1. Introduction
Recently, more and more attention be paid to the study of groups of co-inhabiting and even taxonomically close bat species. In such communities, quite complex and contrasting relationships develop between their members [
1,
2,
3,
4,
5]. Various bat species often occupy the same habitats. In this case, the competitive exclusion principle can be applied, according to which species with the same ecology cannot live in the same space. Species living together should show different preferences in some component of the ecological niche, spatial or trophic, to reduce their competition [
6].
The territory of the European Russia is inhabited by 27 bat species. The bat fauna of the Middle Volga region includes 16 species [
7,
8,
9,
10]. Five bat species of the genus
Myotis (Chiroptera, Vespertilionidae) are among them: the whiskered bat
Myotis mystacinus (Kuhl, 1819), the Daubenton's bat
Myotis daubentonii (Kuhl, 1817), the pond bat
Myotis dasycneme (Boie, 1825), the Brandt's bat
Myotis brandtii (Eversmann, 1845) and the Natterer's bat
Myotis nattereri (Kuhl, 1817). All
Myotis bats are sedentary species, wintering in underground shelters, caves and abandoned adits [
2,
9]. Among sedentary bat species inhabiting the Samarskaya Luka the populations of
M. brandtii and
M. daubentonii reach the highest abundance [
2].
Despite the relative similarity in the habitat preferences, various
Myotis spp. have significant trophic and chorological differences [
7,
11,
12]. The life style differences, in particular, feeding habits and spatial distribution, cause the feathers of the trematode fauna in
Myotis spp. In this regard, it is of considerable interest to study the helminth fauna of syntopic
Myotis bats, and especially their trematodes.
Previously, studies of helminths in various bat species of the genus
Myotis were carried out in many European countries [
13]. The helminth fauna in
M. mystacinus is the most studied and was studied in Poland, Austria, Italy, Switzerland, the Czech Republic, Slovakia, Moldova, Ukraine, Belarus, Georgia and Azerbaijan. In this bat species, 25 species of parasitic worms were registered in Europe and the countries of the former USSR, including 15 trematodes [
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28].
The helminth fauna in
M. dasycneme was studied in Poland and Hungary [15,16, 18,29]. Data on the helminths in
M. daubentonii were obtained in Belarus, Hungary, Germany, Italy, Norway, Poland, Ukraine and Turkey [
15,
16,
29,
30,
31,
32,
33,
34,
35]. Parasitic worms in
M. nattereri were studied in Belarus, Hungary, Poland, Spain and Austria [
15,
16,
29,
34,
36,
37].
Data on
M. brandtii helminths in West and Central Europe are known only from studies by Shimalov et al. [
34,
38]. Probably, the scarce data on the parasitic worms of
M. brandtii in Europe are due to the fact that
M. mystacinus and
M. brandtii, as independent species, began to be distinguished only in the last 40 years [
7,
8]. Thus, the data of helminthological studies of
M. mystacinus apparently refer to both sympatric bat species.
There is little information about trematodes, as well as about helminths in general, of
Myotis aurascens Kuzyakin, 1935 and
Myotis alcathoe Helversen et Heller, 2001, studied only in Turkey [
35,
39];
Myotis oxygnathus (Monticelli, 1885) – in Serbia [
40];
Myotis myotis Borkhausen, 1797 and
Myotis emarginatus (E. Geoffroy, 1806) – in Austria [
36]. Recently Frank with coauthors [
13] presented a summary of parasites (including seven trematode species) in
Myotis bats inhabiting European countries. Unfortunately, this review did not include some works on parasites of bats from Eastern Europe.
In Russia, there are very few works containing data on helminths in
Myotis bats. The first report on the parasitic worms
M. mystacinus in Russia was given by Sten’ko et al. [
41], where three species of trematodes were found in the Crimea. Podvyaznaya [
42,
43] studied one species,
Allassogonoporus amphoraeformis (Mödlinger, 1930), from
M. brandtii in the Voronezh Nature Reserve. Demidova and Vekhnik [
44] studied the trematodes in
M. mystacinus and
M. brandtii from the Samarskaya Luka, in which two and nine species of trematodes were revealed, respectively. Five species of trematodes were found in
M. brandtii from Karelia [
45]. The study by Gulyaev et al. [
46] provides data on one studied specimen of
M. brandtii from the Magadan Oblast, in which was only
Plagiorchis sp. was found. We studied the helminths in
M. dasycneme and
M. daubentonii from Mordovia, where three and six trematode species were identified in bats, respectively [
47,
48]. Our works on parasites in vertebrates from the Middle Volga region include the information on trematodes in all five species of bats [
49,
50,
51,
52,
53].
Thus, the aim of our work was to study the biodiversity of trematodes in syntopic populations of five Myotis species in the Samarskaya Luka (European Russia).
2. Materials and Methods
The material for this research was collected from our own field studies on bat ecology and helminths in the territory of the Samarskaya Luka National Park (Samara Oblast, European Russia), which were conducted in the period from 2005 to 2007. The study area was the coastal zone of the Volga river in the northern part of the Samarskaya Luka (Samara Oblast).
Myotis bats were studied in three trapping stations in the National Park near Solnechnaya Polyana, Bogatyr and Shiryaevo villages (
Figure 1).
Figure 1.
A map of bat trapping places in the Samarskaya Luka National Park. Red crosses in the map show the bat trapping sites.
Figure 1.
A map of bat trapping places in the Samarskaya Luka National Park. Red crosses in the map show the bat trapping sites.
In total, we studied 247
Myotis brandtii, 262
Myotis daubentonii, 135
Myotis dasycneme, 125
Myotis mystacinus, and 98
Myotis nattereri. Bats were caught by mist nets at nights. We used the common method of placing the net between two vertical sticks, which were telescopic fishing rods 6 m long [
54]. Also, we studied bats that died from natural causes in the wintering places.
Chiropterans were examined by the methods of complete helminthological dissection [
55,
56]. Trematodes were collected from bats and fixed in 70% ethanol for further investigations. Digeneans were stained with aceto-carmine, dehydrated in a graded ethanol series (70–96%) and cleared in clove oil. Then parasitic worms were mounted in Canada balsam.
The indexes generally accepted in parasitology were used to characterize the helminth infection of
Myotis bats: the prevalence (
P, %), the mean abundance (
MA), and the intensity range (
I, specimens) [
57]. The Shannon (
H') and Shannon evenness (
E) indexes were calculated to determine the species diversity of bat trematodes. The significance of the differences between the Shannon index values was measured using Student’s t-test. The degree of similarity of the trematode faunas in
Myotis spp. was revealed using the Jaccard (
CJ) (qualitative data) and Sørensen (
CN) (quantitative data) similarity indexes [
58]. The degree of similarity was assessed as low (0–0.33), medium (0.34–0.66), and high (0.67–1).
