1. Introduction
Ependymoma (EPN) is a malignant neuroglial tumor of the central nervous system that can occur in various locations within the central nervous system. Spinal ependymoma is commonly seen in adults above the age of 35. According to data from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute from 1976 to 2006, the incidence rate of pediatric spinal ependymoma is 0.06 to 0.11 cases per 100,000 people. Approximately 50% of adult cases occur in the spinal cord, while nearly 90% of pediatric cases occur intracranially, accounting for 5% to 10% of all pediatric intracranial tumors [1, 2]. According to the World Health Organization (WHO) classification and grading of central nervous system tumors involving ependymoma, Grade I includes subependymoma (SE) and myxopapillary ependymoma (MPE), both of which are more common in adults than in children and are located in the ventricles and conus medullaris of the spinal cord, respectively. Grade II corresponds to the classic ependymoma, which is the most common histological type in children. It is characterized by pseudorosettes (tumor cells arranged in a radiating pattern) around blood vessels, and in approximately 25% of cases, ependymoma exhibits perivascular rosettes (tumor cells arranged around the central lumen). Ependymomas can be further classified into cellular, papillary, clear cell, and tanycytic subtypes. Grade III corresponds to anaplastic ependymoma, which is a malignant tumor characterized by a high nuclear-to-cytoplasmic ratio and a high mitotic count. However, due to the heterogeneity of the tumors, distinguishing between Grades II and III solely based on histopathology can be challenging [3, 4]. In recent years, advances in immunohistochemistry, transcriptome analysis, and methylation profiling have redefined the biological properties of ependymoma. A large international study has identified 9 distinct molecular subtypes of ependymoma based on population demographics, clinical, genetic, and prognostic differences, further dividing posterior fossa (PF), supratentorial (ST), and spinal (SP) ependymomas into 3 subtypes [
5].
As a relatively rare malignant tumor of the central nervous system, ependymoma (EPN) can occur at any age and can involve various locations along the neural axis. Ependymoma accounts for approximately 7% of all neuroglial tumors, with a higher incidence in males than in females [3, 6]. Ependymal tumors have the potential to spread within the nervous system, including the spinal cord, through cerebrospinal fluid dissemination. Approximately 25% occur in the supratentorial brain ventricles, and the remainder occur in the spinal cord [
7]. The incidence of metastasis increases with higher tumor grade, but systemic metastases are rare. Additionally, statistics have shown that approximately 45% of ependymoma patients have poor prognoses [
8]. Complete tumor resection and local radiation therapy have resulted in an overall survival rate of up to 85% [
9]. Despite improvements in survival rates, prognosis remains poor for young children and patients with recurrence or metastasis, with high rates of recurrence. The treatment of ependymoma remains challenging [
10].
The treatment standards for ependymoma still involve surgical removal and adjuvant radiation therapy. The purpose of surgical removal is to obtain tumor tissue for diagnosis, open the cerebrospinal fluid pathway to relieve hydrocephalus, eliminate compression on delicate neural structures, and achieve maximal safe resection. However, for patients in whom complete surgical removal is not possible, systemic chemotherapy can improve their prognosis to some extent. Nearly half of ependymoma patients will experience recurrence, usually occurring in the early postoperative period and often at the site of the original tumor. Recurrent ependymoma generally has a poor prognosis, with a 5-year survival rate of only 25% [11, 12]. Clinical trials have explored the use of various chemotherapy drugs for treating recurrent ependymoma, but the efficacy rates for single-agent (12.9%) or multi-agent (17.4%) chemotherapy are both low, and this strategy has not demonstrated any clear benefits for pediatric patients' survival [
13]. Currently, there are no clear recommendations for using chemotherapy to treat recurrent pediatric intracranial ependymoma. The World Health Organization's treatment standards for grade II or III intracranial ependymoma in children involve receiving focal conformal radiation therapy after tumor resection, with a maximum dose of 59.4 Gy [
14]. In summary, even with surgery, chemotherapy, and/or adjuvant radiation therapy, the recurrence rate of ependymoma can reach as high as 23-66% [15-18]. Currently, there is no standard treatment plan for recurrent ependymoma, and clinicians often choose an appropriate treatment approach (surgery, radiation therapy, or chemotherapy) based on individual circumstances.
Currently, research on the prognosis of childhood ependymoma after recurrence is mostly based on data obtained from studies on overall recurrent gliomas, or on small, heterogeneous institutional reviews and summaries of clinical experience. Therefore, this study utilizes a meta-analytical approach to evaluate the prognosis and influencing factors of recurrent pediatric ependymoma, aiming to provide evidence-based recommendations for clinical practice.
2. MATERIALS AND METHODS
2.1. Literature search strategy
Computer retrieval of Cochrane Library, Embase, PubMed, and Web of Science. The search strategy includes subject terms and free words. The search time is limited from the establishment of the database to September 2023. At the same time, reference literature of included studies is also retrieved to supplement the relevant literature. The search terms include (("Ependymoma"[Mesh]) OR ((((((((((((((((Ependymomas)) OR (Ependymoma, Papillary)) OR (Ependymomas, Papillary)) OR (Papillary Ependymomas)) OR (Papillary Ependymoma)) OR (Ependymoma, Myxopapillary)) OR (Ependymomas, Myxopapillary)) OR (Myxopapillary Ependymoma)) OR (Myxopapillary Ependymomas)) OR (Anaplastic Ependymoma)) OR (Anaplastic Ependymomas)) OR (Ependymoma, Anaplastic)) OR (Ependymomas, Anaplastic)) OR (Cellular Ependymoma)) OR (Clear Cell Ependymoma))) AND (("Neoplasm Recurrence, Local"[Mesh]) OR (((((((((((Local Neoplasm Recurrences) OR (Locoregional Neoplasm Recurrence)) OR (Recurrences, Local Neoplasm)) OR (Neoplasm Recurrences, Local)) OR (Recurrence, Local Neoplasm)) OR (Recurrence, Locoregional Neoplasm)) OR (Local Neoplasm Recurrence)) OR (Neoplasm Recurrence, Locoregional)) OR (Locoregional Neoplasm Recurrences)) OR (Neoplasm Recurrences, Locoregional)) OR (Recurrences, Locoregional Neoplasm)))) AND (("Pediatrics"[Mesh]) OR ((((((((((((((((Adolescents) OR (Adolescence)) OR (Teens)) OR (Teenagers)) OR (Youths)) OR (Female Adolescent)) OR (Female Adolescents)) OR (Adolescents, Male)) OR (Adolescent, Male)) OR (Male Adolescent)) OR (Male Adolescents)) OR (Pediatric)) OR (child)) OR (Children)) OR (Kids)) OR (school-age))). Taking Embase as an example, please refer to
Table 1 for the specific search strategy.
