1. Introduction
White oak (
Quercus alba) is an important tree species both for ecological significance and economic importance across the eastern United States. White oaks are dominating canopy that serves a vital role in forming stand structures and composition in forests. For a wide variety of creatures, including nesting birds and arboreal animals, it's wide, spreading crown provides a friendly microhabitat [
1,
2]. Acorns and fallen leaves provide a wealth of food for wildlife, supporting complex food chains and promoting biodiversity [
3,
4]. Furthermore, white oaks’ vast root systems create essential symbiotic connections with mycorrhizal fungi that aid in nutrient cycling and uptake. The tree’s capacity to store large amounts of carbon helps to reduce greenhouse gas emissions and mitigate the effects of climate change [
5,
6]. The white oak’s vital function in the forest ecosystems of the eastern United States is further demonstrated by its patterns of succession and regeneration. Because of its effective acorn dispersal, the tree offers a plentiful supply of seeds for spontaneous growth, guaranteeing the continuation of its lineage for future generations. However, white oaks population is in danger due to the increased declining situation associated with changes in forest stand dynamics.
Oak mortality is among the most observed phenomenon in oak forests in Eastern U.S. [
7,
8,
9,
10]. The mortality starts with the oak tree browning of leaf, turning black, curl-up, and finally falling to the ground [
11,
12,
13,
14,
15]. Factors responsible for the oak decline are suspected long-term predisposing, short-term inciting, and contributing factors[
13,
15]. Predisposing factors are related to stand longevity and maturity, which are responsible for a tree's natural ability to growth inhibition and lead to injury-inducing agents. The inciting factors are related to physical or biological conditions, which are related to defoliating insects, hail, frost, and drought. Reports from inciting factors showed typical crown dieback, browning, and new leaf emergence in dying trees, which eventually leads to deaths[
16]. The contributing factors are related to pathogenic fungi and boring insects that ultimately kill the trees. This decline has grappled with notable drought outbreaks, late spring frost, the emergence of saprophytic fungus due to climate change [
17], and oak borer attacks on the most vulnerable sites.
Typically, oak mortality has been targeted in red oak group species. More recently, white oak mortality (WOM) becomes a prominent target across the eastern US. It seems that the spatial distribution pattern of WOM is not uniform. Poor resource site such as droughty, poor drainage, and soil nutrient deficiency are more prone to WOM [
18]. It is because low resources had led to declining and widespread regeneration failure in white oaks. Scientists also reported WOM in higher-quality mesic sites where the forest has gone through a high stand density and maturity stage [
19,
20]. The mortality is more prevalent in the self-thinning stage, as the tree species under high stand density struggle to utilize maximum resources [
21]. WOM has been observed in different topographies, from low-lying lands to valley floors (Abrams, 2003). North-facing slopes where sunlight is low are more prone to high oak mortality[
22]. Besides, ecological stressors such as browsing, heavy shade, and disturbances have influenced white oaks in many parts of eastern hardwood forests [
23].
The impacts of white oak mortality are not only limited to individual tree species but also affect entire forest ecosystems and ecological processes. For instance, white oaks were dying in greater numbers i.e., 30% of healthy crowns, which were less than 4m in width across the 516-ha of Ozark Highlands [
24]. Similarly, some 900-ha area of Baskett Wildlife Research and Education Center across the Ozark Border of Central Missouri depicted 10% of white oaks killed by drought-pathogen interactions [
25]. White oak mortality across forest ecosystems can lead to changes in nutrient cycling, water regulation, and carbon sequestration [
26,
27,
28]. White oak is a long-lived species and can sequester large amounts of carbon during its lifetime [
29,
30]. The decline of mature white oaks can disrupt carbon sinks and worsen the situation for climate change and carbon management [
31,
32]. Researchers found that a lack of plant-soil interactions may result in the decreased intensity of carbon sequestration and increase the carbon emissions in the atmosphere [
33]. It is also known that white oak is a dominant canopy species across the eastern US that shape forest structure and composition influencing soil processes, species interactions, and light availability [
34]. Its loss can lead to a change in forest dynamics such as impacting the recruitment of new individuals to alterations of the competitive relationship among plant species [
35,
36]. The decline in white oaks can impact numerous wildlife species and their microhabitats that rely upon shelter and foraging [
37]. Moreover, the white oak decline can have adverse effects on wildlife by altering species distribution and food chain interactions as well [
38].
Soil conditions play an important role in tree species' survival. The study on tree species at local scales may not represent an actual pattern that can be best reflected at the regional level [
39]. While numerous factors influence white oak's spatial pattern and survival, soil properties emerged as significant factors that influence forest ecosystem dynamics [
16]. Eastern US covers a diverse range of soil types and landscapes, which offers a unique opportunity to study the influence of soil properties on WOM patterns. By examining the spatial heterogeneity of soil types and their interactions with stand characteristics, we can understand how soil properties contribute to the spatial patterns of WOM across a broad scale[
40,
41]. The survival and establishment of white oaks as well as their susceptibility to various stressors and disturbance factors can be affected by variations in soil qualities, such as texture, soil moisture, and organic matter [
42]. Therefore, it is necessary to incorporate data from multiple sites from the eastern US and study regional variations in soil types that influence the spatial pattern of WOM.
The aim of this study is (1) to assess the spatial distribution pattern of WOM rate across the eastern US and (2) to evaluate the influence of soil properties (soil texture, organic matter, and total available water) on spatial distribution patterns of WOM rate. The hypothesis was that the observed spatial pattern of the WOM rate is random across the eastern US.
2. Materials and Methods
2.1. Study Area
We selected the eastern United States as our study area, which predominantly covers oak forests [
43]. It is comprised of ten states that cover a land area of 1,273,420,251.33 hectares (
Figure 1). It consists of varied tree species, and a wide range of climate, soil, and terrains [
44,
45,
46]. The land structure in the eastern part of the study area is significantly Appalachian plateaus with low mountains, narrow valleys, and sharp edges. On the western portion, the area varies from highly flat central till plains to interior low plateau along with Ozark highlands. The area consists of a variety of ecological regions such as the Ouachita Mountains of Arkansas to the Northern Cumberland Mountains in West Virginia. In the south, central till plains Oak-Hickory of Illinois to Interior Low Plateau Highland Riff of Alabama.
The climate in this region is long, hot summers and cool winters. The mean annual temperature ranges from 4 degrees to 18 degrees Celsius in the east-west gradient with warmer temperatures in the south. The precipitation ranges from 500 mm in the northwest to 1,650 mm in the southeast. Some Appalachian Mountains precipitation goes up to 2,000 mm during spring and fall. The soil types are mostly mollisols, inceptisols, alfisol, and ultisols. These areas consist of xeric gradient characterized by thin, rocky soils, exposed south, southwest, and wet slopes [
47]. Our study area was historically dominated by oaks, hickories, and pines. Today, most of the forest areas have been replaced by agriculture and rapid urbanization [
44,
48]. In our study area, there are deciduous types of forests such as oaks (
Quercus spp.), hickory (
Carya spp.), american beech (
Fagus grandifolia), ash (
Fraxinus spp.), and maple (
Acer spp.).
Several research concentrated on the spatial patterns of white oaks that utilized climate, topography, and several other factors combined as well as individually [
49,
50]. There is very little research done on spatial patterns of white oaks but those are focused on local level studies. The evidence did not find clear spatial patterns for white oaks across broader scales, which highlights the unique approach for our study [
39]. We incorporated forest inventory data, soil properties, and statistical analysis using point patterns analysis to make it possible to find broad patterns, distinctions, or parallels in several contexts. Other reasons could be to open a new research direction and expand knowledge, the study may develop research areas that have not yet been thoroughly studied.
2.2. Data Acquisition and Processing
2.2.1. Forest Inventory and Analysis Program
FIA program has been monitoring national forest resources across all ownerships in the US [
51,
52]. The inventory data are collected and processed on a relational database based on categorization into multiple phases. The relational database structure facilitates the integration of data from a variety of sources including historical records, satellite imaging, and remote sensing, which adds depth and diversity to the forest inventory dataset. This approach allows for efficient data querying which is required for the development of reports, analysis, and summary statistics where data are organized, accessible, and made consistent for long term forest management planning [
28,
51]. Stratified estimations are used to estimate population parameters for most of the variables depending on the scale and level of information [
53]. The inventoried data have an advantage over other databases as the plots are evenly distributed without any geospatial bias [
54]. In the astern US, FIA plots are recorded with at least one forested condition, which is remeasured again every five years. Each plot measures key attributes of all tree species including plots, surveyed years, and others [
55]. The plot designs in FIA are permanent samples with a fixed radius. The sampling plots are designed with the hexagonal grid having one plot per 2,428 ha (6,000 ac). This plot is based on phase 2 and 3 ground plots, which are clusters of four points. Phases 2 (contains ground plots) and 3 (subsets of phase 2 with the addition of tree health-related attributes such as crown width) are the subplots that can be new plots, remeasured plots with nationally standard and fixed radius plots of 0.40 hectares. The four points i.e., 1,2,3, and 4 is in central, 0 degree, 120 degrees, and 240 degrees azimuth, respectively away from point 1.
