The signalling hormone jasmonic acid (JA) regulates up to 85% of wound- and herbivore-induced genes in Arabidopsis thaliana leaves (Acosta & Farmer, 2010). Besides regulating the response to herbivore attacks, JAs are also involved in regulating plant growth processes, such as primary root growth, reproductive development, and leaf senescence (Wasternack & Hause 2013; Huang et al. 2017). The amino acid conjugate JA-Ile has been identified as the main endogenous bioactive JA molecule (Fonseca et al. 2009). However, recent studies report other endogenous bioactive JA molecules, such as JA conjugated with other amino acids or hydroxylated JA-Ile (Yan et al. 2016).
The JA signalling pathway can integrate primary and specialized metabolism via the regulation of repressor-transcription factor complexes, which may be associated with re-allocation of carbon and recalibration of growth rates (Guo et al. 2018). Therefore, the activation of inducible plant defences often results in reduced growth and development (Karasov et al. 2017). This is commonly referred to as the growth-defence trade-off (Züst et al. 2017). The mechanisms of growth-defence trade-offs have been widely studied in plants against insect herbivory (reviewed in Züst et al. 2017; He et al. 2022). Even knowledge about how to uncouple this mechanism has still been less explored, researchers are always searching for an effective and efficient approaches to solve this scientific question.
A previously described JA-Ile biosynthesis inhibitor, jarin-1, was firstly shown to exclusively inhibit the JA-conjugating enzyme JAR1 in A. thaliana and Cardamine hirsuta. Jarin-1 application prevented active JA signaling by blocking the formation of JA-Ile, the bioactive form of JA (Meesters et al. 2014; Ishimaru et al. 2018). Thereafter, jarin-1 has been used to inhibit JA induced responses in other species, such as strawberry, tomato and potato (Delgado et al. 2018; Liu et al. 2022; Munawar et al. 2023). However, the effect of jarin-1 in inhibiting JA-Ile biosynthesis was not always evidenced as done for A. thaliana (Meesters et al. 2014). This leads to the hypothesis that the function of this chemical inhibitor is highly dependent on the plant species used.
