1. Introduction
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) is a basic biochemical technique universally used for the electrophoretic fractionation of proteins. This technique has only changed marginally over the past five decades [
1]. There are two types of apparatus for protein SDS-PAGE: Vertical and horizontal electrophoresis systems [
2]. They both follow the same electrophoretic principles, but the main difference is in the installation of gels. In the vertical PAGE system, a gel is held within two glass plates and mounted between two buffer chambers. The only electric path between the two buffer chambers is through the gel. In the horizontal PAGE system, an unsupported gel is placed onto the cooling plate, and a pair of electrodes connect with a gel to provide an electric field. Electrolytes are provided from buffer-soaked wicks without using buffer chambers. While the vertical PAGE system is preferred for routine protein separations, the horizontal flatbed PAGE system is better suited for two-dimensional polyacrylamide gel electrophoresis (2D-PAGE). The thin, horizontal, flatbed SDS-PAGE system offered higher resolution and sharper protein spots than the conventional vertical gels in two-dimensional polyacrylamide gel electrophoresis [
3]. The gel handling and sample loading are more effortless in the horizontal system. Therefore, possible SDS-PAGE automation could be achieved with the horizontal system.
For separating a wide range of proteins in a single lane, protein bands must be more focused for higher resolutions. Laemmli’s discontinuous SDS-PAGE system has continuously been improved in many ways. Using a concentration gradient gel instead of a single concentration gel has extensively improved the resolution of complex protein mixtures [
4]. Although even with an extensive gradient range, smaller proteins could not be resolved well with a standard buffer system. Adding urea to the buffer improved the separation of small peptides but hampered proper molecular weight determination [
5]. An alternative was to optimize the ionic constituents in buffers from Laemmli’s original system [
6,
7,
8]. Efficient removal of Joule heating was also crucial to preventing band broadening [
9]. The field-inversion gel electrophoresis (FIGE) was one of the pulsed-filed gel electrophoresis techniques originally invented to separate large DNA molecules [
10]. In the FIGE, the polarity of an electric field is periodically reversed with a longer time or a higher voltage gradient in one direction; therefore, size-dependent resolution can be achieved. The FIGE is the easiest to perform with minimal equipment requirements yet successfully maximizes efficiency and selectivity for large DNA molecules. The FIGE was successfully implemented to protein PAGE to reduce band diffusion and to increase protein concentration in a band [
11].
All commercially available horizontal PAGE systems used electrodes disposed on the top of a gel at both ends. In such a configuration, an electric field was stronger and caused faster migration of proteins in the top portion of a gel. Hence, the protein bands were broadened and not clearly separated from others. In this study, we developed new electrode designs which could rectify this flaw. The FIGE technique was also tested together with the double-deck electrodes to achieve the best resolution for the protein electrophoresis.
2. Materials and Methods
2.1. Reagents
30% Acrylamide (29:1), TEMED, and Precision Plus Protein™ Standards Dual Color were purchased from BIO-RAD (Hercules, CA, USA). Tris-HCl, ammonium persulfate, and sodium dodecyl sulfate were purchased from Sigma (St. Louise, MO, USA). All reagents used were of analytical grade.
2.2. Fabrication of SDS-Polyacrylamide gels
The gels were 10% T, 3.3% C acrylamide, and bis-acrylamide. The gels were made with a size of 8 x 8 cm. The caster for gels was manufactured with glass plates and 3D-printed parts.