The dominance of species in the parasite community was determined using the the Palia-Kovnatsky index of dominance (
D) [
59]. Trematode dominance groups were as follows: 100‒10, dominants; 10‒1, subdominants; and 1‒0.001, adominants. The Mann–Whitney (
U) tests was used to compare the total infestation of
Myotis spp., and to assess the significance of differences in the infection of bats by individual trematode species. The similarity dendrogram of the helminth faunas in
Myotis bats was made using the unweighted pair group method with arithmetic average (UPGMA) and the Morisita index as a distance measure in PAST 2.17 [
60]. To standardize the bat sample size, we used the species richness index (bootstrap estimator), which makes it possible to predict the number of helminth species that were not included in the collections [
61,
62]. The taxonomy of digeneans is given according the Fauna Europaea (
http://www.fauna-eu.org). Trematode voucher specimens are stored in the parasitological collection of the Institute of Ecology of the Volga River Basin of the Russian Academy of Sciences (Togliatti, Russia).
3. Results
In total, 11 species of trematodes were revealed in five species of
Myotis bats in the Samarskaya Luka National Park (
Table 1).
Table 1.
Trematodes in Myotis bats from the Samarskaya Luka National Park.
Table 1.
Trematodes in Myotis bats from the Samarskaya Luka National Park.
| Trematode species |
Myotis brandtii |
Myotis
daubentonii
|
Myotis dasycneme |
Myotis
mystacinus
|
Myotis nattereri |
|
Plagiorchis koreanus (Ogata, 1938) |
81.4(1–111)/8.41
|
28.2(1–26)/1.5 |
63.7(1–52)/6.2 |
43.2(1–10)/1.7 |
40.8(1–13)/1.7 |
|
Plagiorchis mordovii Schaldybin, 1958 |
25.1(1–15)/1.0 |
3.8(1–5)/0.1 |
76.3(1–50)/11.6 |
– |
– |
|
Plagiorchis muelleri Tkach et Sharpilo, 1990 |
24.4(1–9)/0.7 |
– |
– |
13.6(1–6)/0.3 |
10.2(1–5)/0.2 |
|
Plagiorchis vespertilionis (Müller, 1780) |
18.2(1–8)/0.5 |
79.4(1–99)/12.7 |
11.1(1–21)/0.8 |
– |
– |
|
Prosthodendrium ascidia (Beneden, 1873) |
87.5(1–178)/25.9 |
– |
74.8(3–225)/30.4 |
72.0(1–51)/10.5 |
12.2(1–5)/0.4 |
|
Prosthodendrium chilostomum (Mehlis, 1831) |
32.8(1–62)/2.4 |
56.1(1–73)/7.7 |
16.3(1–13)/0.8 |
0.8(27)/0.2 |
6.1(1–3)/0.1 |
|
Prosthodendrium cryptolecithum Zdzitowiecki, 1969 |
39.7(1–70)/4.2 |
– |
53.3(1–94)/5.8 |
– |
– |
|
Prosthodendrium hurkovaae Dubois, 1960 |
– |
22.9(1–60)/2.0 |
29.6(1–21)/2.4 |
– |
5.1(1–3)/0.1 |
|
Prosthodendrium longiforme (Bhalerao, 1926) |
27.5(1–9)/0.8 |
66.8(1–110)/7.9 |
– |
– |
– |
|
Parabascus duboisi (Hurkova, 1961) |
54.7(1–59)/5.8 |
36.6(1–61)/4.6 |
63.9(1–58)/9.6 |
2.4(2–3)/0.1 |
3.1(1–5)/0.1 |
|
Lecithodendrium linstowi Dollfus, 1931 |
9.7(1–31)/0.9 |
76.7(1–125)/27.7 |
– |
1.6(1)/0.02 |
– |
| Total |
10 |
8 |
8(8.0) 2
|
6(6.6) |
6(6.1) |
The greatest richness of the trematode fauna was found in
M. brandtii, including 10 digenean species (
Table 1). The total infestation of
M. brandtii with trematodes reached 100%,
MA = 50.4. According to the Palia-Kovnatsky dominance index, two species,
Pr. ascidia (
D = 44.9) and
P. koreanus (13.5) dominated in the trematode fauna of this bat species. The group of subdominants included
P. duboisi (6.3),
Pr. cryptolecithum (3.3) and
Pr. chilostomum (1.6) (
Figure 2).
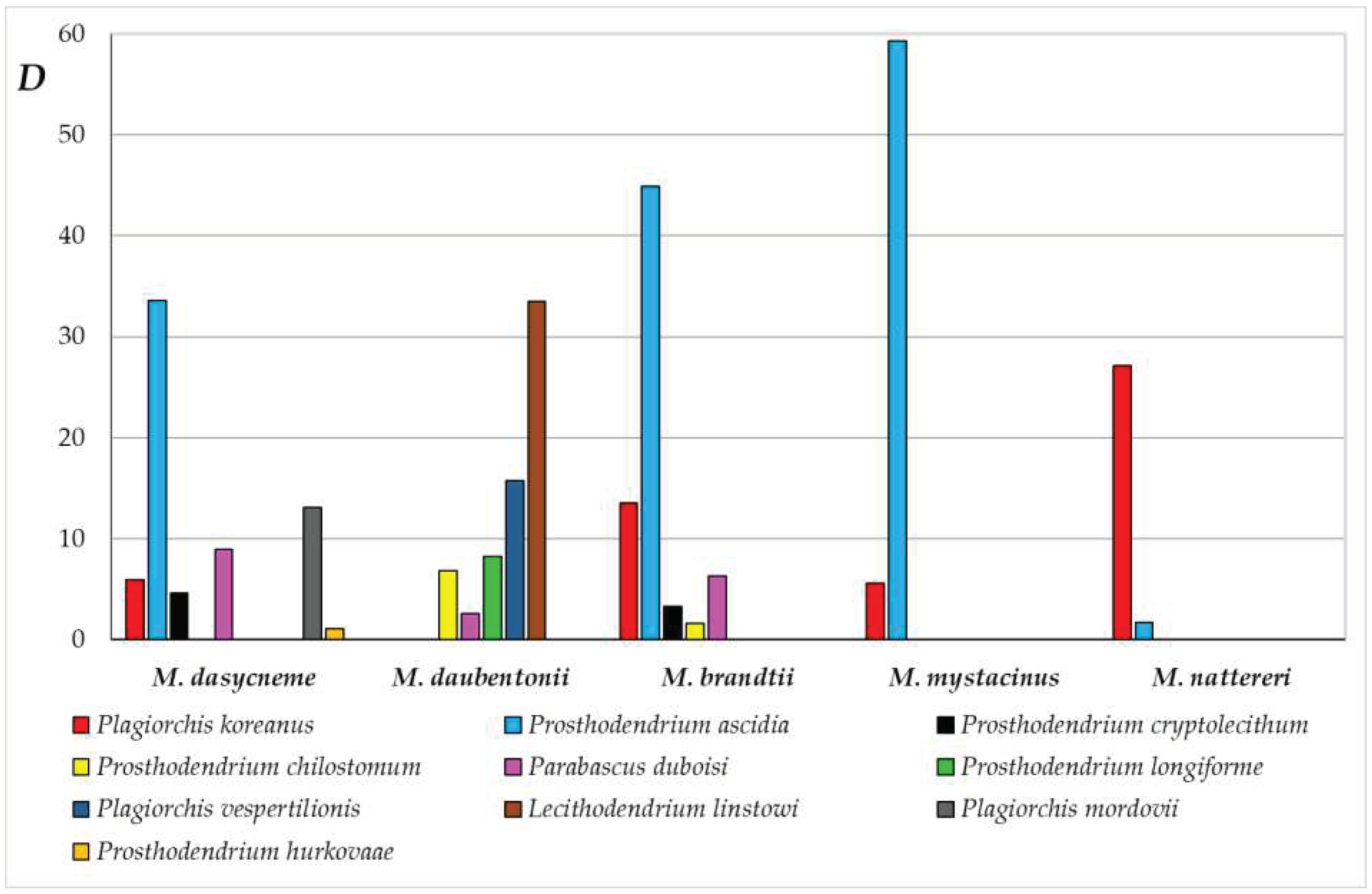
Figure 2.