In this study, we investigated whether jarin-1 does act on JA-Ile biosynthesis in other plant species, such as Medicago truncatula, Solanum lycopersicum and Brassica nigra. We performed the well-established root growth inhibition assay by treatment of seedlings with MeJA and applied jarin-1 simultaneously with MeJA to check, whether jarin-1 prevents the root growth inhibition or not. Our results show clearly that jarin-1 did not rescue the inhibitory effect of MeJA in all plant species tested.
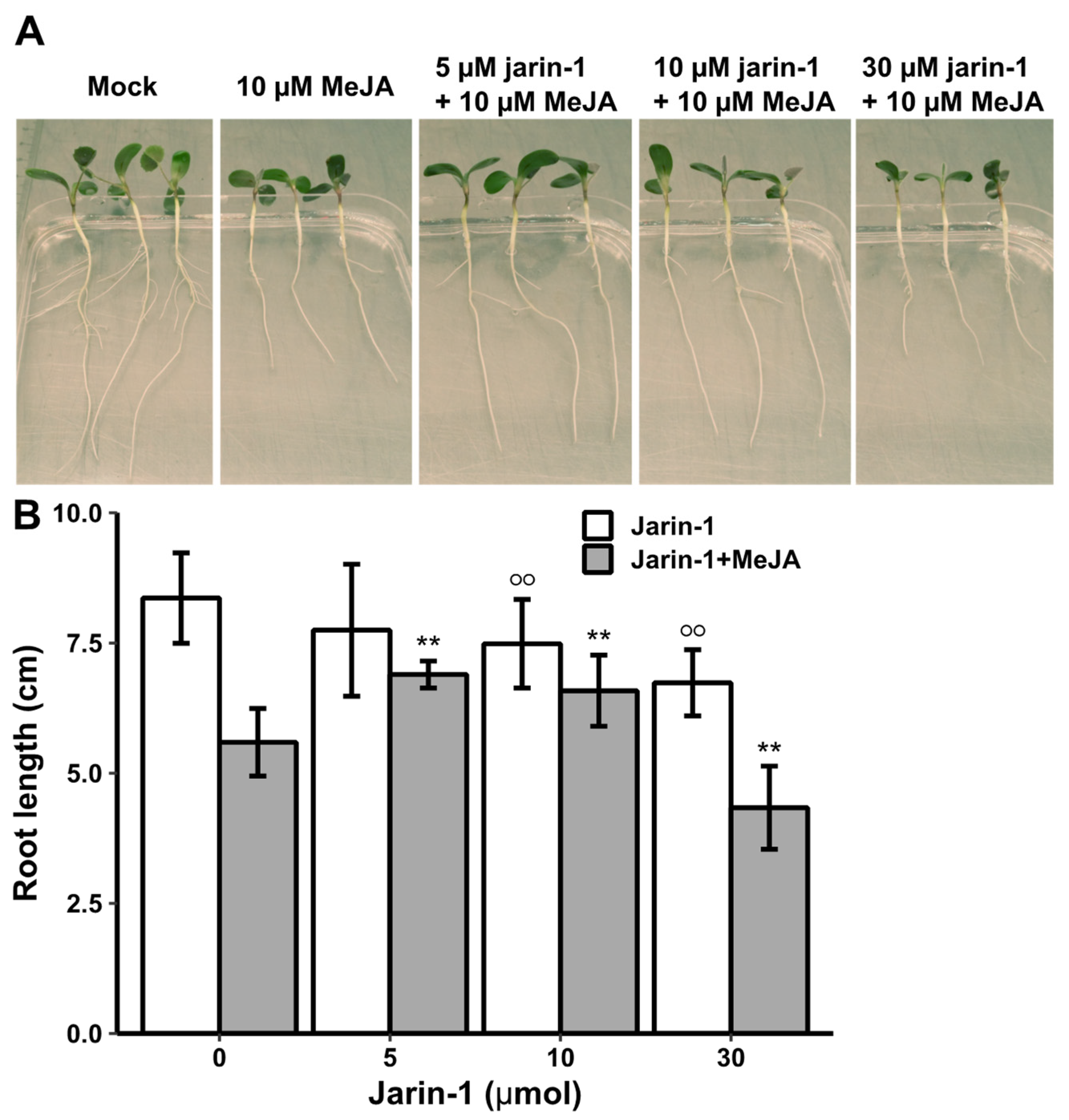
First, to test whether jarin-1 counteracts MeJA function in
M. truncatula, we used seedlings grown in vitro and treated them with different concentrations of jarin-1 (0, 5, 10 or 30 µM). Half of the plants were simultaneously treated with 10 µM MeJA, a treatment leading to significantly decreased root growth in various plant species (Staswick et al. 1992). Root length was determined eight days after treatment (
Figure 1). Treatment with 30 µM jarin-1 alone had a clear negative effect on root growth, which was also observed on seedlings treated with 10 µM jarin-1, although to a smaller extent (
Figure 1). Simultaneous application of 5 or 10 µM jarin-1 partially alleviated the root growth inhibition by MeJA. This demonstrated that, like in
A. thaliana (Meesters et al. 2014), jarin-1 can partially mitigate MeJA-induced root growth inhibition in
M. truncatula. Based on our results and the results from the previous report in
A. thaliana, we selected the 10 µM jarin-1 treatment to test its effect on MeJA-root growth in seedlings of
S. lycopersicum and
B. nigra. Application of 10 µM jarin-1 had, however, no effect on the MeJA-induced root growth inhibition in seedlings of