2.3. Electrodes design
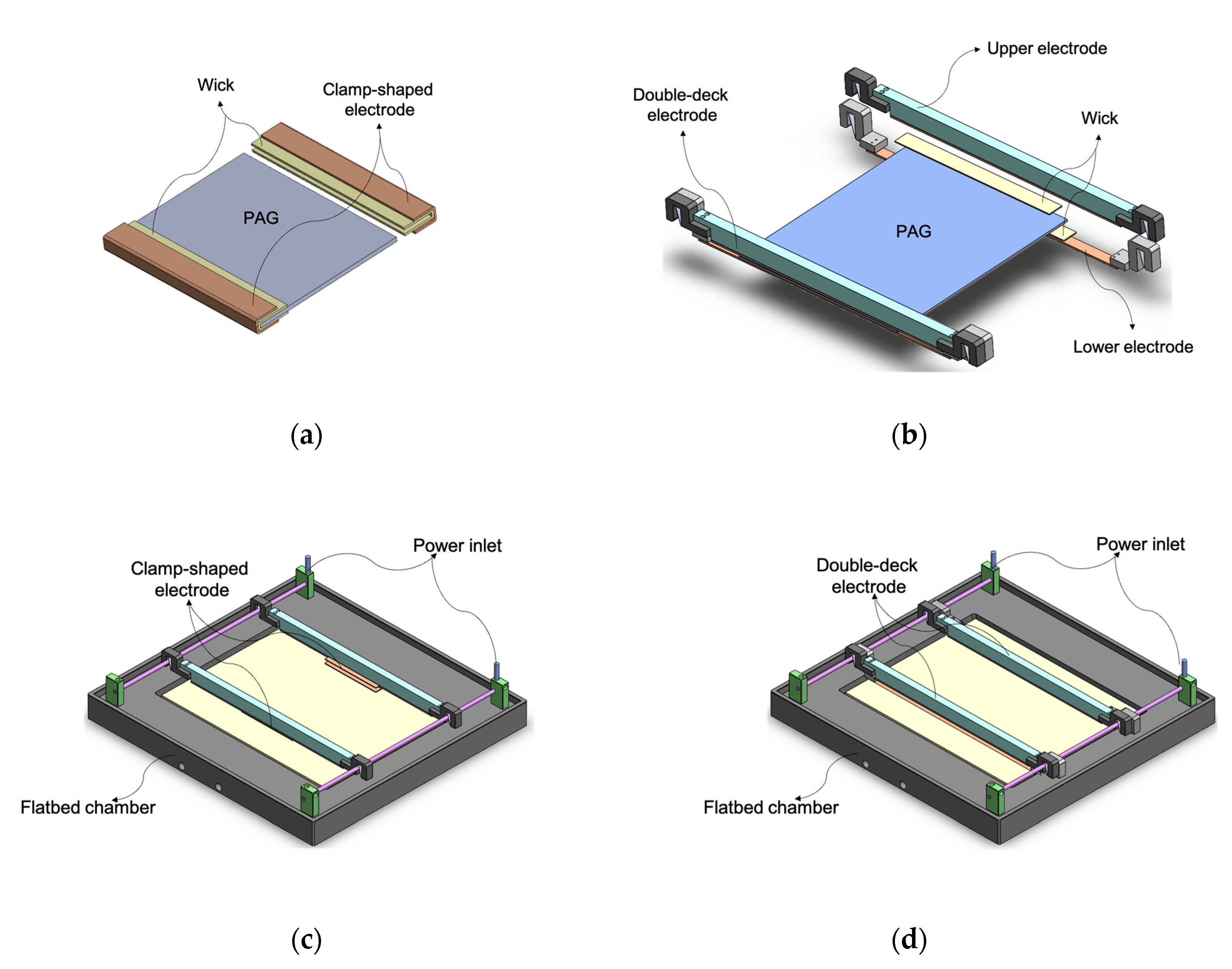
The clamp-shaped electrodes were manufactured by folding platinum-coated titanium plates twice. Both gel ends were covered with wet paper wicks and inserted into the clamp-shaped electrodes. For the double-deck flat electrodes, two pairs of platinum-coated titanium strips were placed on the gels' top and bottom. Wet paper wicks were cut to the shape of electrodes and inserted between gels and electrodes.
