Submitted:
28 September 2023
Posted:
28 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Some elements of rhabdomyosarcoma relevant to SALIQ
2. A. PAX and FOXO1
2. B. Glycogen synthase kinase 3b
2. C. MyoD, myogenin, and myogenesis
3. Simvastatin
4. ATRA
5. Lithium
6. Itraconazole, and Hh
7. Quercetin and onions
8. Pharmacological considerations
8. A. Simvastatin
8. B. ATRA
8. C. Lithium carbonate
8. D. Itraconazole
8. E. Quercetin
8. Discussion and Conclusions
- Frequent meetings in person with the treating physician for a standard review of systems and targeted physical exam. Weekly is a minimum during the uptitration establishment phase unless experience with a given regimen allows longer evaluation intervals.
- Frequent blood test monitoring for bone marrow, liver and kidney function.
- Starting with low doses, adding one drug at a time, reevaluating after several days then increasing dose as tolerated every few days until target dose met or side effects dictate a lower dose.
- Patients must have 24/7 ready direct access to the treating physician by phone.
- Careful attention required to assure patient is taking medicines as directed. People often have trouble keeping these multidrug regimens and the frequent dose changes straight.
- Once stability of dose of all drugs and tolerability established, monthly meetings will suffice.
- When these conditions cannot be met, use of multidrug regimens like SALIQ or CUSP9v3 is not recommended until wider experiences with them can determine more suited monitoring recommendations.
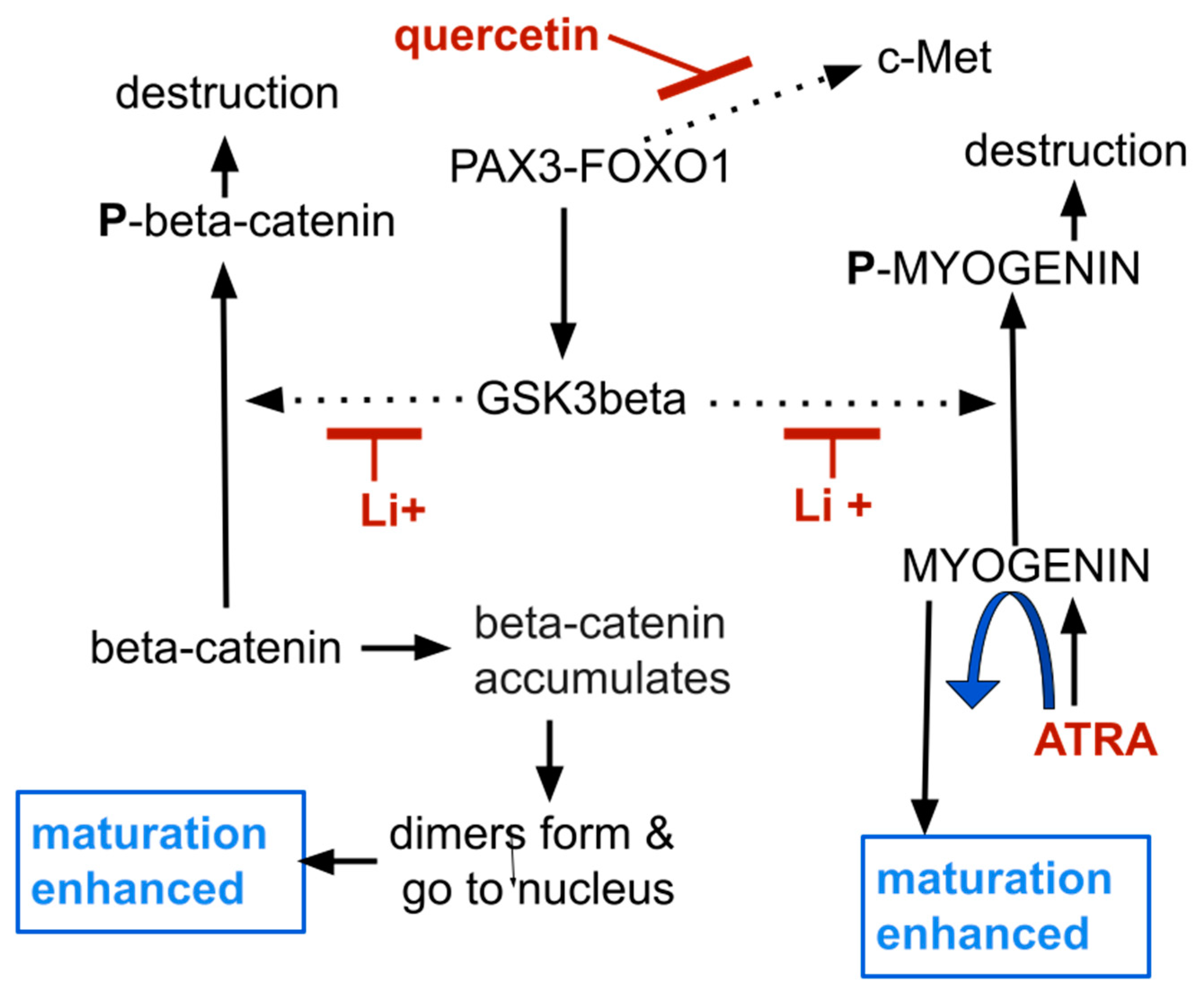
| c-MET | a tyrosine kinase stimulated by HGF, its transcription enhanced by Pax3-FOXO1 |
| FOXO1 | transcription factor directing transcription of target genes |
| GGD | geranylgeranyl diphosphate |
| GSK3 | glycogen synthase kinase 1 beta, a major inhibitor of myogenesis function |
| HGF | hepatocyte growth factor, the cognate ligand of c-Met |
| Hh | Hedgehog signaling |
| HMG-CoA reductase | 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, a statin, the target enzyme simvastatin inhibits |
| MyoD | transcription factor for myocyte differentiation |
| myogenesis | transcription factor necessary for myocyte maturation. |
| Pannexin 1 | an ion channel with lower function in rhabdomyosarcoma |
| PAX | paired box transcription factor, PAX3 or PAX7, protein transcription factor |
| PAX-FOXO1 | PAX3-FOXO1 or PAX7-FOXO1, common abnormal fusion protein driver in rhabdomyosarcoma |
| drug | ped dose | comments |
|---|---|---|
| simvastatin | 10 to 40 mg/d | 0.1% rhabdomyolysis risk over 4 years, CYP3A4 metabolism |
| ATRA | 7 mg/m2/BSA bid | is 30% of standard ATRA ped APL dose, CYP3A4 metabolism |
| lithium carbonate | 300-600 mg/d | little experience with Li+ in children, renal elimination |
| itraconazole | 5 mg/kg/day | strong CYP3A4 inhibition |
| quercetin | 1 to 2 g/d | potentially ineffective |
| drug | starting dose | target dose |
|---|---|---|
| simvastatin | 10 mg/day | 20 to 40 mg/day |
| ATRA | 7 mg/m2/BSA bid | cannot be predicted * |
| lithium carbonate | 300 mg/day | 600 mg/day ** |
| itraconazole | 2.5 mg/kg/day | 5 mg/kg/day |
| quercetin | O.5 g/day | 2 g/day |
| drug | quadpill dose/d (%) | Common dose/d | MOA |
|---|---|---|---|
| irbesartan | 37·5 mg (25%) | 150 mg | ARB |
| amlodipine | 1·25 mg (25%) | 5 mg | Ca++ channel blocker |
| indapamide | 0·625 mg (25%) | 2.5 mg | thiazide diuretic |
| bisoprolol | 2·5 mg (25%) | 10 mg | beta blocker |
Abbreviations
References
- Hettmer, S.; Linardic, C.M.; Kelsey, A.; Rudzinski, E.R.; Vokuhl, C.; Selfe, J.; Ruhen, O.; Shern, J.F.; Khan, J.; Kovach, A.R.; et al. Molecular testing of rhabdomyosarcoma in clinical trials to improve risk stratification and outcome: A consensus view from European paediatric Soft tissue sarcoma Study Group, Children’s Oncology Group and Cooperative Weichteilsarkom-Studiengruppe. Eur. J. Cancer 2022, 172, 367–386. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.; Guttridge, D.C. Mechanisms of impaired differentiation in rhabdomyosarcoma. FEBS J. 2013, 280, 4323–4334. [Google Scholar] [CrossRef] [PubMed]
- Melcón, S.G.; Codina, J.S.d.T. Molecular biology of rhabdomyosarcoma. Clin. Transl. Oncol. 2007, 9, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.J.; Pressey, J.G.; Barr, F.G. Molecular pathogenesis of rhabdomyosarcoma. Cancer Biol. Ther. 2002, 1, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Leiner, J.; Le Loarer, F. The current landscape of rhabdomyosarcomas: an update. Virchows Arch. 2019, 476, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Parham, D.M.; Giannikopoulos, P. Rhabdomyosarcoma: From Obscurity to Clarity in Diagnosis … But With Ongoing Challenges in Management: The Farber-Landing Lecture of 2020. Pediatr. Dev. Pathol. 2021, 24, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.Y.; Guttridge, D.C. Dysregulated Myogenesis in Rhabdomyosarcoma. Curr. Top. Dev. Biol. 2017, 126, 285–297. [Google Scholar] [CrossRef]