2.2. Inclusion criteria and Exclusion criteria
2.2.1. Inclusion criteria:
A search was conducted in the included search engines for all available years of studies. Studies were included if they met the following criteria: (1) Recurrent ependymoma patients under the age of 25; (2) Histologically confirmed ependymoma at initial diagnosis or recurrence; (3) Reporting of median progression-free survival (PFS) or overall survival (OS) at first recurrence. If all participants in a study met the inclusion criteria, cumulative data were included. Studies reporting individual patient data, which allowed calculation of median OS and/or median PFS, were also included. Studies that did not provide standard error or standard deviation estimates of median survival rates, or studies that could not be calculated based on individual data, were excluded based on the requirements of accurate meta-analysis. Case reports or series with less than 5 patients were also excluded to avoid inherent bias due to small sample sizes.
2.2.2. Exclusion criteria
(1) Duplicate data studies; (2) Reviews, conference papers, and comments; (3) Non-Chinese or non-English literature; (4) Literature with unreasonable or poor study design quality; (5) Original data unavailable. (6) Sample size less than 5 cases.
2.3. Literature and data extraction
Two researchers independently screened the literature, extracted data, and cross-checked, with the assistance of a third party in case of disagreements. After excluding duplicate literature, the title and abstract of the remaining articles were read to exclude obviously irrelevant literature. Further reading of the full text was conducted to determine final inclusion. If necessary, authors were contacted via email or telephone to obtain important information that was not confirmed but related to the study. Extracted content mainly included the researcher's name, publication date, country, number of cases in the experimental and control groups, age of the study subjects, intervention measures, and outcome indicators.
2.4. Quality evaluation of included studies
The risk of bias for each study was evaluated using version 5.3.0 of the Cochrane Collaboration's recommended assessment. The content included random allocation methods, allocation concealment, blinding, completeness of outcome data, selective reporting of study results, and other sources of bias. Each item was evaluated as "high risk of bias," "low risk of bias," or "unclear." When a study fully met these requirements, it was rated as Grade A; if it partially met the requirements, it was rated as Grade B; if it did not meet the requirements at all, it was rated as Grade C and excluded. Two researchers independently evaluated the quality of the literature, and a higher score indicated higher quality.
2.5. Statistical methods
Meta-analysis was performed using the official software RevMan 5.4 from the Cochrane Collaboration. As the outcome measures of this study were median overall survival (mOS) and median progression-free survival (mPFS), the hazard ratio (HR) was used as the effect measure with a 95% confidence interval (CI) as the effect size statistic. If there was no heterogeneity among the study results (P>0.1, I2<50%), a fixed-effect model was used for the analysis. If heterogeneity was present (P≤0.1, I2>50%), a random-effects model was used for the analysis, along with subgroup analysis or sensitivity analysis. A P value less than 0.05 was considered statistically significant. If the original study results were reported as medians (interquartile ranges), the formula proposed by Wan et al. [
19] was used to estimate the mean and standard deviation. If the results were reported as mean differences and 95% CI between groups, the HR was calculated using the RevMan 5.4 software calculator [
20].
3. Results
3.1. Literature search results
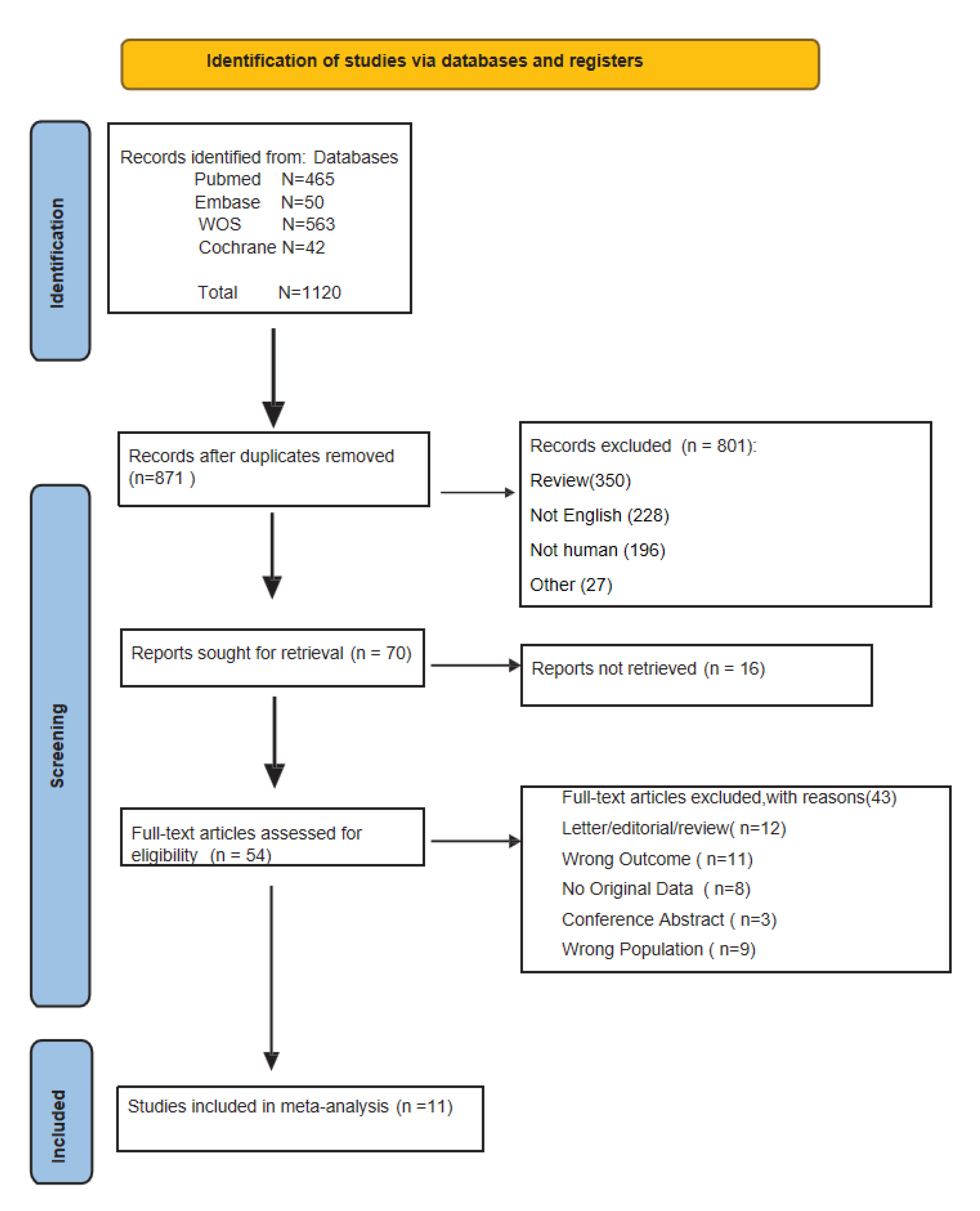
Based on the predetermined inclusion and exclusion criteria and literature search strategy, a total of 1120 articles (465 from PubMed, 50 from Embase, 42 from Cochrane Library, and 563 from Web of Science) were initially identified. After a hierarchical screening process, 11 studies [21-31] with a total of 296 patients were finally included. All 11 studies provided information on the median overall survival (mOS), and three studies provided information on the median progression-free survival (mPFS). The process of literature selection is shown in
Figure 1, and the general characteristics of the included studies are shown in
Table 2.
