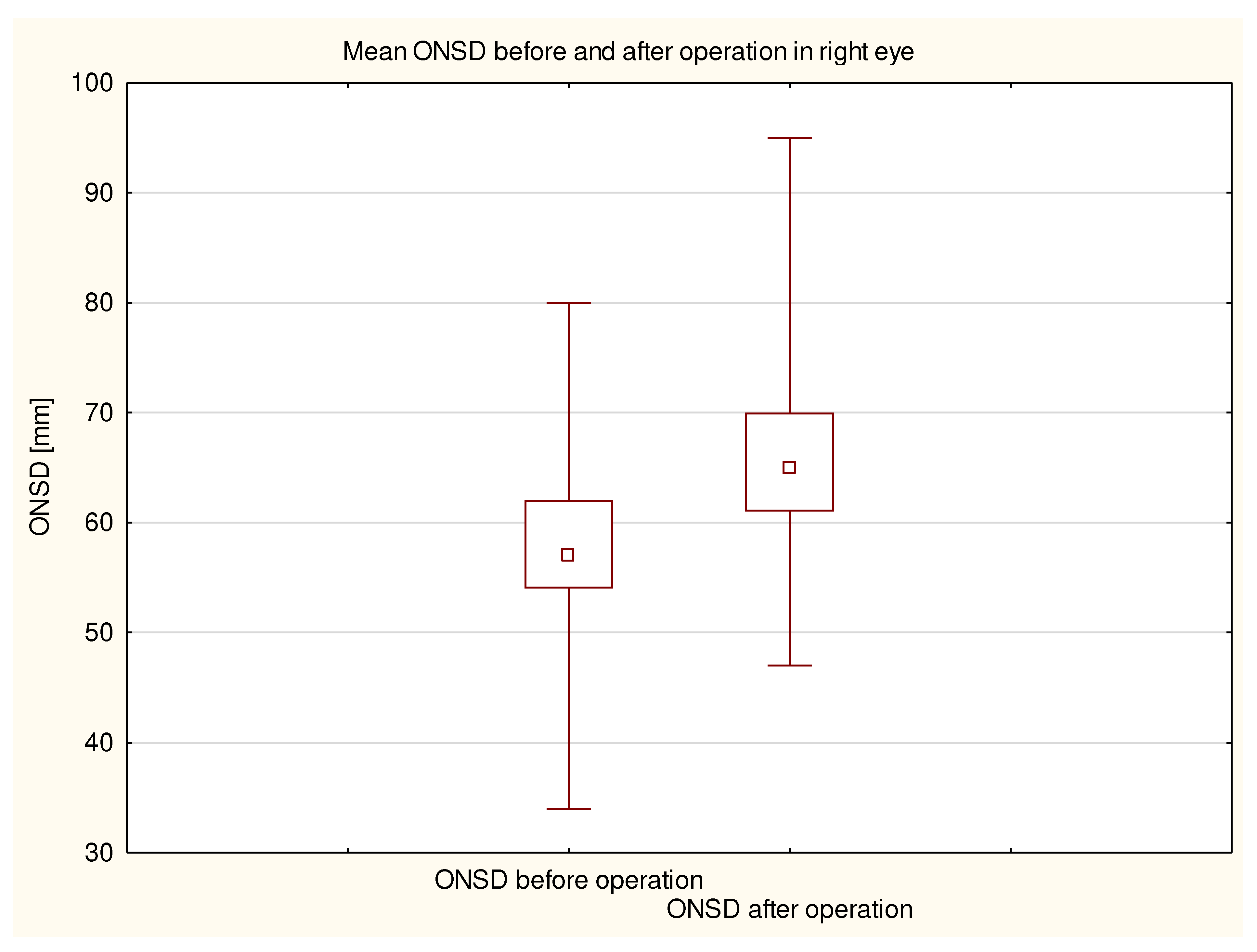
2. The average duration of the procedures was 174 ± 80 minutes. The average amount of fluids obtained during the procedure was 1469 ± 653 ml. The average preoperative ONSD in the right eye was 5.8 ± 0.7 mm (
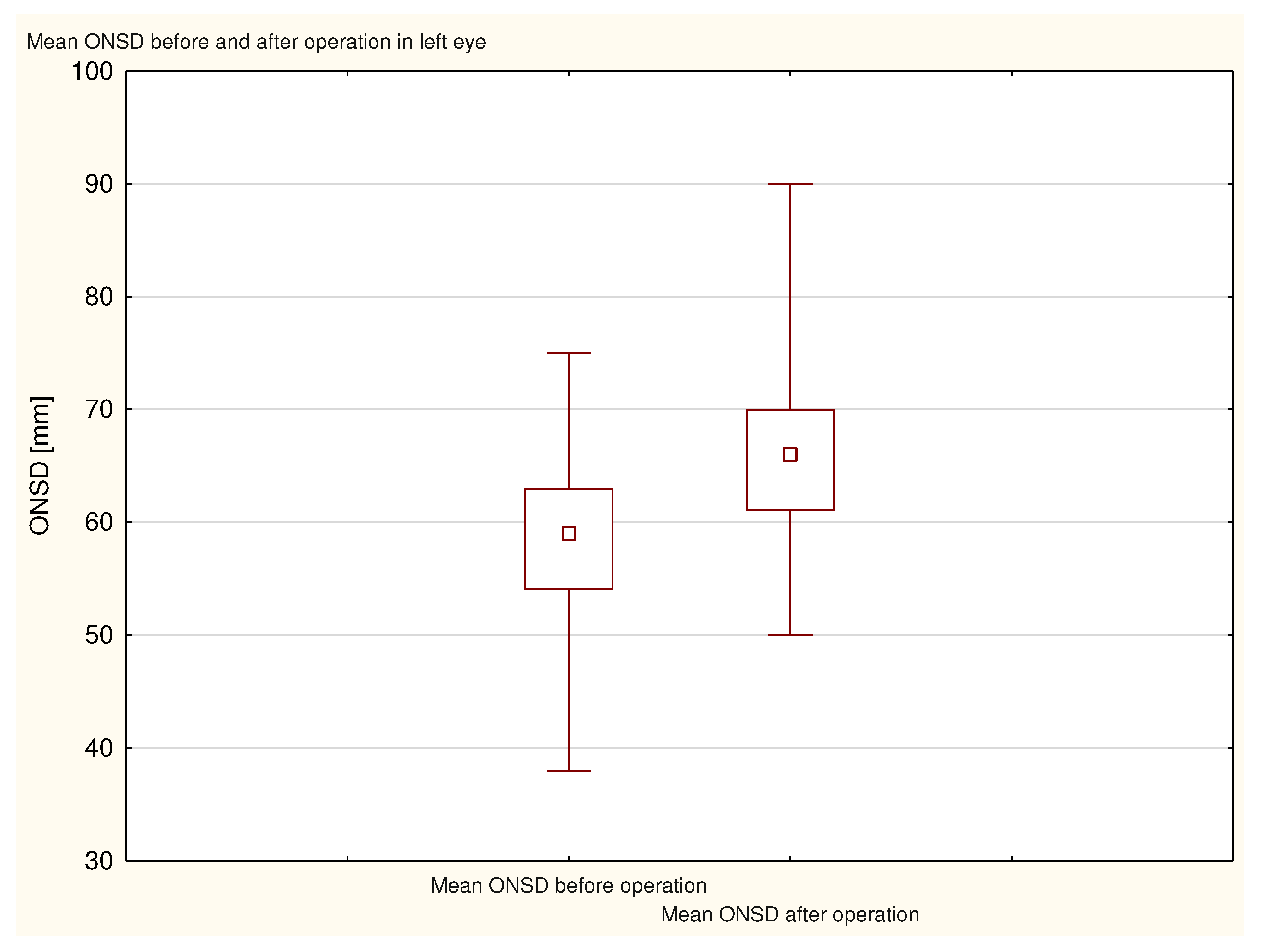
Figure 2), and the average preoperative ONSD in the left eye was 5.8 ± 0.8 mm (
Figure 3). The average postoperative ONSD in the right eye was 6.6 ± 0.8 mm, and the average postoperative ONSD in the left eye was 6.6 ± 0.7 mm. In 33 patients there was a difference in excess of the ONSD norm in one eye before and after operation. All ONSD measurements were statistically significant (p <0.000001;
Table 2).
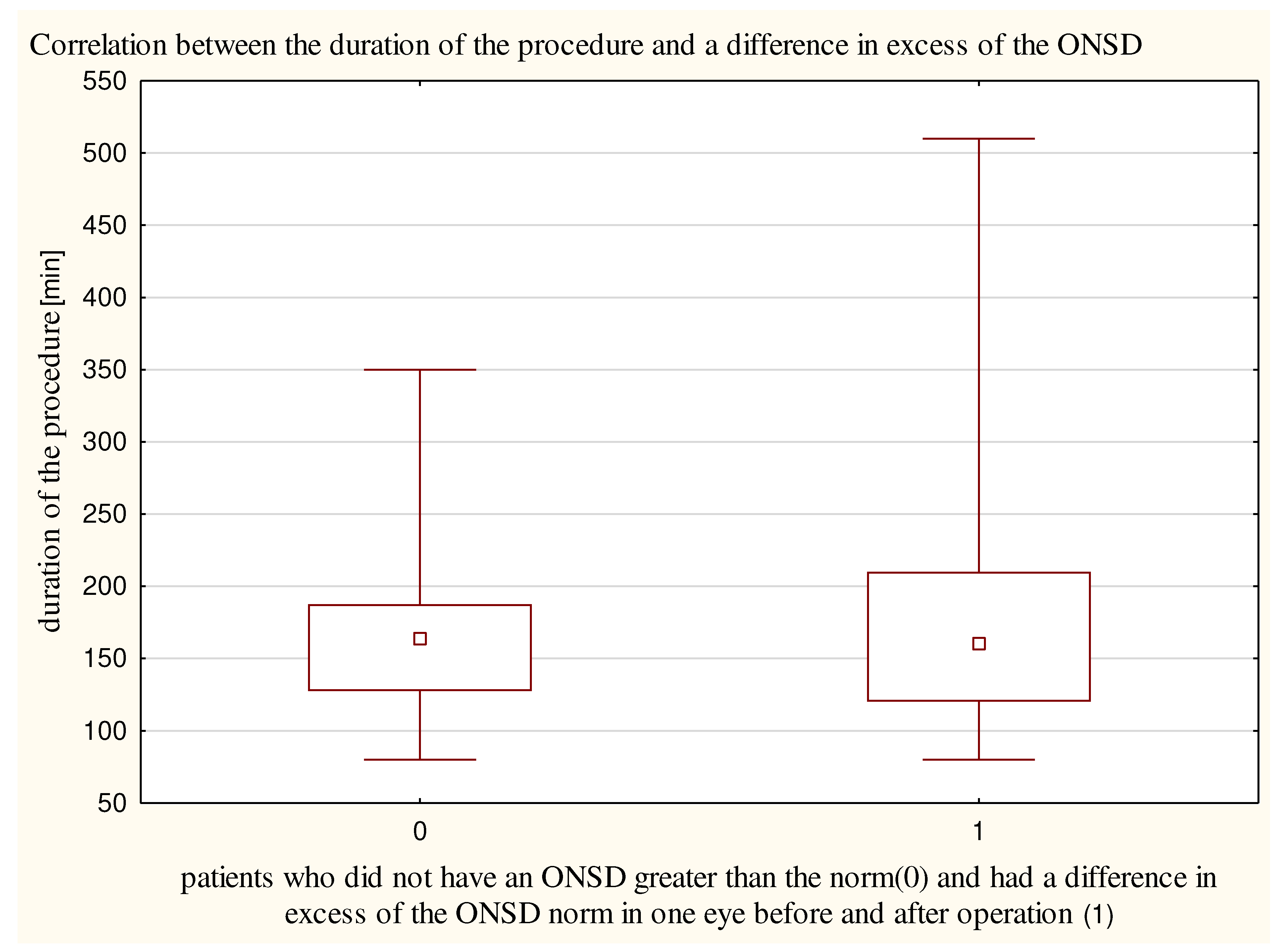
The duration of the procedure did not differ significantly (p = 0.96) between patients who had a difference in excess of the ONSD norm in one eye before and after operation (182±99 minutes) (