- Pomella, S.; Danielli, S.G.; Alaggio, R.; Breunis, W.B.; Hamed, E.; Selfe, J.; Wachtel, M.; Walters, Z.S.; Schäfer, B.W.; Rota, R.; et al. Genomic and Epigenetic Changes Drive Aberrant Skeletal Muscle Differentiation in Rhabdomyosarcoma. Cancers 2023, 15, 2823. [Google Scholar] [CrossRef]
- Agaram, N.P. Evolving classification of rhabdomyosarcoma. Histopathology 2021, 80, 98–108. [Google Scholar] [CrossRef]
- Skapek, S.X.; Anderson, J.; Barr, F.G.; Bridge, J.A.; Gastier-Foster, J.M.; Parham, D.M.; Rudzinski, E.R.; Triche, T.; Hawkins, D.S. PAX-FOXO1 fusion status drives unfavorable outcome for children with rhabdomyosarcoma: A children’s oncology group report. Pediatr. Blood Cancer 2013, 60, 1411–1417. [Google Scholar] [CrossRef]
- Williamson, D.; Missiaglia, E.; Pritchard-Jones, K.; Oberlin, O.; Shipley, J.M.; Delattre, O.; De Reyniès, A.; Pierron, G.; Thuille, B.; Palenzuela, G.; et al. Fusion Gene-Negative Alveolar Rhabdomyosarcoma is Clinically and Molecularly Indistinguishable from Embryonal Rhabdomyosarcoma. J. Clin. Oncol. 2010, 28, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, R.; Gu, J.; Yin, X.; Jin, N.; Xie, S.; Wang, Y.; Chang, H.; Qian, W.; Shi, J.; et al. Cross talk between PI3K-AKT-GSK-3β and PP2A pathways determines tau hyperphosphorylation. Neurobiol. Aging 2015, 36, 188–200. [Google Scholar] [CrossRef]
- Chu, D.; Tan, J.; Xie, S.; Jin, N.; Yin, X.; Gong, C.-X.; Iqbal, K.; Liu, F. GSK-3β is Dephosphorylated by PP2A in a Leu309 Methylation-Independent Manner. J. Alzheimer’s Dis. 2015, 49, 365–375. [Google Scholar] [CrossRef]
- O’connor, C.M.; Perl, A.; Leonard, D.; Sangodkar, J.; Narla, G. Therapeutic targeting of PP2A. Int. J. Biochem. Cell Biol. 2018, 96, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Snitow, M.E.; Bhansali, R.S.; Klein, P.S. Lithium and Therapeutic Targeting of GSK-3. Cells 2021, 10, 255. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Vázquez, E.Y.; Quintas-Granados, L.I.; Cortés, H.; Carmen, M.G.-D.; Leyva-Gómez, G.; Rodríguez-Morales, M.; Bustamante-Montes, L.P.; Silva-Adaya, D.; Pérez-Plasencia, C.; Jacobo-Herrera, N.; et al. Lithium: A Promising Anticancer Agent. Life 2023, 13, 537. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, G.B.; Okutachi, S.; Abankwa, D. Potential of phenothiazines to synergistically block calmodulin and reactivate PP2A in cancer cells. PLOS ONE 2022, 17, e0268635. [Google Scholar] [CrossRef]
- Chien, W.; Sun, Q.-Y.; Lee, K.L.; Ding, L.-W.; Wuensche, P.; Torres-Fernandez, L.A.; Tan, S.Z.; Tokatly, I.; Zaiden, N.; Poellinger, L.; et al. Activation of protein phosphatase 2A tumor suppressor as potential treatment of pancreatic cancer. Mol. Oncol. 2015, 9, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Dionyssiou, M.G.; Ehyai, S.; Avrutin, E.; Connor, M.K.; McDermott, J.C. Glycogen synthase kinase 3β represses MYOGENIN function in alveolar rhabdomyosarcoma. Cell Death Dis. 2014, 5, e1094. [Google Scholar] [CrossRef]
- Myer, A.; Olson, E.N.; Klein, W.H. MyoD Cannot Compensate for the Absence of Myogenin during Skeletal Muscle Differentiation in Murine Embryonic Stem Cells. Dev. Biol. 2001, 229, 340–350. [Google Scholar] [CrossRef]
- Adhikari, A.; Kim, W.; Davie, J. Myogenin is required for assembly of the transcription machinery on muscle genes during skeletal muscle differentiation. PLOS ONE 2021, 16, e0245618. [Google Scholar] [CrossRef] [PubMed]
- Sebire, N.J.; Malone, M. Myogenin and MyoD1 expression in paediatric rhabdomyosarcomas. J. Clin. Pathol. 2003, 56, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Battistelli, C.; Garbo, S.; Maione, R. MyoD-Induced Trans-Differentiation: A Paradigm for Dissecting the Molecular Mechanisms of Cell Commitment, Differentiation and Reprogramming. Cells 2022, 11, 3435. [Google Scholar] [CrossRef] [PubMed]
- Calhabeu, F.; Hayashi, S.; E Morgan, J.; Relaix, F.; Zammit, P.S. Alveolar rhabdomyosarcoma-associated proteins PAX3/FOXO1A and PAX7/FOXO1A suppress the transcriptional activity of MyoD-target genes in muscle stem cells. Oncogene 2012, 32, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Hu, J.-W.; He, X.-R.; Jin, W.-L.; He, X.-Y. Statins: a repurposed drug to fight cancer. J. Exp. Clin. Cancer Res. 2021, 40, 241. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-H.; Liu, C.-H.; Ding, D.-C. Statins as Repurposed Drugs in Gynecological Cancer: A Review. Int. J. Mol. Sci. 2022, 23, 13937. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.-F.; Wang, M.-X.; Chen, Z.-L.; Yang, L. Targeting the Tumor Microenvironment: A Literature Review of the Novel Anti-Tumor Mechanism of Statins. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Xie, L.; Zhu, G.; Shang, J.; Chen, X.; Zhang, C.; Ji, X.; Zhang, Q.; Wei, Y. An overview on the biological activity and anti-cancer mechanism of lovastatin. Cell. Signal. 2021, 87, 110122. [Google Scholar] [CrossRef]
- Duarte, J.A.; de Barros, A.L.B.; Leite, E.A. The potential use of simvastatin for cancer treatment: A review. Biomed. Pharmacother. 2021, 141, 111858. [Google Scholar] [CrossRef]
- Joharatnam-Hogan, N.; Alexandre, L.; Yarmolinsky, J.; Lake, B.; Capps, N.; Martin, R.M.; Ring, A.; Cafferty, F.; E Langley, R. Statins as Potential Chemoprevention or Therapeutic Agents in Cancer: a Model for Evaluating Repurposed Drugs. Curr. Oncol. Rep. 2021, 23, 29. [Google Scholar] [CrossRef]
