Submitted:
28 September 2023
Posted:
29 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
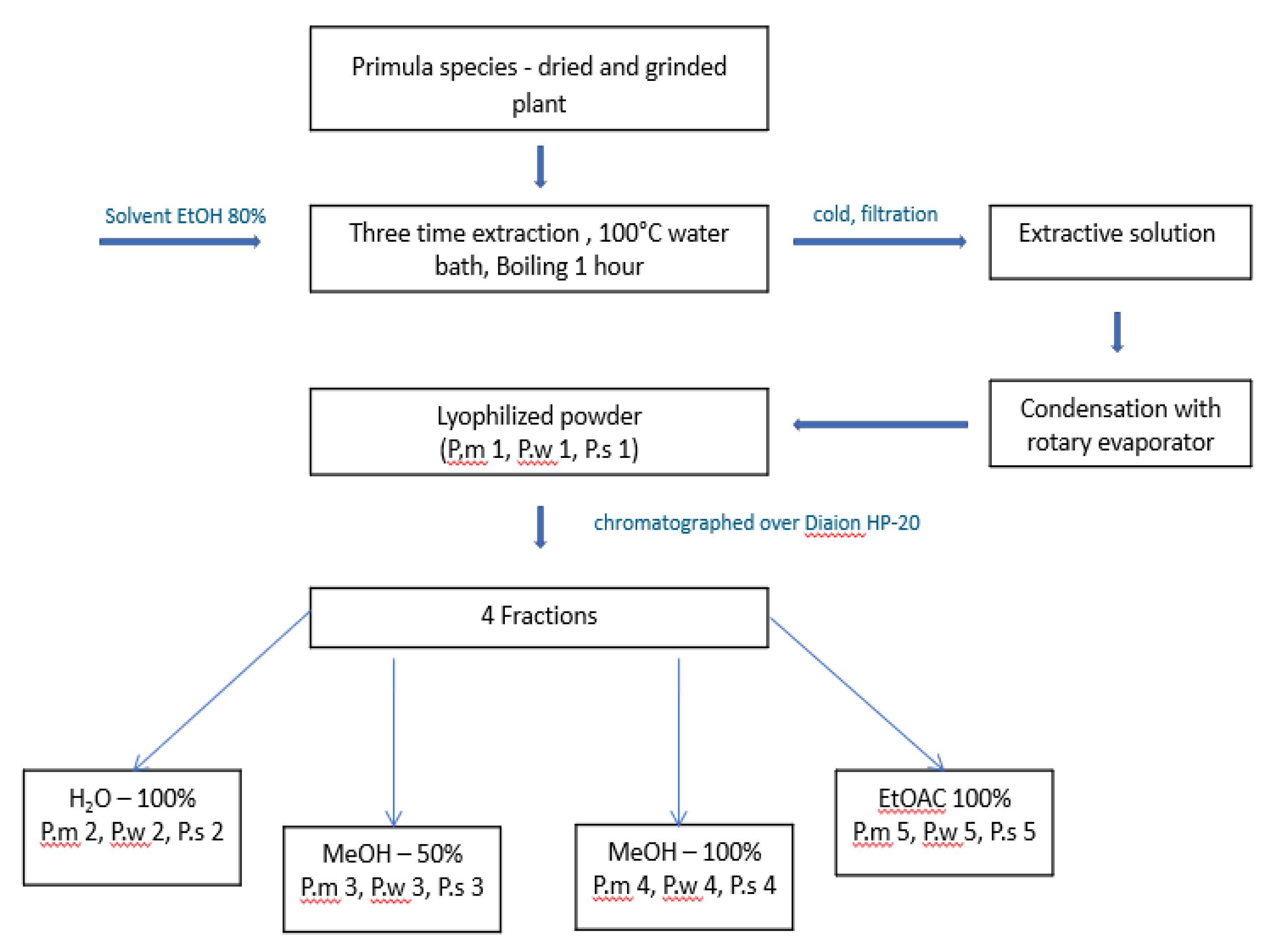
2.1. Screening of secondary metabolites
2.2. Effect of the fractions on the scavenging activity towards radical cation ABTS*+ and DPPH* formation
2.3. Effect of the fractions on ROS production by stimulated neutrophils.
2.4. Effect of the fractions on MPO activity
3. Discussion
4. Materials and Methods
4.1. Chemicals and reagents
4.2. Sample collection
4.3. Extraction procedure and preliminary phytochemicals screening
4.4. ABTS Free Radical Scavenging Activity Assay
4.5. DPPH Free Radical Scavenging Activity Assay
4.6. The SIEFED (“Specific Immunological Extraction Followed by Enzymatic Detection”) and classical enzymatic methods for measurement of equine active myeloperoxidase in biological samples.
4.7. Measurement of ROS production
4.8. Statistical analysis
5. Conclusion
Supplementary Materials
References
- WFO – 7000000497, Primulaceae Batsch ex Borkh. Bot. Wörterb. 2: 240. 1797.
- Microstructural Features of Primula worownoii Losinsk., Primula macrocalyx Bunge. and Primula saguramica Gavr. from Georgian Flora. Nino Sukhishvili1, Ketevan Mchedlidze2, Karen Mulkijanyan3 and Lasha Mskhiladze1. [CrossRef]
- Phytochemistry of European Primula species, Paola S. Colombo, Guido Flamini, Graziella Rodondi, Claudia Giuliani, Laura Santagostini, Gelsomina Fico. Phytochemistry 2017, 143, 132–144.
- Unusual Flavones from Primula macrocalyx as Inhibitors of OAT1 and OAT3 and as Antifungal Agents agains Candida rugosa, Xue Li, Xue Wang, Caiyu Li, Manana Khutsishvili, George Fayvush, Daniel Atha, Youcai Zhang & Robert P. Borris, Published: 25 June 2019.