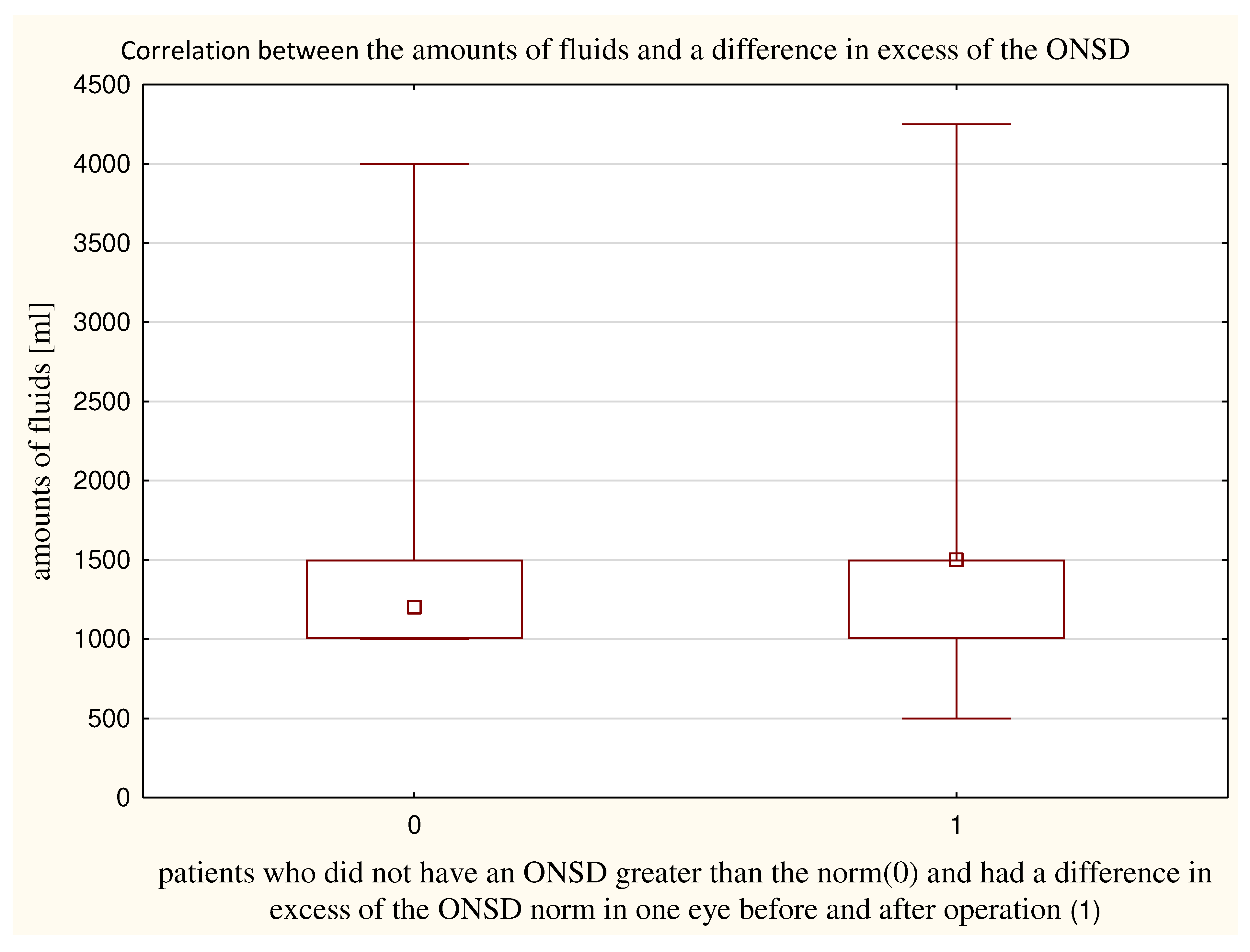
Figure 4) and patients who did not have an ONSD greater than the norm (167±57 minutes). The amounts of fluids obtained did not differ significantly (p = 0.15) between patients who had a difference in exceeding norm of ONSD in one eye before and after (1574±747 ml) and patients who did not exceed the norm of ONSD (1372±561 ml) (
Figure 5).