- Hamelin, B.A.; Turgeon, J. Hydrophilicity/lipophilicity: relevance for the pharmacology and clinical effects of HMG-CoA reductase inhibitors. Trends Pharmacol. Sci. 1998, 19, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Vuu, Y.M.; Shahib, A.K.; Rastegar, M. The Potential Therapeutic Application of Simvastatin for Brain Complications and Mechanisms of Action. Pharmaceuticals 2023, 16, 914. [Google Scholar] [CrossRef] [PubMed]
- Backes, J.M.; A Howard, P.; Ruisinger, J.F.; Moriarty, P.M. Does Simvastatin Cause More Myotoxicity Compared with Other Statins? Ann. Pharmacother. 2009, 43, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.M.; Eisenberg, M.J.; Christopoulos, S. Rhabdomyolysis associated with hydroxymethylglutaryl-coenzyme A reductase inhibitors. Am. Hear. J. 2004, 147, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.R.; A Tobert, J. Simvastatin: a review. Expert Opin. Pharmacother. 2004, 5, 2583–2596. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Midgley, L.; O’Regan, D.J.; E Porter, K. Comparison of the Efficacies of Five Different Statins on Inhibition of Human Saphenous Vein Smooth Muscle Cell Proliferation and Invasion. J. Cardiovasc. Pharmacol. 2007, 50, 458–461. [Google Scholar] [CrossRef]
- Araki, M.; Motojima, K. Hydrophobic statins induce autophagy in cultured human rhabdomyosarcoma cells. Biochem. Biophys. Res. Commun. 2008, 367, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Araki, M.; Maeda, M.; Motojima, K. Hydrophobic statins induce autophagy and cell death in human rhabdomyosarcoma cells by depleting geranylgeranyl diphosphate. Eur. J. Pharmacol. 2012, 674, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Codenotti, S.; Zizioli, D.; Mignani, L.; Rezzola, S.; Tabellini, G.; Parolini, S.; Giacomini, A.; Asperti, M.; Poli, M.; Mandracchia, D.; et al. Hyperactive Akt1 Signaling Increases Tumor Progression and DNA Repair in Embryonal Rhabdomyosarcoma RD Line and Confers Susceptibility to Glycolysis and Mevalonate Pathway Inhibitors. Cells 2022, 11, 2859. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Qiao, G.; Liu, Y.; Tian, L.; Hui, N.; Li, J.; Ma, Y.; Li, H.; Zhao, Q.; Cao, W.; et al. Overview of all-trans-retinoic acid (ATRA) and its analogues: Structures, activities, and mechanisms in acute promyelocytic leukaemia. Eur. J. Med. Chem. 2021, 220, 113451. [Google Scholar] [CrossRef]
- Brown, G. Retinoic acid receptor regulation of decision-making for cell differentiation. Front. Cell Dev. Biol. 2023, 11, 1182204. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.; Matthay, K.K.; Villablanca, J.G.; Maurer, B.J. Retinoid therapy of high-risk neuroblastoma. Cancer Lett. 2003, 197, 185–192. [Google Scholar] [CrossRef]
- Giuli, M.V.; Hanieh, P.N.; Giuliani, E.; Rinaldi, F.; Marianecci, C.; Screpanti, I.; Checquolo, S.; Carafa, M. Current Trends in ATRA Delivery for Cancer Therapy. Pharmaceutics 2020, 12, 707. [Google Scholar] [CrossRef] [PubMed]
- Masetti, R.; Biagi, C.; Zama, D.; Vendemini, F.; Martoni, A.; Morello, W.; Gasperini, P.; Pession, A. Retinoids in Pediatric Onco-Hematology: the Model of Acute Promyelocytic Leukemia and Neuroblastoma. Adv. Ther. 2012, 29, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Hu, G.; Cai, X. The success and the challenge of all trans retinoic acid in the treatment of cancer. Crit Rev Food Sci Nutr. 2019, 59, S71–S80. [Google Scholar] [CrossRef]
- Calleja, E.M.; Warrell, R.P. Differentiating agents in pediatric malignancies: All-trans-retinoic acid and arsenic in acute promyelocytic leukemia. Curr. Oncol. Rep. 2000, 2, 519–523. [Google Scholar] [CrossRef]
- Hsu, J.Y.; Danis, E.P.; Nance, S.; O’Brien, J.H.; Gustafson, A.L.; Wessells, V.M.; Goodspeed, A.E.; Talbot, J.C.; Amacher, S.L.; Jedlicka, P.; et al. SIX1 reprograms myogenic transcription factors to maintain the rhabdomyosarcoma undifferentiated state. Cell Rep. 2022, 38, 110323–110323. [Google Scholar] [CrossRef]
- Yu, Y.; Khan, J.; Khanna, C.; Helman, L.; Meltzer, P.S.; Merlino, G. Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat. Med. 2004, 10, 175–181. [Google Scholar] [CrossRef]
- Ehinger, D.; Frostberg, H.; Larsson, S.; Gisselsson, D. SIX1 as a Novel Immunohistochemical Marker in the Differential Diagnosis of Rhabdomyosarcoma. Fetal Pediatr. Pathol. 2023, 42, 1–12. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, Y.; Zhao, L.; Lin, Z.; Piao, Y. The Significance of SIX1 as a Prognostic Biomarker for Survival Outcome in Various Cancer Patients: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 622331. [Google Scholar] [CrossRef]