- Budzianowski, J.; Wollenweber, E. Rare flavones from the glandular leaf exudate of the Oxlip, Primula elatior L. Natural Product Communications 2007, 2, 267–270. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J Nat Prod. 2000, 63, 1035–42. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Nitration of Flavonoids and Tocopherols as Potential Modulators of Nitrosative Stress—A Study Based on Their Conformational Strutures and Energy Content, Jos Manuel Perez de la Lastra, Celia Andres Juan, Francisco J.Plou and Eduardo Perez-Lebena, Received: 9 April 2022 / Revised: 27 April 2022 / Accepted: 5 May 2022 / Published: 9 May 2022. [CrossRef]
- Walker, E.; Pacold, M.; Perisic, O.; et al. Structural determinations of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell 2000, 6, 909–919. [Google Scholar] [CrossRef]
- Shiba, Y.; Kinoshita, T.; Chuman, H.; Taketani, Y.; Takeda, E.; Kato, Y.; Naito, M.; Kawabata, K.; Ishisaka, A.; Terao, J.; Kawai, Y. Flavonoids as substrates and inhibitors of myeloperoxidase: molecular actions of aglycone and metabolites. Chem Res Toxicol. 2008, 21, 1600–9. [Google Scholar] [CrossRef]
- Dymock, W.; Warden, C.J.; Hand Hooper, D. Pharmacographical Indica., 451. Published by Thacker Spink and company, Calcutta. Reprinted by Hamdard Institute of Health and Tibbi (Medical) Research Karachi, 1972. 1890. [Google Scholar]
- Jager, A.K.; Gauguyn, B.; Adsersen, A.; Gudyksen, L. Screening of plants used in Danish folk medicine to treat epilepsy and convulsions. Journal of Etnopharmacology 2006, 105, 294–300. [Google Scholar] [CrossRef]
- Saqib, N. Phytochemical studies of some Primulaceous leguminous plants. Ph.D. Thesis, university of Karachi, 1980; pp. 4–6. [Google Scholar]
- Pharmacology in ancient Georgia and the ways of its further development from ancient times to XX century, publishing house, “Art’’ Tbilisi - 1987, Sokrat Salukvadze p.188.