One patient who had a difference in exceeding norm of ONSD in one eye before and after had postoperative nausea, and two patients who did not exceed the norm of ONSD had postoperative nausea and vomiting (PONV) (p = 0.94).
Four patients who had a difference in exceeding norm of ONSD in one eye before and after had postoperative delirium, and four patients who did not exceed the norm of ONSD also had postoperative delirium (p = 0.81).
We considered that waking more than 10 minutes from the end of the procedure to be a prolonged awakening time. In both groups, 15 patients had prolonged awakening time (p= 0.29)
4. Discussion
The point of care ultrasound and especially the measurement of the ONSD for the purpose of assessing ICP has generated significant interest in the last decade. The ICP measure is very invasive, that is why less invasive techniques should be use to detect pathophysiological changes in ICP. The clinical implications might include the avoidance of craniectomy or dangerous transport in critically ill or trauma patients at high risk of complications in case of further elevation of the ICP.
We found that ONSD increased in patients who underwent urological procedures performed in the Trendelenburg position. The Trendelenburg position is often used during laparoscopic procedures to improve the visibility of the target organs. In our study, although we proved that this position led to increased ONSD above the norm, the position did not have any significant effect on the incidence of postoperative complications. In a study conducted by Kim et al. [
19] with a group of 64 patients, there were no significant differences in ONSD between patients placed in the Trendelenburg position vs. patients placed in the anti-Trendelenburg position within 30 minutes of the establishment of the pneumoperitoneum. Dip et al. [
20] porved in patients operated laparoscopic without Trendelenburg position the mean ONSD was increasing significantly at 15 min by a median of 0.6 mm and at 30 min by a median of 1.0 mm. Kim et al. [
21] also found that the ONSD increased by 12.5% at 10 and 30 minutes after the establishment of the pneumoperitoneum for 20 patients who underwent robotic laparoscopic radical prostatectomy. Verdonck e al. [
22] concluded that ONSD did not change in 20 patients during robotic laparoscopic radical prostatectomy and the total time in Trendelenburg position was 213 minutes. In Blecha et al. [
23] study 51 patients had a robotic-assisted laparoscopic prostatectomy. The duration of surgery was 218 minuters, and the change of ONSD was not correlate with time of surgery. In our study the average duration of the procedures was similar (174 ± 80 minutes), but we had more patients and differentiation in procedrures. In Yilmaz et al. study [
24] the mean duration of the laparoscopic hysterectomy was 78±12 minutes and it correlate with significant increase in ONSD.
Robba et al. performed a systematic review and meta-analysis of seven studies (320 patients) to evaluate the diagnostic accuracy of sonographic ONSD measurements of adults [
25]. They found that thresholds in the range of 4.80–6.30 mm demonstrated robust prediction ability (AUC of 0.94) for the assessment of intracranial hypertension (applying a threshold of > 20 mmHg or > 25 cmH
20).
Verdonck et al. [
22] stated that ONSD did not change during robotic laparoscopic radical prostatectomy for 20 patients. Intracranial pressure increased during CO
2 pneumoperitoneum and head-down positioning, but no changes were found in ONSD in the head-down position. This lack of change was due to the compensatory mechanisms that respond to the increase in the intracranial blood volume and the resultant ICP increase.
The depth of anesthesia and the average age of patients may be the cause of the inconsistent results. In Blecha et al. [
23] study the ONSD variation was greater in older patients than in patients aged less than 63 years, which may suggest that self-regulation of ICP is better in younger patients. The depth of anesthesia may affect the changes in CBF, and thus the changes in ICP and ONSD. In this study, the ONSD did not significantly exceed the initial value (maximum rise 0.2 mm). The differences in the changes in the ONSD during operation were age-related. Younger patients had a significantly higher baseline ONSD under mechanical ventilation, suggesting higher elasticity of the dura mater. Interestingly, the ONSD was on average 0.21 mm wider (
P = 0.017) in patients aged <63 years (median age) and showed greater variations in diameter than in older patients. In our study most of the patients (n=39) were over 63 years old, it could affected excess of the ONSD norm in one eye before and after operation. In the group over 63 years old, 18 patients there was a difference in excess of the ONSD norm in one eye before and after operation. Elderly patients lack autoregulation of cerebral blood flow and are unable to tolerate a prolonged elevated ICP.