- Huang, S.; Lin, W.; Wang, L.; Gao, Y.; Yuan, X.; Zhang, P.; Chen, Y.; Chu, Q. SIX1 Predicts Poor Prognosis and Facilitates the Progression of Non-small Lung Cancer via Activating the Notch Signaling Pathway. J. Cancer 2022, 13, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhao, Q.; Yang, X.; Wang, T.; Yuan, S.; Meng, Q. SIX1: A Prognostic Biomarker in Uterine Corpus Endometrial Carcinoma. Comb. Chem. High Throughput Screen. 2023, 26, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Huang, Y.; Chen, Y.; Wu, Z.; Xie, H.; Zhou, H.; Xing, C. FOXC2-induced circCASK aggravates colorectal cancer progression by upregulating SIX1 expression. IUBMB Life 2023, 75, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Masuda, M.; Sakakibara, I.; Uchida, T.; Niida, Y.; Mori, Y.; Kamei, Y.; Okumura, Y.; Ohminami, H.; Ohnishi, K.; et al. All-trans retinoic acid changes muscle fiber type via increasing GADD34 dependent on MAPK signal. Life Sci. Alliance 2022, 5, e202101345. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Mertens, T.C.; Shivshankar, P.; Bi, W.; Collum, S.D.; Wareing, N.; Ko, J.; Weng, T.; Naikawadi, R.P.; Wolters, P.J.; et al. Sine oculis homeobox homolog 1 plays a critical role in pulmonary fibrosis. J. Clin. Investig. 2022, 7, e142984. [Google Scholar] [CrossRef] [PubMed]
- Ohi, S. Characterization, anticancer drug susceptibility and atRA-induced growth inhibition of a novel cell line (HUMEMS) established from pleural effusion of alveolar rhabdomyosarcoma of breast tissue. Hum. Cell 2007, 20, 39–51. [Google Scholar] [CrossRef]
- Al-Tahan, A.; Sarkis, O.; Harajly, M.; Bs, O.K.B.; Zibara, K.; Boulos, F.; Dighe, D.; Kregel, S.; Bazarbachi, A.; El-Sabban, M.; et al. Retinoic acid fails to induce cell cycle arrest with myogenic differentiation in rhabdomyosarcoma. Pediatr. Blood Cancer 2011, 58, 877–884. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, T.; Zhang, R.; Qin, X.; Zhao, J. All-trans retinoic acid regulates sheep primary myoblast proliferation and differentiation in vitro. Domest. Anim. Endocrinol. 2019, 71, 106394. [Google Scholar] [CrossRef]
- Ogose, A.; Motoyama, T.; Watanabe, H.; Hotta, T. In vitro differentiation and proliferation in a newly established human rhabdomyosarcoma cell line. Virchows Arch. 1995, 426, 385–391. [Google Scholar] [CrossRef]
- Ramp, U.; Gerharz, C.D.; Doehmer, J.; Oster, O.; Gabbert, H.E. Uniform response of c-raf expression to differentiation induction and inhibition of proliferation in a rat rhabdomyosarcoma cell line. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1990, 59, 271–280. [Google Scholar] [CrossRef]
- Gerharz, C.D.; Bracke, M.E.; Mareel, M.M.; Gabbert, H.E. Modulation of invasive potential in different clonal subpopulations of a rat rhabdomyosarcoma cell line (BA-HAN-1) by differentiation induction. Clin. Exp. Metastasis 1993, 11, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Górski, G.K.; Donaldson, M.H.; McMorrow, L.E. Synergistic inhibition of human rhabdomyosarcoma cells by sodium phenylacetate and tretinoin. Vitr. Cell. Dev. Biol. - Anim. 1993, 29, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Gabbert, H.E.; Gerharz, C.D.; Biesalski, H.K.; Engers, R.; Luley, C. Terminal differentiation and growth inhibition of a rat rhabdomyosarcoma cell line (BA-HAN-1C) in vitro after exposure to retinoic acid. Cancer Res. 1988, 48, 5264–5269. [Google Scholar] [PubMed]
- Ricaud, S.; Vernus, B.; Bonnieu, A. Response of human rhabdomyosarcoma cell lines to retinoic acid: Relationship with induction of differentiation and retinoic acid sensitivity. Exp. Cell Res. 2005, 311, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.W.; Wiley, J.C.; Mous, M.; Narendran, A.; Gee, M.F.; Goldberg, M.; Sexsmith, E.; Malkin, D. Differentiation of rhabdomyosarcoma cell lines using retinoic acid. Pediatr. Blood Cancer 2005, 47, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Palomares, T.; Castro, B.; del Olmo, M.; Iglesias, A.; Bilbao, P.; Alonso-Varona, A. Influence of the level of γ-glutamyltranspeptidase activity on the response of poorly and moderately differentiated rhabdomyosarcoma cell lines to all-trans-retinoic acid. Anti-Cancer Drugs 2006, 17, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Arnold, H.H.; Gerharz, C.D.; E Gabbert, H.; Salminen, A. Retinoic acid induces myogenin synthesis and myogenic differentiation in the rat rhabdomyosarcoma cell line BA-Han-1C. J. Cell Biol. 1992, 118, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Arnold, H.H.; Braun, T.; Bober, E.; Buchberger, A.; Winter, B.; Salminen, A. Regulation of myogenin expression in normal and transformed myogenic cell lines. Symp Soc Exp Biol. 1992, 46, 37–51. [Google Scholar]
- Crouch, G.D.; Helman, L.J. All-trans-retinoic acid inhibits the growth of human rhabdomyosarcoma cell lines. Cancer Res. 1991, 51, 4882–4887. [Google Scholar]
- Miyoshi, K.; Kohashi, K.; Fushimi, F.; Yamamoto, H.; Kishimoto, J.; Taguchi, T.; Iwamoto, Y.; Oda, Y. Close correlation between CXCR4 and VEGF expression and frequent CXCR7 expression in rhabdomyosarcoma. Hum. Pathol. 2014, 45, 1900–1909. [Google Scholar] [CrossRef]