- Zaza Panaskerteli-Tsitsishvili, healing book “karabadine’’, publishing house “Soviet Georgia’’ Tbilisi. 1978, p. 623.
- Kimeridze K. , Georgian Soviet Encyclopedia , vol. 10, ch. , 1986. — p. 429.
- Laboratory techniques in biochemistry and molecular biology. General editors: RH. BURDON and P.H van KNIPPENBERG; techniques in free radical research; Catherine A. Rice-Evan, Anthony T. Diplock, Martyn C.R. Symons. 1991 ELSEVIER. Pg. 30-34.
- Harborne, J.B. Phytochemical methods: A guide to modern techniques of plant analysis, 2nd ed.; Chapman and Hall: London, 1998; pp. 54–84. [Google Scholar]
- Kokate, K.C. Practical pharmacognosy, 4th ed.; Vallabh Prakashan: Delhi, 1997; p. 218. [Google Scholar]
- WAGNERANDBLADT-plant-drug-analysis-a-thin-layer-chromatography-atlas-(359-364) 2001.
- Aslam, K.; Nawchoo, I.A.; Bhat, M.A.; Ganie, A.H.; Aslam, N. Ethno-pharmacological review of genus Primula. International Journal of Advanced Research 2014, 2, 29–34. [Google Scholar]
- Arteaga, J.F.; Ruiz-Montoya, M.; Palma, A.; Alonso-Garrido, G.; Pintado, S.; Rodríguez-Mellado, J.M. Comparison of the simple cyclic voltammetry (CV) and DPPH assays for the determination of antioxidant capacity of active principles. Molecules 2012, 17, 5126–5138. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant Property of Coffee Components: Assessment of Methods that Define Mechanisms of Action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