2.4. FIGE module
A schematic representation of the pulsing circuitry is provided in
Supplementary Figure S1. A picture of the prototype FIGE module used in gel electrophoresis can be found in
Supplementary Figure S2. Forward and reverse switching of the electric field supply to the gel was achieved by interfacing the voltage supply (PS300HC, BIOFACT, Korea) with a 400 V direct current relay (G9EJ-1-P-E, OMRON, Japan) that could handle a current of 15 Amp. The forward and reverse switching rate was controlled by an Arduino Uno microcontroller that could deliver pulses as short as one msec. The performance of this instrumentation regarding square waveform, amplitude, length, and stability was checked and ascertained by an oscilloscope (DSO3152A, Agilent Technologies, USA).
2.5. Horizontal SDS-PAGE
Flatbed Professional, horizontal flatbed chamber (FC-EDCProf-2836, Gel Company, USA) was used as a platform for all electrophoretic separations. Electrophoresis was performed with either its original platinum wire electrodes, the clamp-shaped electrodes, or the double-deck electrodes. The distance between an anode and a cathode electrode was 6 cm. The temperature of the cooling plate was set to 6oC. The running buffer was prepared from 25 mM tris base, 192 mM glycine, and 0.1% SDS with a pH of 8.3. 5 μl of pre-stained protein marker was loaded into four sample wells (2 x 2 x 2 mm) and separated under a constant current of 50 mA for 90 min. Voltage was usually started around 70 V and gradually increased to 120 V. Bromophenol blue dye traveled for about 3 cm. Temperature changes during electrophoretic separations were measured with three digital thermometers with a K-type thermocouple probe. The thermometer probes were inserted into the gel’s top, bottom, and middle portions.
Pulsed-field electrophoresis was achieved by interfacing a power supply with the FIGE module. A constant current of 50 mA was applied with an alternating forward pulsing time of 3 sec and reverse pulsing time of 1 sec. Separation continued until bromophenol blue dye traveled for about 3 cm.
After electrophoresis, the image of a gel was scanned with an optical scanner (SL-C486FW, Samsung, Korea). The gel was also longitudinally cleaved along each lane, and the cross-sectional images were obtained with an optical scanner.
3. Results
We explored the less frequently discussed topic of the electrode design in the horizontal PAGE systems and its effects on the gel resolution. For typical vertical PAGE systems, two electrodes are submerged in separate running buffer reservoirs. An electric field is applied through the running buffer and enters a gel from the ends in migrating direction. This configuration allows the uniform distribution of electrophoretic force inside the gels. For the horizontal PAGE, however, two long electrodes are disposed on the top of a gel at each end. Multiple layers of paper wicks are wetted with the running buffer and placed as interfaces.
The standard electrodes for the horizontal system were made with thin platinum wire, also tested in this study. Recently variation of the electrode was introduced with a thicker titanium rod with a conductive polymer cover that provided better conductivity (Serva Electrophoresis, Heidelberg, Germany). To apply a uniform electric field to a gel, thin metal tapes were cut to the cross-sectional size of a gel, attached to the migration ends, and used as electrodes. This configuration showed some promising upright protein band patterns but was impractical to used. Because it was thin and fragile, the gel got easily wrinkled or even torn off when placing the electrodes. It was also challenging to maintain proper contact with a thin gel during the operation.
Two new electrodes were designed for an even electric field during electrophoresis and easier installation. The clamp-shaped electrode (
Figure 1a) was manufactured by folding a platinum-coated titanium plate twice to a Π shape from side. Each end of a gel was wrapped in running buffer-soaked paper wicks and slid into the electrodes. This electrode provided an electric field from the gel’s top, bottom, and side. However, the insertion of a gel into this electrode was still cumbersome. The double-deck electrode (
Figure 1b) was developed to ease the handling difficulty. It consists of two sets of platinum-coated titanium strip pairs that sandwich the gel from the top and beneath. Since each strip pair was connected to the same power outlet, the same electric fields were applied simultaneously to the top and bottom of a gel. New electrodes were tested in a flatbed electrophoresis unit replacing the original wire electrode (
Figure 1c and 1d).
