Submitted:
22 September 2023
Posted:
28 September 2023
You are already at the latest version
Abstract
Keywords:
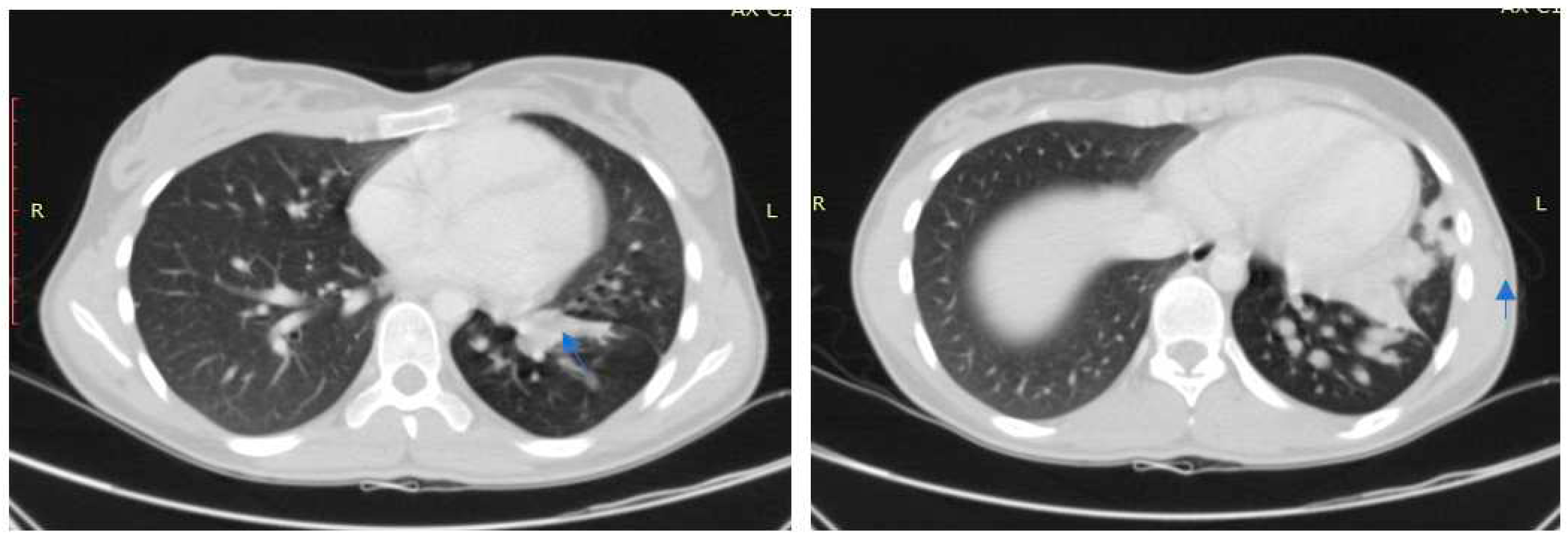
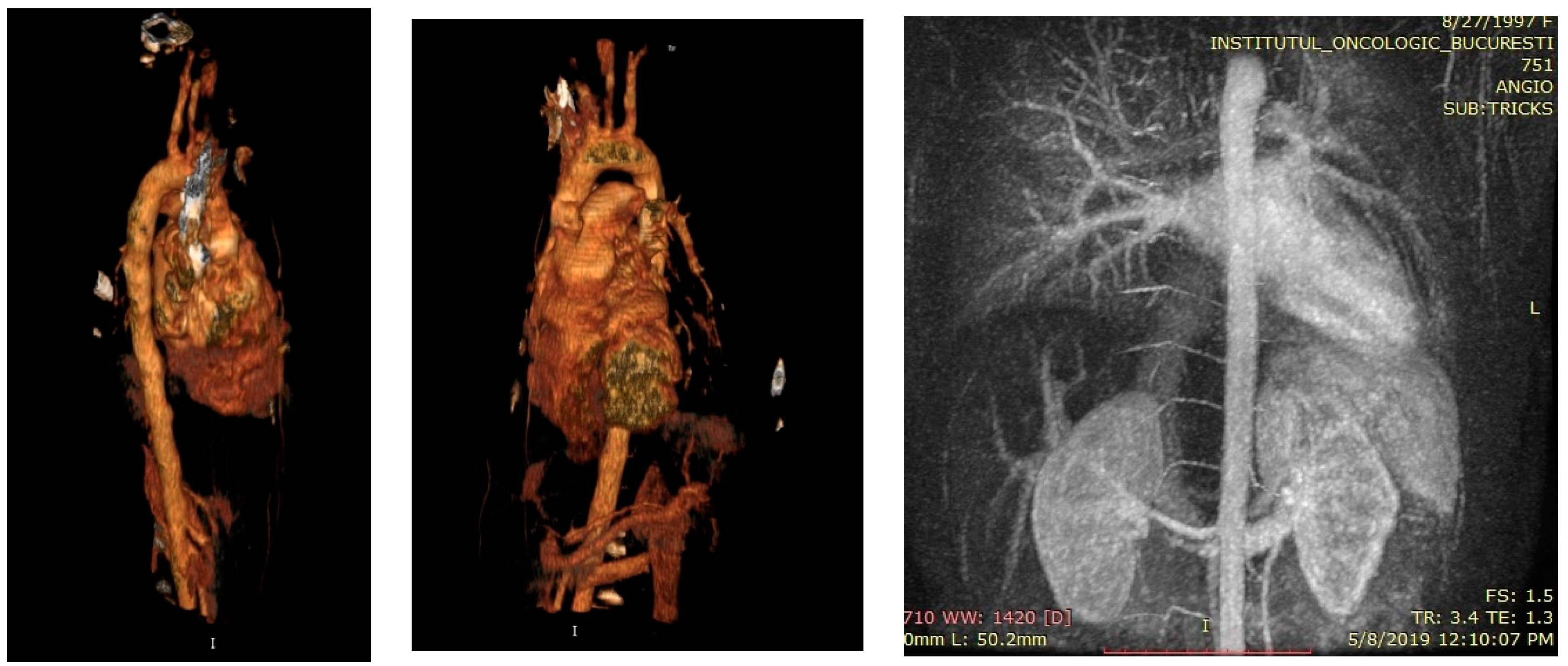