- Noroozisharaf, A.; Samizadeh Lahiji, H.; Hatamzadeh, A.; Bakhshi, D. Phytochemical attributes of endemic endangered primrose (Primula heterochroma Stapf.) accessions grown in Iran. Physiol Mol Biol Plants. 2015, 21, 573–81. [Google Scholar] [CrossRef]
- Tarapatskyy, M.; Gumienna, A.; Sowa, P.; Kapusta, I.; Puchalski, C. Bioactive Phenolic Compounds from Primula veris L.: Influence of the Extraction Conditions and Purification. Molecules. 2021, 26, 997. [Google Scholar] [CrossRef]
- Franck, T.; Mouithys-Mickalad, A.; Robert, T.; et al. Differentiation between stoichiometric and anticatalytic antioxidant properties of benzoic acid analogues: a structure/redox potential relationship study. Chemico-biological Interactions 2013, 206, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Derochette, S.; Franck, T.; Mouithys-Mickalad, A.; Deby-Dupont, G.; Neven, P.; Serteyn, D. Serteyn, Intra- and extracellular antioxidant capacities of the new water soluble form of curcumin (NDS27) on stimulated neutrophils and HL-60 cells. Chem. Biol. Interact. 2013, 201, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Neupane, G.P.; Lee, E.S.; Jeong, B.S.; Park, B.C.; Thapa, P. NADPH oxidase inhibitors: a patent review. Expert Opin. Ther. Pat. 2011, 21, 1147–1158. [Google Scholar] [CrossRef]
- Malle, E.; Furtmüller, P.G.; Sattler, W.; Obinger, C. Myeloperoxidase: a target for new drug development. Br J. Pharmacol. 2007, 152, 838–854. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, H.; Kuppusamy, P.; Roubaud, V.; Zweier, J.L.; Trush, M.A. Validation of lucigenin (bis-N-methylacridinium) as a chemilumigenic probe for detecting superoxide anion radical production by enzymatic and cellular systems. J. Biol. Chem. 1998, 273, 2015–2023. [Google Scholar] [CrossRef]
