1. Introduction
Knee osteoarthritis (OA) is a common progressive joint disease, characterized by chronic pain and functional disability [
1]. The pooled global prevalence of knee OA in the year 2020 was 16.0% in individuals above 15 years of age and 22.9% in those aged 40 years and over [
2]. The prevalence and incidence of knee OA increases with age and is higher in women than in men [
2]. It represents a substantial and increasing health burden with considerable personal, economic, and societal toll [
1,
3].
OA is characterized by the degradation of articular cartilage and bone matrix components [
4]. The aim of treatment is to preserve joints in order to improve pain, restore activity, and delay arthroplasty [
5]. The various treatment options for OA include pharmacological approaches, bone marrow stimulation techniques, cartilage or chondrogenic tissue-based repair, osteochondral autologous transplantation, autologous chondrocyte implantation [
5], intra-articular injections of corticosteroids, hyaluronic acid, polynucleotides, oxygen-ozone therapy, platelet-rich plasma, subchondral injections of platelet-rich plasma or calcium phosphate and intra-meniscal injections of micro-fragmented adipose tissue rich in stromal vascular fraction [
6]. However, none of the aforementioned treatment options effectively arrest structural deterioration of cartilage and bone or successfully reverse existing structural defects [
3,
4,
7]. They also do not enable regeneration of the articular tissue with its distinct functional characteristics [
8]. As OA is a highly heterogeneous disease, targeting a single joint tissue for treatment may not be effective [
4].
Regenerative medicine, which involves the use of biological sources such as stem cells and grafts for repair, regeneration and replacement of cells, tissues or organs in order to restore structure and physiological functions is considered a promising therapy for OA management [
4,
9]. Minimally manipulated mesenchymal stem cells (MSCs) are reported to be safe and have some short-term beneficial effects [
10]. Autologous micrografting technology (AMT
®) involves the use of autologous micrografts to stimulate/enhance regeneration of an impaired or damaged tissue. The key strengths of AMT
® are a good safety profile, non-rejection of the injected micrografts and specificity to recover normal functioning of tissues through specific signaling pathways. Use of autologous micrografts can overcome some of the current limitations of other therapeutic approaches, like invasiveness, donor site morbidity, cell death and allogeneic response [
11].The AMT
® procedure is not considered an advanced therapy medicinal product as it falls under the category of non-substantially manipulated cells or tissues used for the same essential function, which means that the cells when removed from their original environment in the human body are used to maintain the original function(s) in the same anatomical or histological environment under allogeneic or autologous conditions [
12].
The Rigenera
® technology (Regenera Activa Worldwide S.L., Barcelona, Spain, and Human Brain Wave SRL, Torino, Italy) is a novel strategy for tissue mechanical disaggregation, which allows obtaining autologous micrografts [
13,
14] enriched in progenitor cells expressing MSC-like markers and having strong regenerative potential [
15].The AMT
® procedure using Rigenera
® technology has been used worldwide for more than ten years since its discovery and development in 2012 to stimulate and enhance self-regenerative processes for multiple conditions. It has shown clinical efficacy in the management of complex wounds [
16,
17,
18], for the regeneration of the bone in periodontal surgeries [
19], for pinched nose deformity [
20,
21,
22], for improving hair density in patients affected by androgenetic alopecia [
23,
24,
25,
26], for treatment of scars [
27], for treatment of osteochondral lesions of the knee [
28], and for the treatment of knee chondropathy [
5]. The procedure has also shown promise for treatment of osteochondral defects in a preclinical study [
11].
The aim of this study was to determine the clinical effect and outcomes of the AMT® procedure in patients with early stages of knee OA.
2. Materials and Methods
This was a prospective, open-label study conducted between June 2022 to November 2022.
2.1. AMT® procedure
The AMT
® procedure used in this study was like the one used in previous studies in knee-joint-degeneration patients [
5,
24,
27]. The tools used in the AMT
® procedure are Rigeneracons
® SRT (7945RS), Sicurdrill
® 2.0, Sicurlid and Sicurstick (Regenera Activa Worldwide S.L.,
Figure 1a–d).
