Submitted:
27 September 2023
Posted:
28 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plasmids and AAVs
2.2. Animals and intravitreal injections (IVI)
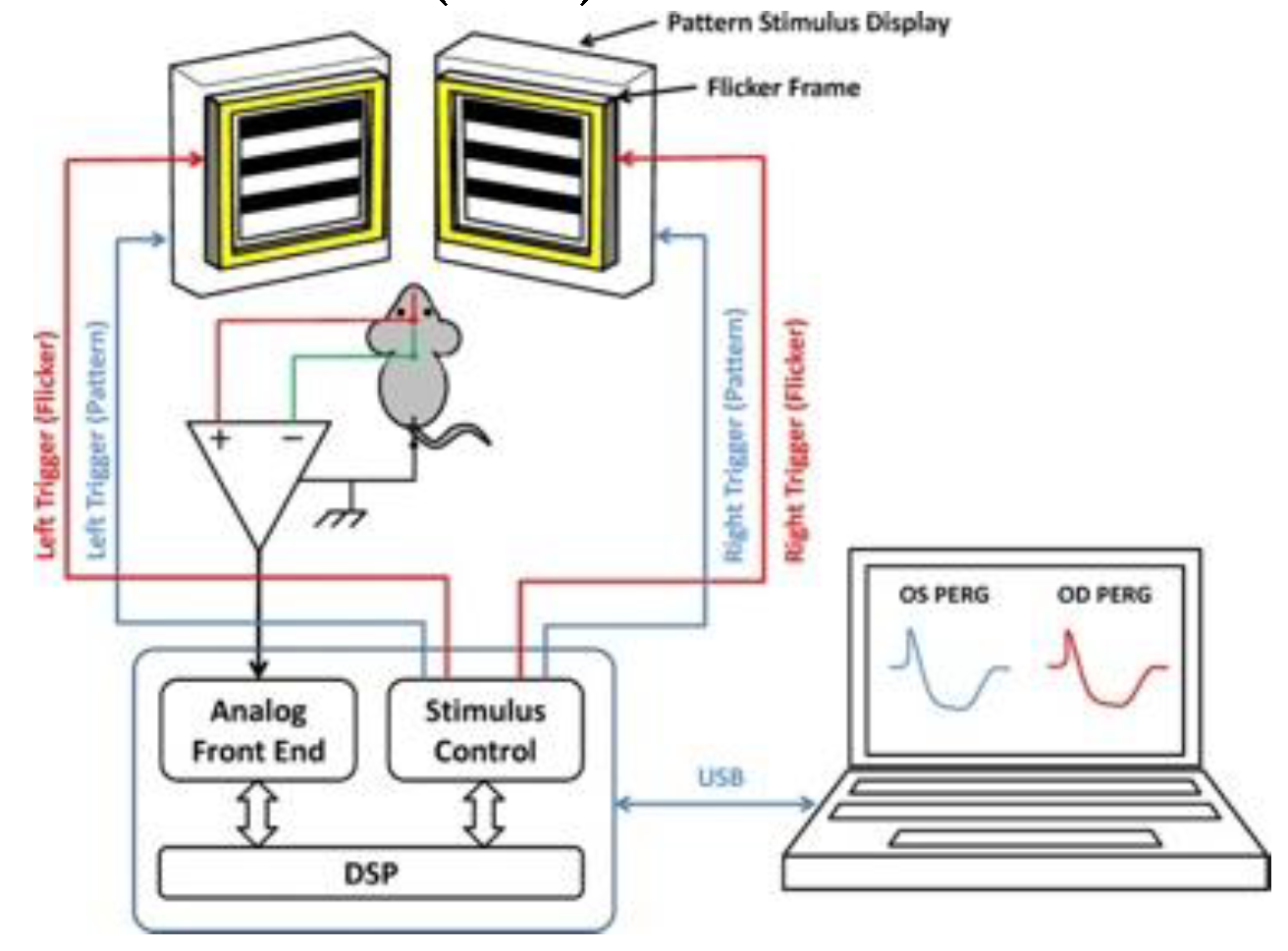
2.3. PERG, FERG, and SD-OCT
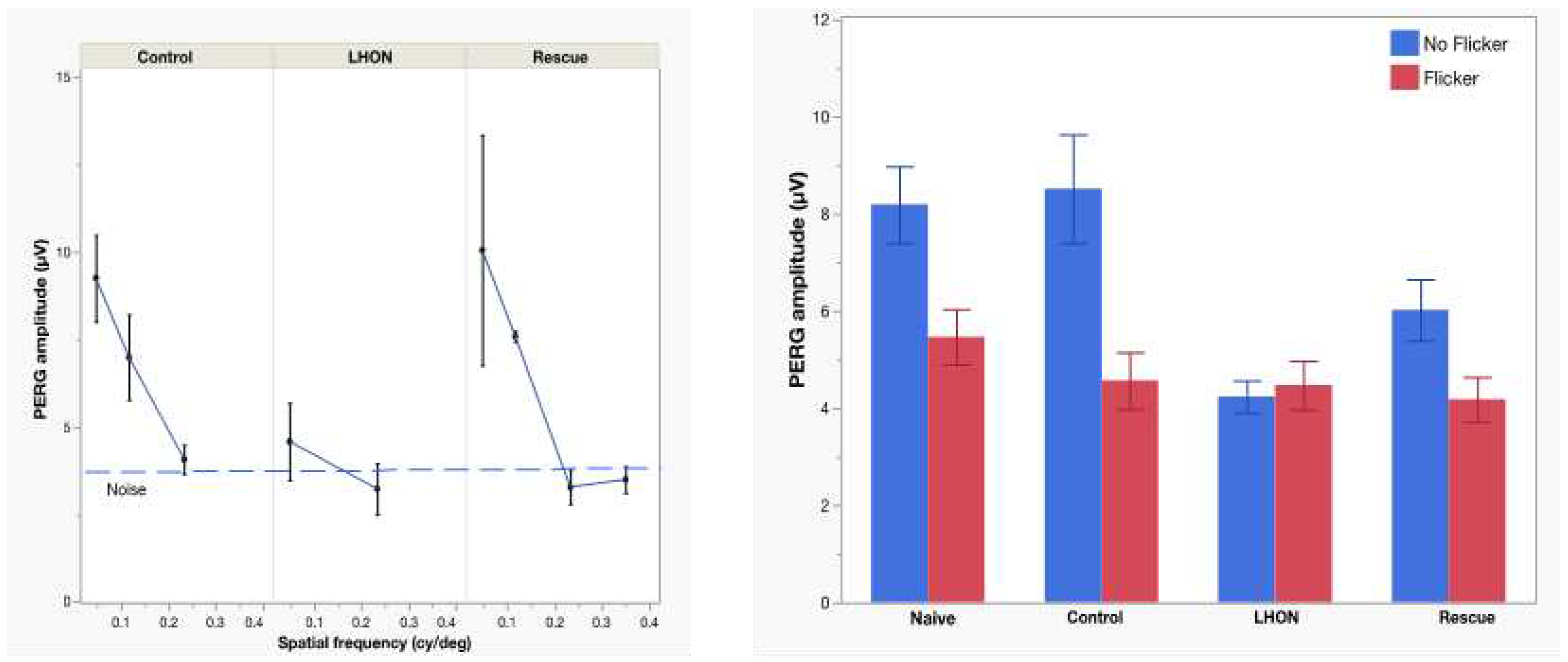
2.4. PERG-based Visual acuity
2.5. Flicker-induced PERG adaptation
2.6. Immunostaining
2.7. Statistical Analysis
3. Results
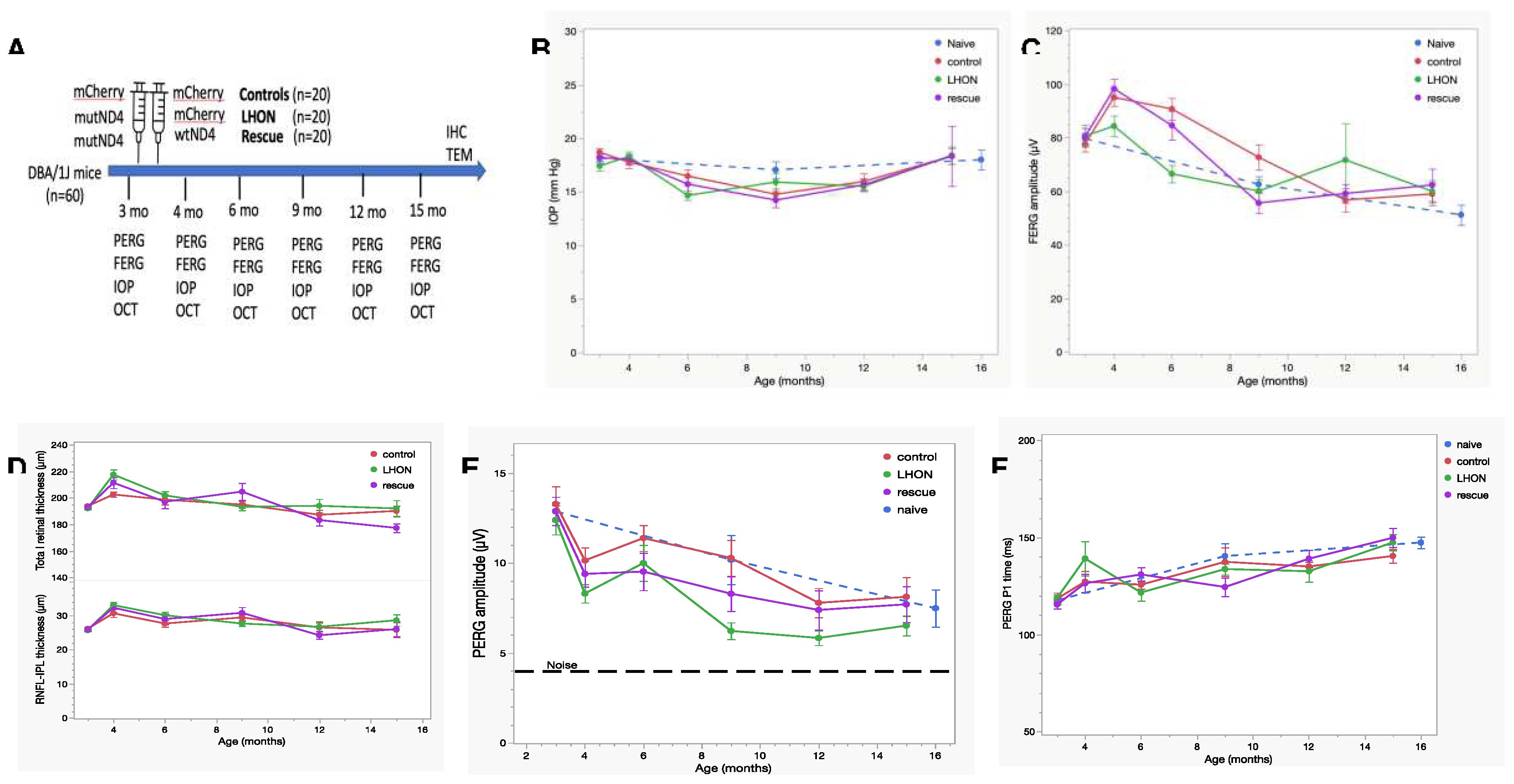
3.1. Wild-type hND4 rescues RGC dysfunction induced by mutant hND4
3.2. Wild-type hND4 rescues loss of visual acuity induced by mutant hND4
3.3. Wild-type hND4 rescues loss of RGC metabolic autoregulation induced by mutant hND4.
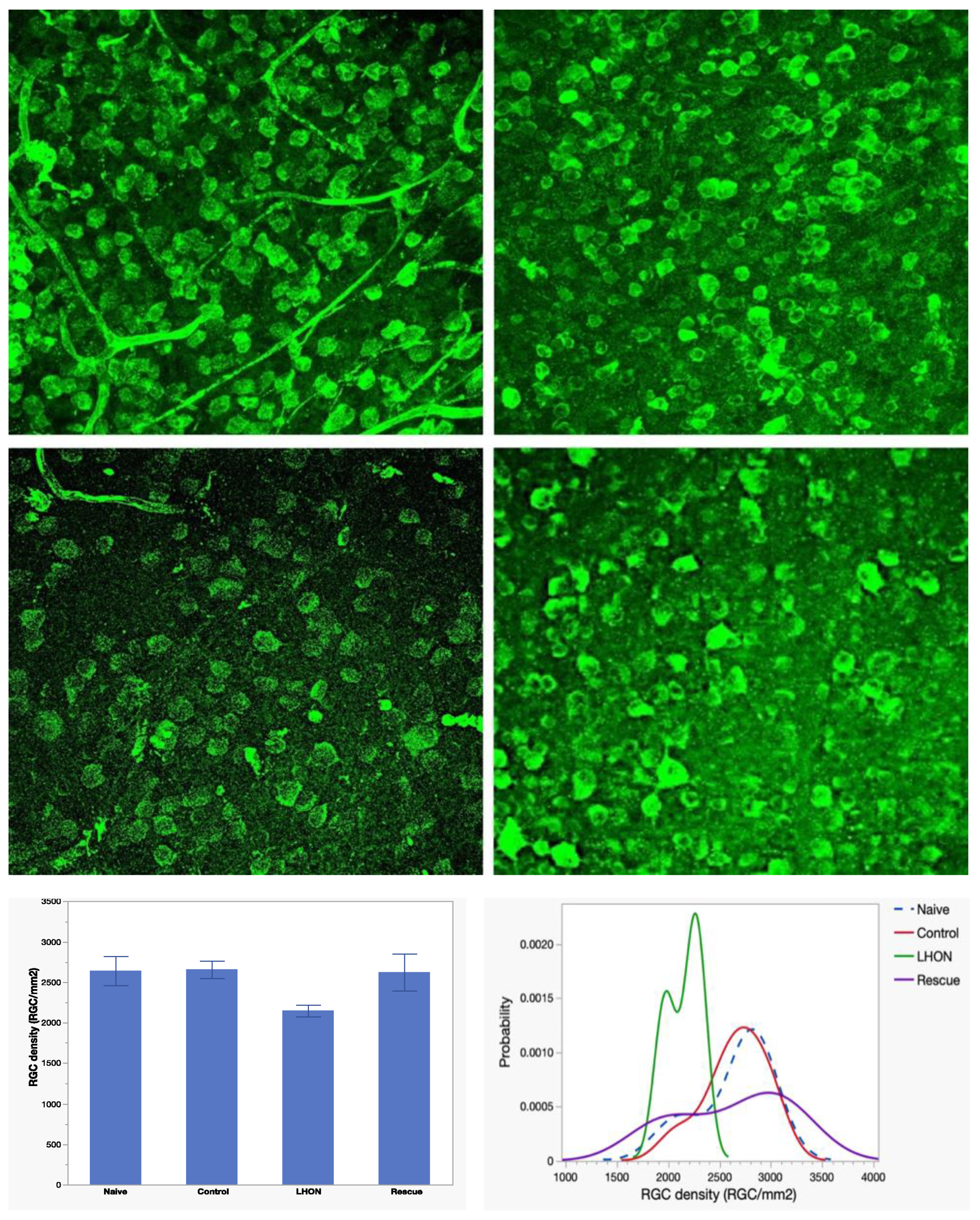
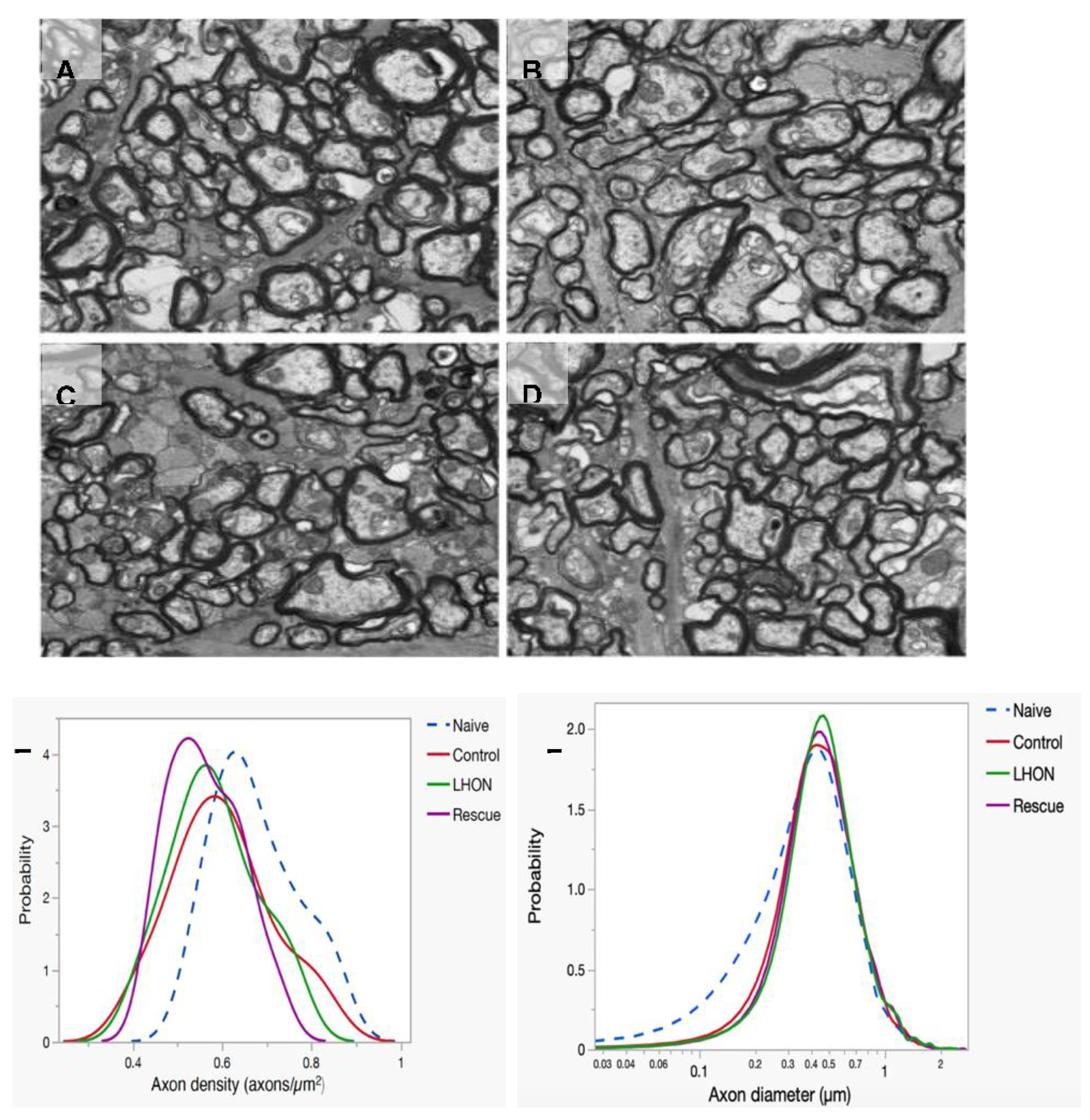
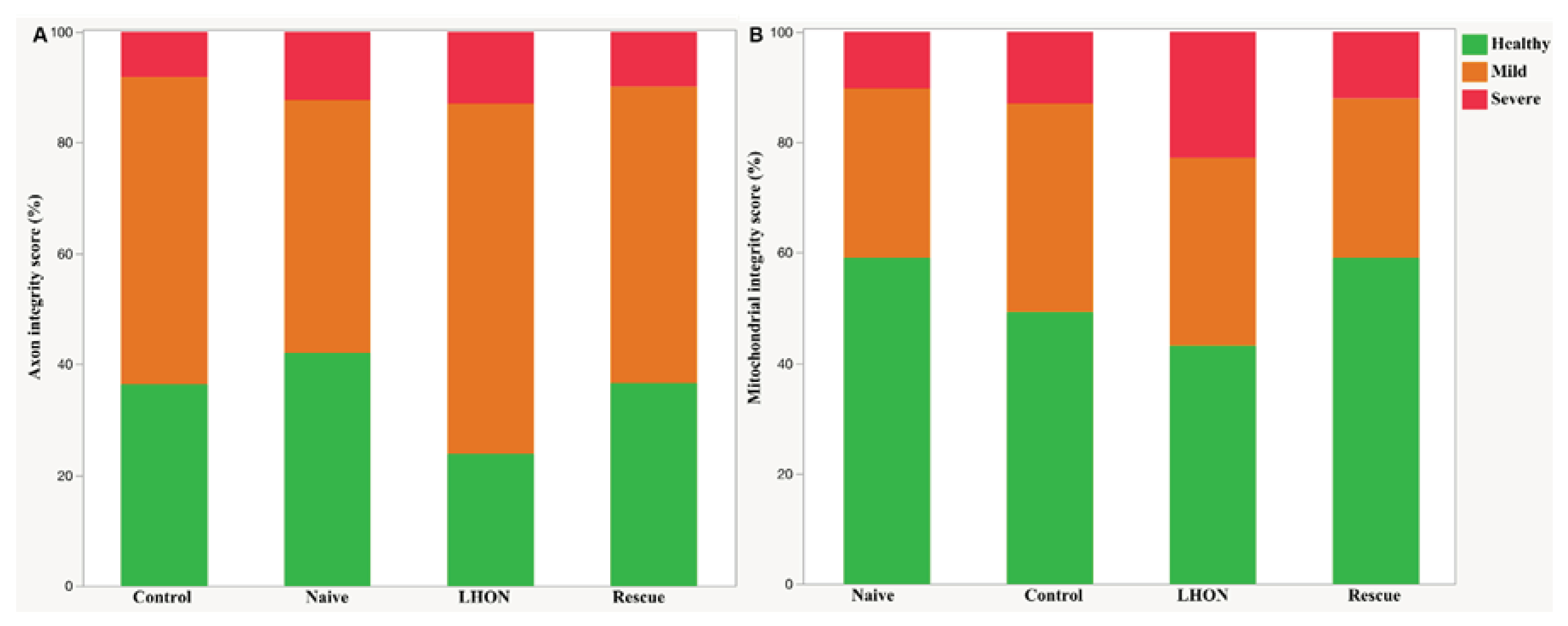
3.4. Wild-type hND4 rescues loss of RGCs induced by mutant hND4.
3.5. Wild-type hND4 rescues optic atrophy induced by mutant hND4
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kauppila, J.H.K.; Baines, H.L.; Bratic, A.; Simard, M.L.; Freyer, C.; Mourier, A.; Stamp, C.; Filograna, R.; Larsson, N.G.; Greaves, L.C.; et al. A Phenotype-Driven Approach to Generate Mouse Models with Pathogenic mtDNA Mutations Causing Mitochondrial Disease. Cell Rep. 2016, 16, 2980–2990. [Google Scholar] [CrossRef] [PubMed]