A Reflects the number of individual patients from the respective study that was used in the survival and meta-analyses. Individual patients were excluded if they did not meet all inclusion criteria for the study (e.g. they did not have recurrent disease).
3.2. The results of literature quality assessment
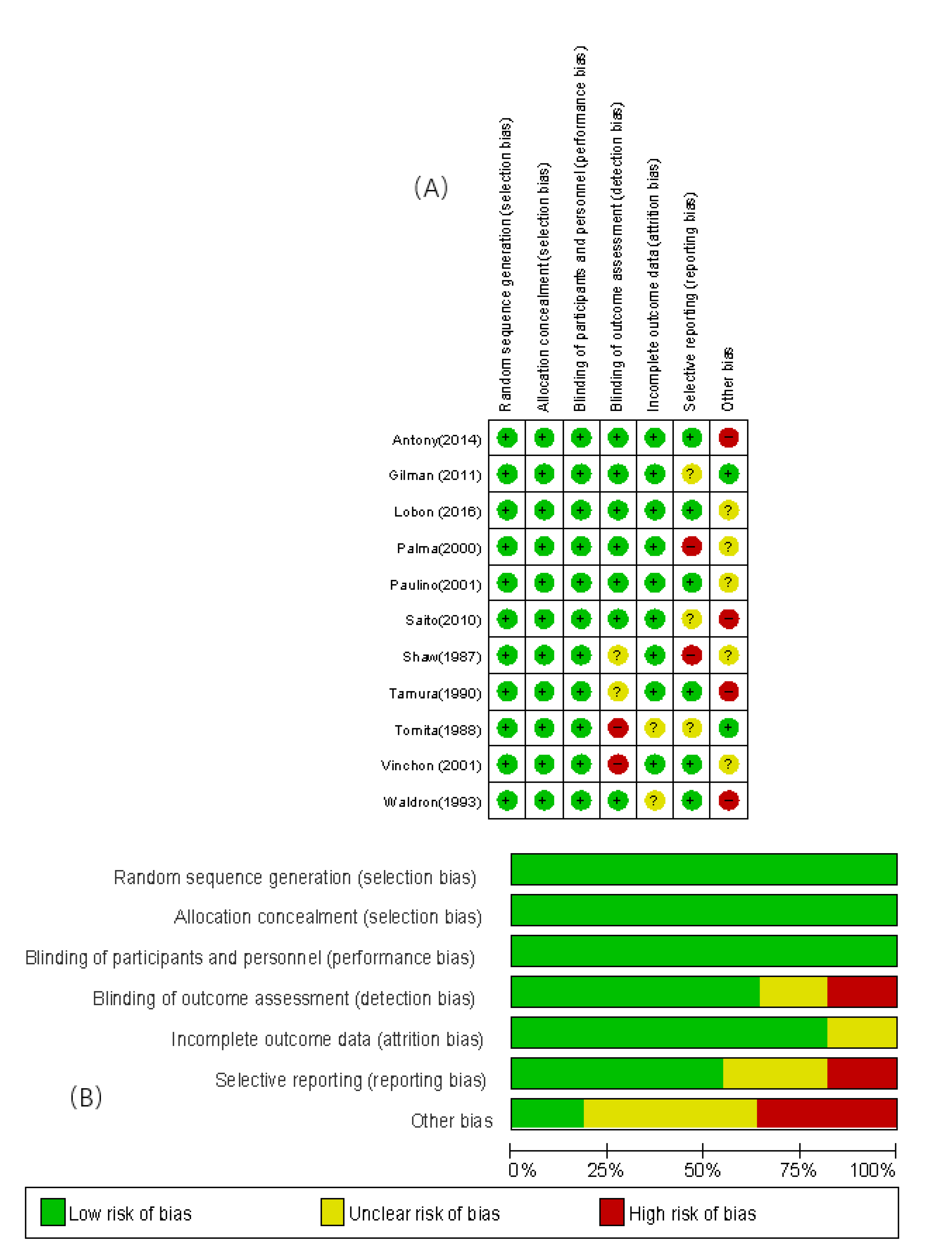
The included 11 studies were assessed for bias using the bias risk assessment tool of the Cochrane Collaboration. The assessment items included: 1) methods of random number generation (selection bias), 2) concealment of allocation results (selection bias), 3) blinding of patients (implementation bias), 4) completeness of outcome data (measurement bias), 5) selective reporting of study results (reporting bias), 6) other sources of bias. The risk bias assessment table was created using Revman 5.4 software, and the overall quality of the literature was good. Please refer to
Figure 2A and 2B for details.