The pre-stained size marker was separated in 10% Tris-Glycine polyacrylamide gels with a flatbed electrophoresis unit to evaluate protein migration patterns in the horizontal PAGE. This electrophoresis instrument design has been used for over 40 years without significant modifications [
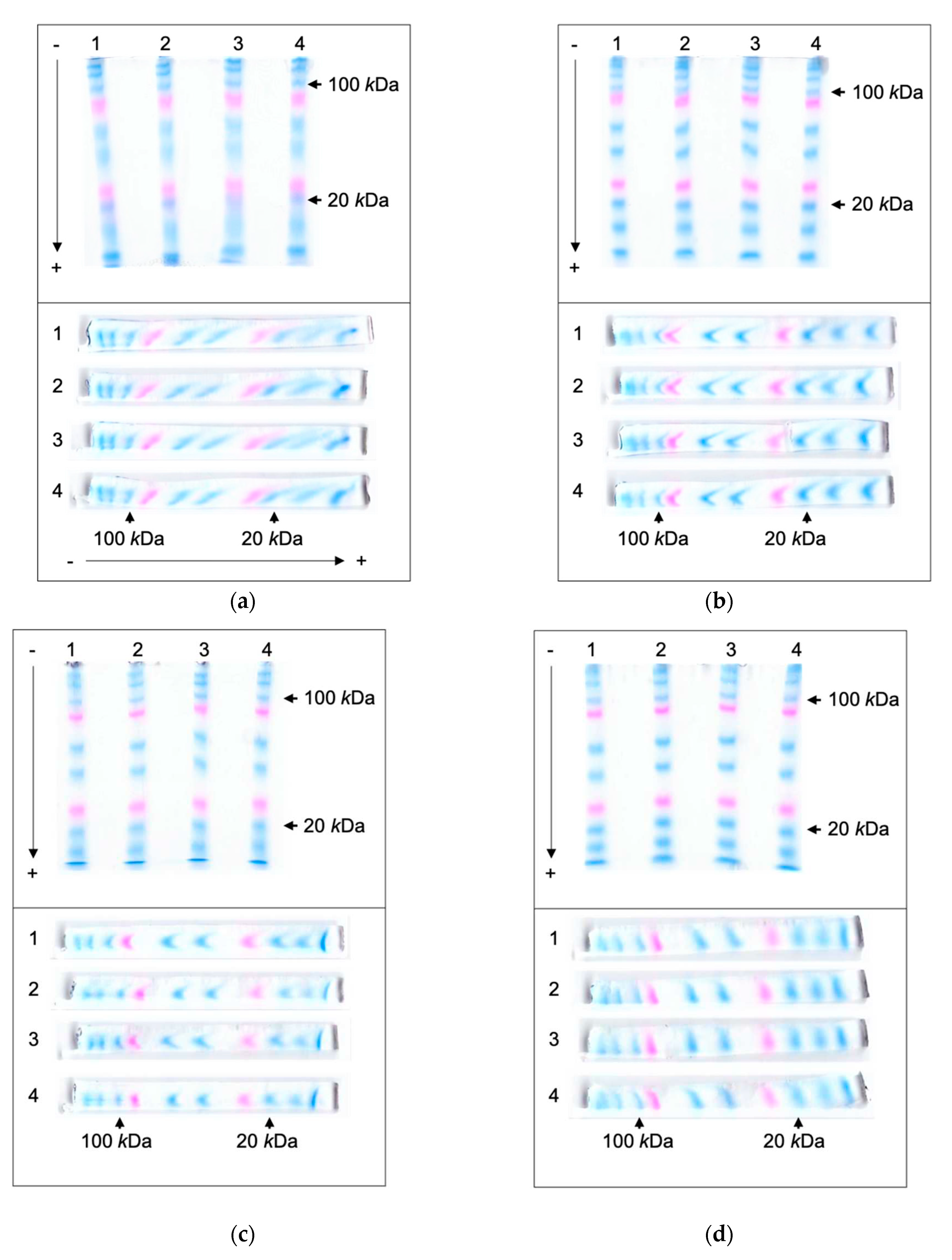
2]. Platinum wire electrodes were placed on the top of a gel at both ends in the migrating direction. After electrophoretic separation, the shapes of protein bands were monitored from the top and the lateral side of a gel (
