1. Introduction
Heavy metals are potential environmental pollutants, with the ability to cause serious health problems (disorders, diseases, organ malformations) [
1,
2,
3]. An important source of heavy metal pollution is agriculture through the inputs used (chemical fertilizers, organic fertilizers, pesticides, irrigation water) [
4,
5,
6]. The systematic and long application of phosphorus-based and zinc fertilizers leads to the increase of cadmium accumulations in soils [
7,
8,
9]. Contamination of animals with heavy metals can be done through direct exposure, contaminated water, contaminated grains used in their food and industrial emissions [
10,
11].
Natural and anthropogenic pollution are the two main types with direct impacts on the environment [
12,
13,
14].
Natural pollution is supported by the geological, geomorphological, biological, atmospheric, hydrological and pedological processes. The main pollutants result from the flow of oxygen, carbon, water, nitrogen, phosphorus and sulfur through the geographical envelope (atmosphere, hydrosphere, lithosphere, pedosphere, and biosphere) [
15,
16]. Fluxes have been more closely studied for the biosphere.
Human civilization is the support of anthropogenic pollution, the phenomenon has been gradually accentuated in a close correlation with the development of intensive agriculture, industrialization, the circulation of goods and people and progressive urbanization [
17,
18]. Human activities eliminate a series of pollutants in the environment: gas emissions, dust, smoke and aerosols in the atmosphere [
19,
20,
21]. The anthropogenic pollution is known as pollution caused by conventional fuels, chemicals, noise, electromagnetic fields and waste pollution [
22,
23,
24].
A third source of atmospheric pollution with heavy metals is the biotic environment, through vegetation, the residues’ storage, and manure [
25,
26,
27].
The appearance of toxic effects on plant metabolism is conditioned by atmospheric deposition on the soil, respectively beyond the tolerance limits of plants [
28,
29]. Studying the accumulation of airborne heavy metals in forest vegetation was concluded that it depends on the species and tolerance of the plant, the age of the vegetative organ and the type of metal (heavy metals are absorbed in the order Pb > Zn > Cu) [
30,
31].
The absorption capacity of metals from the air by the leaves is different [
32,
33]. This depends on air humidity (high humidity favors foliar absorption: Zn and Cu are faster foliar absorbed than Pb, which is more adsorbed on the surface of the leaves) on pH (this factor being very important for wet penetrations), by the state of oxidation, etc. [
34,
35,
36].
The degree of toxicity of heavy metals transferred to the plant by air depends on the concentrations (quantity) of the metal in the environment; the form of exposure vector (ingestion, absorption through the roots after the deposition of metals from the atmosphere on the soil); dose distribution/exposure time; the type and severity of the effect [
37].
The mechanisms by which heavy metals manifest their toxic effect are: blocking the functional groups of molecules with an important biological role: enzymes, polynucleotides, or transport systems for nutrients; substitution of essential metal ions in biomolecules or other functional cellular units; denaturation and inactivation of bio-molecules, especially enzymes - destruction of the integrity of cell membranes (by direct effect on sulfhydryl groups of membrane constituents and by direct or indirect induction of membrane lipid peroxidation using toxic free radicals and cell organelles, oxidative stress [
38,
39,
40,
41,
42].
The directive on environmental quality standards in the field of water and soil policy is the last regulation necessary to support Directive 2000/60/EC establishing the community framework for action in the field of water and soil strategy, known as the WFD Directive (Water Framework Directive), which integrates land and water management in a zonal watershed [
43]. The directive requires the preparation of a watershed management plan (surface and underground) for each area in the European Union.
The WFD Directive requires that all river basins and soils of the European Union reach a good quality stage and establishes a new regime for the prevention and control of their chemical pollution by the year 2025.
According to Romanian legislation, the maximum concentrations allowed for heavy metal ions in water must not exceed the values, regulated by the Order of the Ministry of the Environment and Water Management 161/2006, for the approval of the Normative on the classification of surface water quality to establish the ecological status of bodies of water [
44] (
Table 1).
The Romanian regulation specifying the limits of soil contaminant concentrations is Order no. 756 /1997 [
47] for the approval of the Regulation on environmental pollution assessment of the Ministry of Environment and Forests, supplemented by Order no. 592 of June 25, 2002, for the approval of the Regulation on the establishment of limit values, threshold values and evaluation criteria and methods for sulfur dioxide, nitrogen dioxide and nitrogen oxides, suspended dust, lead, benzene, monoxide of carbon and ozone in the surrounding air [
48].
Reference values regarding soil pollution with heavy metals according to Order no. 756 of 1997 are shown in
Table 2.
Table 3 shows the maximum concentrations of heavy metals allowed in the human body according to international regulations developed by the World Health Organization, as well as the conditions they can cause the human body’s ailments [
49].
The provisional tolerable weekly intake (PTWI) approved by the SCF (Scientific Committee on Food) [
50] for heavy metals are:
2. Materials and Methods
2.1. Soil, water, and food sampling area
Vatra Dornei is a typical mountain depression settlement, placed in the northern part of the Eastern Carpathians, at an average altitude of 800 meters. The city is in the Dornelor Depression, which has the following limits: Rarău and Giumalău Mountains to the northeast; Călimani Mountains to the south, and Suhard Mountains to the north. The geography of the territory consists of crystalline schists (the Suhard Mountains, in the northern part) and volcanic rocks (the Călimăni Mountains, in the southern part).