Acute rise in ICP might be destructive for pa tients with unrecognized intracerebral vascular and mass lesions. Moreover, even in the absence of these pathologies, increase in ICP might lead to PONV. PONV is closely related to patient satisfaction, length of hospital stay, and morbidity. In Yilmaz et al. [
24] study in 61 patients the median ONSD after operation was sig nificantly higher compared to the baseline values (5.1 mm vs. 4.9 mm). Patients experienc ing PONV within 3 hours of recovery had a significantly higher change in ONSD. The results of Yilmaz et al. [
24] study show that the extent of the increase in ONSD during the procedure is significantly correlated with PONV occurring within the first three hours of recovery. In our study only 3 patients had PONV in postoperative period. There was no correlation with PONV and change in ONSD. This discrepancy is probably due to the fact that our study enrolled mostly elderly male patients, and female patients vulnerable to PONV. In Kim et al. study [
26] 42 female patients undergoing robot-assisted laparoscopic surgery concluded the significant increase in the ONSD of patients with a smaller ONSD increase during propofol anesthesia compared to sevoflurane anesthesia. Two patients from the Sevoflurane group experienced PONV, but this number was not statistically significant.
Bang et al.[
27] enrolled 67 patients Undergoing Robot-Assisted Laparoscopic Prostatectomy, 36 patients had an ONSD increase of 10% or more and only five patients showed increased values of ONSD (>5.7 mm). Significant differences in inadequate emergence were observed only after extubation. These results suggest that the increase in ONSD can potentially predict inadequate emergence which suggests that the adverse effects of elevated ICP, persist only for a short period. In our study there was no significance correlation between changes in ONSD and delirium or prolonged awakening time.
In animal studies [
28,
29,
30], investigators found that in peritoneal depression and Trendelenburg position the intracranial pressure increased by 10 mmHg above baseline. According to the Monro-Kellie doctrine, the volume of the intracranial space, composed of brain tissue, blood, and cerebrospinal fluid, is constant. By preserving the balance between these components, clinicians can maintain the correct ICP [
25,
31]. Any increase in blood volume (patient placement and pneumoperitoneum) should decrease the volume of the cerebrospinal fluid, so the ICP remains constant. However the increase in ICP was initially explained by impaired drainage of cerebrospinal fluid at the lumbar plexus level resulting from the increased intraabdominal pressure associated with the pneumoperitoneum applied during laparoscopic surgery. Rosenthal et al. [
32] described a two-staged mechanism of ICP elevation in laparoscopic surgery. They proposed that an increase in intraabdominal pres sure due to pneumoperitoneum compresses inferior vena cava and impairs venous drainage from the lumbar plexus. The in crease in intraabdominal pressure and The compression on inferior vena cava elevate the diaphragm. Because of these the intrathoracic pressure increases which further impairs right atrial and ventricular filling and impairs also the drainage of superior vena cava. As a result of increase in central venous pressure and the resultant de crease in drainage from lumbar plexus and cen tral nervous system are likely to contribute to the elevation of ICP occurring during laparoscopic surgery.
Animal studies by Kalmar et al. [
33] showed that an increase in ICP caused intravenous fluid displacement at the rate of 2 ml / min, which was required to maintain normal ICP. Moreover, ICP changes can be monitored in real time by ultrasound assessment of ONSD. Wang et al. confirmed the legitimacy of using ultrasound to assess ICP by examining the intracranial pressure and ONSD in 60 patients [
6]; they found a strong correlation between both parameters. Compared with CT and MRI, ultrasound is less expensive, easier to access, less time-consuming, and free from causing patient complications [
24,
34]. MRI studies in the paediatric population confirmed the correlation between increased ONSD and increased ICP [
35,
36]. Sekhon et al. assessed the relation between ONSD and CT analysis in a group of 57 patients with post-traumatic brain injury [
37]; the investigators found a strong correlation between ONSD as measured by CT and ICP. Moreover, with a cut-off at 6.0 mm, the ONSD had a sensitivity of 97%, specificity of 42%, a positive predictive value of 67%, and a negative predictive value of 92%. The meta-analysis performed by Kim et al. [
18] with a group of 460 patients revealed that increased ICP during a laparoscopic procedure may result in increased ONSD. The ONSD returned to normal after the pneumoperitoneum was evacuated.
5. Limitation
This study had possible limitations. Anesthetic agents used to maintain anesthesia have variable effects on ICP. Inhaled anesthetics may have influenced the ICP.
Propofol and sevoflurane have different effects on ICP [
38]. Sevoflurane produces an intrinsic cerebral vasodilatory effect resulting from vascular smooth muscle cell relaxation mediated by calcium and potassium ions [
39]. Thus, cerebral blood flow and ICP increase during sevoflurane anesthesia under clinical anesthetic dosages, which are above 1.0 MAC [
39]. Propofol causes cerebral vasoconstriction, and cerebral blood flow is reduced following cerebral metabolic rate suppression [
40]. Cerebral blood flow decreases relative to the dose-dependent depression of cerebral metabolic rate during propofol anesthesia [
40]. Therefore, propofol reduces or maintains ICP.
Dose-related reductions in cerebral blood flow (CBF), ICP, and cerebral metabolic rate have been reported during propofol anesthesia, all volatile anesthetics (such as desflurane and sevoflurane, among others) have a direct, dose-dependent vasodilator effect on the cerebral vessels, resulting in an increase in cerebral blood volume and ICP [
41] Anesthetic agents used to maintain anesthesia have variable effects on ICP.