3.3. Meta-analysis results
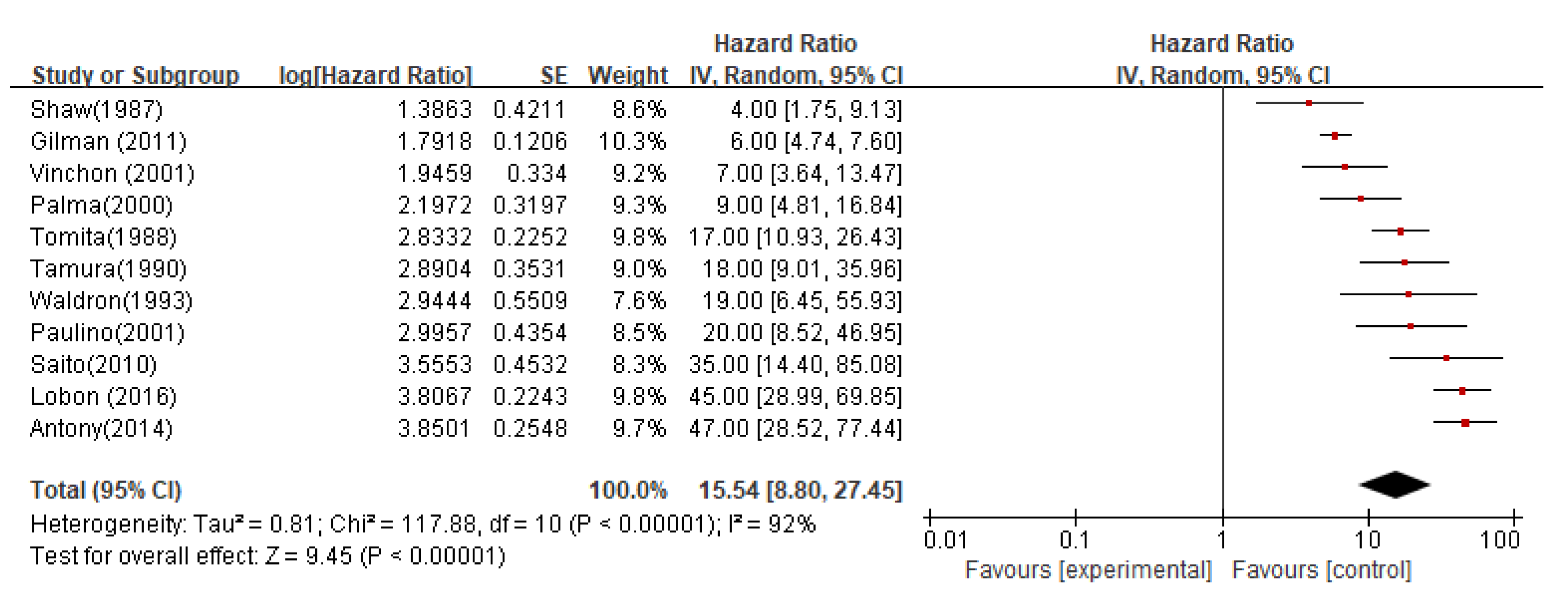
Overall and progression-free survivalThe integrated results of 11 studies showed a significant heterogeneity between the studies (I2=92%). The random effects model was used for combination, and the results showed statistically significant differences (P < 0.00001).
In all studies, the median overall survival (OS) estimate from the date of recurrence was 15.54 months (95%CI 8.80-27.45; P < 0.00001;
Figure 3).
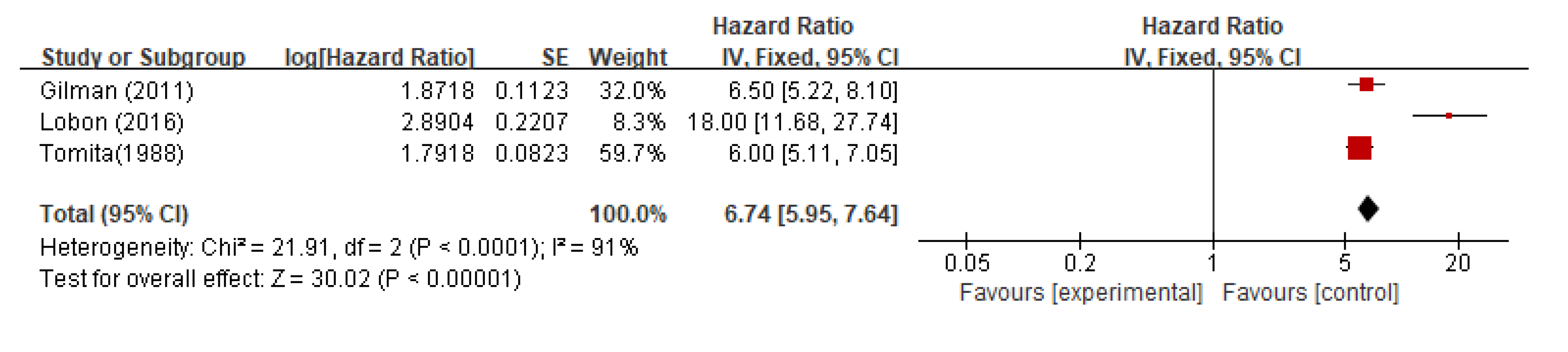
The pooled median progression-free survival (PFS) from all studies was 6.7 months (95%CI 5.59-7.64; P < 0.0001;
Figure 4).
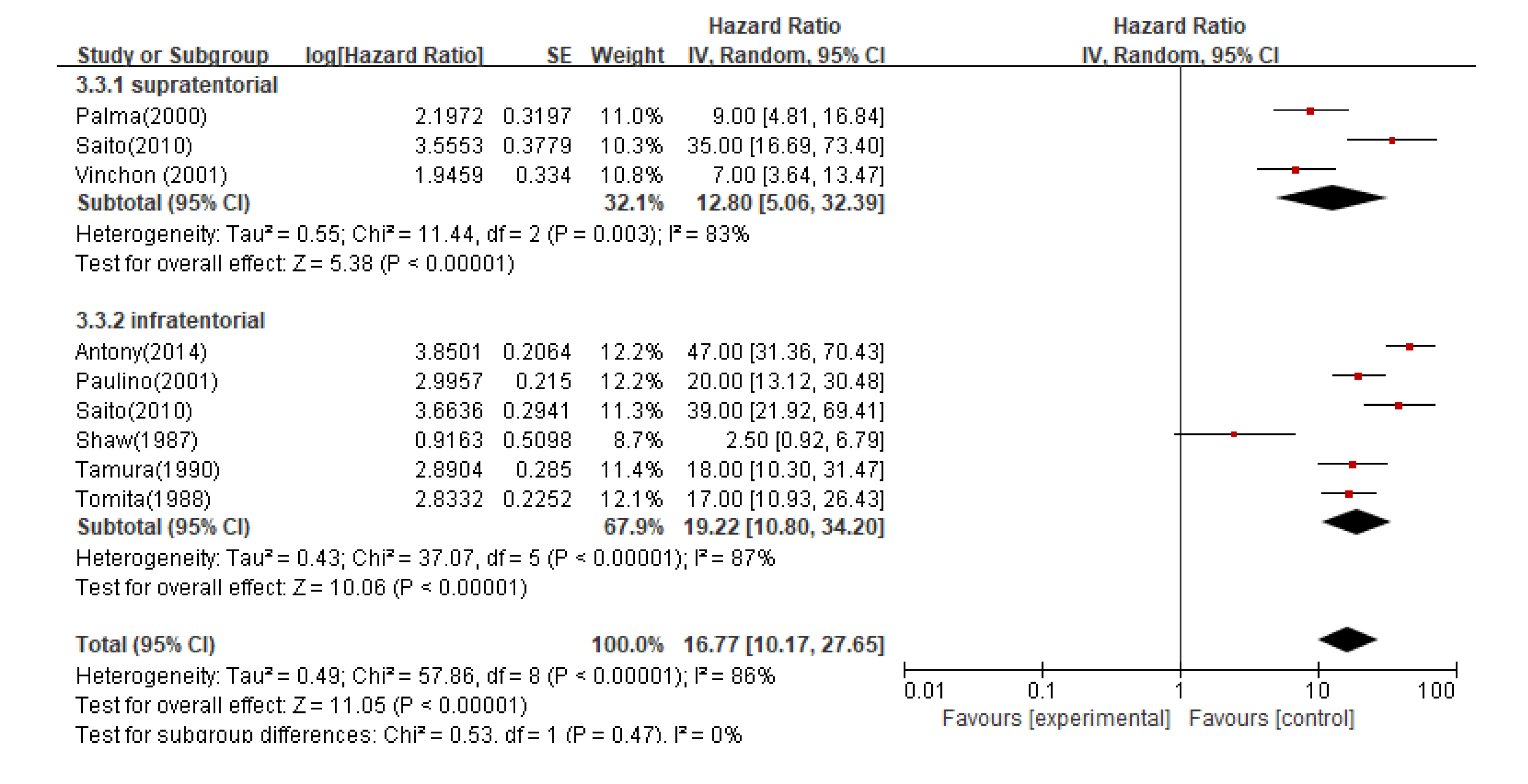
In subgroup analysis, the pooled median OS for infratentorial and supratentorial ependymoma patients was 16.7 months (95%CI 10.17-27.65; P < 0.00001), and the median OS for supratentorial recurrent patients was 12.8 months (95%CI 5.06-32.39; P < 0.005), while the median OS for infratentorial recurrent patients was 19.2 months (95%CI 10.80-34.20; P < 0.0001) (
Figure 5).