In the area of Vatra Dornei, the contrasting altitudes appeared against the background of a great fragmentation of the relief and the early intervention of the anthropic factor (forestry and mining explorations), generated a relative "disorder" within the ecosystem.
The transformations and changes produced in the geographical landscape caused by mining activities are much more severe compared to other effects in mountains. They are due to the opening of quarries for the exploitation of manganese ore, polymetallic, sulfur, and construction rocks, as well as the construction of waste rock storage dumps, from both underground and surface mining. It is appreciated that a category of landscape, called extractive landscape, can be distinguished in the area, with the sub-type of the quarry landscape, through the presence of basins and settling ponds, surrounded by predominantly forest areas.
The human communities in the vicinity of the extractive industrial areas in the Vatra Dornei area (Ciocăneşti, Cârlibaba, Iacobeni, Călimani, Crucea, etc.) feel the full influence of the degradation of environmental factors. For this reason, it is necessary to gather information and evaluate the effects of extractive activities on the environment. The general industrial activity carried out in the area had the effect of damaging the ecosystems, both during the active period and after its partial cessation. Due to the toxic residues emitted into the environment during the exploitation of natural resources, the general area is affected, being exogenously and endogenously eroded. Degradation is also favored by climatic, edaphic and orographic factors, which contribute to the propagation of pollutants in the environment.
2.2. Sampling and processing of samples
2.2.1. Sampling of samples
The experiments took place over six months in the year 2022. Samples of soil, water, plant material (lettuce, spinach), apples, raw meat and milk, as well as products derived from them - smoked meat and cheese, were collected. All food products were procured from the markets in the area, by local producers. The samples were transported in polyethylene bags and bottles were closed and labeled accordingly.
The soil samples were taken from two depths of 0 - 20 cm and 20 - 30 cm, and the used pedological probe was cleaned after each collection, to avoid contamination in the chain. The plant material was taken in its entirety: root and aerial parts (stems and leaves). Each specimen was placed in a polyethylene bag labeled according to the requirements.
2.2.2. Samples processing
This stage includes the procedures that are performed for the preparation of the collected samples to carry out physical-chemical or radiometric analyses.
The primary processing of soil samples includes conditioning; macroscopic soil analysis; drying; sifting; determining the final mass of the soil samples; sampling and storage.
Primary processing of water samples includes conditioning; macroscopic analysis; filtering; sampling and storage (
Figure 1).
The primary processing of plant material includes conditioning; macroscopic examination; washing each plant sample; determining the length of the whole plant; separation of plant morphological parts: root, aerial parts (stem and leaves); removal of water particles remaining after washing; collecting samples for the analyzes to be performed; the drying of samples and storage and preservation of plant material.
Primary fruit processing includes conditioning, macroscopic examination, washing each sample, removal of water particles left after washing, collecting samples for the analyses to be performed and the storage and preservation of the samples.
The primary processing of raw meat, smoked meat and cheese includes conditioning, macroscopic analysis of samples, determination of the sample to be analyzed, sample collection, storage and preservation.
The primary processing of milk includes conditioning, macroscopic analysis, filtering, sampling and storage.
The functional schemes for the preparation of meat, milk and their products are identical to those for plant material/soil.
2.2.3. Biometric determinations for plant material
Biometrics determines the variation of the morphological parameters of the plant depending on different environmental conditions.
For the investigated plant material, the following biometric measurements were performed: lengths of the whole plant and the parts, respectively roots and aerial parts, masses of the whole plant and the parts (
Figure 2).
2.2.4. Analysis of the physico-chemical properties of the soil
The physico-chemical characteristics of the soil are represented by the content of dry substance and humidity, pH, electrical conductivity and heavy metal content.
The humidity was determined by the gravimetric method. The soil was oven-dried at 105°C to constant mass. As soon as they were removed from the oven, the soil samples were placed in the desiccator. The humidity was then calculated, in percentages, respectively the dry substance content:
where:
mi = the initial mass of the sample [g];
mf = the final mass of the sample after drying [g];
U = humidity [%];
The soil pH determination was carried out in laboratory conditions, from the unground but sieved soil. A 1:5 soil solution was prepared from 6 g of soil and 30 mL of distilled water, which was placed in a plastic container with a tight lid. The solution was homogenized by magnetic stirring (Retch magnetic stirrer) for 15 min. The pH was immediately measured by using a pH meter.
The determination of electrical conductivity was carried out 24 hours after the soil pH analysis. During this time, the aqueous solution was decanted, through gravitational sedimentation. Conductivity represents soil’s salinity grade, a characteristic that can be a limiting factor in the development of vegetation. It is the physical quantity that expresses the ability of the soil to transmit an electric charge.
The determination of the heavy metal content was carried out by atomic absorption spectrometry using an AA220 VARIAN spectrophotometer. The principle of the method consists of the penetration of the ions from the solution to be analyzed together with the carrier gas into the high-temperature area, respectively the flame, where they become atoms. In the temperature range 2000 – 3000 0C, the atoms are brought to the energy state favorable for absorption, reducing the emission to a minimum. For this analysis, the preparation of the samples to be investigated is necessary. The soil samples were brought into solution by acid digestion with aqua regia to minimize the interference with the organic matrix of the product. The next step was filtering the mineralized samples. These are brought to a 200 mL volumetric flask, adding distilled water. The solutions obtained must have definite clarity for the mineralization to be complete. The analysis of the samples at the atomic absorption spectrometer requires a minimum of 5 calibration standards in 3 different concentrations for the chosen metals and a blank of acidulated water (1%). The device was calibrated using standard solutions, according to the specifications in the spectrometer user manual.