S. lycopersicum and
B. nigra. In other words, seedlings treated with 10 µM MeJA and 10 µM jarin-1 simultaneously, had similar root lengths as seedlings treated with 10 µM MeJA alone (
Figure 2,
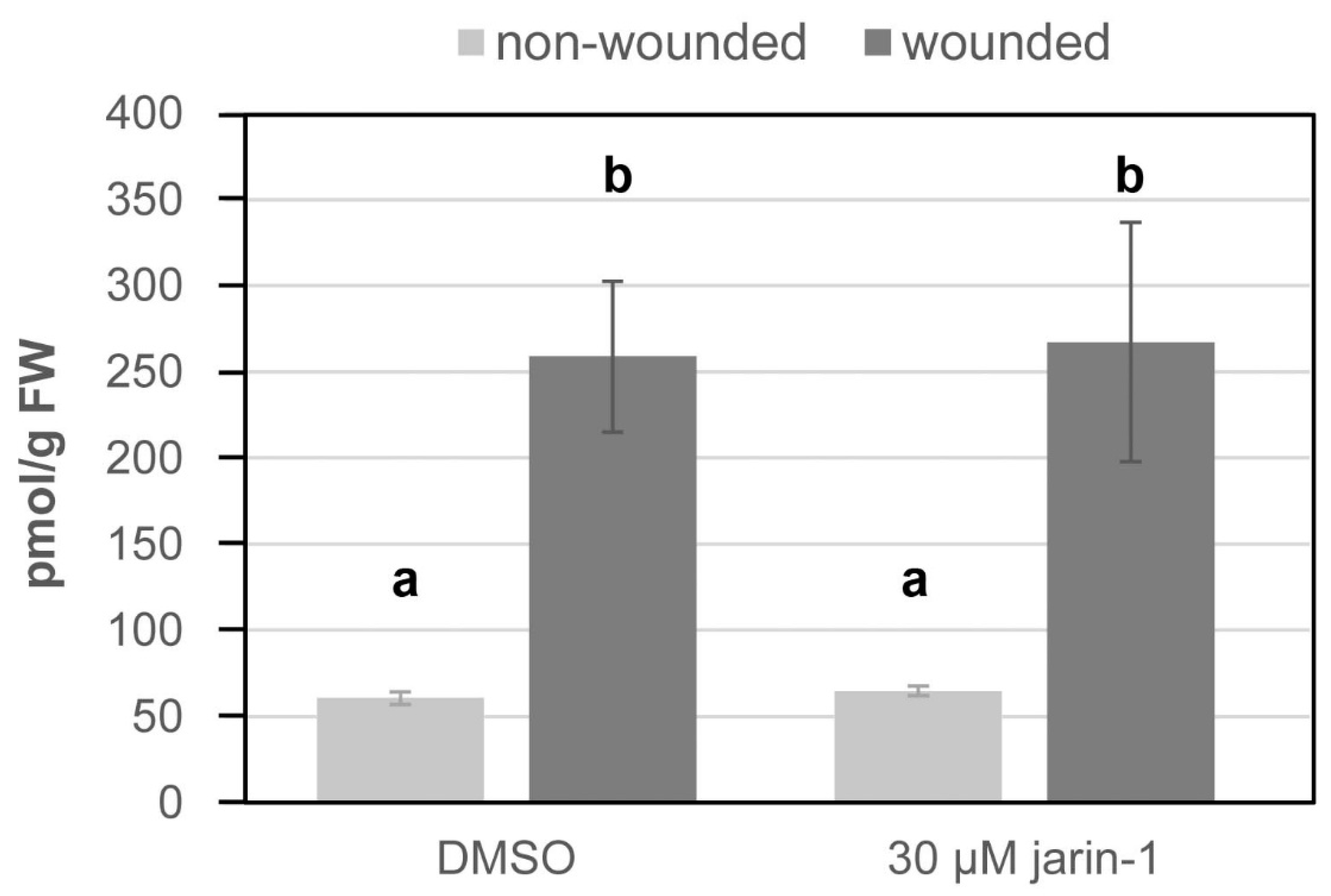
Table 1). This suggests that the function of jarin-1 might dependent on the plant species. To check the effect of jarin-1 on the endogenous rise of JA-Ile in tomato leaves upon wounding, leaf disks were treated with 30 µM jarin-1 for one hour followed by wounding with forceps. As the tomato plants for this bioassay are 6 weeks old, we applied 30 µM jarin-1 instead of 10 µM jarin-1 to make the chemical penetrate into leaf tissues and function effectively. Determination of JA-Ile levels at one hour after wounding revealed that jarin-1 had no effect on the wound-induced biosynthesis of JA-Ile in tomato leaf disks (
Figure 3). Based on our results, we propose that the function of jarin-1 as inhibitor of formation of active jasmonates depends on the plant species. To use it, researchers should test the activity of jarin-1 in their model plants before addressing specific scientific questions related to the function of JAR1 or JA-Ile.
In conclusion, jarin-1 is a small-molecule inhibitor of jasmonate responses that is active in A. thaliana and M. truncatula, but not in S. lycopersium and B. nigra. It still serves as an effective chemical tool in dissecting the complex jasmonate signaling networks to avoid the time-consuming genetic knockout for non-model plants. Its usability has, however, to be tested for other plant species.
Author Contributions
MZ designed and performed the experiments and wrote the first version of this manuscript. BH and NvMD took the lead of revising this manuscript. All authors contributed to the final version of this manuscript.
Data Availability Statement
Data are available from the corresponding authors upon reasonable request.