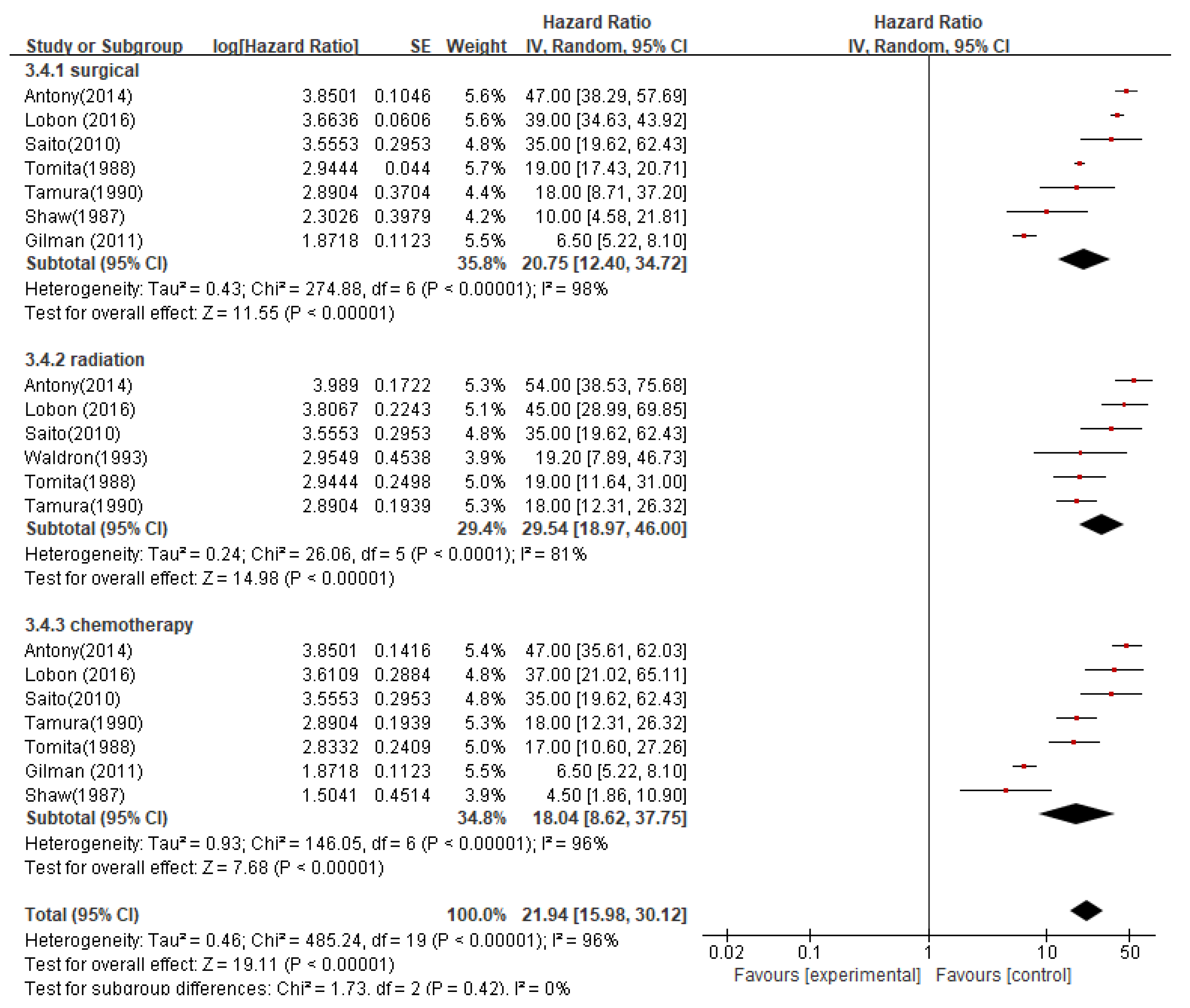
The pooled median OS for patients who received surgical resection at the time of recurrence was 20.7 months (95%CI 12.40-34.72; P < 0.00001), while the mOS for patients who received radiation therapy was 29.5 months (95%CI 18.97-46.00; P < 0.0001), and for patients who received chemotherapy was 18.0 months (95%CI 8.62-37.75; P < 0.00001). The pooled median OS for all three treatment modalities was 21.9 months (95%CI 15.98-30.12; P < 0.00001) (
Figure 6).