Uranium was determined using the spectrophotometric method.
Depending on the uranium content of the sample, 1-10 mL is dosed with the pipette and then dilutions are made to record the extinction values read on the spectrophotometer in the middle of the calibration curve, as recommended by the analytical methods that use this technique. 2 mL HNO3 and 2 mL HClO4 were added to the digested sample. The samples were brought to dry, repeated twice with 4.5 N HCl solution, 2 ml of 1% ascorbic acid solution, and a few granules of metallic zinc; this step of the analysis ensures the reduction of uranium from U6+ to U4+. After approximately 15 min., the time required for uranium reduction, the samples were transferred into 50 mL volumetric flasks. Also here, 2 mL Arsenazo III (laboratory reagent) 0.05% solution was added, and the flasks were filled to the mark with 4.5 N HCl solution.
The calibration was done as follows: from a 10 ppm/mL standard solution, 1, 2, 3, and 4 mL were pipetted into Berzelius glasses, and 4.5 N HCl solution, 2 mL of 1% ascorbic acid solution, and a few grains of metallic zinc, as in the case of samples. After the reduction, the standards were transferred to 50 mL volumetric flasks, 2 mL Arsenazo III 0.05% solution was added, and the flasks were filled to the mark with 4.5N HCl solution.
Both the samples and the standards were read on the CECIL 101 UV-VIS spectrophotometer, at the wavelength of 670 mm, against a sample containing all reagents (minus the sample). The calibration curve was determined using the least squares method and according to this was calculated for all the experiments carried out, each point separately.
2.2.5. Analysis of the physical-chemical properties of plant material
The gravimetric method was used to determine the humidity of plant matter, both for the roots and for the green aerial parts, as well as for apples. 2-3 g of green material sample (stem, leaves, fruit) were weighed and dried in an oven, at 105°C, for one hour, until a constant mass was obtained (the difference should be less than 0.0002 g). The calculation of the percentage of moisture in the plant material was made according to the same formulas as for the soil.
To determine the heavy metal content of the plant material, it was necessary to go through the following steps: grinding the plant samples, mineralization or acid digestion and flame atomic absorption spectrometry.
Grinding of the dry plant samples was carried out separately for the morphological parts of the plant, the root, the aerial parts, and the fruit. Acid digestion or mineralization was carried out with the help of the magnetic stirrer with heating, according to the same method as for the soil.
The next step was filtering the mineralized samples. They were brought to a 200 mL volumetric flask, filled with distilled water. The analysis of the mineralized samples was carried out with the same spectrometer as for the soil. The analysis of the samples at the atomic absorption spectrometer requires a minimum of 5 calibration standards in 5 different concentrations for the chosen metals and a blank of acidified water (1%). The calibration of the device was carried out with the help of standard solutions, according to the specifications in the user manual of the spectrometer.
To determine the uranium content of plants and fruits, the same method as for soil was used.
2.2.6. Analysis of the physico-chemical properties of food products
The determination of the heavy metal content of food products required drying of the samples, grinding of the samples, calcination, mineralization or acid digestion, flame atomic absorption spectrometry and in the case of milk evaporation, mineralization, flame atomic absorption spectrometry. Acid digestion or mineralization was carried out using a magnetic stirrer with heating, according to the same method as for soil.
The next step was filtering the mineralized samples. They were brought to a 200 mL volumetric flask, filled with distilled water. The analysis of the mineralized samples was carried out with the same spectrometer as for the soil. The analysis of the samples at the atomic absorption spectrometer requires a minimum of 5 calibration standards in 3 different concentrations for the chosen metals and a blank of acidified water (1%). The calibration of the device was carried out with the help of standard solutions, according to the specifications in the user manual of the spectrometer.
To determine the uranium content in food pods, the same method is used on the ground.
3. Results
The experiments concerned the following types of materials: soil; water; plant material - spinach, lettuce; fruits - apples; milk; raw meat; smoked meat and cheese. The contaminants in this work are represented by heavy metals (Cu, Cd, Pb, Zn, Mn) and uranium.
3.1. The experimental results obtained for the soil samples.
Soil moisture, pH, and electrical conductivity range are shown in
Table 4.
The high degree of heavy metal pollution of the soil samples is due to the lower humidity, as it is not covered with vegetation and can capture the contaminating compounds more easily. A higher soil moisture has a directly proportional influence.
By averaging all the samples taken, insignificant differences in pH are observed. In general, the pH values of the soil samples vary between 6.25 and 7.25 (60% of the samples fall between these values).
The value of the electrical conductivity of the soil does not follow a pattern in the case of the determinations made and falls within the normal limits of soil conductivity.
To highlight the phenomenon of bioaccumulation, samples taken at a depth of 0-20 cm were analyzed. Cu, Cd, Pb, Zn, and Mn contents were determined by flame atomic absorption spectrometry and U by photocolorimetry. The number of samples is 20.
The reference values are established by the Ministry of Water, Forests and Environmental Protection, by Order no. 756/1997 [
47].