Dominant and subdominant species of trematodes in Myotis bats from the Samarskaya Luka (the Palia-Kovnatsky dominance index).
Figure 2.
Dominant and subdominant species of trematodes in Myotis bats from the Samarskaya Luka (the Palia-Kovnatsky dominance index).
Eight species of trematodes were revealed in both M. daubentonii and M. dasycneme, the total infestation of which in both species was 100%, the abundance index was 64.2 and 67.6, respectively.
The trematode fauna of M. daubentonii is dominated by L. linstowi (33.5) and P. vespertilionis (15.7), while Pr. longiforme (8.2), Pr. chilostomum (6.8) and P. duboisi (2.6) are subdominants. In the trematode fauna of M. dasycneme dominants are Pr. ascidia (33.6) and P. mordovii (13.1); subdominants – P. duboisi (8.9), P. koreanus (5.9), Pr. cryptolecithum (4.6) and Pr. hurkovaae (1.1).
Myotis mystacinus has 6 species of trematodes, as well as M. nattereri. The total infection of M. mystacinus with trematodes was 60%, MA = 12.8. Among trematodes of M. mystacinus dominant is Pr. ascidia (59.3); while P. koreanus (5.6) is subdominant. The total infestation of M. nattereri compared to other Myotis bats is low and amounted to only 32%, and MA is 2.6. In the trematode fauna of M. nattereri dominant is P. koreanus (27.1), and subdominant is Pr. ascidia (1.7). The other four species in the trematode fauna of M. nattereri are adominants.
In the trematode fauna of
Myotis bats, the structure and number of dominant and subdominant species are different (
Figure 2). No common dominant or subdominant trematode species were revealed for all five
Myotis species. Only two species of trematodes,
P. koreanus and
Pr. ascidia are dominant or subdominant species for four bat species, and
P. duboisi for three
Myotis species (
Figure 2).
Only three of 11 trematode species were revealed in all five bat species:
P. koreanus,
Pr. chilostomum and
P. duboisi (
Table 1).
Prosthodendrium ascidia was found in four species of bats.
Plagiorchis vespertilionis, P. mordovii, P. muelleri, Pr. hurkovaae and
L. linstowi have each been recorded in three host species. Two host bat species were noted for
Pr. cryptolecithum and
Pr. longiforme (
Table 1).
Values of trematode species diversity in
Myotis bats from the Samarskaya Luka National Park are shown in the
Table 2.
Table 2.
Values of trematode biodiversity indexes in Myotis bats.
Table 2.
Values of trematode biodiversity indexes in Myotis bats.
| Index |
M. brandtii |
M. daubentonii |
M. dasycneme |
M. mystacinus |
M. nattereri |
| Margalef index, DMg
|
0.955 |
0.719 |
0.768 |
0.678 |
0.906 |
| Shannon index, H' |
1.555 |
1.584 |
1.592 |
0.616 |
1.127 |
| Shannon evenness index, E |
0.675 |
0.762 |
0.766 |
0.344 |
0.629 |
| Simpson index, d |
3.175 |
3.817 |
3.718 |
1.439 |
2.110 |
The diversity indices of the trematode fauna are significantly higher in
M. daubentonii and
M. dasycneme, with the exception of the values of the Margalef index. The values of the Margalef index in these bat species are slightly lower than those in
M. brandtii and
M. nattereri (
Table 2). The trematode fauna of
M. brandtii and
M. nattereri is less diverse. The least diversity of digenean fauna was observed in
M. mystacinus (
Table 2). This bat species has significantly lower Shannon diversity, Shannon evenness, Margalef and Simpson evenness indices than other species (
Table 2). The differences in the Shannon species diversity index of the trematode fauna of the five species of moths are significant (P < 0.001), with the exception of
M. daubentonii –
M. dasycneme pair (P > 0.05).
Comparison of the trematode infection of five
Myotis species using the Kruskal-Wallis test revealed significant differences (H = 425.6, P < 0.0001). Pairwise comparison of infestation of different
Myotis bats by digeneans using the Mann-Whitney test showed significant differences in most cases (P < 0.0001), with the exception of the pair
M. daubentonii –
M. dasycneme (
Table 3).
Table 3.
Validity of differences in infection of Myotis spp. by common trematode species.
Table 3.