- Jurkute, N.; Yu-Wai-Man, P. Leber hereditary optic neuropathy: bridging the translational gap. Curr Opin Ophthalmol. 2017, 28, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Yu-Wai-Man, P.; Griffiths, P.G.; Hudson, G.; Chinnery, P.F. Inherited mitochondrial optic neuropathies. J Med Genet. 2009, 46, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Bacman, S.R.; Williams, S.L.; Pinto, M.; Peralta, S.; Moraes, C.T. Specific elimination of mutant mitochondrial genomes in patient-derived cells by mitoTALENs. Nat Med. 2013, 19, 1111–1113. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Koilkonda, R.D.; Chou, T.H.; Porciatti, V.; Ozdemir, S.S.; Chiodo, V.; Boye, S.L.; Boye, S.E.; Hauswirth, W.W.; Lewin, A.S.; et al. Gene delivery to mitochondria by targeting modified adenoassociated virus suppresses Leber's hereditary optic neuropathy in a mouse model. Proc Natl Acad Sci U S A. 2012, 109, E1238–E1247. [Google Scholar] [CrossRef] [PubMed]
- Chadderton, N.; Palfi, A.; Millington-Ward, S.; Gobbo, O.; Overlack, N.; Carrigan, M.; O'Reilly, M.; Campbell, M.; Ehrhardt, C.; Wolfrum, U.; et al. Intravitreal delivery of AAV-NDI1 provides functional benefit in a murine model of Leber hereditary optic neuropathy. Eur J Hum Genet. 2013, 21, 62–68. [Google Scholar] [CrossRef]
- Liu, H.L.; Yuan, J.J.; Zhang, Y.; Tian, Z.; Li, X.; Wang, D.; Du, Y.Y.; Song, L.; Li, B. Factors associated with rapid improvement in visual acuity in patients with Leber's hereditary optic neuropathy after gene therapy. Acta Ophthalmol. 2020, 98, e730–e733. [Google Scholar] [CrossRef]