The
Table 5 shows the soil heavy metal content range and the reference values. From the total of 20 soil samples taken from the area, 6% of the samples showed values above the allowed limit for Cu, 8% of the samples showed values above the allowed limit for Zn and 10% showed values above the allowed limit for Mn (of these only two samples had Mn content above the alert threshold).
3.2. The experimental results obtained for the water samples.
Samples taken both from the water coming from the wells of the households in the area and from the river and creek were analyzed. Cu, Cd, Pb, Zn, and Mn contents were determined by flame atomic absorption spectrometry and U by photocolorimetry and shown in
Table 6. The number of samples was 30. The reference values are established by the Water Law and NTPA 001/2002 [
51].
Of the total of 30 water samples taken from the area, 1% of the samples showed values above the permissible limit for Cu, and 1.5% showed values above the permissible limit for Pb. The samples that showed increased values for Cu and Pb are samples taken from the river.
Regarding the content of fixed residues, the samples that presented the highest values were taken from the wells of households in the area.
3.3. Experimental results obtained for plant material samples (lettuce, spinach, and apples)
The following plant material was analyzed: 40 salad samples; 40 samples of spinach; 40 samples of apples. Biometric measurements for lettuce and spinach highlighted the fact that the average values of the lengths of spinach and lettuce roots are smaller than the aerial parts (
Table 7).
According to [
52,
53] the maximum allowed limits of heavy metal concentrations in plants are 73.3 mg/kg - Cu, 0.3 mg/kg - Pb, 99.4 mg/kg - Zn, 0.2 mg/kg - Cd, 30 mg/kg - Mn and 0 mg/kg - U.
It is observed that none of the metal elements studied registers an increase above the level allowed by the regulations in force, only Zn is close to the maximum limit allowed. Of the total of 40 salad samples, 30% of them show a maximum concentration of Zn of 81.2 mg/kg. In Regulation No. 420/2011 of the European Commission [
53], the maximum limits allowed only for Pb and Cd are established. Thus, in vegetables - leaves, and fresh herbs, the normal values are 0.3 mg×kg
-1 for Pb and 0.2 mg×kg
-1 for Cd, which is also confirmed by the experimental analysis.
Regarding the radioactivity of plant materials, the reference values are not established for γ radiation, but for radionuclides of major interest, by the United Nations Scientific Committee on the Effect of Atomic Radiation (UNSCEAR [
54]). Some important values would be for the following radionuclides:
214Pb = 0.001 – 0.01 Bq×kg
-1;
226Ra = 0.001 – 0.1 Bq×kg
-1;
235U = 0.001 – 0.1 Bq×kg
-1.
After the Chornobyl nuclear accident, a cumulative radioactivity value of 600 Bq×kg
-1 was established for the radioisotopes
134Cs and
137Cs. The value of 500 Bq×kg
- 1, established after the Fukushima disaster, was taken as the maximum allowed limit [
55]. Fortunately, only one sample out of the 40 samples of spinach leaves has an extremely low content of uranium.
3.4. Experimental results obtained for food samples (milk, cheese, raw meat, and smoked meat)
20 milk samples were analyzed; 20 samples of cheese; 15 samples of fresh meat (chicken breast); and 15 samples of smoked meat (pork). The results are displayed in
Table 8 and the maximum values of heavy metal allowed in food products are in
Table 9.
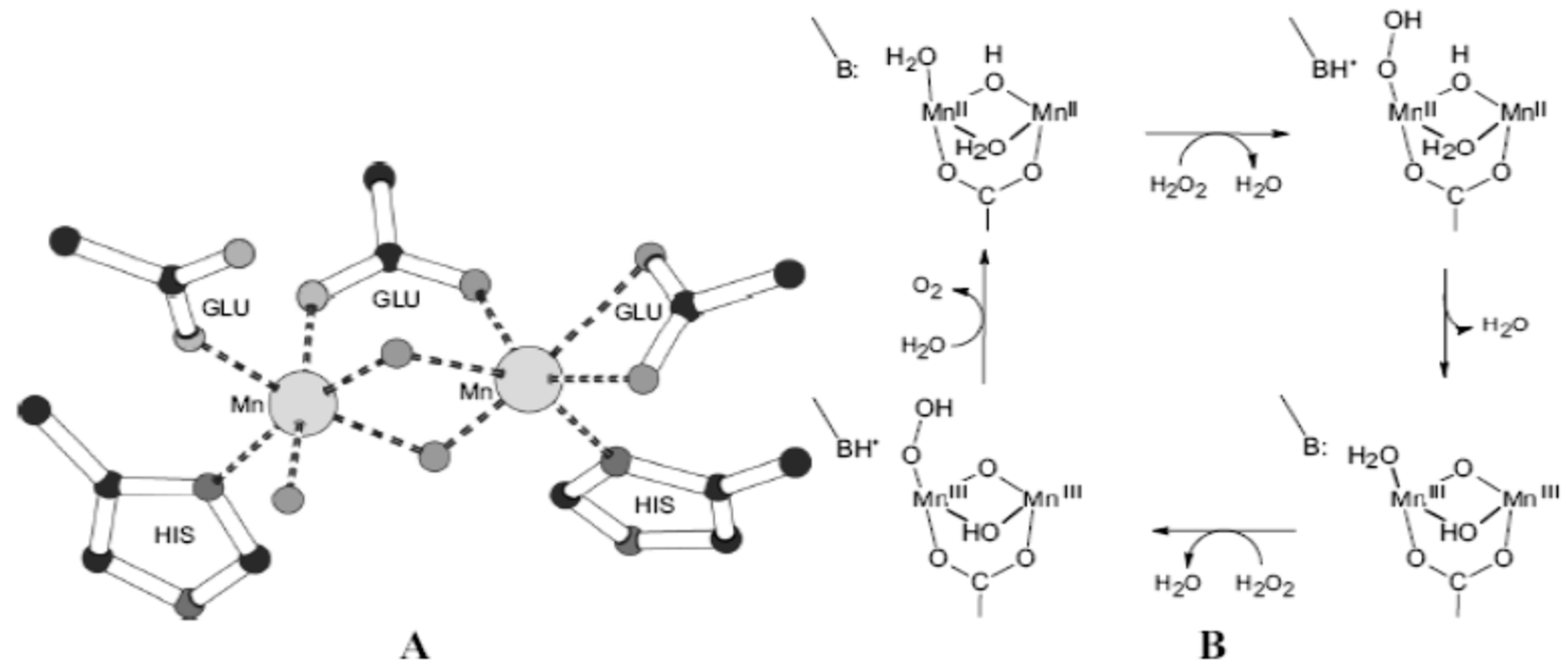
The Mn content in cheese is attributed to the large number of biomolecules containing manganese, namely: superoxide dismutase (Mn-SOD), catalase, Mn-ribonucleotide reductase, Mn-peroxidase, ligninase, the evolving oxygen center (OEC) from the photosystem II(PS-II) and Mn-thiosulfate oxidase.