Validity of differences in infection of Myotis spp. by common trematode species.
| Bat species |
Plagiorchis koreanus |
Parabascus duboisi |
Prosthodendrium chilostomum |
Total infestation |
| |
U |
p |
U |
p |
U |
p |
U |
p |
| M. mystacinus/ M. brandtii |
7549.0 |
0.0001 |
7222.0 |
0.0001 |
10540.0 |
0.0001 |
3895.0 |
0.0001 |
| M. mystacinus / M. daubentonii |
14310.0 |
0.016 |
10650.0 |
0.0001 |
7375.0 |
0.0001 |
2328.0 |
0.0001 |
| M. mystacinus / M. dasycneme |
5496.0 |
0.0001 |
3214.0 |
0.0001 |
7141.0 |
0.0001 |
1156.0 |
0.0001 |
| M. mystacinus / M. nattereri |
6048.0 |
0.858 |
6084.0 |
0.760 |
5740.0 |
0.013 |
2433.0 |
0.0001 |
| M. dasycneme /M. brandtii |
14840.0 |
0.073 |
13760.0 |
0.01 |
13860.0 |
0.001 |
1234.0 |
0.0001 |
| M. dasycneme /M. daubentonii |
10210.0 |
0.0001 |
12500.0 |
0.0001 |
9814.0 |
0.0001 |
17330.0 |
0.7455 |
| M. dasycneme /M. nattereri |
4296.0 |
0.0001 |
2551.0 |
0.0001 |
5970.0 |
0.027 |
69.5 |
0.0001 |
| M. brandtii / M. daubentonii |
13430.0 |
0.0001 |
27540.0 |
0.002 |
22380.0 |
0.0001 |
24650.0 |
0.0001 |
| M. brandtii / M. nattereri |
5873.0 |
0.0001 |
5738.0 |
0.0001 |
8847.0 |
0.0001 |
292.0 |
0.0001 |
| M. daubentonii / M. nattereri |
11390.0 |
0.057 |
8419.0 |
0.0001 |
6124.0 |
0.0001 |
166.5 |
0.0001 |
Analysis of the infestation of bats by common species using the Mann-Whitney test showed significant differences in the infection of bats with
Pr. chilostomum (in all cases),
P. koreanus and
P. duboisi (in most cases) (
Table 3). Differences in the
P. koreanus infestation in pairs
M. brandtii –
M. dasycneme,
M. mystacinus –
M. nattereri,
M. nattereri –
M. daunentonii, as well as in the infection of
M. mystacinus and
M. nattereri with
P. duboisi are not significant. There are no significant differences in the
P. koreanus infestation in the pairs
M. brandtii –
M. dasycneme,
M. mystacinus –
M. nattereri,
M. nattereri –
M. daunentonii, as well as in the infection of
M. mystacinus and
M. nattereri by
P. duboisi (
Table 3).
We conducted a comparative analysis of the helminth faunas in
Myotis species inhabiting the Samarskaya Luka. The similarity dendrogram of the parasite faunas in five bat species is shown in
Figure 3.
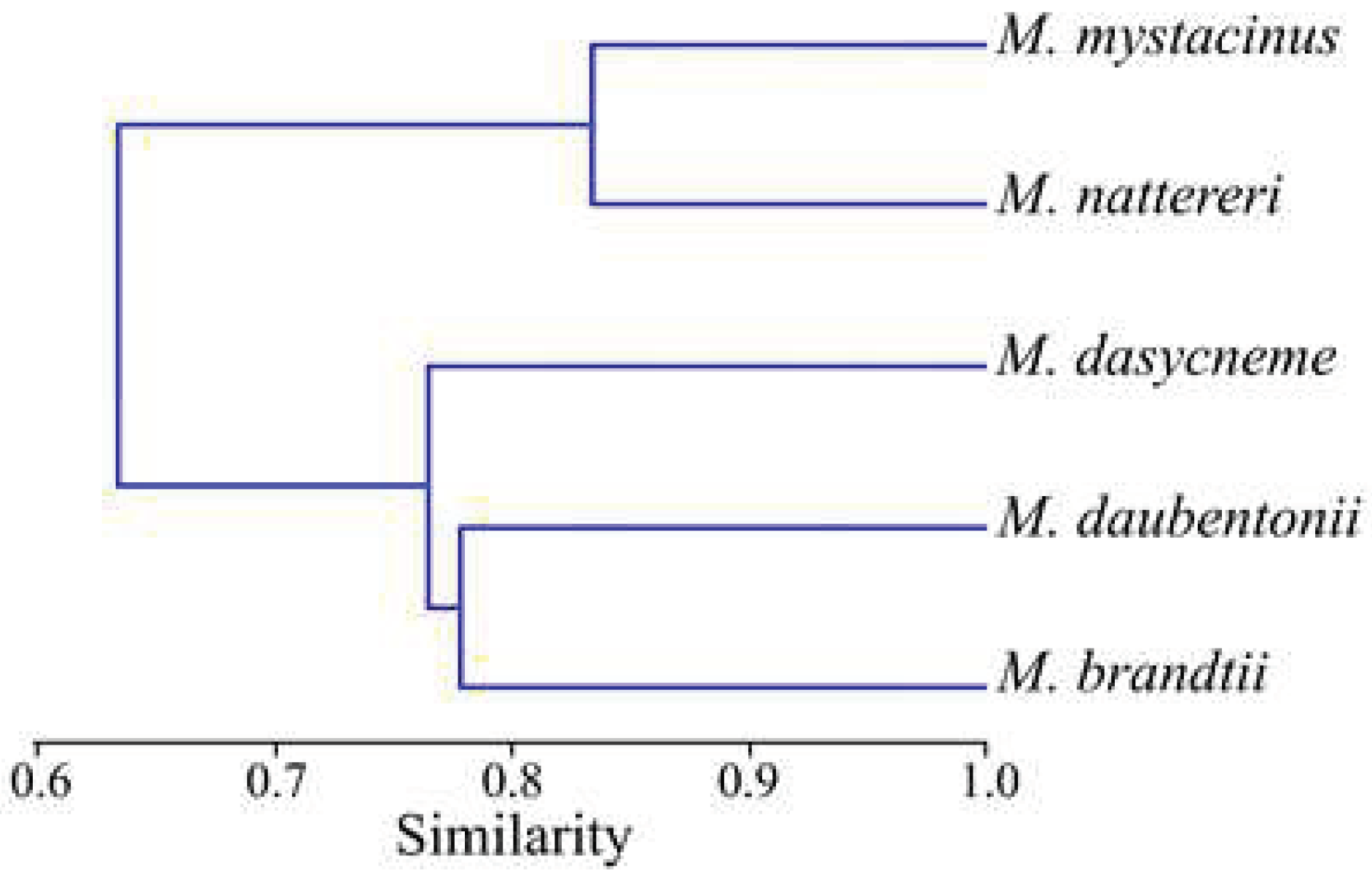
Figure 3.