In Yang et al. [
42] meta-analysis of data from 379 patients in 7 RCTs, were found that the ONSD was significantly lower during propofol anesthesia than during inhalation anesthesia at 30 and 60 minutes after changing to the Trendelenburg position and introducing a pneumoperitoneum. We maintained anaesthesia with sevoflurane, so it could affected the results of the ONSD.
Additionally, we did not have a control group because we investigated our routine protocol as an observational study. There was no assessment of direct measuring of ICP. Prospective randomized studies are warranted to verify our results.
6. Conclusions
Perioperative assessment of ONSD is useful. ONSD increased in patients who underwent procedures performed in the Trendelenburg position. The Trendelenburg position can be considered safe for laparoscopic urological procedures.
Funding
This study was funded by Jagiellonian University – Collegium Medicum, grant number N41/DBS/000891.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Bioethics Committee of the Jagiellonian University in Cracow on 23/02/2018 (No. 1072.6120.30.2018). ClinicalTrials.gov Identifier: NCT03485612.
Conflicts of Interest
All authors do not have any conflict of interest.
References
- Caras RJ, Lustik MB, Kern SQ, Sterbis JR, McMann LP. Laparoscopic radical prostatectomy demonstrates less morbidity than open radical prostatectomy: an analysis of the American College of Surgeons-National Surgical Quality Improvement Program database with a focus on surgical trainee involvement. J Endourol. 2014 Mar;28(3):298-305. Epub 2013 Dec 10. PMID: 24164643. [CrossRef]
- Hargreaves DM. Hypercapnia and raised cerebrospinal fluid pressure. Anaesthesia. 1990 Dec;45(12):1096-7. PMID: 2278350. . [CrossRef]
- Sahay N, Sharma S, Bhadani UK, Singh A, Sinha C, Sahay A, Ranjan A, Agarwal M. Effect of Pneumoperitoneum and Patient Positioning on Intracranial Pressures during Laparoscopy: A Prospective Comparative Study. J Minim Invasive Gynecol. 2018 Jan;25(1):147-152. Epub 2017 Sep 12. PMID: 28918894. [CrossRef]
- Citerio G, Bakker J, Bassetti M, Benoit D, Cecconi M, Curtis JR, Hernandez G, Herridge M, Jaber S, Joannidis M, Papazian L, Peters M, Singer P, Smith M, Soares M, Torres A, Vieillard-Baron A, Timsit JF, Azoulay E. Year in review in Intensive Care Medicine 2013: I. Acute kidney injury, ultrasound, hemodynamics, cardiac arrest, transfusion, neurocritical care, and nutrition. Intensive Care Med. 2014 Feb;40(2):147-159. Epub 2013 Dec 13. PMID: 24337402. [CrossRef]
- Tavakoli S, Peitz G, Ares W, Hafeez S, Grandhi R. Complications of invasive intracranial pressure monitoring devices in neurocritical care. Neurosurg Focus. 2017 Nov;43(5):E6. PMID: 29088962. [CrossRef]
- Wang LJ, Chen LM, Chen Y, Bao LY, Zheng NN, Wang YZ, Xing YQ. Ultrasonography Assessments of Optic Nerve Sheath Diameter as a Noninvasive and Dynamic Method of Detecting Changes in Intracranial Pressure. JAMA Ophthalmol. 2018 Mar 1;136(3):250-256. PMID: 29392301; PMCID: PMC5885896. [CrossRef]
- Rajajee V, Vanaman M, Fletcher JJ, Jacobs TL. Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care. 2011 Dec;15(3):506-15. PMID: 21769456. [CrossRef]
- Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2011 Jul;37(7):1059-68. Epub 2011 Apr 20. PMID: 21505900. [CrossRef]
- Munk PL, Vellet AD, Levin M, Lin DT, Collyer RT. Sonography of the eye. AJR Am J Roentgenol. 1991 Nov;157(5):1079-86. PMID: 1927796. [CrossRef]
- Tayal VS, Neulander M, Norton HJ, Foster T, Saunders T, Blaivas M. Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med. 2007 Apr;49(4):508-14. Epub 2006 Sep 25. PMID: 16997419. [CrossRef]
- Soldatos T, Karakitsos D, Chatzimichail K, Papathanasiou M, Gouliamos A, Karabinis A. Optic nerve sonography in the diagnostic evaluation of adult brain injury. Crit Care. 2008;12(3):R67. Epub 2008 May 13. PMID: 18477382; PMCID: PMC2481450. [CrossRef]
- Amini A, Kariman H, Arhami Dolatabadi A, Hatamabadi HR, Derakhshanfar H, Mansouri B, Safari S, Eqtesadi R. Use of the sonographic diameter of optic nerve sheath to estimate intracranial pressure. Am J Emerg Med. 2013 Jan;31(1):236-9. Epub 2012 Aug 31. PMID: 22944553. [CrossRef]