The action mechanisms of these enzymes are different transfers and include oxo-atom, electron transfer, reduction of ribonucleotide to water and oxidation of thiosulfate to sulfate [
57,
58,
59].
The main defense mechanism of living cells uses superoxide dismutase and catalase to protect the cell structure against harmful and reactive oxygen species such as superoxide radicals or dihydrogen peroxide (
Figure 3) [
57]. Catalases are enzymes that protect cells from oxidative damage. In addition to heme-type catalase, a rare secondary class of manganese catalases has been found in three bacteria:
Lactobacillus plantarum,
Thermus thermophilus, and
Thermoleophilium album. That might explain the higher manganese content in cheese.
3.5. Influence of temperature and time on food samples (spinach, apples, cheese, raw meat, and smoked meat)
The effect of the following factors was studied:
Temperature by thermal preparation: boiling for spinach samples and baking for apples.
Temperature by thermal preparation – frying for fresh chicken meat (breast), boiling for milk samples, boiling for fresh chicken meat samples, freezing for milk samples, freezing for fresh chicken meat samples.
Time – for samples of cheese and smoked meat.
3.5.1. Influence of temperature on spinach and apple samples
The following plant material was analyzed: 40 spinach samples; and 40 samples of apples.
The temperature effect on plant samples was studied by boiling in water of spinach and apple samples.
Spinach samples (only stems and leaves were used in the case of spinach) and apple ones were boiled at a constant temperature (80 0C) in Berzelius glasses for 1 hour. After boiling the samples were drained, dried, and analyzed to find out the heavy metals’ contents.
The experimental results displayed in
Table 10 showed a decrease in the content of heavy metals in the plant material. Thermal preparation positively influences the concentrations of heavy metals in spinach and apples, but we do not know the effect on the quality (vitamin content) of spinach and apples.
A decrease in the copper content after thermal preparation is observed by approximately 40% of the initial content, for Pb a decrease of approximately 25%, for Zn a decrease of approximately 20%, for Cd a decrease of approximately 50%, for Mn a decrease of approximately 30% and the uranium was no longer found.
In the case of apple samples, a decrease in the copper content after thermal preparation is observed by approximately 30% of the initial content for Cu, and for Zn a decrease of approximately 30%.
3.5.2. The influence of temperature on fresh meat (chicken breast) and milk samples
The study of the influence of temperature on fresh meat and milk samples was re-analyzed as follows:
Frying for fresh chicken meat (breast),
Boiling for milk samples,
Boiling for samples of fresh chicken meat,
Freezing for milk samples,
Freezing for fresh chicken meat samples.
15 samples of fresh meat for frying (chicken breast), 20 samples of milk, 15 samples of fresh meat (chicken breast) for boiling, and 20 samples of milk and fresh meat for freezing were analyzed.
The fresh meat samples were subjected to the thermal treatment of frying in vegetable oil, as well as thermal preparation on the grill without the addition of oil (on the stove) and the results are shown in
Table 11.
The experimental results showed a decrease in the content of heavy metals in the meat samples after thermal preparation. Thermal preparation positively influences the concentrations of heavy metals in fresh meat.
A decrease in the copper content after thermal preparation (by frying the chicken meat) is observed by approximately 20% of the initial content, for Mn a decrease of approximately 30%.
The milk samples were subjected to the heat treatment of boiling up to the boiling point of the milk and the results are displayed in
Table 12.
The experimental results showed a decrease in the content of heavy metals in boiled milk. Thermal preparation positively influences the concentrations of heavy metals in milk.
A decrease in copper content is observed after the thermal preparation of milk by boiling by approximately 30% of the initial content, for Pb a decrease of approximately 20%, for Zn a decrease of approximately 30%, for Cd a decrease of approximately 20 %, for Mn a decrease of approximately 100% and uranium was no longer found.
The meat samples were subjected to the thermal treatment of boiling for 1 hour and the results are presented in
Table 13.