The Rigeneracons
® SRT is a sterile disposable class IIa medical device designed to mechanically disaggregate solid human tissue to obtain soluble autologous micrografts, which are injectable (the AMT
® solution). Rigeneracons
® SRT comprises a grid with 100 hexagonal holes, each containing 6 calibrated microblades; a helix that rotates through an internal metal ring at a constant 80 revolutions per minute to disaggregate tissues without causing cellular disruption and maintaining cell viability; and 3 arms. The Sicurdrill
® 2.0 is a class I medical device with a motor that provides the electromechanical impulse to rotate the helix of the Rigeneracons
® SRT. The Sicurdrill
® 2.0 operates in 1-minute cycles, each cycle started by pressing the frontal button. It is specially designed to be easily transportable. The Sicurlid and Sicurstick are specific adapters to secure the Rigeneracons
® SRT to the Sicurdrill
® 2.0. The procedure is shown in
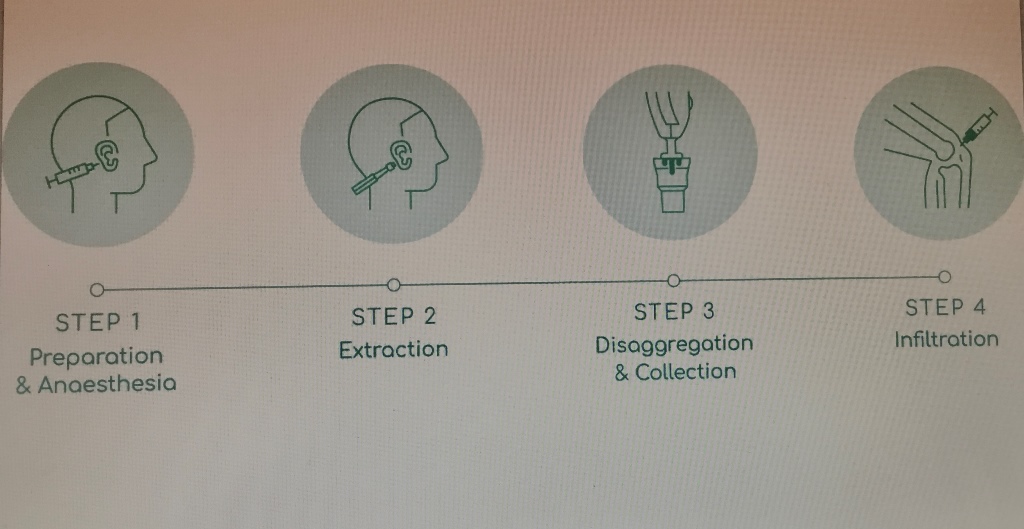
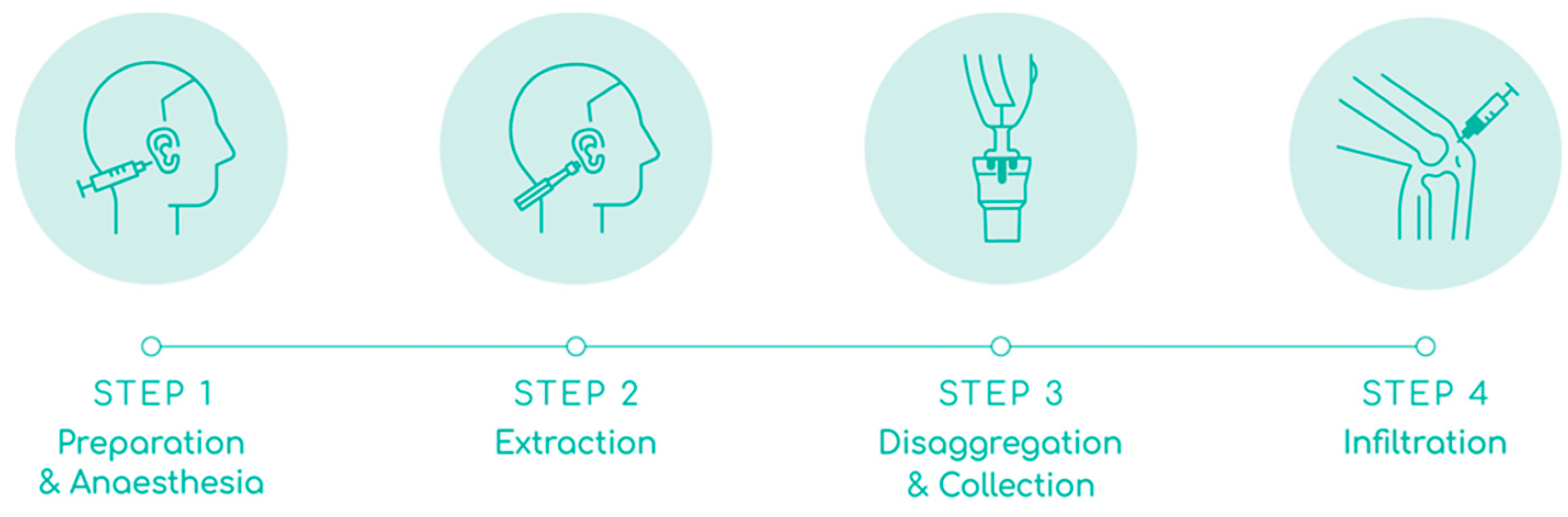
Figure 2 and described below:
Preparation and anesthesia: The auricular concha of the ear was disinfected with chlorhexidine and the area for biopsy extraction was marked with a dermal marker. The area was anaesthetised with 2.5 mL of 2% lidocaine without vasoconstrictors; 0.5 mL in the retroauricular nerve to anesthetise and the rest on both sides of the auricular concha to hydro separate the skin from the cartilage. Adrenaline was used if the bleeding did not stop after applying 5 minutes of haemostatic pressure.
Extraction: Three biopsy punches were made in the marked area with a 2.5 mm dermal punch. The three biopsies were placed on the grid of the Rigeneracons® SRT, and manually rotated to cover them under the helix. The donor area in the ear was covered with a band-aid.