Each point is called a subplot, which is surrounded by a 7.32 m (24 feet) fixed radius. The sub plots are used to measure trees of DBH 0.13 m (5 inches) or greater. The total measurement of all four subplots is 0.07 hectares (1/6 acre). In every subplot, there is a micro plot of a fixed radius of 2.07 m (6.8 feet) where saplings and seedlings of 1 to 0.12 m (4.9 inches) DBH are measured. The four micro plots measure a total of approximately 0.0053 hectares (1/75 acre). These micro plots are exactly offset at 3.66 m (12 feet) with a 90-degree azimuth from subplot centers. Each subplot is surrounded by a macro plot with a fixed radius of 17.95 m (58.9 feet) and used to measure a larger tree or mortality of DBH 1.02 m (40 inches) and greater. Hence, all four macro plot measures 0.40 hectares (1 acre). As combined, they possess tri-areal plots. However, the area where macro plots are not possible can be operated as a bi-areal plot.
FIA also records condition-level information from the sampled data. A condition is based on changes in land use or vegetation that fall inside a plot. Furthermore, it is also determined by the ten percent crown or canopy cover of live tally trees [
56]. A condition is designed in such a way that arbitrary numbers are assigned and defined using discrete variables such as forest type, stand size, species composition, stand structure, stand origin, ownership group, and disturbance history [
56].
The periodic and annual inventory is recorded approximately every ten and five years, respectively. However, both periodic and annual inventories contained differences in plot design, sampling, and measurement protocols. Hence, it is not possible to combine both periodic and annual data with dissimilar samples, and this is the reason we utilized our data only from annualized survey years. For instance, our farthest annual data goes back to 1998 from Virginia, which is the oldest annual inventory among ten states. Also, most of the plot inventories of hardwood forests, especially white oaks, are fully recorded until 2019. Therefore, we chose our timeframe from 1998 to 2019 to investigate WOM spatial patterns across the Eastern US.
2.2.2. Plot Selection for Live and Declining White Oaks
Plot data were acquired from USDA Forest Service DataMart [
111] that had already been assigned to a stratum by forest inventory program across all states. A stratum refers to a set of plots that have similar classifications captured from remotely sensed imagery. Within the estimation unit of plot sampling, the weight of the stratum is based on the proportion of the stratum [
51,
56]. In the same set of plot data, geographic coordinates (latitudes and longitudes) were recorded that captured a 0.40 ha (1 acre) sample area but not for all trees. The plot was located within one mile of the original location that were recorded by the Forest Service. It is because landowners prefer not to disclose their property publicly due to security reasons associated with it [
56,
57]. We utilized sampled i.e., forested conditions for the plot data which captured most of the white oak trees in ten states.
Our data covers only white oak (
Quercus alba). The reason for this is the dearth of study on the regional pattern analysis of white oak in the eastern United States in particular. There are numerous oak species in the sample plot as well, but we limited our selection to plots with white oak trees, and we conducted our analysis from the oldest survey year 1998 up through 2019. The white oak tree data were extracted from USDA Forest Service [
111] in which we utilized attributes such as inventory year (INVYR) as surveyed year, county code (COUNTYCD) refers to a particular county number for a particular state, unique plot number (PLOT) assigned to each white oak, species code (SPCD) representing particular tree species (in our case, we captured white oak trees only), tree status code (STATUSCD) representing live tree (1) assigned by FIA, trees per acre unadjusted (TPA_UNADJ) refers to the number of trees per acre that the sample tree theoretically represents based on the sample design, cycles (CYCLE) refers to a number assigned to a set of plots measured over a particular time; and current diameter (DIA) refers to diameter at breast height i.e., 4.5 feet above the ground line of the sample tree.
2.2.3. Soil Properties Data
We acquired soil variables such as soil texture, soil organic matter, and total available water from Gridded National Soil Survey Geographic Database (gNATSGO) data, initially a resolution of 10 m was acquired which was later resampled to a 1km grid as our spatial pattern analysis covers broader scale, provided by USDA Natural Resources Conservation Service [
113]. Our preliminary analysis included several stand characteristics such as stand age and DBH; and soil variables such as PH, C, N, and soil depth that brought model complexity reducing the performance of important variables. Therefore, the use of soil texture, soil organic matter, and total available water brought a more significant practical impact on white oak mortality rate. Hence, including soil texture is vital in soil characteristics which identify the proportion of sand, silt, and clay affecting organic matter content and water-holding capacity as well [
58,
59].
The soil texture classifications from SSURGO tabular data were combined with the spatial soil data provided with GIS shapefiles. Some regions were null for soil texture classification, which was addressed by obtaining information from the FAO dataset [
112]. We used Soil Texture Triangle as a reference to fill all null values for soil texture by placing SSURGO and FAO spatial soil data into the ArcGIS [
60]. By doing this, we obtained eighteen types of soil texture classification (including inland water) that were common in our study area (
Table 1). We also calculated soil organic matter and total available water using soil horizon thickness; and classified them into five classes.
2.3. Data Processing and Analysis
2.3.1. Selection of Live and Declining White Oak Plots
Annualized data from 1998 to 2019 were processed to locate white oak plots (
Table 2). After data cleaning and processing for different tables i.e., plot, forest condition, and tree, we selected a plot table to narrow our criteria i.e., locating white oaks’ plots across accessible forest land. For instance, Missouri had a plot table that contained useful attributes such as unique plot numbers related to each county, and each cycle, locations (latitudes and longitudes) with surveyed years for a particular date. We selected sampled plots that contained at least one accessible forest land condition on the plot. The condition table consisted of similar attributes except for locations that are useful to link with the plot table. We also processed the condition and tree table with similar criteria i.e., selecting accessible forest land. The tree table has important attributes such as unique plot number, county code, tree species code, diameter, trees per acre, and cycle.
We selected white oak trees identified with species code 802 from the tree table. From the same tree table containing white oaks only, we calculated the basal area i.e., Basal area of white oak = 0.005454 *(Current Diameter)2 * trees per acre unadjusted. We linked the forest condition table and tree table based on unique plot numbers. Then, all the plot numbers from the combined tree and forest condition table were summarized by basal area. Later, we converted the basal area into the standard unit as a square meter per hectare. Then, we joined summarized basal area and plot tables based on unique plot numbers. The combined plot tables now contained white oaks, plot locations, basal area, survey cycles, etc. Our annual data were grouped into multicycles based on a five-survey-year cycle. We selected an annual cycle that contained a specific time frame. For instance, Missouri had cycle 5 which consisted of inventory years grouped from 1999 to 2003 (usually year-wise data). Similarly, other cycles had a set of inventory years that goes until 2019 which was grouped as cycle 6 (2004 to 2008), cycle 7 (2009 to 2013), and cycle 8 (2014 to 2019). We did the same process with other states and extracted individual plot cycles of white oaks based on surveyed years. We created an appropriate formula using county and plot number i.e., COUNTYCD + (PLOT)/100,000 to link with other white oaks’ cycle. We joined each cycle one by one. For instance, Missouri cycle 5 is joined with cycle 6 and we subtracted cycle 5 basal area from cycle 6 which gave us declining plots in terms of negative basal area. We did the same with other cycles as joining 6 to 7, and 7 to 8. From all these cycles, we get common as well as extra declining plots. However, we only utilized the basal area from a single plot so that we can avoid repetition. All these linking processes for spatial tables and calculations were done in ArcGIS. On utilizing basal areas, we extracted 2,220 declining white oak plots from 7,405 live white oak plots across ten states.
2.3.2. White Oak Mortality Rate Analysis
The mortality rate was calculated by using basal areas of surveyed cycles, which were grouped into five years. Some of the white oak plots were remeasured in the period from 1998 to 2019. Hence, we omitted those repeated plots to have accurate results. However, we summed up all those remeasured plots’ basal areas to obtain the true basal area. We selected all the basal areas from declining plots of white oaks concerning surveyed years. The declining plots were chosen based on reduced or basal area change. Reduced basal area refers to the decrease in basal area over given timeframe referring to white oak plots. As the eastern US forest has been gone for self-thinning stage because of forest clearing and agricultural abandonment from last century [
23,
61]. As the trees continue growing, the basal area increases while the number of trees decreases. Therefore, basal area change considers factors such as size and the contribution of individual trees in addition to the quantity of trees present. Using basal area change over time reveal the decline of white oak stands whereas individual lost ignores sizes differences, provides incomplete picture of competition [
62,
63]. For instance, we calculated basal area change as:
We did the same with all remaining cycles and chose decreased basal areas that directly referred to the declining white oak plots. We calculated the mortality rate in terms of the reduced basal area of white oaks divided by the differences in the surveyed year between two extreme cycles. All those declining plots representing mortality rates were extracted and compiled the results. We calculated the mortality rate as:
We assumed oak forests in this region have mostly gone through the self-thinning stage [
64,
65,
66], and thus reduction of the white oak basal area can be attributed to WOM.