4. Discussion
The experimental results showed a decrease in the content of heavy metals in the meat samples after thermal preparation. Thermal preparation positively influences the concentrations of heavy metals in fresh meat.
There is a decrease in the copper content after the heat-cooking of the chicken meat with about 20% of the initial content, for Mn a decrease of about 30%.
It is also observed that both by frying and by boiling the chicken meat, the contents of heavy metals decrease in the same proportions, which shows that the preparation of the meat at elevated temperatures is not influenced by very high temperatures, it is the necessary boiling temperature of water to lower the level of heavy metals.
The milk and raw meat samples were frozen and kept in the freezer: the milk for 7 days, and the raw chicken meat for a month.
After thawing, results like the initial ones were obtained, which indicates that keeping at temperatures below 0oC does not influence the metal content in food products.
Part of the samples were thermally prepared after thawing and similar results to those presented above were obtained after thermal preparation.
To study the influence of time on the food samples, the cheese and smoked meat samples were kept in the refrigerator in airtight boxes for one month (cheese) and 50 days (smoked meat). As a result of the analyses carried out after this time, no changes in the content of heavy metals were observed, which means that time does not influence the concentration of heavy metals in food products.
After the analyses were carried out, it was found that thermal preparation positively influences the concentrations of heavy metals in food products, obtaining decreases in the contents of Cu by 30-40%, of Pb by 25%, of Zn by 20-30%, of Cd by 20-50%, of Mn with 30-100%, of U of 100%.
It was observed that the type of thermal preparation method chosen (roasting or boiling) does not change the amount of heavy metal contents that decrease, without knowing how it affects the quality of the food products undergoing preparation.
Noting that both by frying and by boiling food products, the contents of heavy metals decrease in the same proportion, it can also be highlighted that the decrease in contents is not influenced by very high temperatures, being the temperature of boiling the water to lower the level of heavy metals.
After defrosting the food products studied, results like the initial ones were obtained, which indicates that storage at temperatures below 0 0C does not influence the metal content in the food products, so freezing does not lead to a decrease in the content of heavy metals in the food products.
After the analysis was carried out, it was observed that the time factor does not lead to changes in the content of heavy metals, which means that time does not influence the decrease in the concentration of heavy metals in food products.
5. Conclusions
According to the applicable legislation in force, from the analysis of the soil samples, values exceeding the permissible limits for Cu, Zn and Mn were recorded, the pH recorded insignificant differences and the electrical conductivity fell within the nor-mal limits. Of the water samples, 1% of the samples showed values above the permissible limit for Cu, and 1.5% showed values above the permissible limit for Pb, samples which was taken from the river. Regarding the content of fixed residues, the samples that presented the highest values were taken from the wells of households in the area. At analysis of heavy metal of plants, was observed that none of the metal elements studied registers an increase above the level allowed by the regulations in force, only Zn is close to the maximum limit allowed. Regarding the radioactivity of plant materials, only one sample out of the 40 samples of spinach leaves has an extremely low content of uranium.
The results obtained in this work show that the presence of heavy metals in food products is influenced by temperature, so a thermal preparation of the products de-creases the content of heavy metals in these food products. Keeping food products at temperatures lower than 0 0C does not influence their metal content. Also, time does not influence the content of heavy metals in food products.
The results highlighted that the mining activities carried out during time generated environmental pollution with uranium and heavy metals.
Author Contributions
coordination: V.-M. R.; conceptualization, methodology, writing-original draft preparation: D.-M. B., I-C. P.; sampling and investigation: M.Z. and N.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data is unavailable due to privacy restrictions.
Acknowledgments
The authors would like to thank Dr. Chem. Aura Daniela Radu for all the fruitful discussions, comments and suggestions made during the draft writing. Many thanks to the Ministry of Research, Innovation and Digitization for funding. This research was elaborated by the Ministry of Research, Innovation and Digitization by the contract no. 43N/2019.
Conflicts of Interest
The authors declare no conflict of interest.