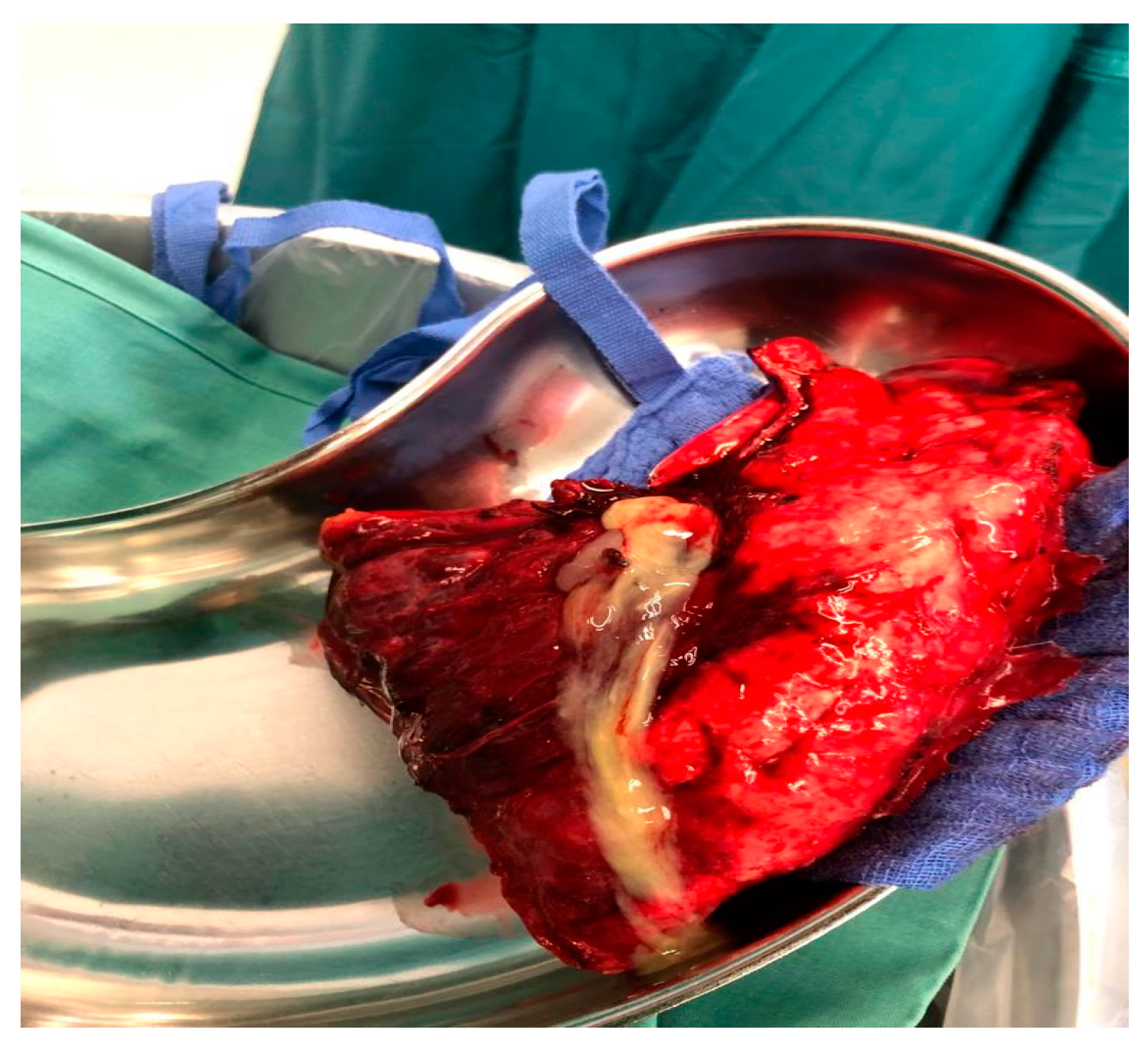
Case no 1.
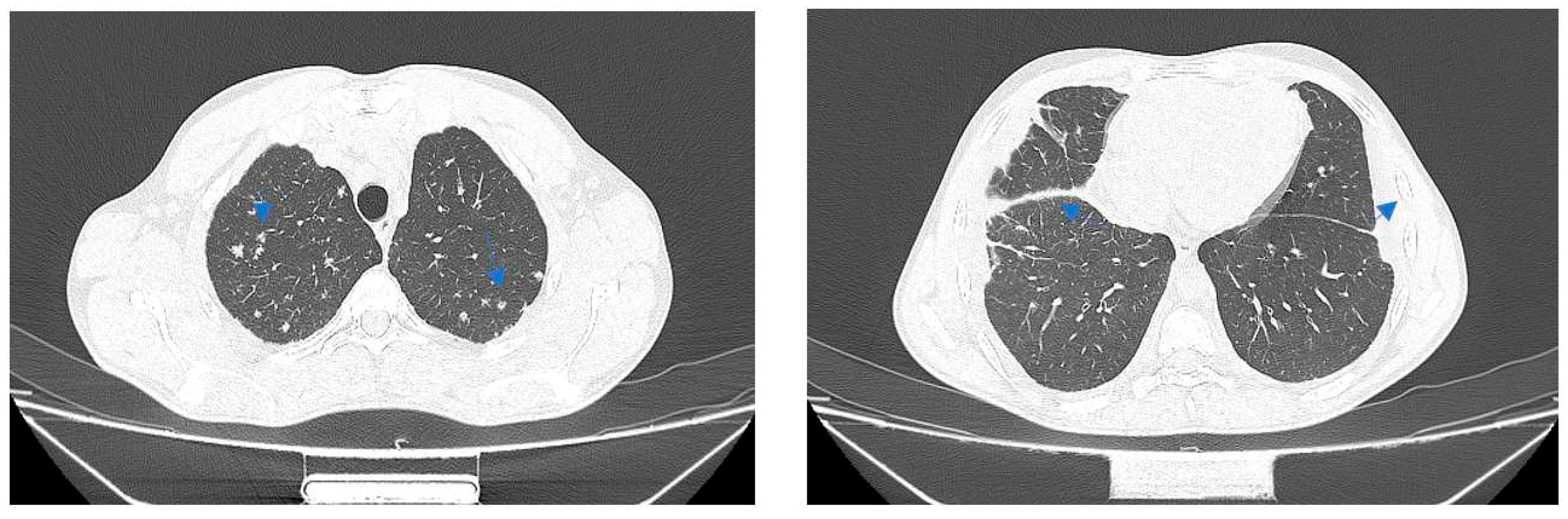
Case no. 2
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seifert, M.; Catanzaro, D.; Catanzaro, A.; Rodwell, T.C. Genetic Mutations Associated with Isoniazid Resistance in Mycobacterium tuberculosis: A Systematic Review. PLOS ONE 2015, 10, e0119628. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report. Geneva, Switzerland; WHO press; 2013.