2.3.3. Spatial Distribution Analysis of WOM by univariate Ripley’s K function
The data for white oak mortality is based on specific point events. Ripley’s K function does a comparison of observed spatial patterns against those expected under complete spatial randomness (CSR) that provides information for clustering, random, and uniform [
67]. The map patterns do not justify the specific patterns such as CSR. Another reason is that edge effects are ignored and significantly impact map patterns that bring biased results [
68,
69]. However, the K function incorporates edge effects and produces valid results mitigating these issues. The use of Ripley’s K function highlights the visualization of spatial patterns at various scales i.e., small scales, larger scales, and their statistical significance whereas map patterns only highlight higher or lower concentrations without indicating particular patterns and statistical significance at varying scales.
We used Multi-Distance Spatial Cluster Analysis-Ripley’s K function tool [
70], which uses nearest neighbor distance to process spatial distribution patterns. ArcMap version 10.7.1 was used for Ripley's K function which defines whether our spatial pattern is cluster, random, or uniform on each analysis scale [
71,
72]. All 2,220 WOM points from 1998 to 2019 were compiled that contained point locations i.e., longitudes and latitudes. In Ripley's k function tool, the initial distance was 2,000 m and the increment distance is 20,000 m. Edge correction was done by using Ripley's edge correction formula [
70], which automatically counts and measures points inside the study area. To test our null hypothesis of CSR, we formed upper and lower confidence envelopes of 99.9% generated from randomizations of 999 plot points using the default random generator in ArcGIS. We examined whether our theoretical curve (K
theo) deviates from CSR or not. If the observed curve (K
obs) is above the theoretical curve and upper envelope, then there is spatial clustering. If it is below the theoretical curve and lower envelope, then WOM points follow spatial dispersion (uniform). There is also a greater chance that both observed and theoretical curves could line up denoting complete spatial randomness or random pattern. We used the transformed K function (L(d)) to represent the graphical interpretation of Ripley's K function.
2.3.4. WOM Rate, Stand Density, and Size Distribution among Soil Variables
We did a log transformation for the WOM rate and stand density of white oaks so that our data distribution could achieve normality. Basal area per area was calculated for stand density from the dying plots of white oak. Soil textures were classified (18 classes in our study area) taking a reference from Food and Agriculture Organization Soils Portal based on World Reference Base (WRB) soil classification system [
70]. We interpolated soil organic matter and total available water and use the interpolated results to compare them with the spatial pattern of the WOM rate. Soil organic matter and total available water percentage were classified into five classes i.e., very low, low, medium, high, and very high using natural breaks (Jenks) from ArcGIS. Our data were analyzed using R studio [
73]. In it, we fitted a linear model (
lm) to find the relationship between WOM rate and soil variables; and between the stand density of WOM and soil variables using significance levels at α = 0.1 and α = 0.05, respectively. The linear regression model equation for WOM rate concerning soil variables can be expressed by:
Where, y = logarithm of WOM rate (dependent variable); β0 = y-intercept; β1, β2, β3 = slope coefficient of each independent variable X; X1 = soil texture class; X2 = soil organic matter class; X3 = total available water class; e = error term.
Similarly, the linear regression model equation for the stand density of WOM across declining plots concerning soil variables can be expressed by:
Where, w = logarithm of stand density (dependent variable); β0 = y-intercept; β1, β2, β3 = slope coefficient of each independent variable Z; Z1 = soil texture class; Z2 = soil organic matter class; Z3 = total available water class; e = error term.
3. Results
3.1. White Oak Mortality Spatial Distribution Patterns
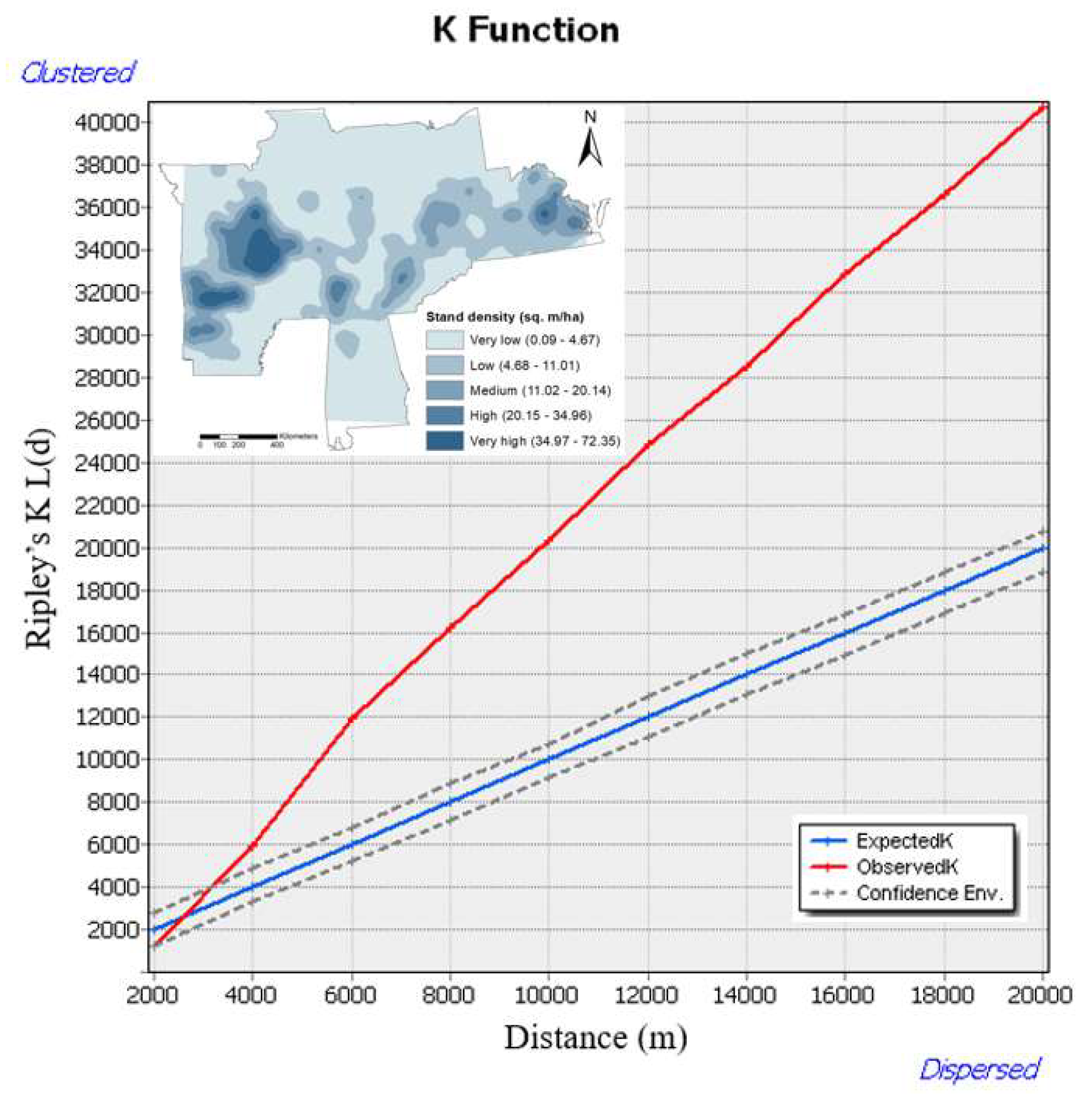
The spatial distribution pattern of the WOM rate showed random patterns up to 3000 m, as the observed K value was inside the confidence envelopes, and we accept the null hypothesis (
Figure 2). Beyond this point, we reject our null hypothesis, the spatial distribution of WOM rate showed clustered until 20,000 m is statistically significant. It is due to the spatial clustering of WOM rates, as indicated by the observed K value being significantly larger than the expected K function and falling outside the confidence envelopes. This clustering pattern of the WOM rate increases as the distance increases. Most of our pattern analysis for the WOM rate depicted clustered (non-random) patterns at a larger distance across the eastern US. Thus, our spatial distribution pattern analysis of WOM showed more clustering patterns than the random pattern as distance increases.
The kernel density maps for white oaks showed the cluster spatial distribution across the declining plots of white oaks (
Figure 2). This density distribution indicated areas for higher, medium, or lower concentration across the eastern US.