References
- García-Navarro, F.J.; Jiménez-Ballesta, R.; Garcia-Pradas, J.; Amoros, J.A.; Perez de los Reyes, C.; Bravo, S. Zinc Concentration and Distribution in Vineyard Soils and Grapevine Leaves from Valdepeñas Designation of Origin (Central Spain). Sustainability 2021, 13, 7390. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. Journal of King Saud University – Science 2022, 34, 101865. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6(9). [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Espinosa, T.; Navarro-Pedreño, J.; Gómez Lucas, I.; Almendro Candel, M.B.; Pérez Gimeno, A.; Jordán Vidal, M.; Papamichael, I.; Zorpas, A.A. Environmental Risk from Organic Residues. Sustainability 2023, 15, 192. [Google Scholar] [CrossRef]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Mubeen, S.; Ni, W.; He, C.; Yang, Z. Agricultural Strategies to Reduce Cadmium Accumulation in Crops for Food Safety. Agriculture 2023, 13, 471. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Suo, L.; Sun, Y.; Sun, N.; Liu, J.; Li, S.; Zou, G.; Liao, S. The Effects of Calcium and Sulfur Fertilizers Accompanied by Different Side Elements on the Growth and Cd Uptake of Spinacia oleracea Grown in Cd-Contaminated Alkaline Soil. Horticulturae 2023, 9, 835. [Google Scholar] [CrossRef]
- Shaaria, N.E.M.; Tajudina, M.T.F.M.; Khandakera, M.M.; Majrashib, A.M.; Alenazic, M.; Abdullahia, U.A. Cadmium toxicity symptoms and uptake mechanism in plants: a review. Brazilian Journal of Biology 2023, 84, e252143. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; He, L. Health risk assessment of heavy metals in soils and food crops from a coexist area of heavily industrialized and intensively cropping in the Chengdu Plain, Sichuan, China. Front. Chem., Sec. Green and Sustainable Chemistry 2022, 10. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. Hindawi, Journal of Chemistry 2019, 6730305. [Google Scholar] [CrossRef]
- Kavsar, N.; Eziz, M.; Sidikjan, N. Pollution and Health Risk Assessment of Hazardous Elements in Surface Dust along an Urbanization Gradient. Sustainability 2023, 15, 11842. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Gaston, K.J. Anthropogenic changes to the nighttime environment. BioScience 2023, 73, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Syakir Ishak, M.I.; Bhawani, S.A.; Umar, K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Osipov, S.; Chowdhury, S.; Crowley, J.N. Severe atmospheric pollution in the Middle East is attributable to anthropogenic sources. Commun Earth Environ 2022, 3, 203. [Google Scholar] [CrossRef]
- Yang, T.; Liu, W. Health Effects of Energy Intensive Sectors and the Potential Health Co-Benefits of a Low Carbon Industrial Transition in China. Int. J. Environ. Res. Public Health 2019, 16, 3022. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Grøntoft, T. Observed Recent Change in Climate and Potential for Decay of Norwegian Wood Structures. Climate 2019, 7, 33. [Google Scholar] [CrossRef]
- González-Hernández, D.L.; Meijles, E.W.; Vanclay, F. Factors that Influence Climate Change Mitigation and Adaptation Action: A Household Study in the Nuevo Leon Region, Mexico. Climate 2019, 7, 74. [Google Scholar] [CrossRef]
- Huang, W.; Yu, P.; Xu, R.; Yang, Z.; Gasevic, D.; Ye, T.; Guo, Y.; Li, S. Long-term impacts of non-occupational wildfire exposure on human health: A systematic review. Environ Pollut 2023, 320, 121041. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. Journal of King Saud University – Science 2022, 34, 101865. [Google Scholar] [CrossRef]
- Nawaz, R.; Aslam, M.; Nasim, I.; Irshad, M.A.; Ahmad, S.; Latif, M.; Hussain, F. Air Pollution Tolerance Index and Heavy Metals Accumulation of Tree Species for Sustainable Environmental Management in Megacity of Lahore. Air 2023, 1, 55–68. [Google Scholar] [CrossRef]
- Kavsar, N.; Eziz, M.; Sidikjan, N. Pollution and Health Risk Assessment of Hazardous Elements in Surface Dust along an Urbanization Gradient. Sustainability 2023, 15, 11842. [Google Scholar] [CrossRef]
- Martínez-Guijarro, R.; Paches, M.; Romero, I.; Aguado, D. Sources, Mobility, Reactivity, and Remediation of Heavy Metal(loid) Pollution: A Review. Adv Environ Eng Res 2021, 2(4), 033. [Google Scholar] [CrossRef]
- Alloway, B.J. Heavy metals in soils, Michigan University, 1990.
- Zhao, H.; Wu, Y.; Lan, X. Comprehensive assessment of harmful heavy metals in contaminated soil in order to score pollution level. Sci Rep 2022, 12, 3552. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, G.; Nagar, V.; Mandzhieva, S.; Minkina, T.; Sankhla, M.S.; Pandit, P.P.; Aseri, V.; Awasthi, K.K.; Rajput, V.D.; Bauer, T.; et al. Sustainable Amelioration of Heavy Metals in Soil Ecosystem: Existing Developments to Emerging Trends. Minerals 2022, 12, 85. [Google Scholar] [CrossRef]
- Báthory, D.; Nicoară, A.; Bercea, V. Heavy metal content in beech (Fagussylvatica L.) and hornbeam (Carpinusbetulus L.) shoots from polluted forest ecosystems, Environment-Research, Protection and Management (in Romanian), Presa Univ. Clujeană, Cluj-Napoca 2003, pp. 35-38.
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Priya, A.K.; Muruganandam, M.; Ali, S.S.; Kornaros, M. Clean-Up of Heavy Metals from Contaminated Soil by Phytoremediation: A Multidisciplinary and Eco-Friendly Approach. Toxics 2023, 11, 422. [Google Scholar] [CrossRef]
- Aydi, S.; Sassi Aydi, S.; Ben Salah, G.; Dekhil, A.; Rahmani, R.; Bouajila, A.; Mohamed, N.A.; Ab-Delly, C. Phytoremediation potential of native plants: Biomonitoring approach in contaminated soils. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2023, 51(2), 13063. [Google Scholar] [CrossRef]
- Yadav, M.; Sharma, P. Current Eco-friendly and Sustainable Methods for Heavy Metals Remediation of Contaminated Soil and Water: Special Emphasis on Use of Genetic Engineering and Nanotechnology. Pollution 2023, 9(3), 1028–1048. [Google Scholar] [CrossRef]
- Litlle, P.; Hartin, M.H. A survey of zinc, lead, and cadmium in soil and natural vegetation around a smelting complex. Environmental Pollution 1972, 3 (3), pp. 241 -254.