- Gee, M.F.W.; Tsuchida, R.; Eichler-Jonsson, C.; Das, B.; Baruchel, S.; Malkin, D. Vascular endothelial growth factor acts in an autocrine manner in rhabdomyosarcoma cell lines and can be inhibited with all-trans-retinoic acid. Oncogene 2005, 24, 8025–8037. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, N.; Butters, R.R.; Brown, E.M. Agonists of the retinoic acid- and retinoid X-receptors inhibit hepatocyte growth factor secretion and expression in U87 human astrocytoma cells. Mol. Brain Res. 2001, 87, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, M.P.; Valvassori, S.S.; Dal-Pont, G.C.; Quevedo, J. Glycogen Synthase Kinase-3β as a Putative Therapeutic Target for Bipolar Disorder. Curr. Drug Metab. 2018, 19, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Outhred, T. Therapeutic Mechanisms of Lithium in Bipolar Disorder: Recent Advances and Current Understanding. CNS Drugs 2016, 30, 931–949. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, A.; Kumar, T.; Kumar, T.; Velagala, V.R.; Velagala, V.R.; Thakre, S.; Thakre, S.; Joshi, A.; Joshi, A. The Actions of Lithium on Glaucoma and Other Senile Neurodegenerative Diseases Through GSK-3 Inhibition: A Narrative Review. Cureus 2022, 14, e28265. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Beaulieu, J.M. Inhibition of glycogen synthase kinase 3 by lithium, a mechanism in search of specificity. Front. Mol. Neurosci. 2022, 15, 1028963. [Google Scholar] [CrossRef]
- Bilir, A.; Aynacioglu, A.S.; Tuna, M.Y. The Possible Interactions and Therapeutic Roles of Lithium Chloride and Midkine on Cancer Treatment. Crit. Rev. Oncog. 2019, 24, 35–45. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, B.; Zhan, M.; Hua, Z.-C. Lithium in Cancer Therapy: Friend or Foe? Cancers 2023, 15, 1095. [Google Scholar] [CrossRef]
- Neofytou, C.; Backlund, A.; Blomgren, K.; Hermanson, O. Irradiation and lithium treatment alter the global DNA methylation pattern and gene expression underlying a shift from gliogenesis towards neurogenesis in human neural progenitors. Transl. Psychiatry 2023, 13, 258. [Google Scholar] [CrossRef]
- Natale, G.; Fini, E.; Calabrò, P.F.; Carli, M.; Scarselli, M.; Bocci, G. Valproate and lithium: Old drugs for new pharmacological approaches in brain tumors? Cancer Lett. 2023, 560, 216125. [Google Scholar] [CrossRef]
- Schleicher, S.B.; Zaborski, J.J.; Riester, R.; Zenkner, N.; Handgretinger, R.; Kluba, T.; Traub, F.; Boehme, K.A. Combined application of arsenic trioxide and lithium chloride augments viability reduction and apoptosis induction in human rhabdomyosarcoma cell lines. PLOS ONE 2017, 12, e0178857. [Google Scholar] [CrossRef] [PubMed]
- Mnatsakanyan, H.; Salmeron-Sanchez, M.; Rico, P. Lithium Directs Embryonic Stem Cell Differentiation Into Hemangioblast-Like Cells. Adv. Biol. 2021, 5, 2000569. [Google Scholar] [CrossRef] [PubMed]
- Pansters, N.A.; Schols, A.M.; Verhees, K.J.; de Theije, C.C.; Snepvangers, F.J.; Kelders, M.C.; Ubags, N.D.; Haegens, A.; Langen, R.C. Muscle-specific GSK-3β ablation accelerates regeneration of disuse-atrophied skeletal muscle. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2015, 1852, 490–506. [Google Scholar] [CrossRef] [PubMed]
- Theeuwes, W.; Gosker, H.; Langen, R.; Pansters, N.; Schols, A.; Remels, A. Inactivation of glycogen synthase kinase 3β (GSK-3β) enhances mitochondrial biogenesis during myogenesis. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2018, 1864, 2913–2926. [Google Scholar] [CrossRef]
- Kurgan, N.; Whitley, K.C.; Maddalena, L.A.; Moradi, F.; Stoikos, J.; Hamstra, S.I.; Rubie, E.A.; Kumar, M.; Roy, B.D.; Woodgett, J.R.; et al. A Low-Therapeutic Dose of Lithium Inhibits GSK3 and Enhances Myoblast Fusion in C2C12 Cells. Cells 2019, 8, 1340. [Google Scholar] [CrossRef]
- Girardi, F.; Le Grand, F. Wnt Signaling in Skeletal Muscle Development and Regeneration. Prog Mol Biol Transl Sci. 2018, 153, 157–179. [Google Scholar] [CrossRef]
- De-Paula, V.J.; dos Santos, C.C.C.; Luque, M.C.A.; Ali, T.M.; Kalil, J.E.; Forlenza, O.V.; Cunha-Neto, E. Acute and chronic lithium treatment increases Wnt/β-catenin transcripts in cortical and hippocampal tissue at therapeutic concentrations in mice. Metab. Brain Dis. 2020, 36, 193–197. [Google Scholar] [CrossRef]
- Ramadan, F.; Fahs, A.; Ghayad, S.E.; Saab, R. Signaling pathways in Rhabdomyosarcoma invasion and metastasis. Cancer Metastasis Rev. 2020, 39, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, E. Wnt Signaling in Rhabdomyosarcoma - A Potential Targeted Therapy Option. Curr. Drug Targets 2016, 17, 1245–1251. [Google Scholar] [CrossRef]
- Annavarapu, S.R.; Cialfi, S.; Dominici, C.; Kokai, G.K.; Uccini, S.; Ceccarelli, S.; McDowell, H.P.; Helliwell, T.R. Characterization of Wnt/β-catenin signaling in rhabdomyosarcoma. Lab. Investig. 2013, 93, 1090–1099. [Google Scholar] [CrossRef]