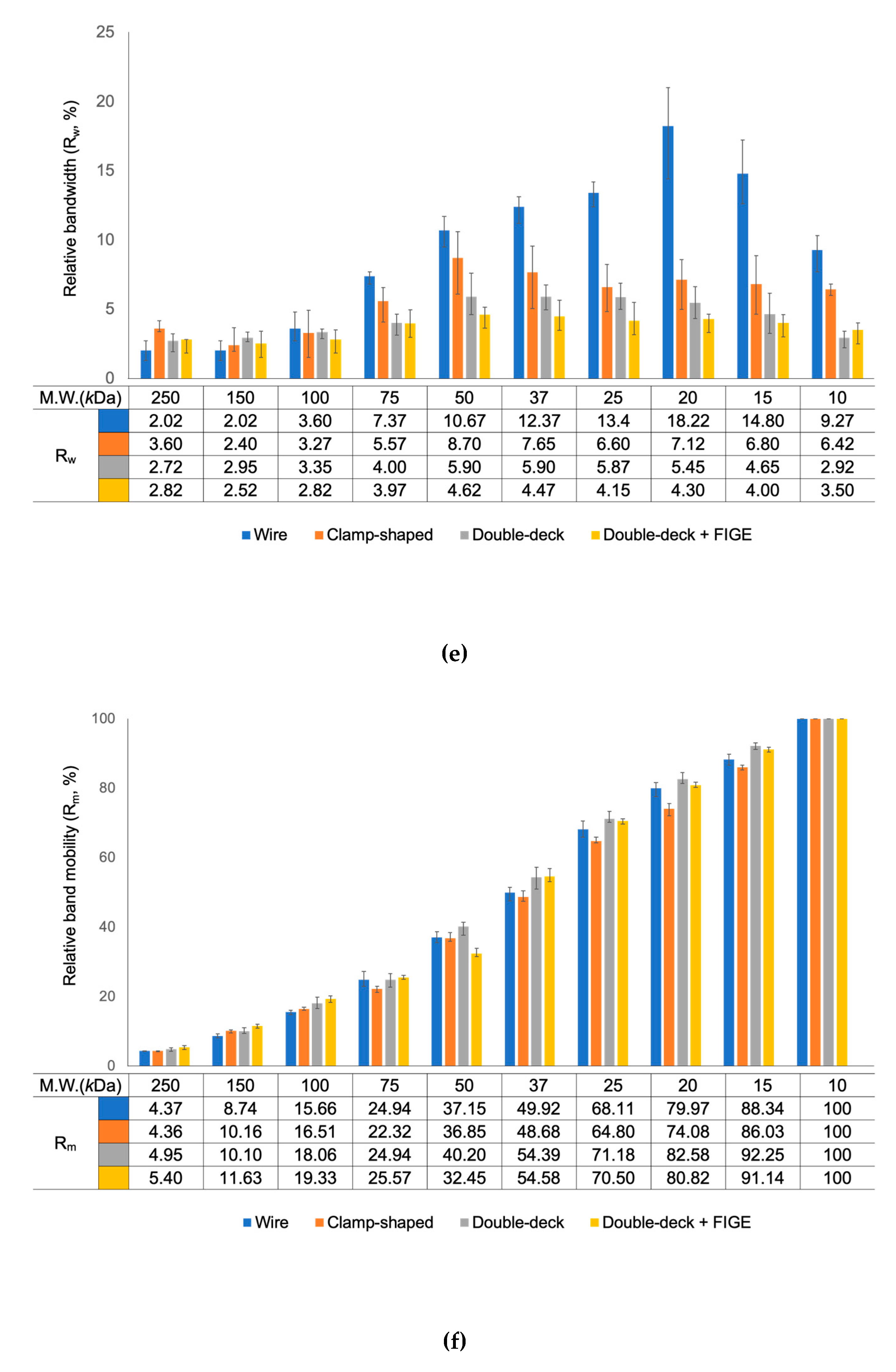
Figure 2a). The protein mobility and the protein separation efficiency (i.e., relative bandwidth in the direction of separation) were determined from the gel images (
Figure 2e and 2f). The relative band mobility (R
m) was defined as the percentile ratio of the target protein's migration distance to the dye front's migration distance. Similarly, relative bandwidth (R
w) was defined as the percentile ratio of the target protein’s bandwidth to the dye front’s migration distance.