3.2. Relationship between WOM rate and Soil Variables
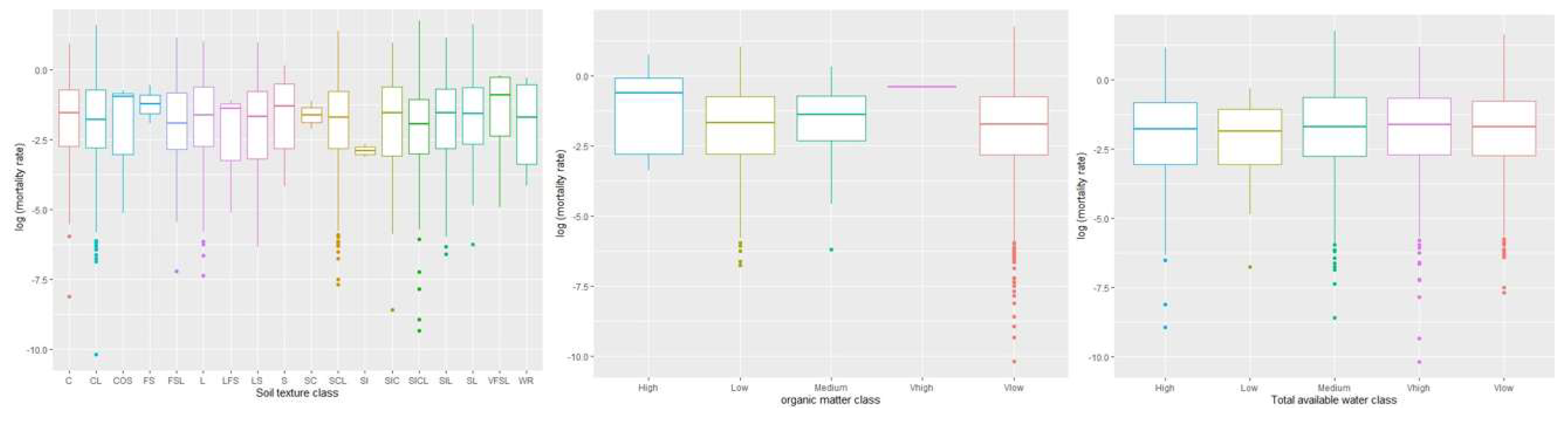
The relationship between logarithmic WOM rate and soil variables was also explained based on central tendency (
Figure 3). Results showed that there was a higher variation among soil texture classes concerning WOM rate. However, the silt (SI) had less variability as compared with other texture classes. We did not see any statistically significant relationship between WOM rate and soil variables other than at p value < 0.1. Hence, we selected those soil variables whose parameters estimates are significant at p value < 0.1 (
Table 3). This significant level is appropriate because of the high complexity of white oak mortality in the eastern U.S. such as effects of several environmental factors on white oak mortality might be subtle. By choosing higher threshold, we were able to address the ecological and anthropogenic factors associated with white oak mortality to explain the patterns of white oak mortality across the eastern U.S. Several studies have used p value <0.1 to address the effects of numerous environmental factors on larger landscape and sample size of plant species [
74,
75]. Our results showed significant effects with a negative linear relationship between silty clay loam (SICL) class and WOM rate (β = -0.39; p = 0.06). This indicates that the content of silt clay loam is negatively related to WOM.
Results from the central tendency of total available water class showed greater variation in WOM rate. The total available water classes were more or less similar in the data distribution with respect to the WOM rate. However, there was a significant and positive linear relationship between very high total available water class and WOM rate (β = 0.20; p= 0.05) indicating that the content of total available water is positively related to WOM rate.
Our results showed that a very high class of soil organic matter had a higher variation concerning the WOM rate. However, the data variation was much higher for the rest of the soil organic matter classes except for very high soil organic matter. Other soil variables such as very low and low organic matter classes were significant and showed a negative linear relationship with WOM rate i.e., β = -0.81, p = 0.08, and β = -0.83, p = 0.07, respectively. These indicate that the content of low and very low organic matter is negatively related to the WOM rate.
3.3. Relationship between Density Distribution of WOM and Soil Variables
The relationship between the density distribution of WOM and most of the soil variables i.e., texture, organic matter, and total available water were significant (p < 0.05;
Table 4). In this regard, the density distribution of soil properties i.e., texture, soil organic matter, and total available water concerning WOM rate was successful in interpreting the spatial pattern of WOM across the eastern US.
The result showed a significant and a negative linear relationship between loamy fine sand and the stand density of WOM (β = -1.50; p = 0.04), indicating that the content of loamy fine sand is negatively related to the density distribution of WOM. Also, fine sandy loam and clay loam showed a significant and a negative relationship with stand density i.e., β = -0.39, p = 0.02; and β = -0.29, p = 0.04, respectively. This indicates that the content of fine sandy loam and clay loam is negatively related to the density distribution of WOM.
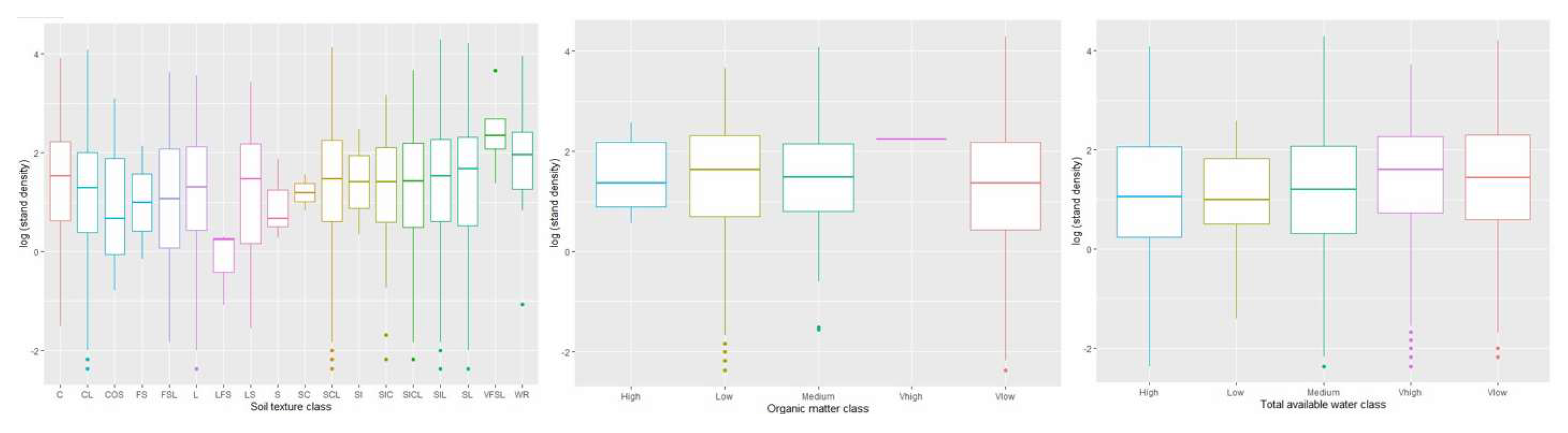
Our result did not show a statistically significant relationship between soil organic matter and stand density of WOM. However, there was a variation of organic matter class along with the stand density of WOM across the eastern US (
Figure 4). The central tendency analysis showed that very high organic matter class played a significant role in the stand density distribution of WOM i.e., 2 m
2/ha. However, the rest of the organic matter class i.e., high, medium, low, and very low classes were positively related to the density distribution of WOM but not quite greater in comparison to very high organic matter.
Our results showed a statistically significant linear relationship between the stand density of white oaks and very low total available water class (β = 0.28 and p = 0.001). However, results showed that a very high total available water class (β = 0.32, p = 0.00) is highly significant with the stand density of white oaks. Our central tendency analysis showed that there was a variation among total available water classes concerning the stand density of WOM (Figure 6). Result showed that very high total available water class had a greater variation concerning the stand density of WOM. We also found that very low total available water class had significant variation in the distribution of stand density of WOM.
4. Discussion
The present study showed that the spatial distribution patterns of white oak mortality in the eastern US are influenced by soil properties, such as texture, organic matter, and soil moisture. The distribution patterns from the K-function indicated that the WOM rate at site scales was random. This confirmed with findings that WOM is reported at various site conditions including poor and good resources sites as well as various topographic and hydrological conditions [
16,
76,
77]. WOM rate showed increasing clustered distribution pattern at broad scales, indicating that mid-level controls such as topography and soil, and regional-scale controls such as climate, drought in particular, may exert some dominant roles over the WOM rate [
25,
78,
79]. The method and analysis used in this study are in accordance with other similar findings that aggregation factors were responsible for the spatial distribution of the WOM rate [
16,
80]. It also appears that site-scale processes such as self-thinning in white oak stands may accumulate across the region, which may result in a region-wide WOM since most white oak stands were regenerated after forest clearing and the subsequent agricultural abandonment nearly a century ago[
81,
82,
83].
The spatial pattern of WOM has been influenced by edaphic factors such as soil variables responsible for clustering. It is because soil conditions determine species establishment and can influence resource availability and forest disturbance [
84,
85]. [
86] found finer soil textures were responsible for oak decline associated with higher levels of soil moisture in the Colombian Amazon. However, our study found coarser soil texture (e.g., silty clay loam) had significant effects on white oaks decline, suggesting a clustered spatial pattern of WOM rate at a larger scale. This may be due to coarser soil textures such as fine sandy loam, clay loam, and loamy fine sand, across our study area, had a higher infiltration rate where water movement occurs through the soil profile resulting reduction in water retention and availability [
30,
87]. Others found that the oak growth was unfavorable across the coarser textured soils due to low water holding capacity and these types of soils may dry out despite the abundant precipitation [
88,
89]. White oaks have vast and frequently deep-reaching root systems. Larger particles and more air spaces in coarser soils can make it difficult for roots to penetrate them[
90]. This may restrict the tree’s access to vital supplies such as water and minerals. In this case, the oak tree may result in stunted growth and can decrease in stand density leading to mortality.