- Halatsch, M.-E.; Kast, R.E.; Karpel-Massler, G.; Mayer, B.; Zolk, O.; Schmitz, B.; Scheuerle, A.; Maier, L.; Bullinger, L.; Mayer-Steinacker, R.; et al. A phase Ib/IIa trial of 9 repurposed drugs combined with temozolomide for the treatment of recurrent glioblastoma: CUSP9v3. Neuro-Oncol. Adv. 2021, 3, vdab075. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-L.; Fang, Z.-X.; Wu, Z.; Hou, Y.-Y.; Wu, H.-T.; Liu, J. Repurposed itraconazole for use in the treatment of malignancies as a promising therapeutic strategy. Biomed. Pharmacother. 2022, 154, 113616. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, W.; Wang, J.Q.; Tang, Z. “Hedgehog pathway”: a potential target of itraconazole in the treatment of cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Bury, D.; Tissing, W.J.E.; Muilwijk, E.W.; Wolfs, T.F.W.; Brüggemann, R.J. Clinical Pharmacokinetics of Triazoles in Pediatric Patients. Clin. Pharmacokinet. 2021, 60, 1103–1147. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.T.H.; Zhao, Z.; Ingham, P.W. Hedgehog signalling. Development 2016, 143, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, B.; Li, X.; Zhao, X.; Huang, T.; Ding, X. The emerging roles of Hedgehog signaling in tumor immune microenvironment. Front. Oncol. 2023, 13, 1171418. [Google Scholar] [CrossRef]
- Zhang, Y.; Beachy, P.A. Cellular and molecular mechanisms of Hedgehog signalling. Nat. Rev. Mol. Cell Biol. 2023, 24, 668–687. [Google Scholar] [CrossRef]
- Ban, L.; Mei, T.; Su, Q.; Li, W.; Huang, Z.; Liu, L.; Wu, Y.; Lv, S.; Wang, A.; Li, S. Anti-fungal drug itraconazole exerts anti-cancer effects in oral squamous cell carcinoma via suppressing Hedgehog pathway. Life Sci. 2020, 254, 117695. [Google Scholar] [CrossRef]
- Deng, H.; Huang, L.; Liao, Z.; Liu, M.; Li, Q.; Xu, R. Itraconazole inhibits the Hedgehog signaling pathway thereby inducing autophagy-mediated apoptosis of colon cancer cells. Cell Death Dis. 2020, 11, 539. [Google Scholar] [CrossRef]
- Gerber, D.E.; Putnam, W.C.; Fattah, F.J.; Kernstine, K.H.; Brekken, R.A.; Pedrosa, I.; Skelton, R.; Saltarski, J.M.; Lenkinski, R.E.; Leff, R.D.; et al. Concentration-dependent Early Antivascular and Antitumor Effects of Itraconazole in Non–Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 6017–6027. [Google Scholar] [CrossRef]
- Freitas, R.D.; Dias, R.B.; Vidal, M.T.A.; Valverde, L.d.F.; Costa, R.G.A.; Damasceno, A.K.A.; Sales, C.B.S.; Rocha, L.d.O.S.d.; dos Reis, M.G.; Soares, M.B.P.; et al. Inhibition of CAL27 Oral Squamous Carcinoma Cell by Targeting Hedgehog Pathway With Vismodegib or Itraconazole. Front. Oncol. 2020, 10, 563838. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Xu, M.; Yang, J.; Ma, X. The role of Hedgehog and Notch signaling pathway in cancer. Mol. Biomed. 2022, 3, 44. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.M.; Paganelli, F.; Truocchio, S.; Palumbo, C.; Chiarini, F.; McCubrey, J.A. Understanding the Roles of the Hedgehog Signaling Pathway during T-Cell Lymphopoiesis and in T-Cell Acute Lymphoblastic Leukemia (T-ALL). Int. J. Mol. Sci. 2023, 24, 2962. [Google Scholar] [CrossRef] [PubMed]
- Suchors, C.; Kim, J. Canonical Hedgehog Pathway and Noncanonical GLI Transcription Factor Activation in Cancer. Cells 2022, 11, 2523. [Google Scholar] [CrossRef]
- Tesanovic, S.; Krenn, P.W.; Aberger, F. Hedgehog/GLI signaling in hematopoietic development and acute myeloid leukemia—From bench to bedside. Front. Cell Dev. Biol. 2022, 10, 944760. [Google Scholar] [CrossRef] [PubMed]
- Meister, M.T.; Boedicker, C.; Linder, B.; Kögel, D.; Klingebiel, T.; Fulda, S. Concomitant targeting of Hedgehog signaling and MCL-1 synergistically induces cell death in Hedgehog-driven cancer cells. Cancer Lett. 2019, 465, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lai, Q.; Wang, D.; Pei, J.; Tian, B.; Gao, Y.; Gao, Z.; Xu, X. Hedgehog signaling regulates the development and treatment of glioblastoma (Review). Oncol. Lett. 2022, 24, 294. [Google Scholar] [CrossRef]
- Yoon, J.W.; Lamm, M.; Chandler, C.; Iannaccone, P.; Walterhouse, D. Up-regulation of GLI1 in vincristine-resistant rhabdomyosarcoma and Ewing sarcoma. BMC Cancer 2020, 20, 511. [Google Scholar] [CrossRef]
- Zarzosa, P.; Garcia-Gilabert, L.; Hladun, R.; Guillén, G.; Gallo-Oller, G.; Pons, G.; Sansa-Girona, J.; Segura, M.F.; de Toledo, J.S.; Moreno, L.; et al. Targeting the Hedgehog Pathway in Rhabdomyosarcoma. Cancers 2023, 15, 727. [Google Scholar] [CrossRef]
- Manzella, G.; Schäfer, B.W. Interfering with Hedgehog Pathway: New Avenues for Targeted Therapy in Rhabdomyosarcoma. Curr. Drug Targets 2016, 17, 1228–1234. [Google Scholar] [CrossRef]
- Chelsky, Z.L.; Paulson, V.A.; Chen, E.Y. Molecular analysis of 10 pleomorphic rhabdomyosarcomas reveals potential prognostic markers and druggable targets. Genes, Chromosom. Cancer 2021, 61, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Drummond, C.J.; Hanna, J.A.; Garcia, M.R.; Devine, D.J.; Heyrana, A.J.; Finkelstein, D.; Rehg, J.E.; Hatley, M.E. Hedgehog Pathway Drives Fusion-Negative Rhabdomyosarcoma Initiated From Non-myogenic Endothelial Progenitors. Cancer Cell 2018, 33, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Almazán-Moga, A.; Zarzosa, P.; Molist, C.; Velasco, P.; Pyczek, J.; Simon-Keller, K.; Giralt, I.; Vidal, I.; Navarro, N.; Segura, M.F.; et al. Ligand-dependent Hedgehog pathway activation in Rhabdomyosarcoma: the oncogenic role of the ligands. Br. J. Cancer 2017, 117, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Lin, W.; Li, C.; Ueki, H.; Xue, R.; Sadahira, T.; Hu, H.; Wada, K.; Li, N.; Liu, C.; et al. Repurposing of posaconazole as a hedgehog/SMO signaling inhibitor for embryonal rhabdomyosarcoma therapy. 2021, 11, 4528–4540.