- Porto, B.N.; Stein, R.T. Neutrophil Extracellular Traps in Pulmonary Diseases: Too Much of a Good Thing? Front Immunol. 2016, 7, 311. [Google Scholar] [CrossRef]
- Seibel, J.; Wonnemann, M.; Werz, O.; et al. A tiered approach to investigate the mechanism of anti-inflammatory activity of an herbal medicinal product containing a fixed combination of thyme herb and primula root extracts. Clin Phytosci 2018, 4, 4. [Google Scholar] [CrossRef]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; Sánchez de Medina, F. Effects of flavonoids and other polyphenols on inflammation. Crit Rev Food Sci Nutr. 2011, 51, 331–62. [Google Scholar] [CrossRef] [PubMed]
- Ciz, M.; Denev, P.; Kratchanova, M.; Vasicek, O.; Ambrozova, G.; Lojek, A. Flavonoids inhibit the respiratory burst of neutrophils in mammals. Oxid Med Cell Longev. 2012, 2012, 181295. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Freitas, M.; Lima, J.L.; Fernandes, E. Flavonoids inhibit the production of cytokines/chemokines and induce apoptosis in human neutrophils. Free Radic Biol Med. 2014, 75 Suppl 1, S46. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Myeloperoxidase-derived oxidation: mechanisms of biological damage and its prevention. J. Clin. Biochem. Nutr. 2011, 48, 8–19. [Google Scholar] [CrossRef]