Similarity dendrogram of trematode fauna in Myotis bats obtained using Morisita index (UPGMA) from the Samarskaya Luka National Park. Cophen. corr.: r = 0.802.
Figure 3.
Similarity dendrogram of trematode fauna in Myotis bats obtained using Morisita index (UPGMA) from the Samarskaya Luka National Park. Cophen. corr.: r = 0.802.
The cophenetic correlation coefficient is 0.802, which confirms the validity of the cluster. As a result of the clustering analysis, the considered
Myotis species were divided into two clusters, with the most similar helminth fauna (
Figure 3).
The first cluster is formed by the trematodes of
M. mystacinus and M.
nattereri, in which the greatest similarity of digenean communities is observed (0.83). They differ maximally in the structure of trematodes from the other three
Myotis species, which form the second cluster (
Figure 3).
The second cluster is formed by the clade of trematode fauna of
M. brandtii and
M. daubentonii (0.78), which is adjacent to the digenean fauna
M. dasycneme, more similar to
M. brandtii (0.78); and less with
M. daubentonii (0.75) (
Figure 3). The other pairs showed an average degree of similarity from 0.57 to 0.63.
4. Discussion
The analysis of the trematode fauna of Myotis bats in the Samarskaya Luka National Park showed that the species composition is the richest in M. brandtii (10 species). Slightly fewer species of digeneans were found in M. daubentonii and M. dasycneme (eight species each), while the smallest number of trematode species was found in M. mystacinus and M. nattereri – six species each.
All species of trematodes were revealed in
Myotis bats at the mature stage and are host-specific parasites of bats. Infection of bats with trematodes occurs entirely when feeding on semi-aquatic insects which serve as the second intermediate hosts of these digeneans. Thus, findings of
P. koreanus and
P. vespertilionis indicate the consumption by bats of adult forms of Diptera, Ephemeroptera, Megaloptera, Trichoptera and Odonata [
25]. The presence of
Prosthodendrium chilostomum in the helminth fauna of
Myotis bats indicates that the animals consume imagoes of Trichoptera and, possibly, Odonata [
25]. Second intermediate hosts of the trematode
Pr. ascidia are chironomid larvae, such as
Chironomis plumosus (Linnaeus, 1758) [
25,
63].
The life cycles of
P. mordovii,
P. muelleri,
L. linstowi,
Pr. longiforme,
Pr. cryptolecithum,
Pr. hurkovaae,
P. duboisi and
P. lepidotus are currently not studied. Probably, the second intermediate hosts of these digeneans, like other bat trematodes, are insects that develop in the aquatic environment [
25].
The present differences in the trematode fauna of various
Myotis species are associated mainly with preferences for certain food items. The widest feeding range was found in
M. daubentonii, whose diet in the Samarskaya Luka includes 10 food items from nine insect orders. The diet of
M. dasycneme,
M. brandtii and
M. mystacinus is slightly smaller, numbering nine food objects from eight insect orders [
12]. Also nine food items, but from seven insect orders are registered for
M. nattereri [
12].
Despite the similar diet composition of bats, the share of various food items in the diet of the five bat species is not the same [
12]. Thus, the diet basis for
M. daubentonii and
M. dasycneme consists of Trichoptera, Lepidoptera and Diptera. Coleoptera are somewhat less represented in the diet of these two species, and in
M. daubentonii, members of the orders Hemiptera and Homoptera are also present. Insects of other orders are rare in the diet of
M. daubentonii and
M. dasycneme [
12].
The diet of
M. brandtii is dominated by Lepidoptera and Diptera; Coleoptera are rather less represented; insects of the orders Trichoptera, Neuroptera, Homoptera and Hymenoptera are rarely found [
12]. In
M. mystacinus, the main diet components are Lepidoptera; Trichoptera are less represented, and Coleoptera, Homoptera and Diptera are rare. The diet of
M. nattereri is dominated by Lepidoptera. And members of Trichoptera, Coleoptera, Diptera, Hymenoptera and Homoptera are present in the diet in equal proportions [
12].
The differences identified in the infestation of chiropterans with common trematode species can be explained by different proportions of food items (second intermediate hosts of trematodes) in the diet of Myotis spp.
The diversity of the trematode fauna in bats, in addition to diet, also depends on the number of hunting grounds that chiropterans visit. Trematodes are parasites with a complicated life cycle, involving the change of hosts. Accordingly, the trematode fauna in Myotis bats is formed depending on the presence/absence of the different second intermediate trematode hosts (insects) in each site. Consequently, visiting a larger number of hunting sites by chiropterans helps to expand the diet of bats, which ultimately increases the likelihood of their infection with various species of trematodes.
Myotis spp. show unequal preferences for different types of hunting grounds. Among
Myotis bats, the largest number of hunting sites was found for
M. brandtii,
M. daubentonii and
M. dasycneme [
64].
Myotis nattereri prefers to hunt in only one type of hunting sites, while
M. mystacinus has two types of hunting grounds [
64]. Our finding of a greater number of trematode species in
M. brandtii,
M. daubentonii and
M. dasycneme, compared to
M. nattereri and
M. mystacinus, confirms these data (
Table 1).
In addition, when hunting sites overlap,
Myotis bats occupy different spatial niches [
64]. So,
M. daubentonii and
M. dasycneme hunt above the water surface,
M. brandtii feeds high in the tree crowns and
M. mystacinus hunts immediately below (the middle and lower parts of the tree crowns).
Myotis nattereri prefers to hunt in open, confined spaces above the ground, such as forest edges, wide forest glades and paths [
64]. The confinement of various
Myotis species to certain hunting grounds also affects the species structure and abundance of insects in the bat diet. Ultimately, this determines the species structure and abundance of trematodes in
Myotis bats.
Analysis of the species diversity of trematodes in
Myotis spp. showed that the trematode fauna is more diverse in
M. daubentonii and
M. dasycneme, despite the fact that a greater number of trematode species were found in
M. brandtii (
Table 2). The value of Shannon diversity index in
M. brandtii is lower, which is associated with the high abundance and dominance of one species in its trematode fauna,
Pr. ascidia. At the same time, the Margalef index, which takes into account species richness and the total number of parasite specimens, is significantly higher in
M. brandtii, from which the largest number of trematodes was collected (
Table 2). Abundance and dominance of
Pr. ascidia in
M. mystacinus also leads to a significant decrease in the Shannon index. As a result, the lowest species diversity is observed in this bat species.