- Urla, C.; Stagno, M.J.; Fuchs, J.; Warmann, S.W.; Schmid, E. Anticancer bioactivity of zerumbone on pediatric rhabdomyosarcoma cells. J. Cancer Res. Clin. Oncol. 2022, 149, 3313–3323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, K.; Wang, C.; Ma, C.; Gong, L.; Zhou, H.; Xue, X.; Peng, C.; Li, Y. Protective effects of dietary quercetin on cerebral ischemic injury: pharmacology, pharmacokinetics and bioavailability-enhancing nanoformulations. Food Funct. 2023, 14, 4470–4489. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, N.; Yousefi, Z.; Golabi, M.; Khalilian, P.; Ghezelbash, B.; Montazeri, M.; Shams, M.H.; Baghbadorani, P.Z.; Eskandari, N. The potential anti-cancer effects of quercetin on blood, prostate and lung cancers: An update. Front. Immunol. 2023, 14, 1077531. [Google Scholar] [CrossRef]
- Shabir, I.; Pandey, V.K.; Shams, R.; Dar, A.H.; Dash, K.K.; Khan, S.A.; Bashir, I.; Jeevarathinam, G.; Rusu, A.V.; Esatbeyoglu, T.; et al. Promising bioactive properties of quercetin for potential food applications and health benefits: A review. Front. Nutr. 2022, 9, 999752. [Google Scholar] [CrossRef]
- Sethi, G.; Rath, P.; Chauhan, A.; Ranjan, A.; Choudhary, R.; Ramniwas, S.; Sak, K.; Aggarwal, D.; Rani, I.; Tuli, H.S. Apoptotic Mechanisms of Quercetin in Liver Cancer: Recent Trends and Advancements. Pharmaceutics 2023, 15, 712. [Google Scholar] [CrossRef]
- Maugeri, A.; Calderaro, A.; Patanè, G.T.; Navarra, M.; Barreca, D.; Cirmi, S.; Felice, M.R. Targets Involved in the Anti-Cancer Activity of Quercetin in Breast, Colorectal and Liver Neoplasms. Int. J. Mol. Sci. 2023, 24, 2952. [Google Scholar] [CrossRef]
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosisio, S.; Allegrini, P. Improved Oral Absorption of Quercetin from Quercetin Phytosome®, a New Delivery System Based on Food Grade Lecithin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 169–177. [Google Scholar] [CrossRef]
- Solnier, J.; Chang, C.; Roh, K.; Du, M.; Kuo, Y.C.; Hardy, M.; Lyon, M.; Gahler, R. Quercetin LipoMicel—A Novel Delivery System to Enhance Bioavailability of Quercetin. J Nat Health Prod Res. 2021, 3, 1–8. [Google Scholar] [CrossRef]
- Xiang, X.; Langlois, S.; St-Pierre, M.-E.; Barré, J.F.; Grynspan, D.; Purgina, B.; Cowan, K.N. Pannexin 1 inhibits rhabdomyosarcoma progression through a mechanism independent of its canonical channel function. Oncogenesis 2018, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Hoang, H.-D.; Gilchrist, V.H.; Langlois, S.; Alain, T.; Cowan, K.N. Quercetin induces pannexin 1 expression via an alternative transcript with a translationally active 5′ leader in rhabdomyosarcoma. Oncogenesis 2022, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, K.; Kusienicka, A.; Szewczyk, B.; Adamus, T.; Lukasiewicz, E.; Miekus, K.; Majka, M. Constitutive activation of MET signaling impairs myogenic differentiation of rhabdomyosarcoma and promotes its development and progression. Oncotarget 2015, 6, 31378–31398. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, Y.; Meng, L.; Liu, Y.; Pang, Y.; Cui, W.; Zhang, L.; Li, Z.; Liu, Q.; Shang, H.; et al. c-MET expression potentially contributes to the poor prognosis of rhabdomyosarcoma. Int J Clin Exp Pathol. 2018, 11, 4083–4092. [Google Scholar]
- Taulli, R.; Scuoppo, C.; Bersani, F.; Accornero, P.; Forni, P.E.; Miretti, S.; Grinza, A.; Allegra, P.; Schmitt-Ney, M.; Crepaldi, T.; et al. Validation of Met as a Therapeutic Target in Alveolar and Embryonal Rhabdomyosarcoma. Cancer Res 2006, 66, 4742–4749. [Google Scholar] [CrossRef] [PubMed]
- Rees, H.; Williamson, D.; Papanastasiou, A.; Jina, N.; Nabarro, S.; Shipley, J.; Anderson, J. The MET receptor tyrosine kinase contributes to invasive tumour growth in rhabdomyosarcomas. Growth Factors 2006, 24, 197–208. [Google Scholar] [CrossRef]
- Perrone, C.; Pomella, S.; Cassandri, M.; Pezzella, M.; Milano, G.M.; Colletti, M.; Cossetti, C.; Pericoli, G.; Di Giannatale, A.; de Billy, E.; et al. MET Inhibition Sensitizes Rhabdomyosarcoma Cells to NOTCH Signaling Suppression. Front. Oncol. 2022, 12, 835642. [Google Scholar] [CrossRef] [PubMed]
- Baby, B.; Antony, P.; Vijayan, R. Interactions of quercetin with receptor tyrosine kinases associated with human lung carcinoma. Nat. Prod. Res. 2017, 32, 2928–2931. [Google Scholar] [CrossRef]
- Cao, H.-H.; Cheng, C.-Y.; Su, T.; Fu, X.-Q.; Guo, H.; Li, T.; Tse, A.K.-W.; Kwan, H.-Y.; Yu, H.; Yu, Z.-L. Quercetin inhibits HGF/c-Met signaling and HGF-stimulated melanoma cell migration and invasion. Mol. Cancer 2015, 14, 103. [Google Scholar] [CrossRef]
- Labbé, D.; Provençal, M.; Lamy, S.; Boivin, D.; Gingras, D.; Béliveau, R. The Flavonols Quercetin, Kaempferol, and Myricetin Inhibit Hepatocyte Growth Factor-Induced Medulloblastoma Cell Migration. J. Nutr. 2009, 139, 646–652. [Google Scholar] [CrossRef]
- Shu, Y.; Xie, B.; Liang, Z.; Chen, J. Quercetin reverses the doxorubicin resistance of prostate cancer cells by downregulating the expression of c-met. Oncol. Lett. 2017, 15, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Jung, N.; Lee, S.; Sohng, J.K.; Jung, H.J. Apigenin Inhibits Cancer Stem Cell-Like Phenotypes in Human Glioblastoma Cells via Suppression of c-Met Signaling. Phytotherapy Res. 2016, 30, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- AbouAitah, K.; Swiderska-Sroda, A.; Farghali, A.A.; Wojnarowicz, J.; Stefanek, A.; Gierlotka, S.; Opalinska, A.; Allayeh, A.K.; Ciach, T.; Lojkowski, W. Folic acid-conjugated mesoporous silica particles as nanocarriers of natural prodrugs for cancer targeting and antioxidant action. Oncotarget 2018, 9, 26466–26490. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.E.; Sheehan, S.M.; Taylor, R.G.; Kendall, T.L.; Rice, G.M. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J. Cell. Physiol. 1995, 165, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Otabe, O.; Kikuchi, K.; Tsuchiya, K.; Katsumi, Y.; Yagyu, S.; Miyachi, M.; Iehara, T.; Hosoi, H. MET/ERK2 pathway regulates the motility of human alveolar rhabdomyosarcoma cells. Oncol. Rep. 2016, 37, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, K.; Kucia, M.; Wysoczynski, M.; Reca, R.; Zhao, D.; Trzyna, E.; Trent, J.; Peiper, S.; Zembala, M.; Ratajczak, J.; et al. Both hepatocyte growth factor (HGF) and stromal-derived factor-1 regulate the metastatic behavior of human rhabdomyosarcoma cells, but only HGF enhances their resistance to radiochemotherapy. Cancer Res. 2003, 63, 7926–7935. [Google Scholar] [PubMed]