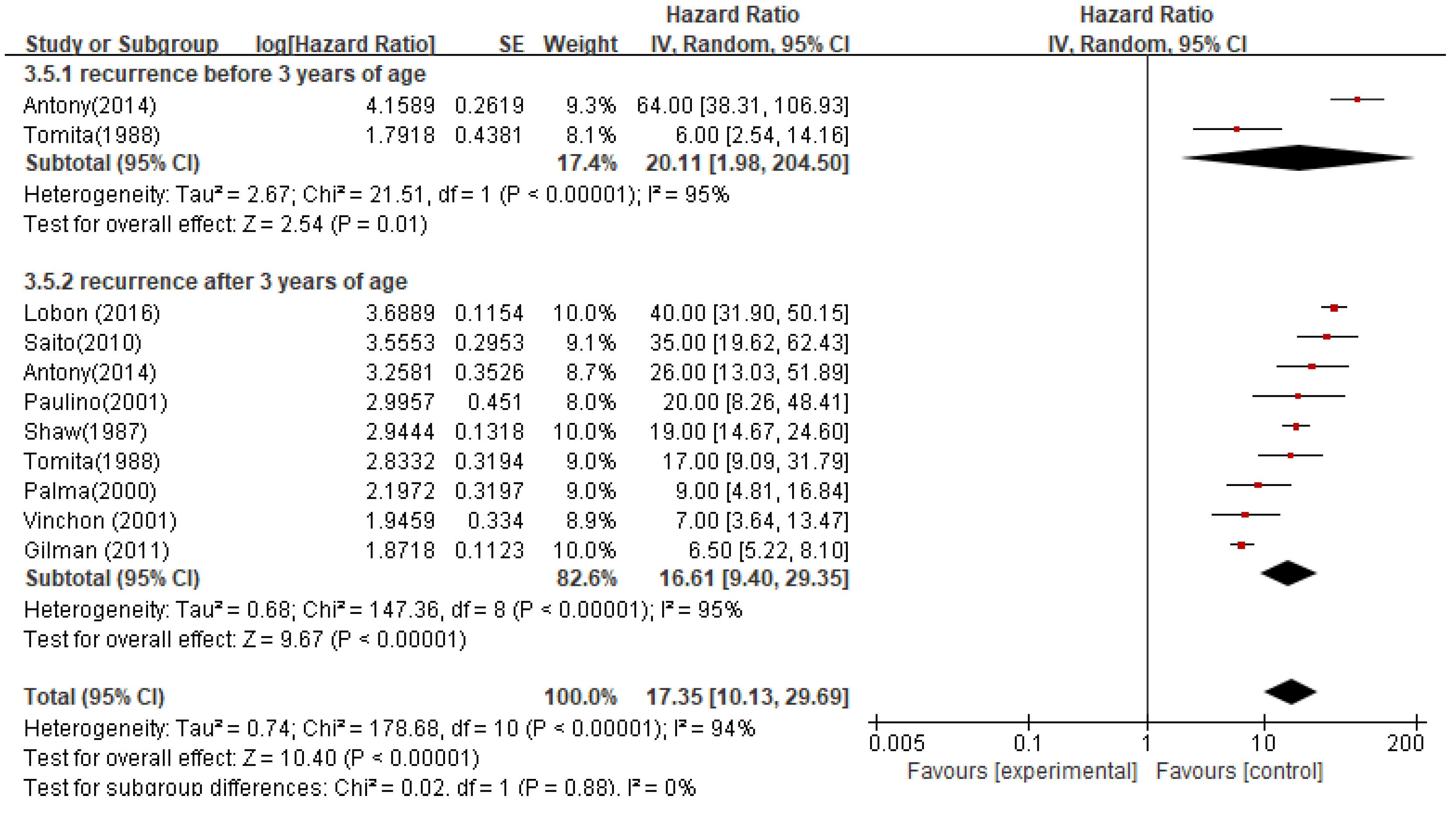
The median OS for patients who relapsed before the age of 3 was 20.1 months (95%CI 1.98-204.50; P < 0.00001), while for patients who relapsed after the age of 3 was 16.6 months (95%CI 9.40-29.35; P < 0.00001). The pooled median OS based on the age of relapse was 17.35 months (95%CI 10.13-29.69; P < 0.00001) (
Figure 7).
3.3. Sensitivity analysis
Sensitivity analysis was conducted by sequentially excluding studies, and the results showed no significant changes in effect size, indicating that the results of this study were relatively stable.
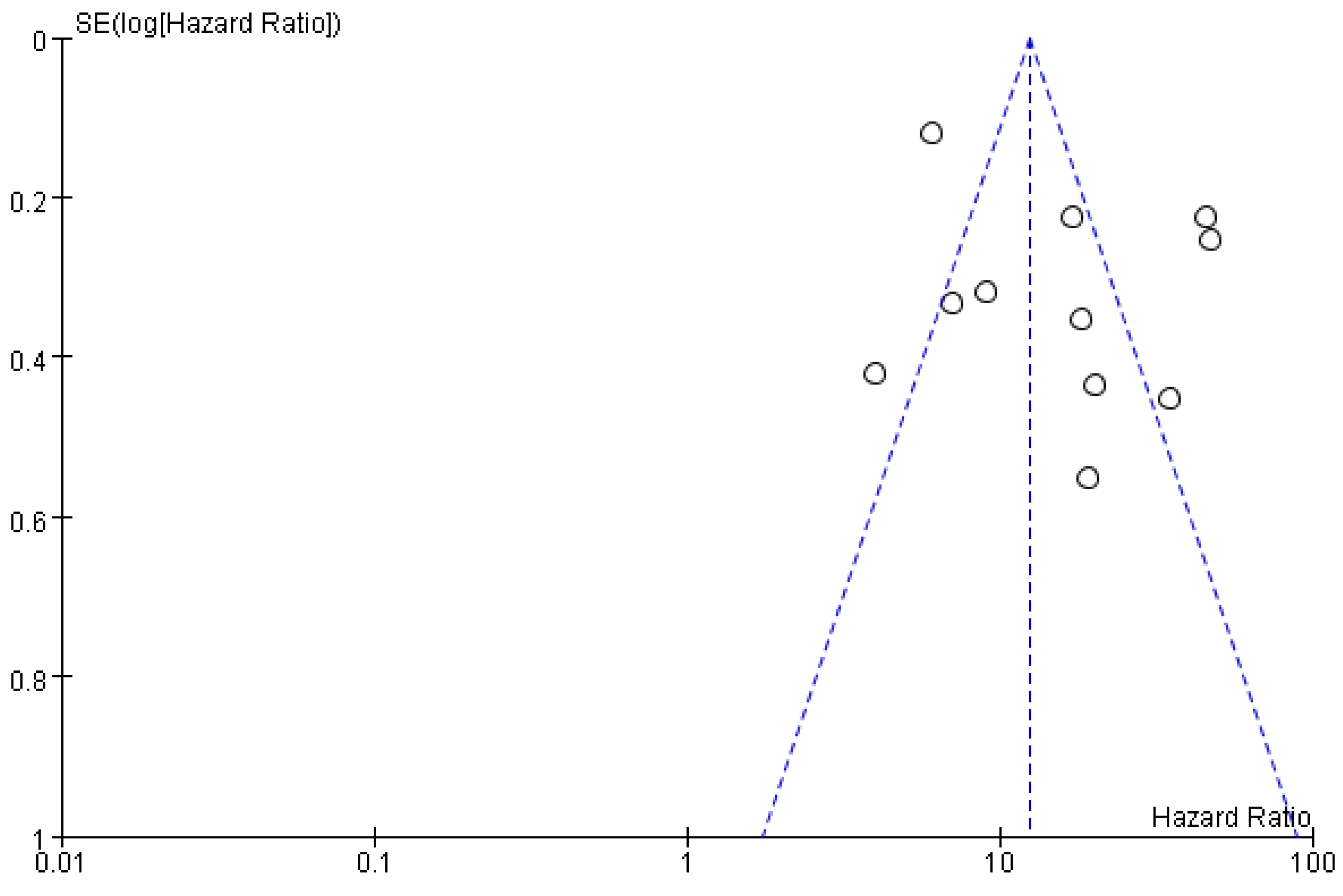
3.4. Publication bias
A funnel plot was constructed to test for publication bias, and the results showed that the scatter plots of the studies were roughly symmetrical, indicating that publication bias in this study was minimal (see
Figure 8).