Disaggregation and collection: Approximately 4.0 mL of saline solution was added through the extraction hole of the Rigeneracons® SRT with a Luer Slip syringe, until the biopsies were slightly embedded in the solution. Then the cap was closed, the Sicurstick inserted in the Rigeneracons® SRT and placed into the Sicurlid. The device was then attached to the Sicurdrill® 2.0. The frontal button on the Sicurdrill® was pressed to start the tissue disaggregation, which was indicated by the yellow flashing LED on the Sicurdrill®. The Sicurdrill® was operated for six consecutive cycles, each cycle of 1-minute duration. At the end of six cycles, 4.0 ml of the AMT® solution was collected with a Luer Slip syringe.
Infiltration: The external femorotibial compartment area of the affected knee was disinfected with chlorhexidine. The AMT® solution was injected with a 21G ½, 0.8 x 40 mm needle into the external femorotibial compartment. The joint was mobilized to distribute the injected AMT® solution evenly across the treated area.
All steps were carried out under sterile conditions and the full Rigeneracons® SRT kit which is intended for single use was safely disposed of after use in accordance with local guidelines.
Joint inflammation, if any, in the first 24-72 hours post-procedure was treated with analgesics (other than nonsteroidal anti-inflammatory drugs which may interfere with the effectiveness of the micrograft). Patients were advised to rest in positions that do not overstrain the treated joint for at least 7 days post-procedure. To ensure healing of the biopsy site in the ear, patients were advised to not shower or have a sauna bath in the first 24-48h post-procedure, to change the dressing of the biopsy site after 24h and to keep the biopsy site clean and dry.
The study was conducted in accordance with the Declaration of Helsinki. Good Manufacturing Practices rules for processing, Good Clinical Practices for the clinical application and ethics committee approval did not apply to the study as it fell under the category of “autologous use in one step surgery, minimal manipulation, monofunctional use (used for the same essential function in the recipient as in the donor), and manipulation with devices in aseptic conditions” [
24,
25].
All patients provided written informed consent before the start of the procedure.
2.2. Eligibility criteria
Patients included in the study were those diagnosed with OA grade 2-3 (except for one patient with OA grade 3-4) on Kellgren and Lawrence scale [
29], meaning that the joint preserved at least part of the articular cartilage.
Patients excluded were those with any autoimmune diseases including rheumatoid arthritis; those with any concomitant uncontrolled metabolic diseases; as well as those with grade 4 advanced OA.
2.3. Assessments
Magnetic resonance imaging (MRI) and X-ray were performed pre-procedure. The Knee Injury and Osteoarthritis Outcome Score (KOOS) questionnaire [
30] recommended by the International Knee Documentation Committee was used for assessing pain, stiffness, and function pre-procedure and at 1- and 6-months post-procedure. The KOOS is a self-reported outcome measure assessing the patient
’s opinion about the health, symptoms, and functionality of their knee. It is a 42-item questionnaire, including 5 subscales: symptoms (seven items), pain (nine items), function in daily living (ADLs [17 items]), sports and recreation function (five items), and quality of life (four items). Each item has five possible answer options scored from 0 (No Problems) to 4 (Extreme Problems) and each of the five scores is calculated as the sum of the items included. Scores are transformed to a 0–100 scale, with zero representing extreme knee problems and 100 representing no knee problems. An aggregate score of all subscales is not calculated since it is regarded desirable to analyze and interpret the five dimensions separately.
Incidence of adverse events was monitored during the study. The biopsied area in the ear was checked for signs of infection and for any abnormality in healing. A clinical evaluation and safety assessment was carried out at 12 months post-procedure.
2.4. Statistical analysis
Data were analyzed with one-way analysis of variance and posterior multiple comparison Bonferroni test. Data were expressed as the mean ± standard deviation (SD). A p ≤ 0.05 was considered statistically significant. RStudio software (RStudio Team (2020). RStudio: Integrated Development for R. RStudio PBC, Boston, Massachusetts) was used for statistical analysis.
3. Results
The study included 10 patients - 4 men and 6 women; all except one patient were aged 53 years and above (
Table 1). Patients reported pain in their affected knee for the past <1 year to 10 years prior to start of study.
3.1. MRI and X-ray findings
MRI at baseline revealed subchondral bone edema, chondropathy patella, and medial compartment narrowing; Baker cyst was noted in one patient (
Table 2). X-ray revealed presence of osteophytes and medial compartment narrowing (
Table 2).
3.2. AMT® procedure outcomes
In all patients, the wound in the ear completely healed within one week without any need for stitches. None of the patients needed adrenaline to stop the bleeding from the biopsied area in the ear. There were no cases of infection or signs of anormal healing of the donor area like “cauliflower ear”. The patients strictly followed physician’s aftercare recommendations for the healing of the donor area.
The AMT
® procedure was successful in all 10 patients. Pain resolution and good recovery of daily activities was seen in all 10 patients, with improvement in mobility being observed as early as 3 weeks post-procedure in 2 patients (
Table 3).