- Delporte, C.; Franck, T.; Noyon, C.; Dufour, D.; Rousseau, A.; Madhoun, P.; Desmet, J.M.; Serteyn, D.; Raes, M.; Nortier, J.; Vanhaeverbeek, M. Simultaneous measurement of protein-bound 3-chlorotyrosine and homocitrulline by LCMS/MS after hydrolysis assisted by microwave: application to the study of myeloperoxidase activity during hemodialysis. Talanta 2012, 99, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Franck, T.; Kohnen, S.; Boudjeltia, K.Z.; Van Antwerpen, P.; Bosseloir, A.; Niesten, A.; Gach, O.; Nys, M.; Deby-Dupont, G.; Serteyn, D. A new easy method for specific measurement of active myeloperoxidase in human biological fluids and tissue extracts. Talanta 2009, 80, 723–729. [Google Scholar] [CrossRef]
- Franck, T.; Kohnen, S.; Deby-Dupont, G.; Grulke, S.; Deby, C.; Serteyn, D. A specific method for measurement of equine active myeloperoxidase in biological samples and in in vitro tests. J. Vet. Diagn. Invest. 2006, 18, 326–334. [Google Scholar] [CrossRef]
- Nyssen, P.; Franck, T.; Serteyn, D.; Mouithys-Mickalad, A.; Hoebeke, M. Propofol metabolites and derivatives inhibit the oxidant activities of neutrophils and myeloperoxidase. Free Radic Biol Med. 2022, 191, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Shiba, Y.; Kinoshita, T.; Chuman, H.; Taketani, Y.; Takeda, E.; Kato, Y.; Naito, M.; Kawabata, K.; Ishisaka, A.; Terao, J.; Kawai, Y. Flavonoids as substrates and inhibitors of myeloperoxidase: molecular actions of aglycone and metabolites. Chem Res Toxicol. 2008, 21, 1600–9. [Google Scholar] [CrossRef]
- Phenolic Compounds: Structure, Classification, and Antioxidant Power; Milena Morandi Vuolo1, Verena Silva Lima1,2, Mário Roberto Maróstica Junior. [CrossRef]