The results of this work showed a strong connection between soil organic matter and tree mortality influencing spatial patterns [
91,
92]. Our study follows similar findings that white oaks exhibited reduced growth [
93], that were dying in clusters across organic matter deficient areas on a larger scale. Another study from [
94] on tree survival and growth in mixed soil types reported that WOM rates were higher in organic matter-scarce areas where the soil was extremely dry. Our results reported low i.e., 0.01%, and very low i.e., no soil organic matter class had significant impacts on WOM rate and shaping the spatial patterns. It is because low or no soil organic matter had combined effects such as reduced nutrient availability, increased susceptibility to insect pests and diseases, and stress from drought that may have contributed to clustered patterns [
58,
95]. Also, our results showed that white oaks are declining randomly at local scales that were associated with low soil fertility. It is because poor nutrient soils do not provide anchorage and nutrient variations can have a significant impact on the oak forest stand dynamics at shorter scales [
96]. However, other research reported differences from our findings that tree mortality across the local scale (e.g., the boreal forest and seasonal rainforest) depicted uniform and somewhat clustered patterns across low nutrient gradients [
97,
98].
Our findings show that the spatial pattern of WOM rate was influenced by variations in soil moisture content. It is because previous studies have found that soil moisture plays a critical role in tree health due to excessive or insufficient moisture levels leading to increased stress and susceptibility to diseases and pests [
99,
100]. For instance, our findings indicated that clustered pattern of WOM rate in low moisture areas (e.g., very low class of total available water), suggesting extremely dry conditions resulting in white oak decline. In contrast to our findings, [
101] found that tree mortality was decreased over an increase in soil moisture that occurred across the driest site during El Nino year in the eastern and central United States. However, our results found compelling evidence suggesting elevated soil moisture levels (e.g., very high class of total available water) are associated with higher mortality rates in white oaks. Elevated soil moisture has adverse conditions on white oaks due to reduced soil aeration and increased waterlogging.[
102] and [
103] reported similar patterns of increased mortality in areas with poor drainage and high soil moisture levels.
The study of the spatial patterns of white oak mortality, specifically in relation to soil texture, soil organic matter, and total available water adds up fresh perspectives to contribute novel insights to the existing body of literature. However, similar relationships have been explored in the past, our study stands out because of its distinctive methodology, large dataset, and insightful dynamics. Unlike earlier studies that often focused on one or two factors, our research employs an integrative strategy focusing on soil properties solely. For instance, [
72] studied spatial patterns of six managed tree species in the central Amazon that were affected by topographic variables such as elevation, slope percentage, and stream distance, and most of the species possessed clustered patterns at smaller scales. However, our study focused on soil properties that influenced white oak mortality and found to be random patterns at shorter scales. While some previous studies depended on finer scales and our study focused on the coarser scale. This coarser scale helped us to identify the overall picture of spatial patterns across the wider landscapes that might have been overlooked. For instance, [
104] studied patterns of oak mortality across the Ozark highlands that did not find any clear white oak declining patterns on a site scale. However, our research found differences in the mortality patterns on the varied scale. Moreover, others have concentrated the oak mortality patterns in the mesic habitats, or dry, upland valleys [
23,
89,
105]. None of these studies considered the larger landscapes across the eastern US. This approach is made more complex by this variety because it lets us investigate how spatial patterns may alter across various types of landscapes. This idea differs from some past research that concentrated on more uniform surroundings.
The practical implication in forestry that soil textures were important factors for white oak mortality can be optimized by selecting white oak declining areas that are better suited to the prevailing soil textures across the eastern US. By utilizing successful forest management practices, forest managers can enhance the establishment and growth of white oaks reducing the risk of mortality [
106,
107]. Areas of white oak mortality associated with particular soil textures can be managed through soil amendments, soil aeration, and fertilization to improve soil structure and nutrient availability reducing mortality [
108,
109]. The information from the influence of soil textures can help in the predictive modeling and assessment tool that can identify areas with higher mortality and spatial patterns based on soil characteristics and management can be done to mitigate white oak losses.
Overall, the influence of soil textures in white oak mortality and its spatial patterns can address the need for a site-specific and holistic approach by incorporating forestry practices to enhance forest health, resilience, and ecosystem services of the white oak-dominated forest across the eastern United States.
5. Conclusions
Our study examined the WOM rate and its spatial patterns across the eastern United States using annual forest inventories and soil variables. The spatial pattern of the WOM rate was clustered at a larger distance and random at a shorter distance. And these patterns were influenced by various sizes of soil variables. Mostly, silty clay loam, fine sandy loam, loamy fine sand, very high and low class of total available water, and very low and low class of soil organic matter played a significant role in the spatial distribution of WOM rate.
The information from WOM rate, stand density of WOM, soil texture, soil organic matter, and total available water revealed mostly clustered spatial patterns, and the patterns differed on the size of scales. Except for some soil textures and total available water, other sizes of organic matter did not show any significant role in the spatial pattern of WOM rate. However, we found that the coarser textured soils are responsible for white oak mortality. The white oak mortality and its spatial pattern are affected by low soil fertility which does not favor white oaks growths and development. High as well as low soil moisture impacted white oak across varied scales and played a role in the spatial patterns. Our study highlighted that the regional pattern analysis of the WOM rate had been influenced by soil characteristics across varying scales. The observed relationship signifies the importance of soil variables in determining the random as well as the clustered spatial pattern of the WOM rate. However, further research is needed to investigate the specific mechanism underlying these relationships and to assess the long-term impacts on oak forest ecosystems. Future studies could explore the effects of multiple factors such as biotic and abiotic factors as well as land use practices, to obtain a more comprehensive understanding of the spatial pattern of WOM.
While our study mainly focused on the relationship between soil variables and WOM rate. It is important to consider other potential factors that can contribute to white oak decline such as climate, topography, stand structure, terrains, insect pest attacks, and others. We were not able to include all these variables because these data required extensive preprocessing and cleaning since we covered ten states from the eastern United States. For instance, our study is based on plot-level analysis that needs to line up with a multi-level dataset of white oak trees, their diameter, forested condition, and surveyed years with respect to all other variables mentioned above. And processing these data required several mathematical calculations and data linkages. We also encountered decreased model performance due to a large number of variables that brought no significant results for spatial patterns of white oak mortality. Similarly, while conducting this research we have limited time and computational power and need to make a choice about which variables to be included based on these constraints. Finally, our study was conducted on specific geographic regions, mainly the eastern US, and the conclusion drawn from our findings to other tree species should be investigated further.
Author Contributions
Conceptualization, S.K.; methodology, S.K.; validation, S.K., and H.H.; writing—original draft preparation, S.K.; writing—review and editing, S.K., H.H., S.B., and A.K.; supervision, H.H.; project administration, H.H., and S.B.; funding acquisition, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
United States Department of Agriculture/National Institute of Food and Agriculture 1890 Capacity Building Grant, Award number 2021-38821-34704.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge Lincoln University College of Agriculture, environment, and Human Science for its facilities and support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wohlleben The Hidden Life of Trees: What They Feel, How They Communicate. Wohlleben, Peter. Irish Forestry 2016, 74, 226–228.