- Yu-Wai-Man, P.; Newman, N.J.; Carelli, V.; Moster, M.L.; Biousse, V.; Sadun, A.A.; Klopstock, T.; Vignal-Clermont, C.; Sergott, R.C.; Rudolph, G.; et al. Bilateral visual improvement with unilateral gene therapy injection for Leber hereditary optic neuropathy. Sci Transl Med. 2020, 12. [Google Scholar] [CrossRef]
- Guy, J.; Feuer, W.J.; Davis, J.L.; Porciatti, V.; Gonzalez, P.J.; Koilkonda, R.D.; Yuan, H.; Hauswirth, W.W.; Lam, B.L. Gene Therapy for Leber Hereditary Optic Neuropathy: Low- and Medium-Dose Visual Results. Ophthalmology 2017, 124, 1621–1634. [Google Scholar] [CrossRef]
- Lam, B.L.; Feuer, W.J.; Davis, J.L.; Porciatti, V.; Yu, H.; Levy, R.B.; Vanner, E.; Guy, J. Leber Hereditary Optic Neuropathy Gene Therapy: Adverse Events and Visual Acuity Results of all Patient Groups. Am J Ophthalmol. 2022. [Google Scholar] [CrossRef]
- Kaeppel, C.; Beattie, S.; Fronza, R.; van Logtenstein, R.; Salmon, F.; Schmidt, S.; Wolf, S.; Nowrouzi, A.; Glimm, H.; von Kalle, C.; et al. AAV Integrates Randomly into the Nuclear and Mitochondrial Genome after LPLD Gene Therapy. Molecular Therapy. 2013, 21, S104–S105. [Google Scholar]
- Yu, H.; Koilkonda, R.D.; Chou, T.H.; Porciatti, V.; Ozdemir, S.S.; Chiodo, V.; Boye, S.L.; Boye, S.E.; Hauswirth, W.W.; Lewin, A.S.; et al. Gene delivery to mitochondria by targeting modified adenoassociated virus suppresses Leber's hereditary optic neuropathy in a mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2012, 109, E1238–E1247. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ozdemir, S.S.; Koilkonda, R.D.; Chou, T.H.; Porciatti, V.; Chiodo, V.; Boye, S.L.; Hauswirth, W.W.; Lewin, A.S.; Guy, J. Mutant NADH dehydrogenase subunit 4 gene delivery to mitochondria by targeting sequence-modified adeno-associated virus induces visual loss and optic atrophy in mice. Mol Vis. 2012, 18, 1668–1683. [Google Scholar] [PubMed]
- Yu, H.; Koilkonda, R.D.; Chou, T.H.; Porciatti, V.; Mehta, A.; Hentall, I.D.; Chiodo, V.A.; Boye, S.L.; Hauswirth, W.W.; Lewin, A.S.; et al. Consequences of zygote injection and germline transfer of mutant human mitochondrial DNA in mice. Proc Natl Acad Sci U S A. 2015, 112, E5689–E5698. [Google Scholar] [CrossRef]
- Liu, Y.; Eastwood, J.D.; Alba, D.E.; Velmurugan, S.; Sun, N.; Porciatti, V.; Lee, R.K.; Hauswirth, W.W.; Guy, J.; Yu, H. Gene therapy restores mitochondrial function and protects retinal ganglion cells in optic neuropathy induced by a mito-targeted mutant ND1 gene. Gene Ther. 2022. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Sant, D.W.; Wang, G.; Guy, J. Mitochondrial Transfer of the Mutant Human ND6T14484C Gene Causes Visual Loss and Optic Neuropathy. Transl Vis Sci Technol. 2020, 9, 1. [Google Scholar] [CrossRef]
- Velmurugan, S.; Chou, T.H.; Eastwood, J.D.; Porciatti, V.; Liu, Y.; Hauswirth, W.W.; Guy, J.; Yu, H. Comparison of different gene-therapy methods to treat Leber hereditary optic neuropathy in a mouse model. Front Neurosci. 2023, 17, 1119724. [Google Scholar] [CrossRef]