Because of the geometry, the electric field strength was diminished from the gel’s top to bottom and caused uneven migration of proteins. The views from the lateral side showed that proteins in the upper part of gels traveled farther; hence the shapes of protein bands from the lateral side were slanted toward the anode (
Figure 2a). Such tendency was more apparent for the proteins with molecular weights lower than 100
kDa, which migrated farther during electrophoresis. Slanting band shapes caused the broadening of bands in the top view and impaired the distinctions between each protein band. This broadening of bands peaked with 20
kDa marker protein with R
w of 18.2.
The protein bands were more focused with these new electrodes and showed better distinctions between them (
Figure 2b, 2c, 2e, and 2f). The side views of gels showed that all bands were thinner and more focused. For all the proteins with molecular weight lower than 100
kDa, which skewed a lot with a wire electrode, R
w values were significantly lower. R
w of 20
kDa marker protein was improved to 7.1 and 5.5 with the clamp-shaped and the double-deck electrodes, respectively. However, the R
m of bands was similar regardless of electrode type.
As the protein bands migrated farther in a gel, they started to form a crescent shape with ends pointing toward the anode. This tendency was more prominent with the double-deck electrodes. It could be assumed that electric fields were stronger and caused faster migration at the gel's top and bottom. Such deformation also caused band broadening. Therefore, the FIGE technique was adopted to remove these pointy trails and to focus the protein bands for enhanced separation (
Figure 2d, 2e, and 2f). The FIGE module interfaced between a power supply and the double-deck electrodes. Since the FIGE module periodically reversed the direction of the electric field, it took longer for the protein bands to migrate for a similar distance as before. All the bands were thinner and aligned nearly vertically for the best resolution. R
w of 20
kDa marker protein was further decreased down to 4.3.
During the electrophoresis, gels had to be cooled down to actively remove heat and minimize the evaporation of the running buffer. Joule heating could affect the electrophoretic mobility of proteins, leading to band broadening [
9]. To monitor the spatial temperature differences, type K thermocouple wire probes were inserted into the gel’s top, middle, and bottom sections, and temperatures were measured during electrophoresis. The temperature at the bottom of the gels was set to 6
oC with the cooling plate. Regardless of the electrode types, temperatures were slightly higher at the top, but the differences were smaller than 2
oC. The measurements remained similar for up to 2 hours.
4. Discussion
This study investigated a long-neglected fault in the electrode design in the horizontal protein PAGE system. How to apply an electric field in a gel was a critical factor for better resolution. Two new electrodes were developed and proven useful to get narrow and focused protein bands. With the expenditure of longer electrophoresis time, the FIGE technique could be used together to get nearly vertical protein band shapes.
In side-by-side comparisons, both the clamp-shaped and double-deck electrodes showed enhanced protein separations similarly. Even for the clamp-shaped electrode, like the double-deck electrode, the major contacts with a gel were made from the gel's top and bottom. The input from the end side should be marginal. It could be assumed that the electric field was applied similarly with both electrodes. Therefore, because of the ease of installation, the double-deck electrode is recommended for laboratory use.
The depicted two electrodes followed the size, the electric connection, and other specifications of Flatbed Professional, Horizontal flatbed chamber unit (FC-EDCProf-2836) from Gel Company in particular. However, when used with other horizontal electrophoresis systems, appropriate changes could be made without departing from the principles and spirit of these embodiments.