A comparative analysis of the trematode fauna in five Myotis species inhabiting the Samarskaya Luka National Park showed a significant similarity in the structure of digeneans in different bat species. This is due to a similar lifestyle and feeding on semi-aquatic insects, which serve as the second intermediate hosts of trematodes. Thus, the finding of three common trematode species in bats (P. koreanus, Pr. chilostomum and P. duboisi) indicates the feeding on the same food objects (insects) – second intermediate hosts of these digenean species. Our data confirm the partial overlap of spatial and trophic niches of various Myotis spp. and their dividing thanks to food preferences and hunting grounds.
5. Conclusions
Thus, 11 trematode species were found in five species of Myotis bats in the Samarskaya Luka National Park. The trematode Prosthodendrium cryptolecithum was recorded for the first time among bats of the Russian fauna. A comparative analysis of the trematode fauna of Myotis bats showed that the species diversity of digeneans is higher in M. daubentonii and M. dasycneme. The trematode fauna in M. brandtii and M. nattereri is less diverse. The least number of trematodes was found in M. mystacinus.
The determining factor in the infection of bats by trematodes is feeding on semi-aquatic insects. The diversity of the trematode fauna in Myotis spp. is caused by the number of hunting sites that bats visit. Various Myotis species visiting the same hunting areas and consuming the same food objects (but in different proportions), have an average degree of similarity in trematode fauna. The revealed differences in the trematode fauna in Myotis spp. are associated both with the food preferences of various bat species and with the variety of hunting grounds used by the bats.
Our parasitological results confirm the data that the ecological niches of the five Myotis species partially overlap in the spatial and trophic components. An analysis of the trematode fauna in Myotis spp. showed that in the Samarskaya Luka there may be weak competition for food items among bats because of their specialization and divergence of home ranges.
Author Contributions
Conceptualization, N.Y.K. and A.A.K.; methodology, N.Y.K. and V.A.V.; software, A.A.K.; validation, N.Y.K., A.A.K. and V.A.V.; formal analysis, A.A.K.; investigation, N.Y.K. and A.A.K.; resources, A.A.K. and V.A.V; data curation, N.Y.K.; writing—original draft preparation, N.Y.K. and A.A.K.; writing—review and editing, A.A.K. and V.A.V.; visualization, N.Y.K. and A.A.K.; supervision, N.Y.K.; project administration, A.A.K.; funding acquisition, A.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, grant number 23-24-1002110021, https://rscf.ru/project/23-24-10021/.
Institutional Review Board Statement
Our research was conducted in compliance with the ethical standards of humane treatment of animals in accordance with the recommended standards described by the Council Directive 86/609/EEC of 24 November 1986 regarding the protection of animals used for experimental and other scientific purposes and with the Commission Recommendation 2007/526/EC of 18 June 2007 on guidelines for the accommodation and care of animals used for experimental and other scientific purposes. The study was carried out in accordance with the Agreement on Scientific Cooperation between the Institute of Ecology of the Volga Basin RAS (IEVB RAS) and the Samarskaya Luka National Park. The research theme, trapping and handling procedures were approved by the Ministry of Natural Resources and Ecology of Russia according to the research topics of the Samarskaya Luka National Park in 2005–2010.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors are deeply grateful to Vladimir Vekhnik (Zhiguli Nature Reserve) for his invaluable help in bat catching and identification.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carter, T.C.; Menzel, M.A.; Chapman, B.R.; Miller, K.V. Partitioning of Food Resources by Syntopic Eastern Red (Lasiurus borealis), Seminole (L. seminolus) and Evening (Nycticeius humeralis) Bats. Amer. Midl. Nat. 2004, 151, 186–191. [Google Scholar] [CrossRef]
- Smirnov, D.G.; Vekhnik, V.P. Abundance and structure of bat communities (Chiroptera: Vespertilionidae), wintering in the artificial caves in the Samarskaya Luka. Rus. J. Ecol. 2011, 1, 64–72. [Google Scholar]
- Mancina, C.A.; García-Rivera, L.; Miller, B.W. Wing morphology, echolocation, and resource partitioning in syntopic Cuban mormoopid bats. J. Mammal. 2012, 93(5), 1308–1317. [Google Scholar] [CrossRef]
- Andreas, M., Reiter A., Cepakova E.; Uhrin M. Body size as an important factor deter-mining trophic niche partitioning in three syntopic rhinolophid bat species. Biologia 2013, 68(1), 170–175. [CrossRef]
- Salinas-Ramos, V.B., Ancillotto L., Bosso L., Sanchez-Cordero V., Russo D. Interspe-cific competition in bats: state of knowledge and research challenges. Mammal Review 2020, 50(1), 68–81. [CrossRef]
- Gause, G.F. The struggle for existence; Williams&Wilkins: Baltimore, USA, 1934; pp. 1–163. [Google Scholar]
- Strelkov, P.P.; Ilyin, V.Yu. Bats of the South of the Middle and Lower Volga regions. Proceedings of Zoological Institute of the Academy of Sciences of the USSR. 1990. 225: 42–167.
- Il’in, V.Yu.; Vekhnik, V.P.; Smirnov, D.G.; Kurmaeva, N.M.; Zolina, N.F.; Matrosova, O.M. Dynamics of abundance of bats (Chiroptera: Vespertilionidae) during hibernation in caves of the Samarian Luka over a 20-year period. Rus. J. Ecol. 1999, 30(6), 428–431. [Google Scholar]
- Smirnov, D.G.; Vekhnik, V.P.; Kurmaeva, N.M.; Shepelev, A.A.; Il’in, V.Yu. Species structure and dynamics of bat communities (Chiroptera: Vespertilionidae) hibernating in artificial caves of Samara Luka. Biol. Bull. 2007, 34(5), 507–516. [Google Scholar] [CrossRef]
- Mammals of Russia. Available online: http://rusmam.ru/mammal/index?sort=sort (accessed on 25 September 2023).