4. Discuss
Ependymomas belong to gliomas and mainly occur in ependymal cells of the ventricular system and central canal of the spinal cord, rarely occurring outside the central nervous system. The most common spinal cord tumor in children is astrocytoma, accounting for 13% of pediatric ependymomas, compared to intracranial ependymomas. The age of onset and anatomical location of ependymomas vary significantly. Pediatric ependymomas mainly occur in the intracranial region, with two-thirds located in the posterior fossa. Supratentorial ependymomas are mainly found in older children and adults. The spinal cord is the most common site for ependymomas in adults, while they are less common in the spinal cord in children. Ependymomas mainly originate from ependymal cells of the ventricular system and central canal of the spinal cord, but a portion of ependymomas occur outside the ventricular system and are not related to it. These ependymomas only occur supratentorially, mainly affecting the frontal and parietal lobes, and are called "cortical ependymomas," "hemispheric ependymomas," or "ectopic ependymomas". Over the past few decades, with the continuous development of imaging technologies such as CT and MRI, the improvement of neurosurgical techniques, and the continuous improvement of various radiation therapy techniques, the mortality rate of intracranial ependymomas has greatly decreased, but the recurrence rate remains high. There is still controversy regarding the treatment strategies and prognostic factors for intracranial ependymomas both domestically and internationally.
Studies have reported that age is a prognostic factor for intracranial ependymoma patients. Many studies believe that younger patients have a poorer prognosis and higher mortality rates. Children over 2 years old and adults have longer overall survival (OS) than children under 2 years old. Age seems to be a strong predictive indicator, with patients under 5 years old or over 59 years old having poor prognosis. Children under 3 years old have worse local control and OS. This study found that children under 3 years old had a shorter median OS after relapse, indicating a worse prognosis (before 3 years old, median OS was 20.1 months, 95% CI 1.98-204.50; P < 0.00001; after 3 years old, median OS was 16.6 months, 95% CI 9.40-29.35; P < 0.00001). The reasons for the poor prognosis in young patients may be: (1) inadequate treatment. Previous studies have considered radiotherapy a taboo for children under 3 years old, as the immature brain is more sensitive to radiation damage, which can cause severe radiation-induced sequelae. Children under 3 years old often avoid or delay radiotherapy until tumor recurrence, and the value of chemotherapy is still unclear; (2) more aggressive histology; (3) age may be associated with certain poor-prognosis molecular subtypes.
Currently, the extent of surgical resection is recognized as a prognostic factor, and the principle is to remove the tumor as completely as possible while preserving neurological function. This applies to all histological grades [
41]. The purpose of surgical resection is to obtain a definite tissue diagnosis and reduce the risk of recurrence. Adjuvant therapy such as radiation and chemotherapy can play their unique roles. Postoperative radiotherapy is the standard treatment for intracranial ependymoma, with longer survival compared to surgery alone [
42]. The prognostic value of chemotherapy for intracranial ependymoma is still unclear. This study did not find that chemotherapy can improve the overall survival (OS) of patients. Although chemotherapy is not the standard treatment for intracranial ependymoma, many centers still use it as supplementary therapy. The meta-analysis in this study suggests that the surgical resection method at the time of recurrence is an independent risk factor for the prognosis of intracranial ependymoma patients. The median OS of patients who underwent surgical resection at the time of recurrence was 20.7 months (95% CI 12.40-34.72; P < 0.00001), while patients who received radiotherapy had a median OS of 29.5 months (95% CI 18.97-46.00; P < 0.0001), both of which were higher than the chemotherapy group (median OS of 18.0 months, 95% CI 8.62-37.75; P < 0.00001).
This study has certain limitations: ① Only Chinese and English literature were included, so there may be incomplete inclusion of articles; ② This study did not include molecular subtypes, which have better prognostic value than histological grading. With the continuous deepening of molecular pathological research, incorporating molecular subtypes into clinical trials will further elucidate their prognostic value and optimize disease risk stratification. ③ This study had a small sample size, so the statistically significant results obtained may be limited.
5. Conclusion
In conclusion, this systematic review and meta-analysis on the prognosis of recurrent pediatric ependymoma indicates that the prognosis of recurrent ependymoma in children is poor, and the prognosis is related to patient age, tumor location, and treatment modality. Future studies should include retrospective investigations and prospective trials with molecular subtype data to effectively predict and treat these tumors, providing more precise treatment options for intracranial ependymoma patients.
Competing Interests
The authors declare that there is no competing interest associated with the manuscript.
Funding
1 National Natural Science Foundation of China grant (No.82272584); 2 2021 Zhejiang Normal University Interdisciplinary Advance Research Fund.