3.3. KOOS subscale scores
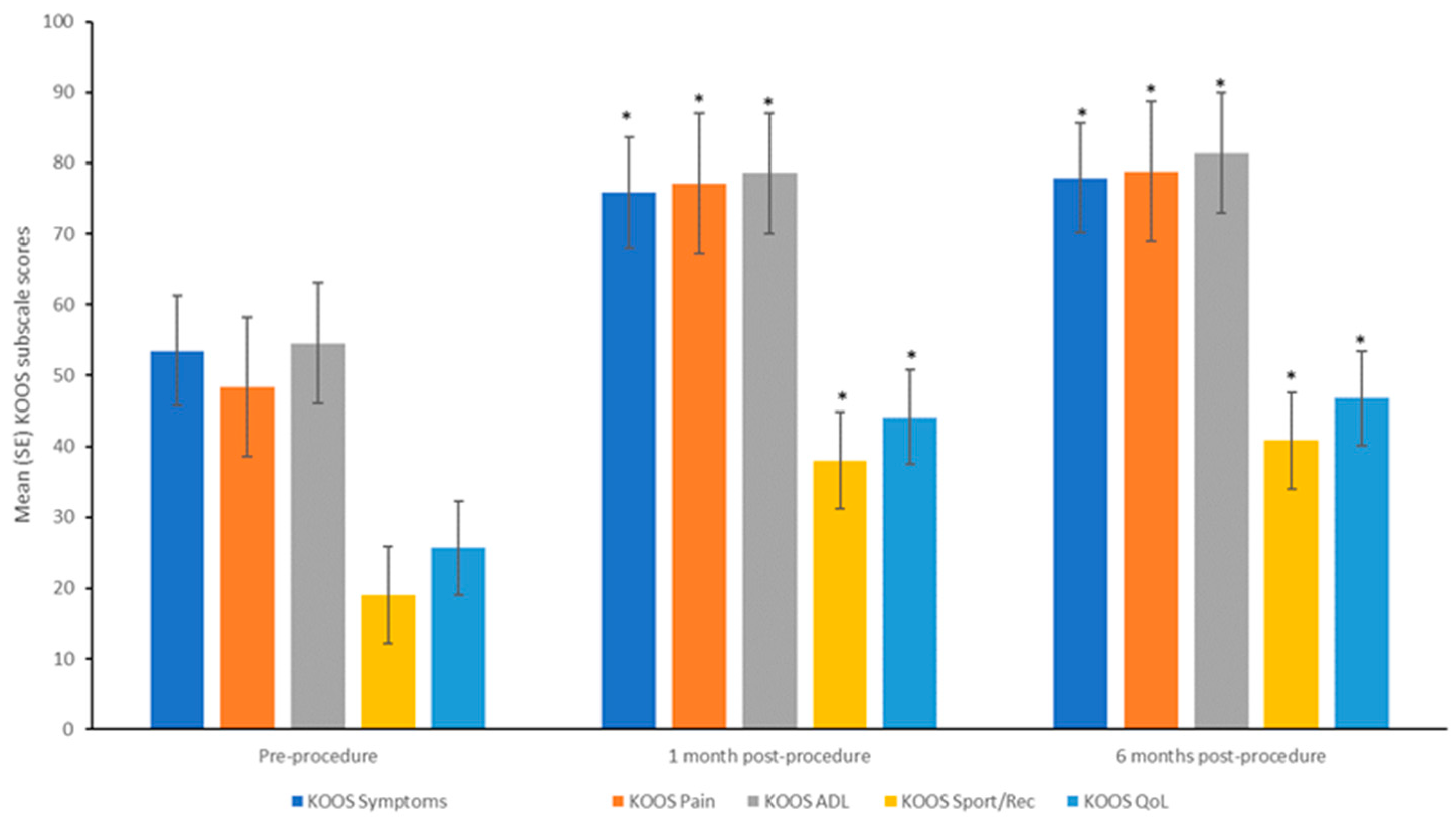
All 10 patients completed the assessments at 1- and 6-months post-procedure. Significant improvements were seen in the mean scores of all five subscales of KOOS (KOOS symptoms, KOOS pain, KOOS ADL, KOOS sport and recreation, and KOOS quality-of-life) between pre-procedure and 1- and 6-month post-procedure (
Figure 3, all p ≤ 0.05). Between 1- and 6-months post-procedure, significant improvements were observed in the mean scores of the subscales of KOOS symptoms, KOOS pain, and KOOS ADL (all p ≤ 0.05), while for the subscales of KOOS sport and recreation, and KOOS quality-of-life, the improvements observed at 1-month were maintained at 6-months. Each one of the KOOS subscales was analyzed and passed the Bonferroni test.
3.4. Safety and long-term follow-up
Clinical evaluation at 12 months post-procedure showed that all 10 patients had continued good mobility and minimal or no pain in the affected knee.
The AMT® procedure showed a good tolerability profile since none of the patients reported any adverse event during the study.