- Robba C, Goffi A, Geeraerts T, Cardim D, Via G, Czosnyka M, Park S, Sarwal A, Padayachy L, Rasulo F, Citerio G. Brain ultrasonography: methodology, basic and advanced principles and clinical applications. A narrative review. Intensive Care Med. 2019 Jul;45(7):913-927. Epub 2019 Apr 25. PMID: 31025061. [CrossRef]
- Wolthers SA, Engelholm CP, Uslu B, Brandt CT. Noninvasive intracranial pressure monitoring in central nervous system infections. Minerva Anestesiol. 2023 Mar;89(3):206-216Epub 2022 Nov 24. PMID: 36422116. . [CrossRef]
- Robba C, Bacigaluppi S, Cardim D, Donnelly J, Bertuccio A, Czosnyka M. Non-invasive assessment of intracranial pressure. Acta Neurol Scand. 2016 Jul;134(1):4-21. Epub 2015 Oct 30. PMID: 26515159. [CrossRef]
- Maissan IM, Dirven PJ, Haitsma IK, Hoeks SE, Gommers D, Stolker RJ. Ultrasonographic measured optic nerve sheath diameter as an accurate and quick monitor for changes in intracranial pressure. J Neurosurg. 2015 Sep;123(3):743-7. Epub 2015 May 8. PMID: 25955869. [CrossRef]
- Blaivas M, Theodoro D, Sierzenski PR. Elevated intracranial pressure detected by bedside emergency ultrasonography of the optic nerve sheath. Acad Emerg Med. 2003 Apr;10(4):376-81. PMID: 12670853. [CrossRef]
- Kim EJ, Koo BN, Choi SH, Park K, Kim MS. Ultrasonographic optic nerve sheath diameter for predicting elevated intracranial pressure during laparoscopic surgery: a systematic review and meta-analysis. Surg Endosc. 2018 Jan;32(1):175-182. Epub 2017 Jun 21. PMID: 28639043. . [CrossRef]
- Kim SH, Kim HJ, Jung KT. Position does not affect the optic nerve sheath diameter during laparoscopy. Korean J Anesthesiol. 2015 Aug;68(4):358-63. Epub 2015 Jul 28. PMID: 26257848; PMCID: PMC4524934. [CrossRef]
- Dip F, Nguyen D, Rosales A, Sasson M, Lo Menzo E, Szomstein S, Rosenthal R. Impact of controlled intraabdominal pressure on the optic nerve sheath diameter during laparoscopic procedures. Surg Endosc. 2016 Jan;30(1):44-9. Epub 2015 Apr 22. PMID: 25899811. [CrossRef]
- Kim MS, Bai SJ, Lee JR, Choi YD, Kim YJ, Choi SH. Increase in intracranial pressure during carbon dioxide pneumoperitoneum with steep trendelenburg positioning proven by ultrasonographic measurement of optic nerve sheath diameter. J Endourol. 2014 Jul;28(7):801-6. Epub 2014 Mar 5. PMID: 24517270. [CrossRef]
- Verdonck P, Kalmar AF, Suy K, Geeraerts T, Vercauteren M, Mottrie A, De Wolf AM, Hendrickx JF. Optic nerve sheath diameter remains constant during robot assisted laparoscopic radical prostatectomy. PLoS One. 2014 Nov 4;9(11):e111916. Erratum in: PLoS One. 2015;10(2):e0118014. PMID: 25369152; PMCID: PMC4219812. [CrossRef]
- Blecha S, Harth M, Schlachetzki F, Zeman F, Blecha C, Flora P, Burger M, Denzinger S, Graf BM, Helbig H, Pawlik MT. Changes in intraocular pressure and optic nerve sheath diameter in patients undergoing robotic-assisted laparoscopic prostatectomy in steep 45° Trendelenburg position. BMC Anesthesiol. 2017 Mar 11;17(1):40. PMID: 28284189; PMCID: PMC5351936. . [CrossRef]
- Yilmaz G, Akca A, Kiyak H, Salihoglu Z. Elevation in optic nerve sheath diameter due to the pneumoperitoneum and Trendelenburg is associated to postoperative nausea, vomiting and headache in patients undergoing laparoscopic hysterectomy. Minerva Anestesiol. 2020 Mar;86(3):270-276. Epub 2019 Oct 28. PMID: 31680498. . [CrossRef]
- Robba C, Santori G, Czosnyka M, Corradi F, Bragazzi N, Padayachy L, Taccone FS, Citerio G. Optic nerve sheath diameter measured sonographically as non-invasive estimator of intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2018 Aug;44(8):1284-1294. Epub 2018 Jul 17. PMID: 30019201. [CrossRef]
- Kim JE, Koh SY, Jun IJ. Comparison of the Effects of Propofol and Sevoflurane Anesthesia on Optic Nerve Sheath Diameter in Robot-Assisted Laparoscopic Gynecology Surgery: A Randomized Controlled Trial. J Clin Med. 2022 Apr 12;11(8):2161. PMID: 35456254; PMCID: PMC9024447. [CrossRef]
- Bang YJ, Jeong H, Heo BY, Shin BS, Sim WS, Kim DK, Lee SH, Kim JS, Shin YH. Effects of Increased Optic Nerve Sheath Diameter on Inadequate Emergence from Anesthesia in Patients Undergoing Robot-Assisted Laparoscopic Prostatectomy: A Prospective Observational Study. Diagnostics (Basel). 2021 Dec 2;11(12):2260. PMID: 34943497; PMCID: PMC8699939. [CrossRef]