- Blois, M. Antioxidant determinations by the use of a stable free radical. Nature. 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH• free radical method. Lebensmittel-Wissenschaft und -Technologie- Food Science and Technology. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Etsè, K.S.; Etsè, K.D.; Nyssen, P.; Mouithys-Mickalad, A. Assessment of anti-inflammatory-like, antioxidant activities and molecular docking of three alkynyl-substituted 3-ylidene-dihydrobenzo[d]isothiazole 1,1-dioxide derivatives. Chem Biol Interact. 2021, 344, 109513. [Google Scholar] [CrossRef] [PubMed]
- Benbarek, H.; Deby-Dupont, G.; Deby, C.; Caudron, I.; Mathy-Hartert, M.; Lamy, M.; Serteyn, D. Experimental model for the study by chemiluminescence of the activation of isolated equine leucocytes. Res. Vet. Sci. 1996, 61, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Franck, T.; Kohnen, S.; De la Rebière, G.; Deby-Dupont, G.; Deby, C.; Niesten, A.; Serteyn, D. Activation of equine neutrophils by phorbol myristate acetate or N-formyl-methionyl-leucyl-phenylalanine induces a different response in reactive oxygen species production and release of active myeloperoxidase. Vet. Immunol. Immunopathol. 2009, 130, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clinical Science. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Goel, G.; Makkar, H.P.S.; Francis, G.; Becker, K. Phorbol Esters: Structure, Biological Activity, and Toxicity in Animals. Int. J. Toxicol. 2007, 26, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Pagani, A.; Gaeta, S.; Savchenko, A.I.; Williams, C.M.; Appendino, G. An Improved Preparation of Phorbol from Croton Oil. Beilstein J. Org. Chem. 2017, 13, 1361–1367. [Google Scholar] [CrossRef]