4. Discussion
Use of biological strategies such as endogenous stem cells or tissue-specific progenitor cells to enable patient’s own cartilage regeneration ability represents an important advance in the treatment of cartilage defects and OA [
31,
32].
Chondrocytes, which are highly specialized cells in the articular cartilage of the knee constitute approximately 1% of total volume [
33] and supply the extracellular matrix (ECM) with the elements such as collagen and proteoglycans that constitute the cartilage function [
34]. Chondrocytes are responsible for replacement of ECM molecules lost through degradation over the years. However, aging decreases the ability of chondrocytes to replace ECM molecules resulting in progressive degeneration of articular cartilage leading to joint pain and dysfunction, clinically identified as OA [
35]. Also, while articular cartilage can withstand intensive and repetitive physical stress, impact forces caused by falls, sports injuries and road accidents lead to substantial damage [
8], but the articular cartilage has limited ability to repair and self-renew as it is an avascular tissue [
8,
36,
37]. Autologous chondrocytes are an effective cell therapy source for repair of chondral defects, but the challenges of using them are the paucity of donor sites, de-differentiation during expansion in culture and early apoptosis [
38]. MSCs are another treatment option with a potential for cartilage regeneration but their use is limited by their tendency to differentiate into various cell lineages and the need for specific induction manipulation to promote chondrogenesis [
38].
Perichondrial tissue which is present in all adult cartilage tissues, except for articular and fibrocartilage [
39], has the potential to produce hyaline-like cartilage [
40]. The clinical relevance of perichondrium was recognized more than a century ago [
39], and it is possibly among the first tissues to attract clinical interest for its regenerative potential, with initial reports on its role in cartilage regeneration made as early as the 19th century [
40,
41,
42]. Interest in the perichondrium was revived in recent years owing to its possible role as a microenvironment containing stem and chondrogenic progenitor cells [
38,
39]. Findings from multiple animal and human studies both in vivo and in vitro indicate that perichondrium can facilitate and is essential for cartilage regeneration and has the clinical potential to treat various cartilage-associated diseases and traumas [
39]. Perichondrium contains a pool of mesenchymal progenitor cells called chondroblasts. Chondroblasts contribute to tissue homeostasis by replicating and differentiating into chondrocytes. An in-vitro study showed that the progenitor cells in perichondrium had paracrine effects on prolonging the lifespan and promoting the proliferation and cartilaginous expression of chondrocytes [
38]. Subcutaneous implantation of progenitor cells and chondrocytes together in nude mice was found to promote formation of cartilaginous tissue [
38].
Auricular cartilage as a source of perichondrial progenitor cells has the advantages of ease of harvest and minimal donor-site morbidity [
38]. Animal studies have demonstrated that autologous micrografts generated from auricular cartilage were positive for cartilage progenitor cell markers such as CD44, CD90, and CD117, and the ribonucleic acid derived from micrografts was positive for tissue cartilage markers such as Sox-9 and COL2A1 [
22]. In comparison with murine MSCs, these levels indicate presence of chondrocyte-progenitor cells with a mesenchymal phenotype in auricular micrografts [
22]. Autologous micrografts from human auricular cartilage cultured in-vitro with human chondrocytes were found to positively influence chondrocyte differentiation as shown by both increased glycosaminoglycans deposition and the presence of collagen II and stimulate secretion of cartilage trophic factors such as insulin-like growth factor 1 and transforming growth factor β, without affecting chondrocyte viability [
31]. The principle behind AMT
® procedure with auricular cartilage for knee OA is to transfer the signalling potential of chondroprogenitor cells, chondrocytes and signalling factors together with ECM components to enhance the restoration of joint function and reduce pain and stiffness [
5].
In this study, use of AMT
® procedure with auricular micrografts was found to be effective and safe in the treatment of early-stage knee OA. Post-procedure, all patients reported minimal or no pain. Improvement in mobility was observed as early as 3 weeks post-procedure, which was maintained even after 12 months post-procedure, indicating early benefits maintained over the long-term. These observations were supported by the significant improvements observed in all five subscales of KOOS, a patient-reported outcome (PRO) measure, at 1- and 6-months post-procedure. PROs are increasingly recognized by regulators, clinicians, and patients as valuable tools to collect patient-centered data [
43,
44]. The KOOS scale, an extension of the Western Ontario and McMaster Universities Arthritis Index (WOMAC) is mainly focused on the knee and is better adapted for younger, more active populations [
43]. It has adequate internal consistency, test-retest reliability and construct validity in young and old adults with knee injuries and/or OA [
45]. A change of 8–10 points is suggested to represent the minimal perceptible clinical improvement of the KOOS [
43]. In this study, the change in KOOS subscale scores from pre-procedure ranged between 20 to 30 points at 6-month post-procedure.
The findings from this study in patients with early-stage knee OA strengthen the observation from in-vitro and animal studies on the use of autologous auricular micrografts for joint pain management. The observations from this study and that of Marcarelli et al
. [
5] suggest a potential role for AMT
® procedure in the treatment of various articular cartilage defects as well as cartilage defects in other joints in the body. Future studies in larger patient populations are needed to validate such clinical application. Limitations of the study are the small sample size, absence of a control group, lack of long-term follow-up and findings being based on a single AMT
® procedure.