- World Health Organization; Multidrug and Extensively Drug-Resistant TB 2010 Global Report of Surveillance and Response. Geneva, Switzerland; WHO press; 2010.
- Sougakoff, W. Molecular epidemiology of multidrug-resistant strains of Mycobacterium tuberculosis. Clin. Microbiol. Infect. 2011, 17, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Narmandakh, E.; Tumenbayar, O.; Borolzoi, T.; Erkhembayar, B.; Boldoo, T.; Dambaa, N.; Burneebaatar, B.; Nymadawa, N.; Mitarai, S.; Jav, S.; et al. Genetic Mutations Associated with Isoniazid Resistance in Mycobacterium tuberculosis in Mongolia. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef] [PubMed]
- Jindani, A.; Aber, V.R.; Edwards, E.A.; Mitchison, D.A. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis 1980, 121, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Heym, B.; Allen, B.; Young, D.; Cole, S. The catalase—peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature 1992, 358, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Dubnau, E.; Quemard, A.; Balasubramanian, V.; Um, K.S.; Wilson, T.; Collins, D.; de Lisle, G.; Jacobs, W.R., Jr. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 1994, 263, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Vilchèze, C.; Jacobs, W.R. Resistance to isoniazid and ethionamide in Mycobacterium tuberculosis: genes, mutations, and causalities. Microbiol Spectr 2014, 2, MGM2. [Google Scholar] [CrossRef] [PubMed]
- Lempens, P.; Meehan, C.J.; Vandelannoote, K.; Fissette, K.; de Rijk, P.; Van Deun, A.; Rigouts, L.; de Jong, B.C. Isoniazid resistance levels of Mycobacterium tuberculosis can largely be predicted by high-confidence resistance-conferring mutations. Sci. Rep. 2018, 8, 3246. [Google Scholar] [CrossRef] [PubMed]
- Vilcheze, C.; Jacobs, W.R., Jr. Resistance to isoniazid and ethionamide in mycobacterium tuberculosis: genes, mutations, and causalities. Microbiology Spectrum. 2014, 2, MGM2-0014-2013. [Google Scholar] [CrossRef] [PubMed]
- Seifert, M.; Catanzaro, D.; Catanzaro, A.; Rodwell, T.C. Genetic Mutations Associated with Isoniazid Resistance in Mycobacterium tuberculosis: A Systematic Review. PLOS ONE 2015, 10, e0119628. [Google Scholar] [CrossRef] [PubMed]
- Böttger, E. C. In Antituberculosis Chemotherapy Vol. 40 (eds P. R. Donald & P. D. van Helden) Ch. 14, 128–144 (Karger, 2011).
- Huyen, M.N.T.; Cobelens, F.G.J.; Buu, T.N.; Lan, N.T.N.; Dung, N.H.; Kremer, K.; Tiemersma, E.W.; van Soolingen, D. Epidemiology of Isoniazid Resistance Mutations and Their Effect on Tuberculosis Treatment Outcomes. Antimicrob. Agents Chemother. 2013, 57, 3620–3627. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, S.V.; Reich, R.; Dou, S.-J.; Jasperse, L.; Pan, X.; Wanger, A.; Quitugua, T.; Graviss, E.A. Single Nucleotide Polymorphisms in Genes Associated with Isoniazid Resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2003, 47, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Jagielski, T.; Grzeszczuk, M.; Kamiński, M.; Roeske, K.; Napiórkowska, A.; Stachowiak, R.; Augustynowicz-Kopeć, E.; Zwolska, Z.; Bielecki, J. Identification and Analysis of Mutations in the KatG Gene in Multidrug-Resistant Mycobacterium tuberculosis Clinical Isolates. Adv. Respir. Med. 2013, 81, 298–307. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