- Smirnov, D.G.; Vekhnik, V.P. Biotopic structure of bat communities inhabiting flood plain ecosystems of the Samarskaya Luka. Proc. Samara Sci. Cent. RAS 2012, 14(1), 177–179. [Google Scholar]
- Smirnov, D.G.; Vekhnik, V.P. Ecology of nutrition and differentiation of the trophic niches of bats (Chiroptera: Vespertilionidae) in Floodplain Ecosystems of the Samara Bend. Biol. Bull. 2014, 1, 53–64. [Google Scholar]
- Frank, R.; Kuhn, T.; Werblow, A.; Liston, A.; Kochmann, J.; Klimpel, S. Parasite diversity of European Myotis species with special emphasis on Myotis myotis (Microchiroptera, Vespertilionidae) from a typical nursery roost. Parasite & Vectors 2015, 8, 101–114. [Google Scholar] [CrossRef]
- Matsaberidze, G.V.; Khotenovsky, I.A. To the trematode fauna of bats in Georgia. In Helminth fauna of animals and plants in Georgia; Kurashvili, B.E., Ed.; Metsniereba: Tbilisi, Georgia, 1967; pp. 83–94. [Google Scholar]
- Zdzietowiecki, K. Helminths of bats in Poland. II. Trematodes of the subfamily Lecithodendriinae. Acta Parasitol. Pol. 1969, 16(24), 208–226. [Google Scholar]
- Zdzietowiecki, K. Helminths of bats in Poland. III. Trematodes of the subfamily Lecithodendriidae, except for Lecithodendriinae. Acta Parasitol. Pol. 1969, 16(24), 227–237. [Google Scholar]
- Skvortsov, V.G. Trematodes of the genus Prosthodendrium (family Lecithodendriidae) from bats in Moldavia. In Parasites of vertebrate animals; Spassky, А.А., Ed.; Stiintsa: Kishinev, Moldova, 1969; pp. 87–97. [Google Scholar]
- Zdzietowiecki, K. Helminths of bats in Poland. I. Cestodes and trematodes of the family Plagiorchiidae. Acta Parasitol. Pol. 1970, 17(20), 175–188. [Google Scholar]
- Khotenovsky, I.A. Family Pleurogenidae Looss, 1899. In Trematodes of animals and man. Essentials of trematodology; Skryabin, K.I., Ed.; Nauka: Moscow, Russia, 1970; Volume 23, pp. 135–306. [Google Scholar]
- Skvortsov, V.G. Trematodes of the family Lecithodendriidae from bats in Moldavia. In Parasites of animals and plants in Moldavia, Spassky A.A., Ed.; Stiintsa: Kishinev, Moldova, 1970; Volume 5, pp. 17–36. [Google Scholar]
- Skvortsov, V.G. Nematodes of bats from Moldavia (Report 2). In Parasites of animals and plants in Moldavia, Spassky A.A., Ed.; Stiintsa: Kishinev, Moldova, 1971; Volume 7, pp. 75–93. [Google Scholar]
- Shakhtakhtinskaya, Z.M.; Mustafaev, Yu.Sh.; Sailov, D.I. About helminths of some bats of Azerbaijan. Proc. Azerb. St. Univ. Biol. Ser. 1971, 2, 25–30. [Google Scholar]
- Skvortsov, V.G. Ecological and faunistic analysis of helminth fauna of bats in Moldavia. In Parasites of animals and plants, Spassky A.A., Ed.; Stiintsa: Kishinev, Moldova, 1973; Volume 9, pp. 92–155. [Google Scholar]
- Melnichenko, E.D.; Panasenko, N.A. To the helminth fauna of bats in the Middle Dnieper region. Vest. zool. 1979, 3, 76–78. [Google Scholar]
- Sharpilo, V.P.; Iskova, N.I. Trematodes. Plagiorchiata. Fauna of Ukraine; Vol. 34(30); Naukova Dumka: Kiev, Ukraine, 1989; pp. 1–280. [Google Scholar]
- Tkach, V.V. First finding of males of Pterygodermatites bovieri (Nematoda, Rictulariidae) parasitizing bats. Zool. Zhurn. 1991, 70(9), 125–127. [Google Scholar]
- Tkach, V.V. Helminths of bats from the fauna of Ukraine. In: Bats: materials of 6th meeting on bats of the CIS countries. Khujand State University: Khujand, Tajikistan, 1995; pp. 90–95.
- Bychkova, E.I.; Akimova, L.N.; Degtyarik, S.M.; Yakovich, M.M. Helminths of vertebrares and man in Belarus; Belarusskaya Nauka Publish.: Minsk, Belarus, 2017; pp. 1–316. [Google Scholar]
- Matskási, I. The Systematico–faunistical survey of the Trematode fauna of Hungarian bats. I. Annales Hist.-Nat. Mus. Nation. Hung. Pars Zool. 1967, (59), 217–238. [Google Scholar]
- Odening, K. Exkretionssystem und systematische stellung einiger fledermaustrematoden aus Berlin und umgebung nebst bemerkungen zum Lecithodendrioiden komplex. Zeitsch. Parasitenk. 1964, 24(5), 453–483. [Google Scholar] [CrossRef] [PubMed]
- Bakke, T.A.; Mehl, R. Two species of fluke recorded in bats in Norway. Fauna, Oslo, Norway 1977, 30(4), 224–226. [Google Scholar]
- Ricci, M. Report on trematode parasites of bats in Italy. Parasitologia 1995, 37(2-3), 199–214. [Google Scholar]
- Tkach, V.V.; Pawlowski, J.; Sharpilo, V.P. Molecular and morphological differentiation between species of the Plagiorchis vespertilionis group (Digenea, Plagiorchiidae) occurring in European bats, with a re-description of P. vespertilionis (Muller, 1780). Syst. Parasitol. 2000, 47, 9–22. [Google Scholar] [CrossRef]
- Shimalov, V.; Demyanchik, M.; Demyanchik, V. A study on the helminth fauna of the bats (Mammalia, Chiroptera: Vespertilionidae) in Belarus. Parasitol. Res. 2002, 88(11), 1011. [Google Scholar] [PubMed]
- Sumer, N.; Yildirimhan, H.S. Intestinal Helminth Parasites of Two Bat Species of the Genus Myotis Kaup, 1829 (Chiroptera: Vespertilionidae) from Turkey, with DNA Sequencing of Helminth Nuclear LSR rDNA. Acta Zool. Bulg. 2019, 71(2), 273–278. [Google Scholar]
- Kochseder, G. Untersuchungen über trematoden und cestoden aus fledermäusen in der Steiermark. In Untersuchungen über Trematoden und Cestoden aus Fledermäusen in der Steiermark. Sitzungsberichten der Österr. Akademie der Wissenschaften; Springer: Berlin, Heidelberg, Germany, 1969; Volume 8, pp. 205–232. [Google Scholar] [CrossRef]
- Alvarez, F.; Rey, J.; Quinterios, P.; Iglesias, R.; Santos, M.; Sanmartin, M.L. Helminth parasites in some Spanish bats. Wiad. Parazytol. 1991, 37(3), 321–329. [Google Scholar] [PubMed]