- Mavrocordatos P, Bissonnette B, Ravussin P. Effects of neck position and head elevation on intracranial pressure in anaesthetized neurosurgical patients: preliminary results. J Neurosurg Anesthesiol. 2000 Jan;12(1):10-4. PMID: 10636614. [CrossRef]
- Josephs LG, Este-McDonald JR, Birkett DH, Hirsch EF. Diagnostic laparoscopy increases intracranial pressure. J Trauma. 1994 Jun;36(6):815-8; discussion 818-9. PMID: 8015003. [CrossRef]
- Rosenthal RJ, Hiatt JR, Phillips EH, Hewitt W, Demetriou AA, Grode M. Intracranial pressure. Effects of pneumoperitoneum in a large-animal model. Surg Endosc. 1997 Apr;11(4):376-80. PMID: 9094281. [CrossRef]
- Eklund A, Smielewski P, Chambers I, Alperin N, Malm J, Czosnyka M, Marmarou A. Assessment of cerebrospinal fluid outflow resistance. Med Biol Eng Comput. 2007 Aug;45(8):719-35. Epub 2007 Jul 17. PMID: 17634761. [CrossRef]
- Rosenthal RJ, Friedman RL, Kahn AM, Martz J, Thiagarajah S, Cohen D, Shi Q, Nussbaum M. Reasons for intracranial hypertension and hemodynamic instability during acute elevations of intra-abdominal pressure: observations in a large animal model. J Gastrointest Surg. 1998 Sep-Oct;2(5):415-25. PMID: 9843600. [CrossRef]
- Kalmar AF, De Ley G, Van Den Broecke C, Van Aken J, Struys MM, Praet MM, Mortier EP. Influence of an increased intracranial pressure on cerebral and systemic haemodynamics during endoscopic neurosurgery: an animal model. Br J Anaesth. 2009 Mar;102(3):361-8. Epub 2009 Feb 2. PMID: 19189987. [CrossRef]
- Helmke K, Hansen HC. Fundamentals of transorbital sonographic evaluation of optic nerve sheath expansion under intracranial hypertension II. Patient study. Pediatr Radiol. 1996 Oct;26(10):706-10. PMID: 8805600. [CrossRef]
- Singhal A, Yang MM, Sargent MA, Cochrane DD. Does optic nerve sheath diameter on MRI decrease with clinically improved pediatric hydrocephalus? Childs Nerv Syst. 2013 Feb;29(2):269-74. Epub 2012 Oct 27. PMID: 23103958. [CrossRef]
- Padayachy LC, Kilborn T, Carrara H, Figaji AA, Fieggen GA. Change in optic nerve sheath diameter as a radiological marker of outcome from endoscopic third ventriculostomy in children. Childs Nerv Syst. 2015 May;31(5):721-8. Epub 2015 Mar 4. PMID: 25735849. [CrossRef]
- Sekhon MS, Griesdale DE, Robba C, McGlashan N, Needham E, Walland K, Shook AC, Smielewski P, Czosnyka M, Gupta AK, Menon DK. Optic nerve sheath diameter on computed tomography is correlated with simultaneously measured intracranial pressure in patients with severe traumatic brain injury. Intensive Care Med. 2014 Sep;40(9):1267-74. Epub 2014 Jul 18. Erratum in: Intensive Care Med. 2015 Jan;41(1):177. Erratum in: Intensive Care Med. 2015 Jan;41(1):177. PMID: 25034476. [CrossRef]
- Kuzkov VV, Obraztsov MY, Ivashchenko OY, Ivashchenko NY, Gorenkov VM, Kirov MY. Total Intravenous Versus Volatile Induction and Maintenance of Anesthesia in Elective Carotid Endarterectomy: Effects on Cerebral Oxygenation and Cognitive Functions. J Cardiothorac Vasc Anesth. 2018 Aug;32(4):1701-1708. Epub 2018 Jan 5. PMID: 29402628. [CrossRef]
- Iida H, Ohata H, Iida M, Watanabe Y, Dohi S. Isoflurane and sevoflurane induce vasodilation of cerebral vessels via ATP-sensitive K+ channel activation. Anesthesiology. 1998 Oct;89(4):954-60PMID: 9778013. [CrossRef]
- Klein KU, Stadie A, Fukui K, Schramm P, Werner C, Oertel J, Engelhard K, Fischer G. Measurement of cortical microcirculation during intracranial aneurysm surgery by combined laser-Doppler flowmetry and photospectrometry. Neurosurgery. 2011 Aug;69(2):391-8. PMID: 21430590. [CrossRef]
- Hassan WMNW, Nasir YM, Zaini RHM, Shukeri WFWM. Target-controlled Infusion Propofol Versus Sevoflurane Anaesthesia for Emergency Traumatic Brain Surgery: Comparison of the Outcomes. Malays J Med Sci. 2017 Oct;24(5):73-82. Epub 2017 Oct 26. PMID: 29386974; PMCID: PMC5772817. [CrossRef]
- Yang J, Yang X, Li X, Ou S. Effects of propofol and inhalational anesthetics on the optic nerve sheath diameter in patients undergoing surgery in the steep Trendelenburg position: a systematic review and meta-analysis. Ann Palliat Med. 2021 Oct;10(10):10475-10485. PMID: 34763494. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).