References
- Liang, M.L. , et al., Abemaciclib, A Selective CDK4/6 Inhibitor, Restricts the Growth of Pediatric Ependymomas. Cancers (Basel) 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S. , et al., Incidence patterns for primary malignant spinal cord gliomas: a Surveillance, Epidemiology, and End Results study. J Neurosurg Spine 2011, 14, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Tensaouti, F. , et al., Feasibility of Dose Escalation in Patients With Intracranial Pediatric Ependymoma. Front Oncol 2019, 9, 531. [Google Scholar] [CrossRef] [PubMed]
- Pessôa, I.A. , et al., Detection and Correlation of Single and Concomitant TP53, PTEN, and CDKN2A Alterations in Gliomas. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Kresbach, C., S. Neyazi, and U. Schüller, Updates in the classification of ependymal neoplasms: The 2021 WHO Classification and beyond. Brain Pathol 2022, 32, e13068. [Google Scholar] [CrossRef] [PubMed]
- Li, Z. and S.A. Langhans, In Vivo and Ex Vivo Pediatric Brain Tumor Models: An Overview. Front Oncol 2021, 11, 620831. [Google Scholar] [CrossRef]
- Zhou, M. , et al., Delineation of molecular characteristics in pediatric PFA ependymoma involving rare osseous and pulmonary metastases: A case report and literature review. Front Oncol 2022, 12, 1001118. [Google Scholar] [CrossRef]
- Sood, D. , et al., 3D extracellular matrix microenvironment in bioengineered tissue models of primary pediatric and adult brain tumors. Nat Commun 2019, 10, 4529. [Google Scholar] [CrossRef]
- Sabnis, D.H. , et al., A role for ABCB1 in prognosis, invasion and drug resistance in ependymoma. Sci Rep 2019, 9, 10290. [Google Scholar] [CrossRef]
- Ozawa, T. , et al. , A De Novo Mouse Model of C11orf95-RELA Fusion-Driven Ependymoma Identifies Driver Functions in Addition to NF-κB. Cell Rep 2018, 23, 3787–3797. [Google Scholar]
- Merchant, T.E. , et al., A retrospective study of surgery and reirradiation for recurrent ependymoma. Int J Radiat Oncol Biol Phys 2008, 71, 87–97. [Google Scholar] [CrossRef]
- Tsang, D.S. , et al., Outcomes After Reirradiation for Recurrent Pediatric Intracranial Ependymoma. Int J Radiat Oncol Biol Phys 2018, 100, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Bouffet, E., M. Capra, and U. Bartels, Salvage chemotherapy for metastatic and recurrent ependymoma of childhood. Childs Nerv Syst 2009, 25, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Rudà, R. , et al., EANO guidelines for the diagnosis and treatment of ependymal tumors. Neuro Oncol 2018, 20, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Merchant, T.E. , et al., Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol 2009, 10, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Grundy, R.G. , et al., Primary postoperative chemotherapy without radiotherapy for intracranial ependymoma in children: the UKCCSG/SIOP prospective study. Lancet Oncol 2007, 8, 696–705. [Google Scholar] [CrossRef]
- van Veelen-Vincent, M.L. , et al., Ependymoma in childhood: prognostic factors, extent of surgery, and adjuvant therapy. J Neurosurg 2002, 97, 827–835. [Google Scholar] [CrossRef]
- Massimino, M. , et al., Final results of the second prospective AIEOP protocol for pediatric intracranial ependymoma. Neuro Oncol 2016, 18, 1451–1460. [Google Scholar] [CrossRef]
- Wan, X. , et al., Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014, 14, 135. [Google Scholar] [CrossRef]
- Koo, C.H. , et al., The Effect of Virtual Reality on Preoperative Anxiety: A Meta-Analysis of Randomized Controlled Trials. J Clin Med 2020, 9. [Google Scholar] [CrossRef]
- Paulino, A.C. , The local field in infratentorial ependymoma: does the entire posterior fossa need to be treated? Int J Radiat Oncol Biol Phys 2001, 49, 757–761. [Google Scholar] [CrossRef]
- Vinchon, M. , et al., Supratentorial ependymoma in children. Pediatr Neurosurg 2001, 34, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Saito, R. , et al., Dissemination limits the survival of patients with anaplastic ependymoma after extensive surgical resection, meticulous follow up, and intensive treatment for recurrence. Neurosurg Rev 2010, 33, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Gilman, A.L. , et al., Phase I study of tandem high-dose chemotherapy with autologous peripheral blood stem cell rescue for children with recurrent brain tumors: a Pediatric Blood and MarrowTransplant Consortium study. Pediatr Blood Cancer 2011, 57, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Antony, R. , et al., A retrospective analysis of recurrent intracranial ependymoma. Pediatr Blood Cancer 2014, 61, 1195–1201. [Google Scholar] [CrossRef]
- Lobón, M.J. , et al., Re-irradiation of recurrent pediatric ependymoma: modalities and outcomes: a twenty-year survey. Springerplus 2016, 5, 879. [Google Scholar] [CrossRef]
- Shaw, E.G. , et al., Postoperative radiotherapy of intracranial ependymoma in pediatric and adult patients. Int J Radiat Oncol Biol Phys 1987, 13, 1457–1462. [Google Scholar] [CrossRef]
- Tomita, T. , et al., Benign ependymomas of the posterior fossa in childhood. Pediatr Neurosci 1988, 14, 277–285. [Google Scholar] [CrossRef]
- Tamura, M. , et al., Adjunctive treatment for recurrent childhood ependymoma of the IV ventricle: chemotherapy with CDDP and MCNU. Childs Nerv Syst 1990, 6, 186–189. [Google Scholar] [CrossRef]
- Waldron, J.N. , et al., Spinal cord ependymomas: a retrospective analysis of 59 cases. Int J Radiat Oncol Biol Phys 1993, 27, 223–229. [Google Scholar] [CrossRef]
- Palma, L. , et al., The importance of surgery in supratentorial ependymomas. Long-term survival in a series of 23 cases. Childs Nerv Syst 2000, 16, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Saito, S. , et al., Radiation necrosis following proton therapy successfully treated by low-dose bevacizumab in a patient with relapsed anaplastic ependymoma. Pediatr Blood Cancer 2018, 65, e27088. [Google Scholar] [CrossRef]
- Shlien, A. , et al., A common molecular mechanism underlies two phenotypically distinct 17p13.1 microdeletion syndromes. Am J Hum Genet 2010, 87, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Wetmore, C. , et al., Phase II evaluation of sunitinib in the treatment of recurrent or refractory high-grade glioma or ependymoma in children: a children's Oncology Group Study ACNS1021. Cancer Med 2016, 5, 1416–1424. [Google Scholar] [CrossRef]
- Hosoya, T. , et al., Pediatric Case of Li-Fraumeni Syndrome Complicated with Supratentorial Anaplastic Ependymoma. World Neurosurg 2018, 120, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Rushing, E.J. , et al., Subependymoma revisited: clinicopathological evaluation of 83 cases. J Neurooncol 2007, 85, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, M.K. , et al., Melanotic ependymoma and subependymoma. Am J Surg Pathol 1990, 14, 729–736. [Google Scholar] [CrossRef]
- Tiwari, N., S. Z. Powell, and H. Takei, Recurrent subependymoma of fourth ventricle with unusual atypical histological features: A case report. Pathol Int 2015, 65, 438–442. [Google Scholar] [CrossRef]
- Gupta, R. , et al., Composite phaeochromocytoma with malignant peripheral nerve sheath tumour and rhabdomyosarcomatous differentiation in a patient without von Recklinghausen disease. J Clin Pathol 2009, 62, 659–661. [Google Scholar] [CrossRef]
- Vajtai, I. , et al. , Rapid spontaneous malignant progression of supratentorial tanycytic ependymoma with sarcomatous features - "Ependymosarcoma". Pathol Res Pract 2010, 206, 493–498. [Google Scholar]
- Pajtler, K.W. , et al., Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell 2015, 27, 728–743. [Google Scholar] [CrossRef] [PubMed]
- Formentin, C. F. Joaquim, and E. Ghizoni, Posterior fossa tumors in children: current insights. Eur J Pediatr, 2023. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).