- Newell, F.L.; Rodewald, A.D. Role of Topography, Canopy Structure, and Floristics in Nest-Site Selection and Nesting Success of Canopy Songbirds. For Ecol Manage 2011, 262, 739–749. [Google Scholar] [CrossRef]
- Parola, A.C.; Vesely, W.S.; Croasdaile, M.A. Geomorphic Characteristics of Streams in the Bluegrass Physiographic Region of Kentucky. Kentucky Division of 2007, 319. [Google Scholar]
- Tallamy, D.W. The Nature of Oaks: The Rich Ecology of Our Most Essential Native Trees; Timber Press, 2021. [Google Scholar]
- Creutzburg, M.K.; Scheller, R.M.; Lucash, M.S.; Evers, L.B.; Leduc, S.D.; Johnson, M.G. Bioenergy Harvest, Climate Change, and Forest Carbon in the Oregon Coast Range. GCB Bioenergy 2016, 8, 357–370. [Google Scholar] [CrossRef]
- Littlefield, C.E.; D’Amato, A.W. Identifying Trade-Offs and Opportunities for Forest Carbon and Wildlife Using a Climate Change Adaptation Lens. Conserv Sci Pract 2022, 4, 1–14. [Google Scholar] [CrossRef]
- Tainter, F.H.; Retzlaff, W.A.; Starkey, D.A.; Oak, S.W. Decline of Radial Growth in Red Oaks Is Associated with Short-Term Changes in Climate. European Journal of Forest Pathology 1990, 20, 95–105. [Google Scholar] [CrossRef]
- Mccracken, K.E.; Witham, J.W.; Hunter, M.L. Relationships between Seed Fall of Three Tree Species and Peromyscus Leucopus and Clethrionomys Gapperi during 10 Years in an Oak-Pine Forest. J Mammal 1999, 80, 1288–1296. [Google Scholar] [CrossRef]
- Starkey, D.A.; Oliveria, F.; Mangini, A.; Mielke, M. NATURAL PHENOMENA, SEVERE OCCURRENCES. In Proceedings of the Upland Oak Ecology Symposium: History, Current Conditions, and Sustainability, Fayetteville, Arkansas, October 7-10, 2002; 2004; Vol. 73, p. 217. [Google Scholar]
- Haavik, L.J.; Jones, J.S.; Galligan, L.D.; Guldin, J.M.; Stephen, F.M. Oak Decline and Red Oak Borer Outbreak: Impact in Upland Oak-Hickory Forests of Arkansas, USA. Forestry: An International Journal of Forest Research 2012, 85, 341–352. [Google Scholar] [CrossRef]
- Spetich, M.A. Upland Oak Ecology Symposium: History, Current Conditions, and Sustainability. Uplannd Oak Ecology Symposium: History, Current Conditions, and Sustainability, 2002; 318. [Google Scholar]
- Heitzman, E.; Shelton, M.G.; Grell, A. Species Composition, Size Structure, and Disturbance: History of an Old Growth Bottomland Hardwood Loblolly Pine (Pinus Taeda) Forest in Arkansas, USA. Natural Areas Journal 2000, 24, 177–187. [Google Scholar]
- Shifley, S.R.; Fan, Z.; Kabrick, J.M.; Jensen, R.G. Oak Mortality Risk Factors and Mortality Estimation. For Ecol Manage 2006, 229, 16–26. [Google Scholar] [CrossRef]
- Greenberg, Cathryn H.; Colllins, S.B. Natural Disturbances and Historic Range of Variation. Natural Disturbances and Historic Range of Variation 2016, 32, 167–202. [Google Scholar]
- Reed, S.E.; English, J.T.; Muzika, R.M. Phytophthora Species Detected in Two Ozark Forests with Unusual Patterns of White Oak Mortality. Plant Dis 2019, 103, 102–109. [Google Scholar] [CrossRef]
- Nagle, A.M.; Long, R.P.; Madden, L. V.; Bonello, P. Association of Phytophthora Cinnamomi with White Oak Decline in Southern Ohio. Plant Dis 2010, 94, 1026–1034. [Google Scholar] [CrossRef]
- Wang, C.; He, H.S.; Kabrick, J.M. A Remote Sensing-Assisted Risk Rating Study to Predict Oak Decline and Recovery in the Missouri Ozark Highlands, USA. GIsci Remote Sens 2008, 45, 406–425. [Google Scholar] [CrossRef]
- McConnell, M.E.; Balci, Y. Phytophthora Cinnamomi as a Contributor to White Oak Decline in Mid-Atlantic United States Forests. Plant Dis 2014, 98, 319–327. [Google Scholar] [CrossRef]
- Dey, D. Dey DC 2002 Chapter 5 Oak Book for Oak Silviculture in Eastern North America. 2009. [Google Scholar]
- Aldrich, P.R.; Parker, G.R.; Romero-severson, J.; Michler, C.H. Forest : 75 Years of Data. Forest Science 2005, 51. [Google Scholar]
- Greenberg, C.H.; Keyser, T.L.; Speer, J.H. Temporal Patterns of Oak Mortality in a Southern Appalachian Forest (1991-2006). Natural Areas Journal 2011, 31, 131–137. [Google Scholar] [CrossRef]
- Balci, Y.; Long, R.P.; Mansfield, M.; Balser, D.; MacDonald, W.L. Involvement of Phytophthora Species in White Oak (Quercus Alba) Decline in Southern Ohio. For Pathol 2010, 40, 430–442. [Google Scholar] [CrossRef]
- Abrams, M.D. Where Has All the White Oak Gone? Bioscience 2003, 53, 927–939. [Google Scholar] [CrossRef]
- Fan, Z.; Kabrick, J.M.; Spetich, M.A.; Shifley, S.R.; Jensen, R.G. Oak Mortality Associated with Crown Dieback and Oak Borer Attack in the Ozark Highlands. For Ecol Manage 2008, 255, 2297–2305. [Google Scholar] [CrossRef]
- Wood, J.D.; Knapp, B.O.; Muzika, R.M.; Stambaugh, M.C.; Gu, L. The Importance of Drought-Pathogen Interactions in Driving Oak Mortality Events in the Ozark Border Region. Environmental Research Letters 2018, 13. [Google Scholar] [CrossRef]
- Chastain, R.A.; Currie, W.S.; Townsend, P.A. Carbon Sequestration and Nutrient Cycling Implications of the Evergreen Understory Layer in Appalachian Forests. For Ecol Manage 2006, 231, 63–77. [Google Scholar] [CrossRef]
- Peltzer, D.A.; Allen, R.B.; Lovett, G.M.; Whitehead, D.; Wardle, D.A. Effects of Biological Invasions on Forest Carbon Sequestration. Glob Chang Biol 2010, 16, 732–746. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Dietze, M.C.; Jackson, R.B.; Phillips, R.P.; Rhoades, C.C.; Rustad, L.E.; Vose, J.M. Forest Biogeochemistry in Response to Drought. Glob Chang Biol 2016, 22, 2318–2328. [Google Scholar] [CrossRef]
- Profft, I.; Mund, M.; Weber, G.E.; Weller, E.; Schulze, E.D. Forest Management and Carbon Sequestration in Wood Products. Eur J For Res 2009, 128, 399–413. [Google Scholar] [CrossRef]
- Wang, D.Y.; Yan, D.H.; Song, X.S.; Wang, H. Impact of Biochar on Water Holding Capacity of Two Chinese Agricultural Soil. Adv Mat Res 2014, 941–944, 952–955. [Google Scholar] [CrossRef]
- de Vries, W.; Du, E.; Butterbach-Bahl, K. Short and Long-Term Impacts of Nitrogen Deposition on Carbon Sequestration by Forest Ecosystems. Curr Opin Environ Sustain 2014, 9, 90–104. [Google Scholar] [CrossRef]
- Hui, D.; Deng, Q.; Tian, H.; Luo, Y. Handbook of Climate Change Mitigation and Adaptation; 2016; ISBN 9781461464310. [Google Scholar]
- Weed, A.S.; Ayres, M.P.; Hicke, J.A. Consequences of Climate Change for Biotic Disturbances in North American Forests. Ecol Monogr 2013, 83, 441–470. [Google Scholar] [CrossRef]
- Martin, K.L.; Goebel, P.C. The Foundation Species Influence of Eastern Hemlock (Tsuga Canadensis) on Biodiversity and Ecosystem Function on the Unglaciated Allegheny Plateau. For Ecol Manage 2013, 289, 143–152. [Google Scholar] [CrossRef]
- Frelich, L.E.; Hale, C.M.; Scheu, S.; Holdsworth, A.R.; Heneghan, L.; Bohlen, P.J.; Reich, P.B. Earthworm Invasion into Previously Earthworm-Free Temperate and Boreal Forests. Biol Invasions 2006, 8, 1235–1245. [Google Scholar] [CrossRef]
- McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.; Aukema, B.H.; Bond-Lamberty, B.; Chini, L.; Clark, J.S.; Dietze, M.; Grossiord, C.; Hanbury-Brown, A.; et al. Pervasive Shifts in Forest Dynamics in a Changing World. Science (1979) 2020, 368, eaaz9463. [Google Scholar] [CrossRef] [PubMed]
- Hall, M. The Effects of Prescribed Burns on Oak ( Quercus Spp.) and Red Maple ( Acer Rubrum ) Stump Sprouts in Southeastern Ohio. 2011, 1–25. [Google Scholar]
- Muhly, T.B.; Hebblewhite, M.; Paton, D.; Pitt, J.A.; Boyce, M.S.; Musiani, M. Humans Strengthen Bottom-Up Effects and Weaken Trophic Cascades in a Terrestrial Food Web. PLoS One 2013, 8. [Google Scholar] [CrossRef]
- Dale, M.R.T. Spatial Pattern Analysis in Plant Ecology; Cambridge university press, 2000. [Google Scholar]
- Guisan, A.; Thuiller, W. Predicting Species Distribution: Offering More than Simple Habitat Models. Ecol Lett 2005, 8, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Milbau, A.; Stout, J.C.; Graae, B.J.; Nijs, I. A Hierarchical Framework for Integrating Invasibility Experiments Incorporating Different Factors and Spatial Scales. Biol Invasions 2009, 11, 941–950. [Google Scholar] [CrossRef]