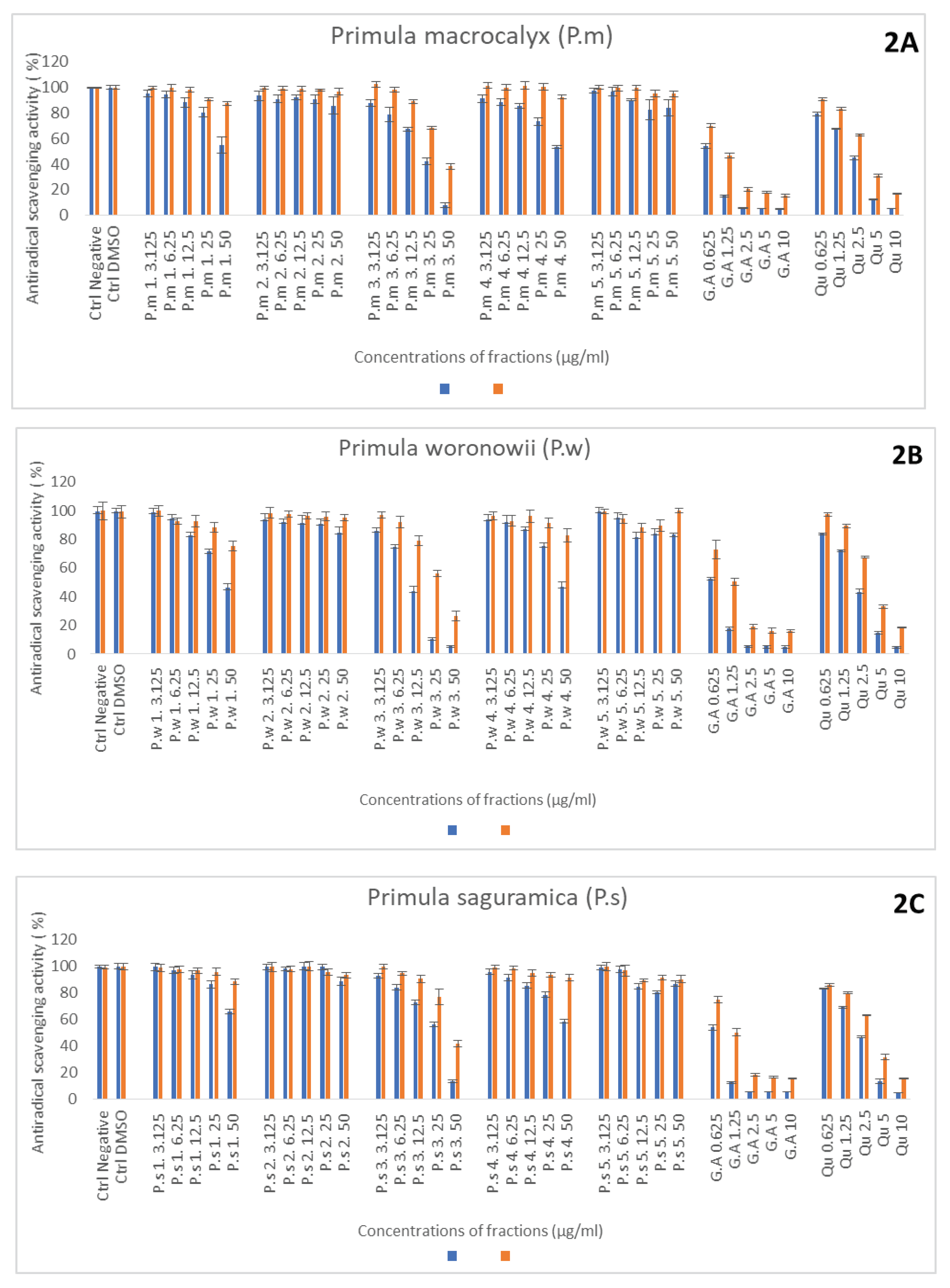
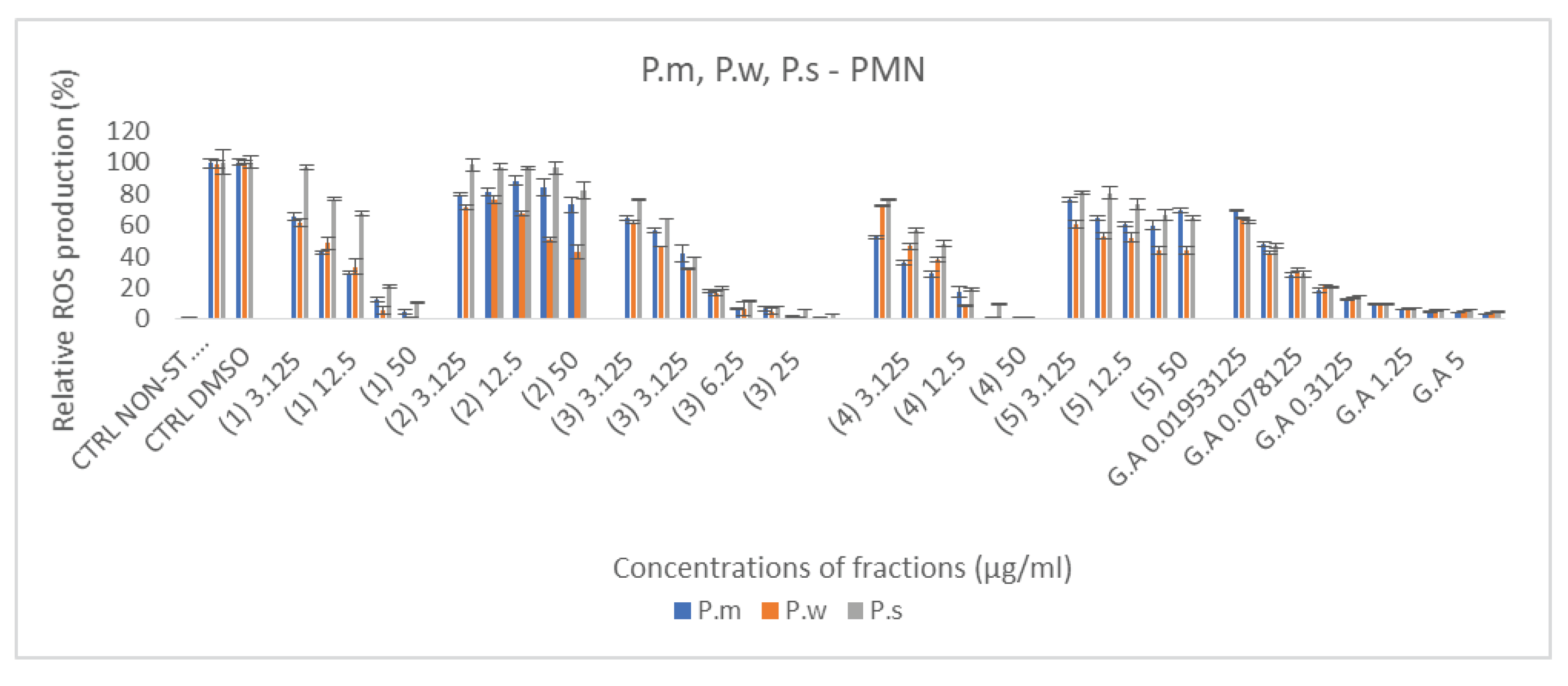
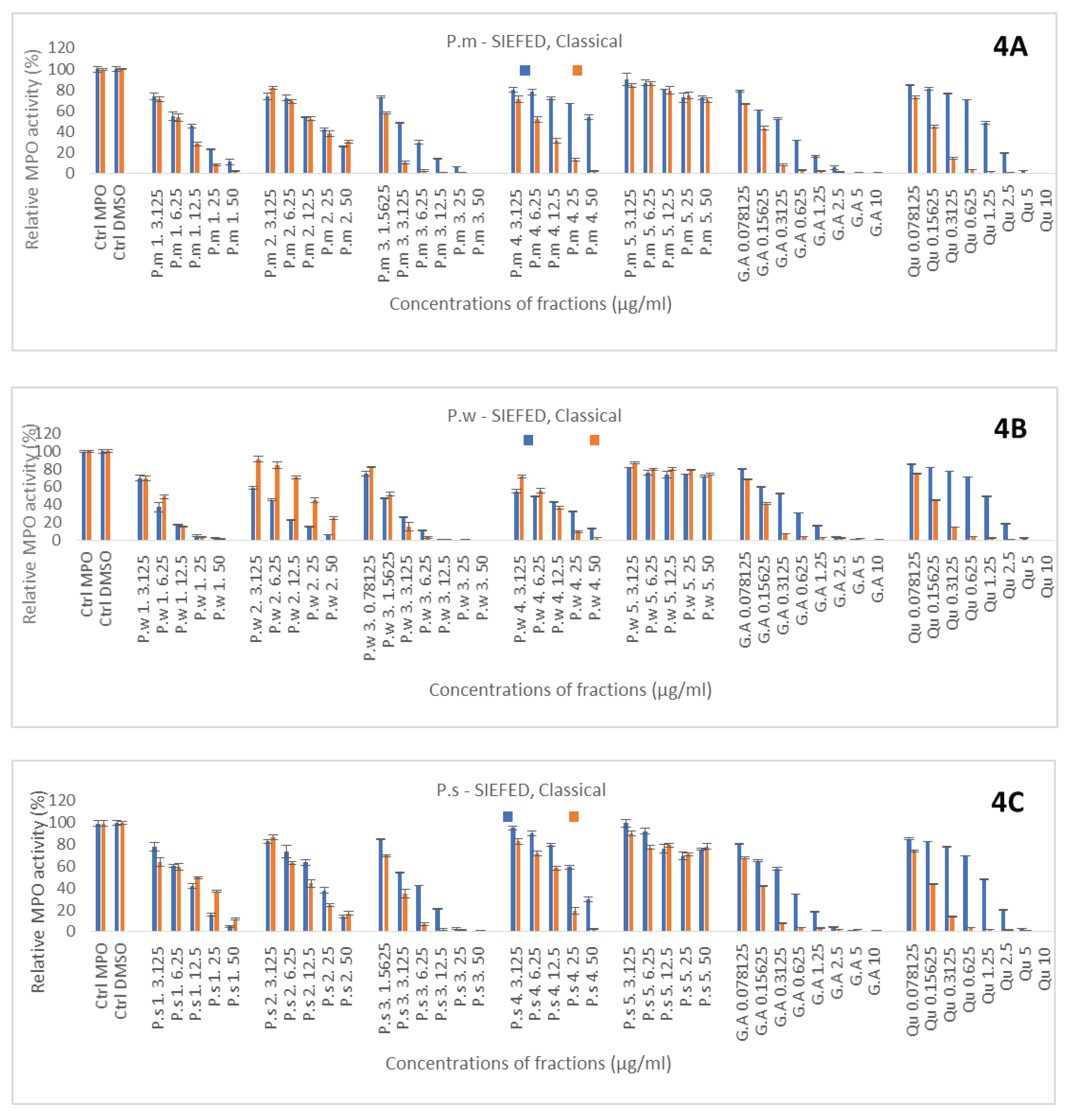
| Name | ABTS | DPPH | SIEFED | Classical | PMN |
| P.m 1 | > 50 | > 50 | 11.89 ± 0.6 | 9.8 ± 0.1 | 5.1 ± 0.3 |
| P.w 1 | 45.4 ± 2.9 | > 50 | 4.8 ± 0.4 | 5.9 ± 0.7 | 6.44 ± 0.2 |
| P.s 1 | > 50 | > 50 | 11.58 ± 0.7 | 14.1 ± 0.1 | 16.8 ±0.02 |
| P.m 2 | > 50 | > 50 | 17.28 ± 0.8 | 11.9 ± 0.1 | > 50 |
| P.w 2 | > 50 | > 50 | 5.2 ± 0.3 | 17.8 ± 0.1 | 25.4 ± 0.01 |
| P.s 2 | > 50 | > 50 | 22.32 ± 1.02 | 9.25 ± 0.04 | > 50 |
| P.m 3 | 12.88 | 36.47 ± 0.13 | 4.44 ± 0.4 | 1.96 ± 0.2 | 1.17 ± 0.07 |
| P.w 3 | 8.51 ± 0.18 | 34.57 ± 0.47 | 1.49 ± 0.7 | 1.89 ± 0.01 | 0.68 ± 0.04 |
| P.s 3 | 27.9 | 48.88 ± 0.5 | 4.89 ± 0.9 | 2.71 ± 0.9 | 1.1 ± 0.03 |
| P.m 4 | > 50 | > 50 | > 50 | 10.51 ± 0.1 | 1.4 ± 0.03 |
| P.w 4 | 48.1 ± 2.1 | > 50 | 6.02 ± 0.2 | 11.53 ± 0.3 | 2.8 ± 0.2 |
| P.s 4 | > 50 | > 50 | 33.9 ± 0.9 | 15.68 ± 0.1 | 5.3 ± 0.1 |
| P.m 5 | > 50 | > 50 | > 50 | > 50 | > 50 |
| P.w 5 | > 50 | > 50 | > 50 | > 50 | > 50 |
| P.s 5 | > 50 | > 50 | > 50 | > 50 | > 50 |
| Galic acid | 0.66 ± 0.15 | 1.09 ± 0.02 | 0.53 ± 0.04 | 0.18 ± 0.02 | 0.03 ± 0.01 |
| Quercetin | 2.07 ± 0.01 | 2.59 ± 0.07 | 1.37 ± 0.02 | 0.18 ± 0.02 | 0.14 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