Our intention was that two electrodes sandwiched a gel from the same location. As shown in
Figure 2d as an example, the gels might still have bands slightly leaned forward or even backward. This seemed to be affected by many characteristics of the gels, e.g., the thickness of gels, the acrylamide concentrations, and the dryness of gels by joule heat. As a remedy, the strength of the electric field at the top and the bottom of the gel might be independently adjusted. When the distances between two electrodes on the gel narrowed, the electric currents were measured increased (
Supplementary Figure S3). Therefore, the electric field could be easily fine-tuned by adjusting the interval between electrodes at the top or the bottom of the gel. For the flatbed electrophoresis unit used in this study, electrodes were electrically connected via guiding rails on the side. The positions of electrodes could be easily changed by sliding them on the guiding rails.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figures S1: Electric circuit of the pulse generator in the FIGE module; Figure S2: The physical setup of the horizontal PAGE system with the FIGE module; Figure S3: The intervals between the electrodes could adjust electric currents.
Author Contributions
Conceptualization, S.K., D.W.L, T.-S.Y. and H.-S.S.; methodology, S.K., D.W.L, K.H.H. and H.S.; validation, S.S., K.H.H, and T.-S.Y.; writing—original draft preparation, D.W.L. and S.S.; investigation, D.W.L., K.H.H. and T-S.Y.; writing—review and editing, S.K., K.H.H, and T.-S.Y.; visualization, D.W.L.; supervision, S.K. and H.-S.S.; project administration, S.K. and H.-S.S.; funding acquisition, S.K. and H.-S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Daejeon Technopark, grant number 2022-CCR-R04 and the National Research Council of Science & Technology (NST), grant number, CRC22021-500.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No additional data were created or analyzed in this study. Data sharing is not applicable to this article. The authors can be contacted for any further information regarding the data within the article.
Conflicts of Interest
The authors have declared no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Westermeier, R. Electrophoresis. In Electrophoresis in Practice; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 7–56. [Google Scholar] [CrossRef]
- Moche, M.; Albrecht, D.; Maaß, S.; Hecker, M.; Westermeier, R.; Büttner, K. The New Horizon in 2D Electrophoresis: New Technology to Increase Resolution and Sensitivity. Electrophoresis 2013, 34, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Margolis, J.; Kenrick, K.G. Polyacrylamide Gel-Electrophoresis across a Molecular Sieve Gradient. Nature 1967, 214, 1334–1336. [Google Scholar] [CrossRef] [PubMed]
- Swank, R.T.; Munkres, K.D. Molecular Weight Analysis of Oligopeptides by Electrophoresis in Polyacrylamide Gel with Sodium Dodecyl Sulfate. Anal Biochem 1971, 39, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Jovin, T.M. Multiphasic Zone Electrophoresis. II. Design of Integrated Discontinuous Buffer Systems for Analytical and Preparative Fractionation. Biochemistry 1973, 12, 879–890. [Google Scholar] [CrossRef]
- Schägger, H.; Aquila, H.; von Jagow, G. Coomassie Blue-Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis for Direct Visualization of Polypeptides during Electrophoresis. Anal Biochem 1988, 173, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Khalkhali–Ellis, Z. An Improved SDS–Polyacrylamide Gel Electrophoresis for Resolution of Peptides in the Range Of 3.5-200kDa. Prep Biochem 1995, 25, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Goharshadi, E.K.; Ahmadzadeh, H.; Samiee, S. Improvement of Heat Dissipation in Agarose Gel Electrophoresis by Metal Oxide Nanoparticles. RSC Adv 2015, 5, 88655–88665. [Google Scholar] [CrossRef]
- Carle, G.F.; Frank, M.; Olson, M. v. Electrophoretic Separations of Large DNA Molecules by Periodic Inversion of the Electric Field. Science (1979) 1986, 232, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.; Low, T.Y.; Freeby, S.; Paulus, A.; Ramnarayanan, K.; Cheng, C.P.P.; Leung, H.C.E. Increase in Local Protein Concentration by Field-Inversion Gel Electrophoresis. Proteome Sci 2007, 5, 18. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).