5. Conclusions
Autologous auricular cartilage micrografts obtained by AMT® procedure (using Rigenera® technology) is an effective and safe protocol in the treatment of early-stage knee OA and could be a valid treatment approach. The findings need to be validated in a larger patient population.
Author Contributions
Conceptualization, Dimitrous Tsoukas, MD; Ilie Muntean, MD and Christos Simos, MD; Methodology, Ilie Muntean, MD; Software, Ruben Sabido-Vera MSs; Validation, Dimitrous Tsoukas, MD and Christos Simos, MD; Statistical analysis, Ruben Sabido-Vera MSs; Investigation, Dimitrous Tsoukas, MD and Ilie Muntean, MD; Resources, Dimitrous Tsoukas, MD; Data curation, Dimitrous Tsoukas, MD and Christos Simos, MD; Writing—original draft preparation, Dimitrous Tsoukas, MD; Writing—review and editing, Dimitrous Tsoukas, MD; Christos Simos, MD and Ilie Muntean, MD; Visualization, Dimitrous Tsoukas, MD; Christos Simos, MD and Ilie Muntean, MD; Supervision, Dimitrous Tsoukas, MD; Christos Simos, MD and Ilie Muntean, MD; Project administration, Dimitrous Tsoukas, MD and Ilie Muntean, MD. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The kits for the AMT® procedure were provided by Regenera Activa Worldwide S.L.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Ethical review and approval were waived for this study as it fell under the category of “autologous use in one step surgery, minimal manipulation, monofunctional use (used for the same essential function in the recipient as in the donor), and manipulation with devices in aseptic conditions”.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is contained within the article.
Acknowledgments
Lakshmi Venkatraman, PhD provided medical writing support which was funded by Regenera Activa Worldwide S.L.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. The Lancet. 2019, 393, 1745-1759. [CrossRef]
- Cui, A.; Li H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, Regional Prevalence, Incidence And Risk Factors Of Knee Osteoarthritis In Population-Based Studies. Eclinical Medicine. 2020, 29-30:100587. [CrossRef]
- Hunter, D.J.; March, L.; Chew, M. Osteoarthritis in 2020 And Beyond: a Lancet Commission. The Lancet. 2020, 396, 1711-1712. [CrossRef]
- Grässel, S.; Muschter, D. Recent Advances In The Treatment Of Osteoarthritis. F1000Res. 2020, 9. [CrossRef]
- Marcarelli, M.; Zappia, M.; Rissolio, L.; Baroni, C.; Astarita, C.; Trovato, L.; Graziano, A. Cartilage Micrografts as a Novel Non-Invasive and Non-Arthroscopic Autograft Procedure for Knee Chondropathy: Three-Year Follow-Up Study. J. Clin. Med. 2021, 10, 322. https://doi. org/doi:10.3390/jcm10020322.
- Fusco, G.; Gambaro, F.M.; Di Matteo, B.; Kon, E. Injections In The Osteoarthritic Knee: A Review Of Current Treatment Options. EFORT Open Rev. 2021, 6, 501-509. [CrossRef]
- Siddiq, M.A.B.; Clegg, D.; Jansen, T.L.; Rasker, J.J. Emerging And New Treatment Options For Knee Osteoarthritis. Curr Rheumatol Rev. 2022, 18, 20-32. [CrossRef]
- Liu, Y.; Shah, K.M.; Luo, J. Strategies For Articular Cartilage Repair And Regeneration. Frontiers In Bioengineering And Biotechnology. 2021, 9, 770655. [CrossRef]
- Roseti, L.; Desando, G.; Cavallo, C.; Petretta, M.; Grigolo, B. Articular Cartilage Regeneration In Osteoarthritis. Cells. 2019, 8, 1305. [CrossRef]
- Di Matteo, B.; Vandenbulcke, F.; Vitale, N.D.; Iacono, F.; Ashmore, K.; Marcacci, M.; Kon, E. Minimally Manipulated Mesenchymal Stem Cells For The Treatment Of Knee Osteoarthritis: A Systematic Review Of Clinical Evidence. Stem Cells Int. 2019, 1735242. [CrossRef]
- Desando, G.; Grigolo, B.; Deangelles Pereira Florentino, A.; Teixeira, M.W.; Barbagallo, F.; Naro, F.; da Silva-Junior, V.A.; Soares, A.F. Preclinical Evidence of Intra-Articular Autologous Cartilage Micrograft for Osteochondral Repair: Evaluation in a Rat Model. Cartilage. 2021, 13, 1770S-1779S. [CrossRef]
- European Medicines Agency. Reflection Paper On Classification Of Advanced Therapy Medicinal Products. EMA/CAT/600280/2010 rev.1. 2015. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-classification-advanced-therapy-medicinal-products_en-0.pdf. (Accessed on 21 July 2023).