- Chou, T.H.; Bohorquez, J.; Toft-Nielsen, J.; Ozdamar, O.; Porciatti, V. Robust Mouse Pattern Electroretinograms Derived Simultaneously From Each Eye Using a Common Snout Electrode. Investigative Ophthalmology & Visual Science 2014, 55, 2469–2475. [Google Scholar]
- Porciatti, V. The mouse pattern electroretinogram. Doc Ophthalmol. 2007, 115, 145–153. [Google Scholar] [CrossRef]
- Yang, X.; Chou, T.H.; Ruggeri, M.; Porciatti, V. A new mouse model of inducible, chronic retinal ganglion cell dysfunction not associated with cell death. Invest Ophthalmol Vis Sci. 2013, 54, 1898–1904. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Garvin, M.K.; Sonka, M. Retinal imaging and image analysis. IEEE Rev Biomed Eng. 2010, 3, 169–208. [Google Scholar] [CrossRef] [PubMed]
- Dysli, C.; Enzmann, V.; Sznitman, R.; Zinkernagel, M.S. Quantitative Analysis of Mouse Retinal Layers Using Automated Segmentation of Spectral Domain Optical Coherence Tomography Images. Translational Vision Science & Technology 2015, 4. [Google Scholar]
- Braha, M.; Porciatti, V.; Chou, T.H. Retinal and cortical visual acuity in a common inbred albino mouse. Plos One 2021, 16. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.H.; Toft-Nielsen, J.; Porciatti, V. Adaptation of retinal ganglion cell function during flickering light in the mouse. Sci Rep. 2019, 9, 18396. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Teo, Y.Y.; Saw, S.M. Application of advanced statistics in ophthalmology. Invest Ophthalmol Vis Sci. 2011, 52, 6059–6065. [Google Scholar] [CrossRef] [PubMed]