Acknowledgements
This research was supported by iDiv funded by the German Research Foundation (DFG–FZT 118, 202548816). We gratefully acknowledge Ainhoa Martínez-Medina for reading a previous version of this manuscript. We gratefully acknowledge Jessil Ann Pajar for providing tomato and Brassica seeds.
Conflicts of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
References
- Acosta I.F., Farmer E.E. (2010) Jasmonates. Arabidopsis Book, 8, e0129.
- Balcke G.U., Handrick V., Bergau N., Fichtner M., Henning A., Stellmach H., Tissier A., Hause B., Frolov A. (2012) An UPLC-MS/MS method for highly sensitive high-throughput analysis of phytohormones in plant tissues. Plant Methods, 8, 47.
- Delgado L.D., Zúñiga P.E., Figueroa N.E., Pastene E., Escobar-Sepúlveda H.F., Figueroa P.M., Garrido-Bigotes A., Figueroa C.R. (2018) Application of a JA-Ile biosynthesis inhibitor to methyl jasmonate-treated strawberry fruit induces upregulation of specific MBW complex-related genes and accumulation of proanthocyanidins. Molecules, 23, 1433.
- Fonseca S., Chini A., Hamberg M., Adie B., Porzel A., Kramell R., Miersch O., Wasternack C., Solano R. (2009) (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nature Chemical Biology, 5, 344–350.
- Guo Q., Major I.T., Howe G.A. (2018) Resolution of growth–defense conflict: mechanistic insights from jasmonate signalling. Current Opinion in Plant Biology, 44, 72–81. mechanistic insights from jasmonate signalling: , Major I.T., Howe G.A. (2018) Resolution of growth–defense conflict.
- He Z., Webster S., He S.Y. (2022) Growth–defense trade-offs in plants. Current Biology, 32, R634–R639.
- Howe G.A., Major I.T., Koo A.J. (2018) Modularity in jasmonate signalling for multistress resilience. Annual Review of Plant Biology, 69, 387–415.
- Huang H., Liu B., Liu L., Song S. (2017) Jasmonate action in plant growth and development. Journal of Experimental Botany, 68, 1349–1359.
- Ishimaru Y., Hayashi K., Suzuki T., Fukaki H., Prusinska J., Meester C., Quareshy M., Egoshi S., Matsuura H., Takahashi K., Kato N., Kombrink E., Napier R.M., Hayashi K.I., Ueda M. (2018) Jasmonic acid inhibits auxin-induced lateral rooting independently of the CORONATINE INSENSITIVE1 receptor. Plant Physiology, 177, 1704–1716.
- Karasov T.L., Chae E., Herman J.J., Bergelson J. (2017) Mechanisms to mitigate the trade-off between growth and defense. Plant Cell, 29, 666–680.
- Liu X., Cheng L., Li R., Cai Y., Wang X., Fu X., Dong X., Qi M., Jiang C.Z., Xu T., Li T. (2022) The HD-Zip transcription factor SlHB15A regulates abscission by modulating jasmonoyl-isoleucine biosynthesis. Plant Physiology, 189, 2396–2412. 2396.
- Meesters C., Mönig T., Oeljeklaus J., Krahn D., Westfall C.S., Hause B., Jez J.M., Kaiser M., Kombrink E. (2014) A chemical inhibitor of jasmonate signalling targets JAR1 in Arabidopsis thaliana. Nature Chemical Biology, 10, 830–836.
- Munawar A., Xu Y., Abou El-Ela A.S., Zhang Y., Zhong J., Mao Z., Chen X., Guo H., Zhang C., Sun Y., Zhu Z., Baldwin I.T., Zhou W. (2023) Tissue-specific regulation of volatile emissions moves predators from flowers to attacked leaves. Current Biology, 33, 2321-2329.
- Staswick P.E., Sut W., Howell S.H. (1992) Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proceedings of the National Academy of Sciences.
- Wasternack C., Hause B. (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Annals of Botany, 111, 1021–1058.
- Yan J., Li S., Gu M., Yao R., Li Y., Chen J., Yang M., Tong J., Xiao L., Nan F., Xie D. (2016) Endogenous bioactive jasmonate is composed of a set of (+)-7-iso-JA-amino acid conjugates. Plant Physiology, 172, 2154–2164.
- Züst T., Agrawal A.A. (2017) Trade-offs between plant growth and defense against insect herbivory: an emerging mechanistic synthesis. Annual Review of Plant Biology, 68, 513–534.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).