- Getzin, S.; Wiegand, T.; Wiegand, K.; He, F. Heterogeneity Influences Spatial Patterns and Demographics in Forest Stands. Journal of Ecology 2008, 96, 807–820. [Google Scholar] [CrossRef]
- Clark, F.B.; Hutchinson, J.G. Central Hardwood Notes; US Department of Agriculture, Forest Service, North Central Forest, 1989. [Google Scholar]
- Fralish, J.S. The Central Hardwood Forest: Its Boundaries and Physiographic Provinces; General Technical Report - North Central Research Station, USDA Forest Service, 2003; pp. 1–20. [Google Scholar]
- Cleland, D.T.; Freeouf, J.A.; Keys, J.E.; Nowacki, G.J.; Carpenter, C.A.; McNab, W.H. Ecological Subregions: Sections and Subsections for the Conterminous United States. Gen. Tech. Rep. WO-76D; 2007; p. 242. [Google Scholar]
- Wang, W.J.; He, H.S.; Thompson, F.R.; Fraser, J.S.; Hanberry, B.B.; Dijak, W.D. Importance of Succession, Harvest, and Climate Change in Determining Future Composition in U.S. Central Hardwood Forests. Ecosphere 2015, 6, 1–18. [Google Scholar] [CrossRef]
- Wang, W.J.; He, H.S.; Thompson, F.R.; Fraser, J.S.; Hanberry, B.B.; Dijak, W.D. Importance of Succession, Harvest, and Climate Change in Determining Future Composition in U.S. Central Hardwood Forests. Ecosphere 2015, 6, 1–18. [Google Scholar] [CrossRef]
- Stambaugh, M.C.; Knapp, B.O.; Dey, D.C. Fire Ecology and Management of Forest Ecosystems in the Western Central Hardwoods and Prairie-Forest Border; 2021; ISBN 9783030732677. [Google Scholar]
- Fearer, T.M.; Norman, G.W.; Pack, J.C.; Bittner, S.; Healy, W.M. Influence of Physiographic and Climatic Factors on Spatial Patterns of Acorn Production in Maryland and Virginia, USA. J Biogeogr 2008, 35, 2012–2025. [Google Scholar] [CrossRef]
- LeBlanc, D.C.; Terrell, M.A. Comparison of Growth-Climate Relationships between Northern Red Oak and White Oak across Eastern North America. Canadian Journal of Forest Research 2011, 41, 1936–1947. [Google Scholar] [CrossRef]
- Bechtold, W.A.; Patterson, P.L. The Enhanced Forest Inventory and Analysis Program--National Sampling Design and Estimation Procedures; USDA Forest Service, Southern Research Station, 2005; Vol. 80. [Google Scholar]
- Tinkham, W.T.; Mahoney, P.R.; Hudak, A.T.; Domke, G.M.; Falkowski, M.J.; Woodall, C.W.; Smith, A.M.S. Applications of the United States Forest Inventory and Analysis Dataset: A Review and Future Directions. Canadian Journal of Forest Research 2018, 48, 1251–1268. [Google Scholar] [CrossRef]
- McRoberts, R.E.; Miles, P.D. United States of America. In National Forest Inventories: Assessment of Wood Availability and Use; Vidal, C., Alberdi, I.A., Hernández Mateo, L., Redmond, J.J., Eds.; Springer International Publishing: Cham, 2016; pp. 829–842. ISBN 978-3-319-44015-6. [Google Scholar]
- Smith, W.B. Forest Inventory and Analysis: A National Inventory and Monitoring Program. Environmental Pollution 2002, 116, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.; Brandeis, T.; Shaw, J.; McWilliams, W.; Miles, P. Forest Inventory and Analysis Database of the United States of America (FIA). Biodiversity & Ecology 2012, 4, 225–231. [Google Scholar] [CrossRef]
- Burrill, E.A.; Wilson, A.M.; Turner, J.A.; Pugh, S.A.; Menlove, J.; Christiansen, G.; Conkling, B.L.; David, W. The Forest Inventory and Analysis Database: Database Description and User Guide Version 8.0 for Phase 2. US Department of Agriculture, Forest Service 2018, 946. [Google Scholar]
- Khadka, S.; Gyawali, B.R.; Shrestha, T.B.; Cristan, R.; Banerjee, S. “Ban”; Antonious, G.; Poudel, H.P. Exploring Relationships among Landownership, Landscape Diversity, and Ecological Productivity in Kentucky. Land use policy 2021, 111, 105723. [Google Scholar] [CrossRef]
- Bot, A.; Benites, J. The Importance of Soil Organic Matter: Key to Drought-Resistant Soil and Sustained Food Production.; 2005. [Google Scholar]
- Yang, H.; Yoo, H.; Lim, H.; Kim, J.; Choi, H.T. Impacts of Soil Properties, Topography, and Environmental Features on Soil Water Holding Capacities (SWHCs) and Their Interrelationships. Land (Basel) 2021, 10, 1290. [Google Scholar] [CrossRef]
- Johnston, K.; Ver Hoef, J.M.; Krivoruchko, K.; Lucas, N. Using ArcGIS Geostatistical Analyst. Analysis 2001, 300, 300. [Google Scholar]
- King, D.I.; Schlossberg, S. Synthesis of the Conservation Value of the Early-Successional Stage in Forests of Eastern North America. For Ecol Manage 2014, 324, 186–195. [Google Scholar] [CrossRef]
- Yang, Y.; Titus, S.J.; Huang, S. Modeling Individual Tree Mortality for White Spruce in Alberta. Ecol Modell 2003, 163, 209–222. [Google Scholar] [CrossRef]
- De Toledo, J.J.; Magnusson, W.E.; Castilho, C. V.; Nascimento, H.E.M. How Much Variation in Tree Mortality Is Predicted by Soil and Topography in Central Amazonia? For Ecol Manage 2011, 262, 331–338. [Google Scholar] [CrossRef]
- Iverson, L.R.; Hutchinson, T.F.; Prasad, A.M.; Peters, M.P. Thinning, Fire, and Oak Regeneration across a Heterogeneous Landscape in the Eastern U.S.: 7-Year Results. For Ecol Manage 2008, 255, 3035–3050. [Google Scholar] [CrossRef]
- Garnas, J.R.; Ayres, M.P.; Liebhold, A.M.; Evans, C. Subcontinental Impacts of an Invasive Tree Disease on Forest Structure and Dynamics. Journal of Ecology 2011, 99, 532–541. [Google Scholar] [CrossRef]
- Yang, S.-I.; Brandeis, T.J. Estimating Maximum Stand Density for Mixed-Hardwood Forests among Various Physiographic Zones in the Eastern US. For Ecol Manage 2022, 521, 120420. [Google Scholar] [CrossRef]
- Robeson, S.M.; Li, A.; Huang, C. Point-Pattern Analysis on the Sphere. Spat Stat 2014, 10, 76–86. [Google Scholar] [CrossRef]
- Haase, P. Spatial Pattern Analysis in Ecology Based on Ripley’s K-Function: Introduction and Methods of Edge Correction. Journal of Vegetation Science 1995, 6, 575–582. [Google Scholar] [CrossRef]
- Gavin, D.G. K1D: Multivariate Ripley’s K-Function for One-Dimensional Data. Oikos 2007, 1, 1–8. [Google Scholar]
- Ripley, B.D. Spatial Statistics.; 2005. [Google Scholar]
- Wehenkel, C.; Brazão-Protázio, J.M.; Carrillo-Parra, A.; Martínez-Guerrero, J.H.; Crecente-Campo, F. Spatial Distribution Patterns in the Very Rare and Species-Rich Picea Chihuahuana Tree Community (Mexico). PLoS One 2015, 10, 1–19. [Google Scholar] [CrossRef]
- Miron, A.C.; Bezerra, T.G.; Nascimento, R.G.M.; Emmert, F.; Pereira, R.S.; Higuchi, N. Spatial Distribution of Six Managed Tree Species Is Influenced by Topography Conditions in the Central Amazon. J Environ Manage 2021, 281. [Google Scholar] [CrossRef]
- Team, R.C. ; others R Development Core Team. R: A language and environment for statistical computing 2016, 55, 275–286. [Google Scholar]
- Radcliffe, D.C.; Hix, D.M.; Matthews, S.N. Predisposing Factors’ Effects on Mortality of Oak (Quercus) and Hickory (Carya) Species in Mature Forests Undergoing Mesophication in Appalachian Ohio. For Ecosyst 2021, 8. [Google Scholar] [CrossRef]
- Kremen, C.; Williams, N.M.; Bugg, R.L.; Fay, J.P.; Thorp, R.W. The Area Requirements of an Ecosystem Service: Crop Pollination by Native Bee Communities in California. Ecol Lett 2004, 7, 1109–1119. [Google Scholar] [CrossRef]
- McCarthy, B.C.; Small, C.J.; Rubino, D.L. Composition, Structure and Dynamics of Dysart Woods, an Old-Growth Mixed Mesophytic Forest of Southeastern Ohio. For Ecol Manage 2001, 140, 193–213. [Google Scholar] [CrossRef]
- Rubino, D.L.; McCarthy, B.C. Evaluation of Coarse Woody Debris and Forest Vegetation across Topographic Gradients in a Southern Ohio Forest. For Ecol Manage 2003, 183, 221–238. [Google Scholar] [CrossRef]
- Wang, W.J.; He, H.S.; Thompson, F.R.; Fraser, J.S.; Dijak, W.D. Landscape- and Regional-Scale Shifts in Forest Composition under Climate Change in the Central Hardwood Region of the United States. Landsc Ecol 2016, 31, 149–163. [Google Scholar] [CrossRef]