- Zeger, S.L.; Liang, K.Y. An overview of methods for the analysis of longitudinal data. Stat Med. 1992, 11, 1825–1839. [Google Scholar] [CrossRef] [PubMed]
- Lietze, A. The role of particulate insoluble substances in food allergy. 3. Heat labile antibody to wheat starch in sera of wheat sensitive patients. Ann Allergy 1969, 27, 9–12. [Google Scholar]
- Sadowsky, C.; Muhl, Z.F.; Sakols, E.I.; Sommerville, J.M. Temporomandibular joint sounds related to orthodontic therapy. J Dent Res. 1985, 64, 1392–1395. [Google Scholar] [CrossRef]
- Shi, C.; Yuan, X.; Chang, K.; Cho, K.S.; Xie, X.S.; Chen, D.F.; Luo, G. Optimization of Optomotor Response-based Visual Function Assessment in Mice. Sci Rep. 2018, 8, 9708. [Google Scholar] [CrossRef]
- Kretschmer, F.; Sajgo, S.; Kretschmer, V.; Badea, T.C. A system to measure the Optokinetic and Optomotor response in mice. J Neurosci Methods. 2015, 256, 91–105. [Google Scholar] [CrossRef]
- Jeffery, G.; Brem, G.; Montoliu, L. Correction of retinal abnormalities found in albinism by introduction of a functional tyrosinase gene in transgenic mice and rabbits. Brain Res Dev Brain Res. 1997, 99, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Braha, M.; Porciatti, V.; Chou, T.H. Retinal and cortical visual acuity in a common inbred albino mouse. PLoS One 2021, 16, e0242394. [Google Scholar] [CrossRef] [PubMed]
- Porciatti, V.; Pizzorusso, T.; Cenni, M.C.; Maffei, L. The visual response of retinal ganglion cells is not altered by optic nerve transection in transgenic mice overexpressing Bcl-2. Proc Natl Acad Sci U S A 1996, 93, 14955–14959. [Google Scholar] [CrossRef] [PubMed]