- Jo, H.; Kim, G.; Chang, J.; Lee, K.; Lee, C.; Lee, B. Chronic Exposure to Lead and Cadmium in Residents Living near a Zinc Smelter. Int. J. Environ. Res. Public Health 2021, 18, 1731. [Google Scholar] [CrossRef]
- Cao, X.; Yuan, M.; Zhang, Y. Heavy Metals Pollution in Soil Around the Lead-zinc Smelting Plant in guanzhong Plain. E3S Web of Conferences 2023, 394, 01012. [Google Scholar] [CrossRef]
- Jin, J.; Mi, R.; Li, Q.; Lang, J.; Lan, Y.; Huang, N.; Yang, G. Bacillus Thuringiensis Enhances the Ability of Ryegrass to Remediate Cadmium-Contaminated Soil. Sustainability 2023, 15, 5177. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Bi, X. Lead, Zn, and Cd in slags, stream sediments, and soils in an abandoned Zn smelting region, southwest of China, and Pb and S isotopes as source tracers. J Soils Sediments 2010, 10, 1527–1539. [Google Scholar] [CrossRef]
- Sun, R.; Gao, Y.; Xu, J.; Yang, Y.; Zhang, Y. Contamination Features and Source Apportionment of Heavy Metals in the River Sediments around a Lead-Zinc Mine: A Case Study in Danzhai, Guizhou, China, Hindawi, Journal of Chemistry 2021, ID 9946026. [CrossRef]
- Bouida, L.; Rafatullah, M.; Kerrouche, A.; Qutob, M.; Alosaimi, A.M.; Alorfi, H.S.; Hussein, M.A. A Review on Cadmium and Lead Contamination: Sources, Fate, Mechanism, Health Effects and Remediation Methods. Water 2022, 14, 3432. [Google Scholar] [CrossRef]
- Bride, Mc. Toxic metals in sewage sludge-amended soils: Has the promotion of beneficial use discounted the risks? Advances in Environmental Research 2003, 8, 5–19. [Google Scholar]
- Zanin, E.; Scapinello, J.; de Oliveira, M.; Dal Magro, J. Adsorption of heavy metals from wastewater graphic industry using clinoptilolite zeolite as adsorbent. Process Safety and Environmental Protection 2017, 105, pp. 194–200. [Google Scholar] [CrossRef]
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Com-munity action in the field of water policy.
- Ministerial Order No 161/2006 for the Approval of the Norm Concerning the Reference Objectives for the Surface Water Quality Classification (Including Quality Standards for Sediments).
- Law, No. 311 of 2004 for the amendment and completion of Law no. 458/2002 regarding the quality of drinking water.
- Order, no. 1146/2002 for the approval of the Normative on the reference objectives for the classification of surface water quality.
- Order, No. 756 of November 3, 1997, for the approval of the Regulation on environmental pollution assessment, Ministry of Water, Forests and Environmental Protection.
- Order, no. 592 of June 25, 2002, for the approval of the Regulation on the establishment of limit values, threshold values and evaluation criteria and methods for sulfur dioxide, nitrogen dioxide and nitrogen oxides, suspended dust, lead, benzene, monoxide of carbon and ozone in the surrounding air.
- World Health Organization (WHO). 10 Chemicals of Public Health Concern. Available online: https://www.who.int/news-room/photo-story/photo-story-detail/10-chemicals-of-public-health-concern (accessed on 10 August 2023).
- Scientific Committee on Food. Available online: https://food.ec.europa.eu/horizontal-topics/expert-groups/scientific-committees/scientific-committee-food-archive_en (accessed on 12 August 2023).
- Normative NTPA-001/2002 of February 28, 2002, regarding the establishment of pollutant loading limits of industrial and urban wastewater when discharged into natural receivers and Water Law no. 107/1996.
- Fao/WHO – Codex Alimentarius Commissios, 2001. Available online: https://www.fao.org/fao-who-codexalimentarius/en/ (accessed on 12 August 2023).
- Regulation, no. 420/2011 of the European Commission.
- The United Nations Scientific Committee on the Effects of Atomic Radiation-UNSCEAR 2008. Available online: https://www.unscear.org/unscear/en/publications/2008_1.html (accessed on 12 August 2023).
- National Health and Nutrition Survey Japan, 2012. Available online: https://www.nibiohn.go.jp/eiken/kenkounippon21/en/eiyouchousa/ (accessed on 12 August 2023).
- Order, no. 975 of December 16, 1998, on the approval of the sanitary-sanitary norms for food, 1998.
- Godbole, M.D. Coordination chemistry of manganese and iron with N,O-donor ligands: oxidation catalysis and magnetochemistry of clusters. 2006, Doctoral Thesis. Available online: https://hdl.handle.net/1887/4333 (accessed on 12 August 2023).
- Baca, S.; Kögerler, P. Cluster-Based Coordination Polymers of Mn/Fe-Oxo Pivalates and Isobutyrates. Chemistry 2021, 3, 314–326. [Google Scholar] [CrossRef]
- Guo, M. .; Yong-Min, G.; Ranjana, S.; Mi Sook, O.; Wang, T.; Hua-Hua, L.; Hai-Yang, D.; Sunder, N.; Ritimukta, S.; Shunichi, F.; Wonwoo, N. Dioxygen Activation and O–O Bond Formation Reactions by Manganese Corroles. J. Am. Chem. Soc. 2017, 139, 44, 15858–15867. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).