- Miekus, K.; Lukasiewicz, E.; Jarocha, D.; Sekula, M.; Drabik, G.; Majka, M. The decreased metastatic potential of rhabdomyosarcoma cells obtained through MET receptor downregulation and the induction of differentiation. Cell Death Dis. 2013, 4, e459. [Google Scholar] [CrossRef]
- Chen, Y.; Takita, J.; Mizuguchi, M.; Tanaka, K.; Ida, K.; Koh, K.; Igarashi, T.; Hanada, R.; Tanaka, Y.; Park, M.-J.; et al. Mutation and expression analyses of theMET andCDKN2A genes in rhabdomyosarcoma with emphasis onMET overexpression. Genes, Chromosom. Cancer 2007, 46, 348–358. [Google Scholar] [CrossRef]
- Diomedi-Camassei, F.; McDowell, H.P.; De Ioris, M.A.; Uccini, S.; Altavista, P.; Raschellà, G.; Vitali, R.; Mannarino, O.; De Sio, L.; Cozzi, D.A.; et al. Clinical Significance of CXC Chemokine Receptor-4 and c-Met in Childhood Rhabdomyosarcoma. Clin. Cancer Res. 2008, 14, 4119–4127. [Google Scholar] [CrossRef]
- Yan, D.; Da Dong, X.; Chen, X.; Wang, L.; Lu, C.; Wang, J.; Qu, J.; Tu, L. MicroRNA-1/206 Targets c-Met and Inhibits Rhabdomyosarcoma Development. J. Biol. Chem. 2009, 284, 29596–29604. [Google Scholar] [CrossRef] [PubMed]
- Lukasiewicz, E.; Miekus, K.; Kijowski, J.; Drabik, G.; Wilusz, M.; Bobis-Wozowicz, S.; Majka, M. Inhibition of rhabdomyosarcoma’s metastatic behavior through downregulation of MET receptor signaling. Folia Histochem. et Cytobiol. 2010, 47, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Saini, M.; Verma, A.; Mathew, S.J. SPRY2 is a novel MET interactor that regulates metastatic potential and differentiation in rhabdomyosarcoma. Cell Death Dis. 2018, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, J.P.; Davis, R.J.; Bennicelli, J.L.; Nauta, L.E.; Barr, F.G. Up-regulation of MET but not neural cell adhesion molecule expression by the PAX3-FKHR fusion protein in alveolar rhabdomyosarcoma. Cancer Res. 1998, 58, 3542–3546. [Google Scholar]
- Ferracini, R.; Olivero, M.; DI Renzo, M.F.; Martano, M.; De Giovanni, C.; Nanni, P.; Basso, G.; Scotlandi, K.; Lollini, P.L.; Comoglio, P. Retrogenic expression of the MET proto-oncogene correlates with the invasive phenotype of human rhabdomyosarcomas. Oncogene 1996, 12, 1697–1705. [Google Scholar] [PubMed]
- Fu, R.; Jiang, S.; Li, J.; Chen, H.; Zhang, X. Activation of the HGF/c-MET axis promotes lenvatinib resistance in hepatocellular carcinoma cells with high c-MET expression. Med Oncol. 2020, 37, 24. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Wang, P. Lenvatinib in Management of Solid Tumors. Oncologist 2020, 25, e30. [Google Scholar] [CrossRef]
- Wagner, J.; Abdel-Rahman, S.M. Jonathan Wagner, DO1,2,3 and Susan M. Abdel-Rahman, PharmD2,3 1Ward Family Heart Center and 2Division of Clinical Pharmacology, Toxicology and Therapeutic Innovation, Children’s Mercy Hospital, Kansas City, Missouri 3Department of Pediatrics, University o; Do; D, P. Pediatric Statin Administration: Navigating a Frontier with Limited Data. J. Pediatr. Pharmacol. Ther. 2016, 21, 380–403. [Google Scholar] [CrossRef]
- Voorberg, A.N.; Kamphuis, E.; Christoffers, W.A.; Romeijn, G.L.E.; Oosterhaven, J.A.F.; Schuttelaar, M.L.A. Efficacy and safety of oral alitretinoin versus oral azathioprine in patients with severe chronic hand eczema: Results from a prematurely discontinued randomized controlled trial. Contact Dermat. 2022, 87, 366–368. [Google Scholar] [CrossRef]
- Findling, R.L.; McNamara, N.K.; Pavuluri, M.; Frazier, J.A.; Rynn, M.; Scheffer, R.; Kafantaris, V.; Robb, A.; DelBello, M.; Kowatch, R.A.; et al. Lithium for the Maintenance Treatment of Bipolar I Disorder: A Double-Blind, Placebo-Controlled Discontinuation Study. J. Am. Acad. Child Adolesc. Psychiatry 2018, 58, 287–296. [Google Scholar] [CrossRef]
- Piérard, G.; Arrese, J.; Piérard-Franchimont, C. Itraconazole. Expert Opin. Pharmacother. 2000, 1, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Lestner, J.; Hope, W.W. Itraconazole: an update on pharmacology and clinical use for treatment of invasive and allergic fungal infections. Expert Opin. Drug Metab. Toxicol. 2013, 9, 911–926. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E.; Alfieri, A.; Assi, H.I.; Burns, T.C.; Elyamany, A.M.; Gonzalez-Cao, M.; Karpel-Massler, G.; Marosi, C.; Salacz, M.E.; Sardi, I.; et al. MDACT: A New Principle of Adjunctive Cancer Treatment Using Combinations of Multiple Repurposed Drugs, with an Example Regimen. Cancers 2022, 14, 2563. [Google Scholar] [CrossRef] [PubMed]
- Halatsch, M.-E.; Dwucet, A.; Schmidt, C.J.; Mühlnickel, J.; Heiland, T.; Zeiler, K.; Siegelin, M.D.; Kast, R.E.; Karpel-Massler, G. In Vitro and Clinical Compassionate Use Experiences with the Drug-Repurposing Approach CUSP9v3 in Glioblastoma. Pharmaceuticals 2021, 14, 1241. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.C.; Chidley, C.; Sorger, P.K. A curative combination cancer therapy achieves high fractional cell killing through low cross-resistance and drug additivity. eLife 2019, 8, e50036. [Google Scholar] [CrossRef] [PubMed]
- Nogales, C.; Mamdouh, Z.M.; List, M.; Kiel, C.; Casas, A.I.; Schmidt, H.H. Network pharmacology: curing causal mechanisms instead of treating symptoms. Trends Pharmacol. Sci. 2021, 43, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Kilmister, E.J.; Koh, S.P.; Weth, F.R.; Gray, C.; Tan, S.T. Cancer Metastasis and Treatment Resistance: Mechanistic Insights and Therapeutic Targeting of Cancer Stem Cells and the Tumor Microenvironment. Biomedicines 2022, 10, 2988. [Google Scholar] [CrossRef]
- Lindsey, B.A.; Markel, J.E.; Kleinerman, E.S. Osteosarcoma Overview. Rheumatol. Ther. 2016, 4, 25–43. [Google Scholar] [CrossRef]
- Cosio, T.; Di Prete, M.; Campione, E. Arsenic Trioxide, Itraconazole, All-Trans Retinoic Acid and Nicotinamide: A Proof of Concept for Combined Treatments with Hedgehog Inhibitors in Advanced Basal Cell Carcinoma. Biomedicines 2020, 8, 156. [Google Scholar] [CrossRef]
- Chow, C.K.; Atkins, E.R.; Hillis, G.S.; Nelson, M.R.; Reid, C.M.; Schlaich, M.P.; Hay, P.; Rogers, K.; Billot, L.; Burke, M.; et al. Initial treatment with a single pill containing quadruple combination of quarter doses of blood pressure medicines versus standard dose monotherapy in patients with hypertension (QUARTET): a phase 3, randomised, double-blind, active-controlled trial. Lancet 2021, 398, 1043–1052. [Google Scholar] [CrossRef]
- Hu, L.; Wang, D.; Liu, H.; Zhang, Q.; Sun, D.; Zhang, L.; Chen, X.; Chang, G.; Wang, J. A double-blind, placebo-controlled trial on the antihypertensive treatment effect of a quadruple single-pill combination. J. Clin. Hypertens. 2021, 23, 815–822. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