- Riva, C.E.; Logean, E.; Falsini, B. Visually evoked hemodynamical response and assessment of neurovascular coupling in the optic nerve and retina. Prog Retin Eye Res. 2005, 24, 183–215. [Google Scholar] [CrossRef] [PubMed]
- Albanna, W.; Kotliar, K.; Luke, J.N.; Alpdogan, S.; Conzen, C.; Lindauer, U.; Clusmann, H.; Hescheler, J.; Vilser, W.; Schneider, T.; et al. Non-invasive evaluation of neurovascular coupling in the murine retina by dynamic retinal vessel analysis. PLoS One. 2018, 13, e0204689. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, P.; Triolo, G.; Toft-Nielsen, J.; Bohorquez, J.; Henderson, A.D.; Delgado, R.; Miskiel, E.; Ozdamar, O.; Feuer, W.J.; Porciatti, V. Next Generation PERG Method: Expanding the Response Dynamic Range and Capturing Response Adaptation. Transl Vis Sci Technol. 2017, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.H.; Romano, G.L.; Amato, R.; Porciatti, V. Nicotinamide-Rich Diet in DBA/2J Mice Preserves Retinal Ganglion Cell Metabolic Function as Assessed by PERG Adaptation to Flicker. Nutrients 2020, 12. [Google Scholar] [CrossRef]
- Cepurna, W.O.; Kayton, R.J.; Johnson, E.C.; Morrison, J.C. Age related optic nerve axonal loss in adult Brown Norway rats. Exp Eye Res. 2005, 80, 877–884. [Google Scholar] [CrossRef]
- Deng, W.; Hedberg-Buenz, A.; Soukup, D.A.; Taghizadeh, S.; Wang, K.; Anderson, M.G.; Garvin, M.K. AxonDeep: Automated Optic Nerve Axon Segmentation in Mice With Deep Learning. Transl Vis Sci Technol. 2021, 10, 22. [Google Scholar] [CrossRef]