- Wang, J.; Maduako, I.N. Spatio-Temporal Urban Growth Dynamics of Lagos Metropolitan Region of Nigeria Based on Hybrid Methods for LULC Modeling and Prediction. Eur J Remote Sens 2018, 51, 251–265. [Google Scholar] [CrossRef]
- Novick, K.; Jo, I.; D’Orangeville, L.; Benson, M.; Au, T.F.; Barnes, M.; Denham, S.; Fei, S.; Heilman, K.; Hwang, T.; et al. The Drought Response of Eastern US Oaks in the Context of Their Declining Abundance. Bioscience 2022, 72, 333–346. [Google Scholar] [CrossRef]
- Wang, W.J.; He, H.S.; Fraser, J.S.; Thompson, F.R.; Shifley, S.R.; Spetich, M.A. LANDIS PRO: A Landscape Model That Predicts Forest Composition and Structure Changes at Regional Scales. Ecography 2014, 37, 225–229. [Google Scholar] [CrossRef]
- Wang, W.J.; He, H.S.; Spetich, M.A.; Shifley, S.R.; Thompson III, F.R.; Larsen, D.R.; Fraser, J.S.; Yang, J. A Large-Scale Forest Landscape Model Incorporating Multi-Scale Processes and Utilizing Forest Inventory Data. Ecosphere 2013, 4, 1–22. [Google Scholar] [CrossRef]
- De Bruijn, A.; Gustafson, E.J.; Sturtevant, B.R.; Foster, J.R.; Miranda, B.R.; Lichti, N.I.; Jacobs, D.F. Toward More Robust Projections of Forest Landscape Dynamics under Novel Environmental Conditions: Embedding PnET within LANDIS-II. Ecol Modell 2014, 287, 44–57. [Google Scholar] [CrossRef]
- Clinton, B.D. Light, Temperature, and Soil Moisture Responses to Elevation, Evergreen Understory, and Small Canopy Gaps in the Southern Appalachians. For Ecol Manage 2003, 186, 243–255. [Google Scholar] [CrossRef]
- Small, C.J.; McCarthy, B.C. Spatial and Temporal Variation in the Response of Understory Vegetation to Disturbance in a Central Appalachian Oak Forest. Journal of the Torrey Botanical Society 2002, 136–153. [Google Scholar] [CrossRef]
- Corcobado, T.; Solla, A.; Madeira, M.A.; Moreno, G. Combined Effects of Soil Properties and Phytophthora Cinnamomi Infections on Quercus Ilex Decline. Plant Soil 2013, 373, 403–413. [Google Scholar] [CrossRef]
- Verheijen, F.G.A.; Zhuravel, A.; Silva, F.C.; Amaro, A.; Ben-Hur, M.; Keizer, J.J. The Influence of Biochar Particle Size and Concentration on Bulk Density and Maximum Water Holding Capacity of Sandy vs Sandy Loam Soil in a Column Experiment. Geoderma 2019, 347, 194–202. [Google Scholar] [CrossRef]
- Chapman, K.A.; Brewer, R. Prairie and Savanna in Southern Lower Michigan: History, Classification, Ecology; Michigan Botanical Club, 2008. [Google Scholar]
- Johnson, P.S.; Shifley, S.R.; Rogers, R.; Dey, D.C.; Kabrick, J.M. The Ecology and Silviculture of Oaks; Cabi, 2019. [Google Scholar]
- Rebertus, A.J.; Meier, A.J. Blowdown Dynamics in Oak-Hickory Forests of the Missouri Ozarks. Journal of the Torrey Botanical Society 2001, 362–369. [Google Scholar] [CrossRef]
- Wunder, J.; Brzeziecki, B.; Zybura, H.; Reineking, B.; Bigler, C.; Bugmann, H. Growth-Mortality Relationships as Indicators of Life-History Strategies: A Comparison of Nine Tree Species in Unmanaged European Forests. Oikos 2008, 117, 815–828. [Google Scholar] [CrossRef]
- Barbeito, I.; Dawes, M.A.; Rixen, C.; Senn, J.; Bebi, P. Factors Driving Mortality and Growth at Treeline: A 30-Year Experiment of 92 000 Conifers. Ecology 2012, 93, 389–401. [Google Scholar] [CrossRef]
- Bargali, K.; Joshi, B.; Bargali, S.S.; Singh, S.P. Oaks and the Biodiversity They Sustain. International Oaks 2015, 26, 65–76. [Google Scholar]
- Lyczak, S.J.; Kabrick, J.M.; Knapp, B.O. Long-Term Effects of Organic Matter Removal, Compaction, and Vegetation Control on Tree Survival and Growth in Coarse-Textured, Low-Productivity Soils. For Ecol Manage 2021, 496, 119428. [Google Scholar] [CrossRef]
- Lee, C.A.; Holdo, R.M.; Muzika, R.-M. Feedbacks between Forest Structure and an Opportunistic Fungal Pathogen. Journal of Ecology 2021, 109, 4092–4102. [Google Scholar] [CrossRef]
- Akana, P.R. Patterns, Mechanisms, and Implications of Spatial Variability in the Ecological Processes Regulating Nutrient Access by Forest Trees; Columbia University, 2022. [Google Scholar]
- Rouvinen, S.; Kuuluvainen, T.; Siitonen, J. Tree Mortality in a Pinus Sylvestris Dominated Boreal Forest Landscape in Vienansalo Wilderness, Eastern Fennoscandia. Silva Fennica 2002, 36, 127–145. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, H.Y.H.; Wang, B.; Xiang, W.; Li, D.; Li, X.; Mallik, A.U.; Ding, T.; Huang, F.; Lu, S.; et al. Conspecific and Heterospecific Crowding Facilitate Tree Survival in a Tropical Karst Seasonal Rainforest. For Ecol Manage 2021, 481, 118751. [Google Scholar] [CrossRef]
- Ghorbani, R.; Wilcockson, S.; Koocheki, A.; Leifert, C. Soil Management for Sustainable Crop Disease Control: A Review. Environ Chem Lett 2008, 6, 149–162. [Google Scholar] [CrossRef]
- Patz, J.A.; Githeko, A.K.; McCarty, J.P.; Hussein, S.; Confalonieri, U.; De Wet, N. ; others Climate Change and Infectious Diseases. Climate change and human health: risks and responses 2003, 2, 103–132. [Google Scholar]
- Dietze, M.C.; Moorcroft, P.R. Tree Mortality in the Eastern and Central United States: Patterns and Drivers. Glob Chang Biol 2011, 17, 3312–3326. [Google Scholar] [CrossRef]
- Beylich, A.; Oberholzer, H.-R.; Schrader, S.; Höper, H.; Wilke, B.-M. Evaluation of Soil Compaction Effects on Soil Biota and Soil Biological Processes in Soils. Soil Tillage Res 2010, 109, 133–143. [Google Scholar] [CrossRef]
- Shah, A.N.; Tanveer, M.; Shahzad, B.; Yang, G.; Fahad, S.; Ali, S.; Bukhari, M.A.; Tung, S.A.; Hafeez, A.; Souliyanonh, B. Soil Compaction Effects on Soil Health and Cropproductivity: An Overview. Environmental Science and Pollution Research 2017, 24, 10056–10067. [Google Scholar] [CrossRef]
- Haavik, L.J.; Billings, S.A.; Guldin, J.M.; Stephen, F.M. Emergent Insects, Pathogens and Drought Shape Changing Patterns in Oak Decline in North America and Europe. For Ecol Manage 2015, 354, 190–205. [Google Scholar] [CrossRef]
- Reed, S.E.; English, J.T.; Muzika, R.M. Phytophthora Species Detected in Two Ozark Forests with Unusual Patterns of White Oak Mortality. Plant Dis 2019, 103, 102–109. [Google Scholar] [CrossRef]
- Arthur, M.A.; Alexander, H.D.; Dey, D.C.; Schweitzer, C.J.; Loftis, D.L. Refining the Oak-Fire Hypothesis for Management of Oak-Dominated Forests of the Eastern United States. J For 2012, 110, 257–266. [Google Scholar] [CrossRef]
- Park, A.; Puettmann, K.; Wilson, E.; Messier, C.; Kames, S.; Dhar, A. Can Boreal and Temperate Forest Management Be Adapted to the Uncertainties of 21st Century Climate Change? CRC Crit Rev Plant Sci 2014, 33, 251–285. [Google Scholar] [CrossRef]
- Löf, M.; Dey, D.C.; Navarro, R.M.; Jacobs, D.F. Mechanical Site Preparation for Forest Restoration. New For (Dordr) 2012, 43, 825–848. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R. Valorizing Biomass to Engineered Biochar and Its Impact on Soil, Plant, Water, and Microbial Dynamics: A Review. Biomass Convers Biorefin 2022, 12, 4183–4199. [Google Scholar] [CrossRef]
- Multi-Resolution Land Characteristics Consortium. USGS. Available online: https://www.mrlc.gov/data?f%5B0%5D=year%3A2019 (accessed on 2 February 2023).
- USDA Forest Inventory and Analysis DataMart. Available online: https://apps.fs.usda.gov/fia/datamart/datamart.html (accessed on 23 June 2021).
- Food and Agriculture Organization of the United Nations. FAO Soils Portal – World Reference Base Map. Available online: https://www.fao.org/soils-portal/data-hub/soil-maps-and-databases/other-global-soil-maps-and-databases/en/ (accessed on 22 April 2023).
- USDA Natural Resource Conservation Service. Gridded National Soil Survey Geographic Database (gNATSGO). Available online: https://nrcs.app.box.com/v/soils/folder/191785692827 (accessed on 4 April 2023).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).