- Shimalov, V.V.; Demyanchik, M.G.; Demyanchik, V.T. The helminth fauna of bats (Microchiroptera) in the Republic of Belarus. Proc. Nation. Acad. Sci. Belarus. Biol. Ser. 2011, 3, 104–110. [Google Scholar]
- Sumer, N.; Yildirimhan, H.S. Helminth parasites of the Whiskered brown bat, Myotis aurescens (Kuzyakin, 1935) (Chiroptera: Vespertilionidae) from Turkey. Acta Zoo. Bulg. 2018, 70(1), 113–116. [Google Scholar]
- Horvat, Z.; Cabrilo, B., Paunovic, M., Karapandza, B., Jovanovic, J., Budinski, I., Bjelic Cabrilo, O. Gastrointestinal digeneans (Platyhelminthes: Trematoda) of horseshoe and vesper bats (Chiroptera: Rhinolophidae and Vespertilionidae) in Serbia. Helminthologia 2017, 54(1), 17–25. [CrossRef]
- Sten’ko, R.P.; Dulitskiy, A.I.; Karpenko, O.V.; Dushevskiy, V.P. Helminth fauna of Crimean chiropterans. Zool. Zhurn. 1986, 65(8), 1133–1139. [Google Scholar]
- Podvyaznaya, I.M. The fine structure of the digestive tract of Allassogonoporus amphoraeformis (Trematoda: Allassogonoporidae). Parazitologiya 1994, 28(5), 403–412. [Google Scholar]
- Podvyaznaya, I.M. The fine structure of the male reproductive system and genital atrium of bat parasite Allassogonoporus amphoraeformis (Trematoda: Allassogonoporidae). Parazitologiya 1996, 30(3), 229–235. [Google Scholar]
- Demidova, T.N.; Vekhnik, V.P. Trematodes (Trematoda, Monorchiidae) of Myotis brandtii and M. mystacinus (Chiroptera, Vespertilionidae) from the Samarskaya Luka (Russia). Vestn. Zool. 2004, 38(5), 71–74. [Google Scholar]
- Lebedeva, D.I.; Belkin, V.V.; Stanyukovich, M.K.; Bespyatova, L.A.; Bugmyrin, S.V. First records of bat parasites in Karelia. Transact. Karel. Res. Cent. RAS 2020, 8, 120–125. [Google Scholar] [CrossRef]
- Gulyaev, V.D.; Orlovskaya, O.M.; Dokuchaev, N.Е. Helminths of bats from Magadan region. Plecotus et al. 2002, 5, 86–92. [Google Scholar]
- Kirillov, A.A.; Ruchin, A.B.; Artaev, O.N. Helminths of bats (Chiroptera) from Mordovia. Bull. Volzhsk. Univ. Tatisch. 2015, 4(19), 319–328. [Google Scholar]
- Ruchin, A.B.; Kirillov, A.A.; Chikhlyaev, I.V.; Kirillova, N.Y. Parasitic worms of land vertebrates of the Mordovia Nature Reserve; Flora and fauna of reserves; Volume 124; Сommittee of RAS for the Conservation of Biological Diversity: Moscow, Russia, 2016; pp. 1–72. [Google Scholar]
- Kirillov, A.A.; Kirillova, N.Yu.; Chikhlyaev, I.V. Trematodes of terrestrial vertebrates of the Middle Volga region; Cassandra: Togliatti, Russia, 2012; pp. 1–329. [Google Scholar]
- Kirillov, A.A.; Kirillova, N.Yu.; Vekhnik, V.P. Trematodes (Trematoda) of bats (Chiroptera) from the Middle Volga region. Parazitologiia 2012, 46(5), 384–413. [Google Scholar]
- Kirillova N.Yu., Kirillov A.A., Vekhnik V.P. Comparative analysis of the helminth fauna of Myotis brandtii and Myotis mystacinus (Сhiroptera, Vespertiluonidae) in the Samarskaya Luka National Park (Russia). Nat. Conserv. Res. 2022, 7(3), 1–14. [CrossRef]
- Kirillova, N.Yu.; Shchenkov, S.V.; Kirillov, A.A.; Ruchin, A.B. Trematodes of genera Gyrabascus and Parabascus from bats in European Russia: morphology and molecular phylogeny. Biology 2022, 11, 878. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, N.Yu.; Kirillov, A.A.; Vekhnik, V.A.; Ruchin, A.B. Trematodes of small mammals (Soricomorpha, Erinaceomorpha, Chiroptera, Rodentia) in the Middle-Volga region (European Russia). 2023. Version 1.6. Institute of Ecology of the Volga river basin of Russian Academie of Sciences. Occurrence dataset. https://doi.org/10.15468/gmt9ct accessed via GBIF.org on 2023-09-26. [CrossRef]
- Jones, C.; McShea, W.J; Conroy, M.J.; Kunz, J.H. Capturing mammals. In Measuring and Monitoring Biological Diversity: Standard Methods for Mammals; Wilson, D.E., Cole, F.R., Nichils, J.D., Rudran, R., Foster, M.S., Eds.; Smithsonian Institution Press: Washington DC, USA, 1996; pp. 115–155. [Google Scholar]
- Ivashkin, V.M.; Kontrimavichus, V.L.; Nasarova, N.S. Methods of the collection and studies of helminths of land mammals; Nauka: Moscow, Russia, 1971; pp. 3–123. [Google Scholar]
- Anikanova, V.S.; Bugmyrin, S.V.; Ieshko, E.P. Methods of the collection and studies of helminths of small mammals; Karelian Scientific Center of RAS: Petrozavodsk, Russia, 2007; pp. 3–145. [Google Scholar]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological diversity and its measurement. Mir: Moscow, Russia, 1992, pp. 3–182.
- Bakanov, A.I. Quantitated estimation of dominance in ecological communities. Borok, Russia, 1987, 64 p. The manuscript was deposited in All-Union Institute of Scientific and Technical Information (VINITI) by 08.12.1987. No. 8593‒В87.
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Version 2.16. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Smith, E.P.; van Belle, G. Nonparametric estimation of species richness. Biometrics 1984, 40, 119–129. [Google Scholar] [CrossRef]
- Poulin, R. Comparison of three estimators of species richness in parasite component communities. J. Parasitol. 1998, 84(3), 485–490. [Google Scholar] [CrossRef] [PubMed]
- Lühe, M. Parasitische Plattwurmer. I. Trematodes; Die Susswasserfauna Deutschlands. Verlag von Gustav Fischer: Jena, Germany, 1909; Volume 17, pp. 1–218. [Google Scholar]
- Smirnov, D.G.; Vekhnik, V.P. Association of bats to different types of hunting grounds in floodplain ecosystems in the Samarskaya Luka. In Recent problems of biological research in western Siberia and adjacent territories; Starikov, V.P., Ed.; Timer Publish.: Surgut, Russia, 2011; pp. 98–101. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).