- Zhu, Y.; Pappas, A.C.; Wang, R.; Seifert, P.; Sun, D.; Jakobs, T.C. Ultrastructural Morphology of the Optic Nerve Head in Aged and Glaucomatous Mice. Invest Ophthalmol Vis Sci. 2018, 59, 3984–3996. [Google Scholar] [CrossRef]
- Guy, J.; Feuer, W.J.; Porciatti, V.; Schiffman, J.; Abukhalil, F.; Vandenbroucke, R.; Rosa, P.R.; Lam, B.L. Retinal ganglion cell dysfunction in asymptomatic G11778A: Leber hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 2014, 55, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhang, Y.; Liu, H.; Wang, D.; Du, Y.; Tian, Z.; Li, X.; Yang, S.; Pei, H.; Wan, X.; et al. Seven-Year Follow-up of Gene Therapy for Leber's Hereditary Optic Neuropathy. Ophthalmology. 2020, 127, 1125–1127. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.G.; Williams, B.P. Evolutionary Inference across Eukaryotes Identifies Specific Pressures Favoring Mitochondrial Gene Retention. Cell Syst. 2016, 2, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.F. Why chloroplasts and mitochondria retain their own genomes and genetic systems: Colocation for redox regulation of gene expression. Proc Natl Acad Sci U S A 2015, 112, 10231–10238. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.L.; Palmer, J.D. Evolution of mitochondrial gene content: gene loss and transfer to the nucleus. Mol Phylogenet Evol. 2003, 29, 380–395. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