- Giaccone, M.; Brunetti, M.; Camandona, M.; Trovato, L.; Graziano, A. A New Medical Device, Based On Rigenera® Protocol, In The Management Of Complex Wounds. J. Stem Cells Res. Rev. Rep. 2014, 1, 3.
- Purpura, V.; Bondioli, E.; Graziano, A.; Trovato, L.; Melandri, D.; Ghetti, M.; Marchesini, A.; Cusella De Angelis, M.G.; Benedetti, L.; Ceccarelli, G.; et al. Tissue Characterization After A New Disaggregation Method For Skin Micro-Grafts Generation. J. Vis. Exp. 2016, 4, e53579. [CrossRef]
- Trovato, L.; Monti, M.; Del Fante, C.; Cervio, M.; Lampinen, M.; Ambrosio, L.; Redi, C.A.; Perotti, C.; Kankuri, E.; Ambrosio, G.; et al. A New Medical Device Rigeneracons Allows to Obtain Viable Micro-Grafts From Mechanical Disaggregation of Human Tissues. J. Cell. Physiol. 2015, 230, 2299-2303. [CrossRef]
- Marcarelli, M.; Trovato, L.; Novarese, E.; Riccio, M.; Graziano, A. Rigenera® Protocol In The Treatment Of Surgical Wound Dehiscence. Int. Wound J. 2016, 14, 277–281. [CrossRef]
- De Francesco, F.; Graziano, A.; Trovato, L.; Ceccarelli, G.; Romano, M.; Marcarelli, M.; Cusella De Angelis, G.M.; Cillo, U.; Riccio, M.; Ferraro, G.A. A Regenerative Approach with Dermal Micrografts in the Treatment of Chronic Ulcers. Stem Cell Rev. 2017, 13, 139–148. [CrossRef]
- Riccio, M.; Marchesini, A.; Zingaretti, N.; Carella, S.; Senesi, L.; Onesti, M.G.; Parodi, P.C.; Ribuffo, D.; Vaienti, L.; De Francesco, F. A Multicentre Study: The Use of Micrografts in the Reconstruction of Full-Thickness Posttraumatic Skin Defects of the Limbs—A Whole Innovative Concept in Regenerative Surgery. Stem Cells International. 2019, 5043518. [CrossRef]
- Graziano, A.; Carinci, F.; Scolaro, S.; D’Aquino, R. Periodontal Tissue Generation Using Autologous Dental Ligament Micro-Grafts: Case Report With 6 Months Follow-Up. Ann. Oral. Maxillofac. Surg. 2013, 1, 20. [CrossRef]
- Gentile, P.; Scioli, M.G.; Bielli, A.; Orlandi, A.; Cervelli, V. A Combined Use Of Chondrocytes Micro Grafts (CMG) Mixed With Platelet Rich Plasma (PRP) In Patien ts Affected By Pinch Nose Deformity. J. Regen. Med. 2016, 5, 2. [CrossRef]
- Gentile, P.; Scioli, M.G.; Bielli, A.; Orlandi, A.; Cervelli, V. Reconstruction of Alar Nasal Cartilage Defects Using a Tissue Engineering Technique Based on a Combined Use of Autologous Chondrocyte Micrografts and Platelet-rich Plasma: Preliminary Clinical and Instrumental Evaluation. Plast. Reconstr. Surg. Glob. Open. 2016, 4, e1027. [CrossRef]
- Ceccarelli, G.; Gentile, P.; Marcarelli, M.; Balli, M.; Ronzoni, F.L.; Benedetti, L.; Cusella De Angelis, M.G. In Vitro and In Vivo Studies of Alar-Nasal Cartilage Using Autologous Micro-Grafts: The Use of the Rigenera® Protocol in the Treatment of an Osteochondral Lesion of the Nose. Pharmaceuticals (Basel). 2017, 10, 53; [CrossRef]
- Zanzottera, F.; Lavezzari, E.; Trovato, L.; Icardi, A.; Graziano, A. Adipose Derived Stem Cells And Growth Factors Applied On Hair Transplantation. Follow-Up Of Clinical Outcome. J. Cosmetics Dermatol. Sci. Appl. 2014, 2014. [CrossRef]
- Gentile, P.; Scioli, M.G.; Bielli, A.; Orlandi, A.; Cervelli, V. Stem Cells From Human Hair Follicles: First Mechanical Isolation For Immediate Autologous Clinical Use In Androgenetic Alopecia And Hair Loss. Stem Cell Investig. 2017, 4, 58. [CrossRef]
- Gentile, P.; Scioli, M.G.; Cervelli, V.; Orlandi, A.; Garcovich, S. Autologous Micrografts from Scalp Tissue: Trichoscopic and Long-Term Clinical Evaluation in Male and Female Androgenetic Alopecia. Biomed Res. Int. 2020, 7397162. [CrossRef]
- Zari, S. Short-Term Efficacy of Autologous Cellular Micrografts in Male and Female Androgenetic Alopecia: A Retrospective Cohort Study. Clin. Cosmet. Investig. Dermatol. 2021,14, 1725-1736. [CrossRef]
- Svolacchia, F.; De Francesco, F.; Trovato, L.; Graziano, A.; Ferraro, G. A. An Innovative Regenerative Treatment of Scars With Dermal Micrografts. J. Cosmet. Dermatol. 2016, 15, 245–253.
- Fernández, A.D.; Luengo, A.B. Biostimulation of Knee Cartilage Using Autologous Micro-Grafts: A Preliminary Study of the Rigenera Protocol in Osteochondral Lesions of the Knee. Rehabilitation Sciences. 2018, 3, 8-12. [CrossRef]
- Kellgren, J.; Lawrence, J. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494-502. [CrossRef]
- Roos, E.M.; Roos, H.P.; Lohmander, L.S.; Ekdahl, C.; Beynnon, B.D. Knee Injury and Osteoarthritis Outcome Score (KOOS)--Development Of A Self-Administered Outcome Measure. J. Orthop. Sports Phys. Ther. 1998, 28, 88-96. [CrossRef]
- Vigano, M.; Tessaro, I.; Trovato, L.; Colombini, A.; Scala, M.; Magi, A.; Toto, A.; Peretti, G.; de Girolamo, L. Rationale and Pre-Clinical Evidences For The Use Of Autologous Cartilage Micrografts In Cartilage Repair. J Orthop. Surg. Res. 2018, 13, 279. [CrossRef]
- Vágó, J.; Takács, R.; Kovács, P.; Hajdú, T.; van der Veen, D.R.; Matta, C. Combining Biomechanical Stimulation And Chronobiology: A Novel Approach For Augmented Chondrogenesis? Front. Bioeng. Biotechnol. 2023, 11, 1232465. [CrossRef]
- Erggelet, C.; Mandelbaum, B. R. Principles of Cartilage Repair. Steinkopff Verlag, Springer. 2008. Available online: Principles Of Cartilage Repair [PDF] [5vq3fnpc02g0] (vdoc.pub). (Accessed on 24 July 2023).
- Pearle, A.D.; Warren, R.F.; Rodeo, S.A. Basic Science of Articular Cartilage and Osteoarthritis. Clin. Sports Med. 2005, 24, 1-12. [CrossRef]
- Buckwalter, J.A.; Mankin, H.J.; Grodzinsky, A.J. Articular Cartilage And Osteoarthritis. Instr Course Lect. 2005, 54, 465-480.
- Buckwalter, J.A.; Mankin, H.J. Articular Cartilage: Degeneration And Osteoarthritis, Repair, Regeneration, And Transplantation. Instr Course Lect. 1998, 47, 487-504.
- Armiento, A.R.; Alini, M.; Stoddart, M.J. Articular Fibrocartilage - Why Does Hyaline Cartilage Fail To Repair? Advanced Drug Delivery Reviews. 2019, 146, 289-305. [CrossRef]
- Ho, C.L.; Huang, L.L.H.; Shieh, S.J. Perichondrial Progenitor Cells Promote Proliferation And Chondrogenesis Of Mature Chondrocytes. Regen Biomater. 2022, 9, rbab078. [CrossRef]
- Gvaramia, D.; Kern, J.; Jakob, Y.; Zenobi-Wong, M.; Rotter, N. Regenerative Potential of Perichondrium: A Tissue Engineering Perspective. Tissue Engineering Part B: Reviews. 2021, 28, 531-541. [CrossRef]
- Bruns, J.; Meyer-Pannwitt, U.; Silbermann, M. The Rib Perichondrium. An Anatomical Study In Sheep Of A Tissue Used As Transplant In The Treatment Of Hyaline-Cartilage Defects. Acta. Anat. (Basel). 1992, 144, 258-266.
- Mori, M. Studies On Cartilage Regeneration, Based On Experimental Studies On Rabbit Ear [in German]. Dtsch. Z. Chir. 1905, 76, 220.
- Skoog, T.; Ohlsén, L.; Sohn, S.A. Perichondrial Potential For Cartilagenous Regeneration. Scand. J. Plast. Reconstr. Surg. 1972, 6,123-125. [CrossRef]
- Roos, E.M.; Lohmander, L.S. The Knee injury and Osteoarthritis Outcome Score (KOOS): From Joint Injury To Osteoarthritis. Health Qual Life Outcomes. 2003, 1, 64. [CrossRef]
- Mercieca-Bebber, R.; King, M.T.; Calvert, M.J.; Stockler, M.R.; Friedlander, M. The Importance Of Patient-Reported Outcomes In Clinical Trials And Strategies For Future Optimization. Patient Relat. Outcome Meas. 2018, 9, 353-367. [CrossRef]
- Collins, N.J.; Prinsen, C.A.C.; Christensen, R.; Bartels, E.M.; Terwee, C.B.; Roos, E.M. Knee Injury and Osteoarthritis Outcome Score (KOOS): Systematic Review and Meta-Analysis Of Measurement Properties. Osteoarthritis and Cartilage. 2016, 24, 1317-1329.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).